Abstract
Summary: The human indusium griseum (IG), the paired dorsal continuation of the hippocampus, was investigated with high-field (3.0T) MR imaging. The IG was clearly visible in 16 out of 20 healthy volunteers. The most common pattern was a single lateralized strip. The classical neuroanatomic pattern of paired symmetric strips along the midline was found in one case. The study clearly demonstrates that diminutive, hitherto overlooked structures such as the IG now can be readily investigated in vivo by noninvasive high-field MR imaging.
The indusium griseum (IG), a dorsal extension of the hippocampus, forms a symmetric pair of narrow strips of gray matter running caudal-rostrally along the dorsal aspect of the corpus callosum in the subcingulate region (1–5). In rodents, the IG retains the neuronal subtypes and lamination patterns of the hippocampus, and is considered to play an important role as an outpost of the hippocampus. In humans, however, neurons in the IG are loosely organized, and their connectivity is not clearly defined. This apparent lack of organization is often cited as evidence for the notion that the IG in humans is vestigial and nonfunctional. This customary benign neglect of the IG in humans is further augmented by the lack of noninvasive methods with which to conduct clinical investigation in live patients.
Recent advancements in MR technologies have introduced various new applications capable of providing substantially higher anatomic resolution. Accordingly, we investigated the IG in healthy volunteers with high-resolution T2-reversed (T2R) imaging on a high-field (3.0 T) MR system.
Methods
A Signa 3 T (GE Medical System, Waukesha, Wisconsin, USA) research imaging system with a superconductive magnet (Magnex, Abingdon, Oxon, UK). Informed consent was obtained from all subjects. Twenty healthy volunteers, aged 18–22 years, were imaged according to the human research guidelines of the Internal Review Board of the University of Niigata.
Data were obtained from a fast spin-echo (FSE) sequence with the parameters 4000/17 (TR/TE); 8 acquisitions, a matrix of 512 × 512 pixels, a field of view of 12 cm, and a 12-echo train. After conventional two-dimensional (2D) Fourier transformation was performed, the gray scale of images was inverted and given an expanded window range. Coronal views of individual subjects were analyzed at the level of the crus fornix, which optimally revealed the IG.
Results
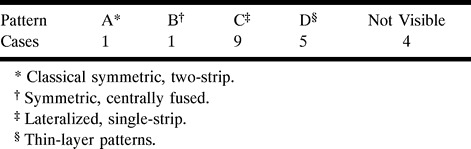
The IG was visible in 16 of 20 subjects. Representative high-resolution coronal images of the subcingulate area at the level of the crus fornix exhibited four representative patterns of the IG: classic symmetric two-strip; symmetric centrally fused; lateralized single-strip; and thin-layer (Fig 1). Table 1 summarizes these results.
fig 1.
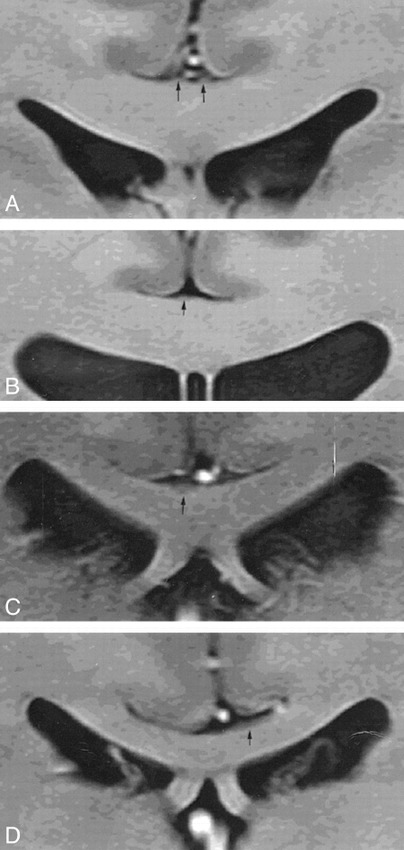
Representative MR images of the IG show classical, symmetric two-strip (A), symmetric, but centrally fused (B), lateralized, single-strip (C), and thin-layer (D) patterns
TABLE: Summary of MR-imaged IG patterns
Discussion
The IG is a component of the limbic system that is identified neuroanatomically as a symmetric pair of thin ribbons of gray matter running caudal-rostrally along the dorsal aspect of the corpus callosum. Developmentally, its cytoarchitecture is related to the hippocampus and the dentate gyrus. Although considerable investigations of the IG have been performed in nonhuman mammalian systems, these have focused on its connectivity and neurochemical characteristics. The precise function of the IG in humans remains unclear (2–5). Investigations in humans have been limited to postmortem studies. Limited pathologic studies comparing the IG and hippocampus in Alzheimer's and healthy patients have shown that the IG is infrequently involved with the typical histopathologic changes associated with Alzheimer's disease (6, 7). The current study demonstrates that the IG can now be assessed clinically with noninvasive imaging techniques.
The gross structural pattern of the IG visible in the limited population of this study indicated that the human IG was often asymmetric. Although it has been widely recognized that asymmetries in the brain serve higher functions unique to humans such as language, gross asymmetry is unusual in brain architecture and deserves further investigation (1, 8).
The main advantage of a high-field MR system is its inherently higher signal-to-noise ratio (S/N) compared to conventional systems. Newer MR techniques such as functional MR, diffusion-based fiber tract analysis, or microscopy can significantly benefit from the higher S/N (9–11). Clinical neuroscientific research has made great advancements aided by the development of a variety of noninvasive imaging techniques such as CT, positron emission tomography (PET), and MR imaging. Of these, MR provides the highest anatomic resolution. The current study exemplifies the extent to which fine anatomic resolution is possible in the clinical setting with the use of high-field imaging systems.
Acknowledgments
The author thanks Dr. Ingrid L. Kwee at the University of California, Davis, for her critical review of the manuscript. The study was supported by grants from the Ministry of Education (Japan), U. S. Public Health Service, and Veterans Administration Research Service.
Footnotes
Address reprint requests to Tsutomu Nakada, the Department of Integrated Neuroscience, Brain Research Institute, University of Niigata, 1 Asahimachi, Niigata 951, Japan.
References
- 1.Carpenter MB. Human Neuroanatomy. Baltimore, MD: Williams & Wilkins;1976:532
- 2.Wyss JM. Sripanidkulchai K. , The indusium griseum and anterior hippocampal continuation in the rat. J Comp Neurol 1983;219:251-272 [DOI] [PubMed] [Google Scholar]
- 3.Adamek GD, Shipley MT, Sanders MS. The indusium griseum in the mouse: architecture, Timm's histochemistry and some afferent connections. Brain Res Bull 1984;12:657-668 [DOI] [PubMed] [Google Scholar]
- 4.Hyland BI, Sirett NE, Hubbard JI. Electrophysiological evidence for a projection from medial prefrontal and anterior limbic cortex toward the medial preoptic area in the cat. Exp Brain Res 1986;63:205-215 [DOI] [PubMed] [Google Scholar]
- 5.Cassell MD, Brown MW. The distribution of Timm's stain in the nonsulphide-perfused human hippocampal formation. J Comp Neurol 1984;222:461-471 [DOI] [PubMed] [Google Scholar]
- 6.Lippa DF, Smith TW. The indusium griseum in Alzheimer's disease: an immunocytochemical study. J Neurol Sci 1992;111:39-45 [DOI] [PubMed] [Google Scholar]
- 7.Lippa CF, Smith TW, Degirolami U, Drachman DA. The indusium griseum: is it involved in Alzheimer's disease? Neurobiol Aging 1990;11:5,551-554 [DOI] [PubMed] [Google Scholar]
- 8.Geschwind N, Levitsky W. Human-brain: left right asymmetries in temporal speech region. Science 1968;161:186-187 [DOI] [PubMed] [Google Scholar]
- 9.Nakada T. High-field (3.0T) MR system: advantages in functional analysis. Nippon Rinsho 1997;55:7,1628-1632 [PubMed] [Google Scholar]
- 10.Nakada T, Matsuzawa H. Three dimensional anisotropy magnetic resonance imaging of the rat nervous system: MR axonography. Neurosci Res 1995;22:389-398 [DOI] [PubMed] [Google Scholar]
- 11.Narasimhan PT, Jacobs RE. Neuroanatomical micromagnetic resonance imaging. In: Toga AW, Mazziotta JC, eds. Brain Mapping: The Methods San Diego, CA: Academic Press; 1996: 147-167


