Abstract
Summary: The MR imaging findings of fungal spinal osteomyelitis in three recipients of organ transplants showed hypointensity of the vertebral bodies on T1-weighted sequences in all cases. Signal changes and enhancement extended into the posterior elements in two cases. Multiple-level disease was present in two cases (with a total of five intervertebral disks involved in three cases). All cases lacked hyperintensity within the disks on T2-weighted images. In addition, the intranuclear cleft was preserved in four of five affected disks at initial MR imaging. MR features in Candida and Aspergillus spondylitis that are distinct from pyogenic osteomyelitis include absence of disk hyperintensity and preservation of the intranuclear cleft on T2-weighted images. Prompt recognition of these findings may avoid delay in establishing a diagnosis and instituting treatment of opportunistic osteomyelitis in the immunocompromised patient.
The MR imaging appearance of spinal osteomyelitis and diskitis has been well described. Typically, it consists of hypointensity of the vertebral bodies and intervertebral disk on T1-weighted images, hyperintensity of the intervertebral disk on T2-weighted images with an abnormal configuration (ie, absent intranuclear cleft), and hyperintensity of the vertebral endplates at the abnormal disk level on T2-weighted images (1). Relative preservation of the intervertebral disk morphology and signal has been reported in some types of nonpyogenic spondylitis, particularly tuberculosis (TB) and certain fungal infections (ie, coccidioidomycoses) (2, 3). MR features of the most common causes of mycotic infection, Candida and Aspergillus, have been the subject of only a few case reports (4, 5). The purpose of this study is to describe the MR imaging findings of Candida and Aspergillus spondylitis in three immunocompromised patients.
Case Reports
Case 1
A 49-year-old man with a history of end-stage liver disease underwent orthotopic liver transplantation and immunosuppression therapy (tacrolimus and prednisone). One week after transplantation, the white blood cell count became elevated. Subsequent blood and urine cultures were positive for Candida albicans. After 1 month of treatment for Candida with amphotericin, he reported progressive pain in the lumbosacral region.
An MR study of the lumbar spine 5 weeks after transplantation showed minimal signal change in the endplates of the second, third, and fourth lumbar vertebrae with corresponding enhancement (Fig 1). The intervertebral disks were normal. A follow-up MR examination performed 10 days later showed increased hypointense signal in the vertebrae on T1-weighted images. Twenty-two days after the initial MR study, a biopsy was performed of the L3 vertebra and L2–L3 disk. Culture and sensitivity demonstrated Candida, which was confirmed with further microscopic analysis after L2–L3 and L3–L4 diskectomy and vertebral debridement.
fig 1.
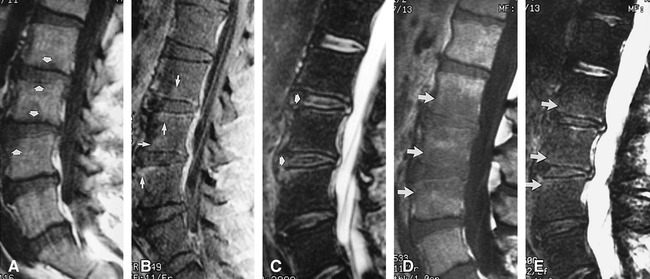
Case 1: 49-year-old man with Candida albicans infection. Biopsy was performed of the L2–L3 disk and L3 vertebra.
A, Sagittal T1-weighted (416/19/2 [TR/TE/excitations]) spin-echo image in the midline of the lumbar spine shows minimal hypointensity within the L2, L3, and L4 vertebral bodies (arrows).
B, Corresponding contrast-enhanced sagittal T1-weighted (749/11/2) image shows enhancement of the vertebral bodies and endplates (arrows).
C, Sagittal T2-weighted (3000/102/4) fast spin-echo image with fat saturation shows minimal hyperintensity within the vertebral endplates at the L2, L3, and L4 levels. Isointense signal with preserved intranuclear clefts (arrows) is present in the L2–L3 and L3–L4 disks as compared with the minimally degenerated disks at the L4–L5 and L5–S1 levels.
D, Sagittal T1-weighted (533/11/2) image 10 days after the initial study shows increased hypointense signal in the L2, L3, and L4 vertebral bodies (arrows).
E, Corresponding sagittal T2-weighted (2600/102/2) fast spin-echo image with fat saturation shows only minimally increased hyperintense signal hyperintense signal in the L2, L3, and L4 vertebral bodies (arrows).
Case 2
A 51-year-old man who had had an orthotopic liver transplantation for hepatitis C was treated with routine immunosuppression and had an unremarkable postoperative course until he presented to the emergency room with dyspnea, fever, and chills 1 month after transplantation. He was admitted and progressively decompensated after respiratory failure developed. A protracted stay in the intensive care unit ensued. A CT study of the abdomen revealed a retrocaval abscess, of which a biopsy specimen was positive for Candida. The patient was subsequently treated with amphotericin.
His medical condition gradually improved, but 11 weeks after transplantation he reported lower back pain. An MR examination of the lumbar spine revealed hypointense signal in the T12 and L1 vertebrae on T1-weighted images, isointense signal relative to marrow on T2-weighted images, and faint enhancement after contrast administration (Fig 2). The T12–L1 intervertebral disk appeared to be spared with an intact intranuclear cleft. Two days, later a CT-guided biopsy of the T12 vertebral body was performed. Microscopic analysis confirmed Candida osteomyelitis.
fig 2.
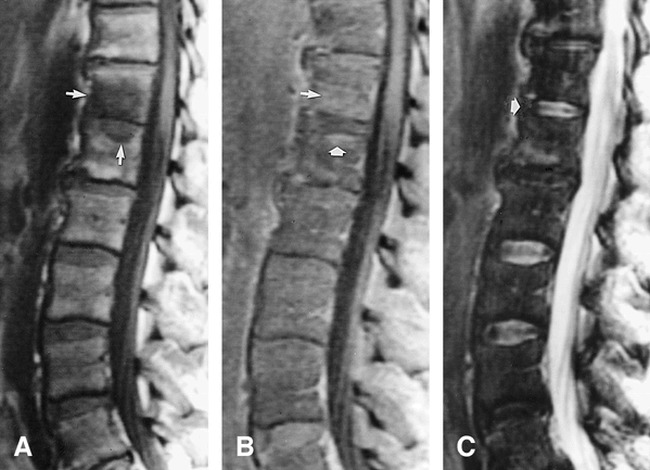
Case 2: 51-year-old man with Candida albicans osteomyelitis. Biopsy was performed at the T12 vertebral body.
A, Sagittal T1-weighted (500/10/2) image of the lower thoracic and lumbar spine shows hypointense signal within the T12 and L1 vertebral bodies (arrows).
B, Contrast enhanced T1-weighted (633/10/2) image shows enhancement of the vertebral marrow producing isointense to hyperintense signal (arrows).
C, Corresponding T2-weighted (4000/102/2) fast spin-echo image shows the normal signal and intranuclear cleft of the T12–L1 disk (arrow).
The patient was treated with a 6-week course of intravenous amphotericin followed by oral fluconazole. During this time his medical condition fluctuated as a result of mild cellular rejection and neutropenia. The patient eventually improved and was discharged home 5 months after admission.
Case 3
A 54-year-old woman who had undergone double lung transplantation owing to bronchiectasis was treated with routine immunosuppression therapy. Infection with Aspergillus developed in the anastomosis, and she was treated with amphotericin. The remaining postoperative course was significant only for mild cellular rejection, bronchitis, and lower back pain. The pain had progressed since transplantation, and by 8 months after surgery had become debilitating and associated with fecal and urinary incontinence. In addition, she reported a 30-pound weight loss over the preceding 4 months.
MR imaging of the lumbar spine 8 months after transplantation showed multilevel disease with hypointense signal on T1-weighted images in the L2–L3 vertebral bodies and superior endplate of L4, with corresponding hyperintense signal on T2-weighted images and enhancement after contrast administration (Fig 3). Paraspinal and epidural inflammation was present and extended from the L2–L4 level. A CT-guided biopsy of the paraspinal inflammation at the L2–L3 level followed by a core biopsy of the posterior inferior aspect of the L2 vertebral body was performed the next day. Microscopic analysis revealed Aspergillus. The patient was subsequently treated with an extended course of amphotericin.
fig 3.
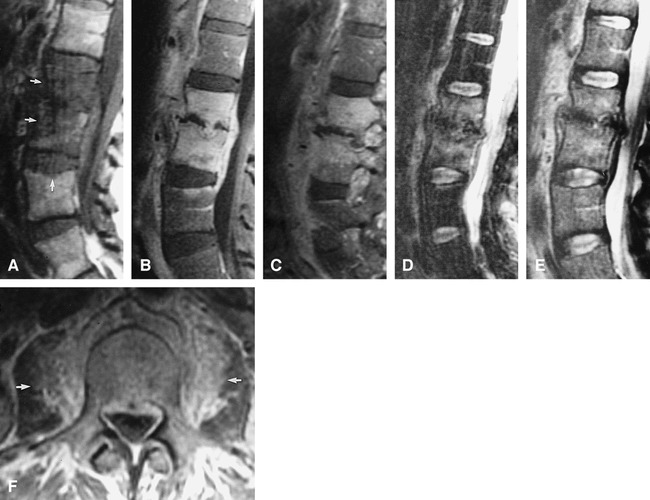
Case 3: 54-year-old woman with Aspergillus osteomyelitis. Biopsy was performed of the L2–L3 paraspinal inflammation and the L3 vertebra.
A, Sagittal T1-weighted (666/15/2) image of the lumbar spine shows multilevel disease with hypointensity in the L2, L3, vertebrae and superior endplate of L4 (arrows). Paraspinal and epidural disease is also present.
B and C, Sagittal and parasagittal contrast-enhanced T1-weighted (616/10/2) images show enhancing paraspinal and epidural inflammation. Disease also extends into the pedicles.
D and E, Sagittal T2-weighted (3500/102/2) fast spin-echo images with (D) and without (E) fat-saturation technique show hyperintense signal within the L2, L3, and L4 vertebrae and superior endplate of L4. Note the increased conspicuity of signal change with fat-saturation. In addition, the L3–L4 disk is normal in signal with a preserved intranuclear cleft. The L2–L3 disk is collapsed.
F, Axial T1-weighted (500/18/1.5) spin-echo image at the L3 level depicts epidural and paraspinal inflammation (arrows).
Four months after diagnosis of Aspergillus osteomyelitis, diskectomy and vertebral debridement of the L2–L3 and L3–L4 levels were performed. Histologic examination revealed chronic osteomyelitis with numerous hyphae, consistent with Aspergillus.
Discussion
Vertebral osteomyelitis and diskitis are commonly due to pyogenic infection, particularly Staphylococcus aureus, which accounts for 60% of spinal infections (6). Nonpyogenic origins of vertebral osteomyelitis and diskitis include Mycobacterium tuberculosis and fungi. The rate of occurrence of fungal infections has risen over the last decade as the population of immunocompromised patients has increased (7). Candida and Aspergillus are the most common causes of mycotic infections in these persons (8).
Few reports have described the MR imaging appearance of fungal spondylitis, particularly in immunocompromised patients (4, 9, 10). Although it may be inferred that the MR imaging characteristics of Candida and Aspergillus spondylitis resemble pyogenic infections on the basis of previous reports, the well-known appearance of pyogenic spondylitis may not accurately reflect that of fungal spinal osteomyelitis in the immunocompromised host (1, 6).
In our cases, hypointensity was seen on T1-weighted images in the vertebral bodies, as is characteristic of pyogenic infection. Interestingly, at three of five levels of osteomyelitis in all three cases there was minimal hyperintensity or isointensity of the vertebrae on T2-weighted images. Minimal hyperintensity may be caused by an absence of an acute inflammatory reaction in immunocompetent patients and in patients with pyogenic infections, since histopathologic features of infection with Candida or Aspergillus depend largely on the immune status of the host. Patients who are immunocompromised may be incapable of mounting a cellular response with a corresponding histologic appearance of fungi accompanied by a sparse mononuclear cell infiltrate (11). The clinical usefulness of minimal signal change on T2-weighted images may, however, be limited, since a recent review of MR imaging findings in spinal osteomyelitis revealed hyperintensity on T2-weighted images in the vertebral bodies in only 56% of cases (10). The low prevalence of hyperintensity may be misleading though, since fat-saturation techniques were not applied in the T2-weighted sequences (10). In studies performed without fat saturation, hyperintense marrow signal on fast spin-echo T2-weighted images may be difficult to distinguish from hyperintense marrow abnormalities. Fat-saturation techniques may help circumvent this problem and may increase conspicuity of vertebral marrow abnormalities, as suggested by Mirowitz et al (12) and demonstrated in one of our cases (Fig 3).
The pedicles were involved in two of our three cases. Involvement of the posterior elements is unusual in pyogenic spondylitis and is more commonly seen in nonpyogenic infections, such as TB (2). The posterior location of disease, whether in the vertebral body or posterior elements, may have therapeutic implications and lead to epidural abscess, meningitis, or myelitis, thus prompting early surgical intervention (13, 14).
Paraspinal inflammation was present in each case. This was minimal to moderate in extent and was commensurate with the amount of bone destruction and disk space involvement. This pattern is in contrast to TB, which is characteristically associated with a large paraspinal inflammatory component (15).
None of our cases showed the hyperintensity on T2-weighted images within the intervertebral disks that is typical of pyogenic infection. In addition, the intranuclear cleft was preserved in all disks in which the disk height was maintained. The absence of hyperintensity within the intervertebral disks on T2-weighted images and the preservation of the intranuclear cleft are findings associated with nonpyogenic or TB spondylitis (2) (Fig 2). These are, however, nonspecific findings that have been reported in cases of pyogenic disease (1, 9, 10). Other nonpyogenic infections caused by Nocardia, Actinomycosis, Echinococcus, and Coccidioidomycosis may also spare the intervertebral disk (2, 6). The pathogenesis for an absence of hyperintensity within the intervertebral disk is unclear and may be multifactorial. The imaging findings may reflect an absence of fungal invasion, an altered inflammatory reaction within the infected disk, or an intrinsic characteristic of either the disk before infection (eg, degenerated disk) or of the invading fungi (eg, paramagnetic elements). Similar to TB, the intervertebral disk may be spared from fungal invasion and inflammatory change. Proposed mechanisms include a lack of proteolytic enzymes within the mycobacterium or variations in vertebral microcirculation that may influence the site of infection and spread of disease (16, 17). Invasion of the disk by fungi with minimal corresponding hyperintensity due to a blunted immune response, as reported in fungal osteomyelitis in immunocompromised patients, may also account for the lack of hyperintensity on T2-weighted images (11). Variation in imaging findings due to host immune response may explain why fungal osteomyelitis may also present with hyperintense signal in a disk on T2-weighted images (4).
Factors intrinsic to fungi that may contribute to an absence of disk hyperintensity on T2-weighted images may include the presence of paramagnetic and ferromagnetic elements within fungi similar to the proposed mechanism for T2 hypointensity in fungal sinusitis (18). This explanation seems unlikely, since the intervertebral disks in our study were often isointense, rather than hypointense, relative to normal disks. Early imaging in the disease course of pyogenic infections is also unlikely, since fungal infections are typically insidious in onset (19). In addition, a preexisting abnormality in an infected intervertebral disk, such as a calcified/mineralized disk or severely degenerated disk with an associated “vacuum” phenomenon, may potentially mask typical signal changes of infection.
Conclusion
MR imaging features of Candida and Aspergillus spondylitis that are distinct from pyogenic osteomyelitis include an absence of disk hyperintensity and preservation of the intranuclear cleft on T2-weighted images. Recognition of MR characteristics of fungal spondylitis should prompt biopsy and may alter management of spondylitis in the immunocompromised host.
Acknowledgments
We express our appreciation to Sabrina L. Jennings for assistance with manuscript preparation.
Footnotes
Address reprint requests to Robert L. Williams, MD, University of Pittsburgh Medical Center, Department of Radiology, Rm D132 PUH, 200 Lothrop St, Pittsburgh, PA 15213.
References
- 1.Modic MT, Feiglin DH, Piraino DW, et al. Vertebral osteomyelitis: assessment using MR. Radiology 1985;157:157-166 [DOI] [PubMed] [Google Scholar]
- 2.Smith AS, Weinstein MA, Mizushima A, et al. MR imaging characteristics of tuberculous spondylitis vs vertebral osteomyelitis. AJNR Am J Neuroradiol 1989;10:619-625 [DOI] [PubMed] [Google Scholar]
- 3.Erly WK, Seeger JF, Carmody RF. Magnetic resonance imaging of coccidioidal spondylitis. Presented at the annual meeting of the American Society of Neuroradiology, Nashville, May 1994
- 4.Munk PL, Lee MJ, Poon PY, et al. Candida osteomyelitis and disc space infection of the lumbar spine. Skeletal Radiol 1997;26:42-46 [DOI] [PubMed] [Google Scholar]
- 5.Morgenlander JC, Rossitch E, Rawlings CE. Aspergillus disc space infection: case report and review of the literature. Neurosurgery 1989;25:126-129 [PubMed] [Google Scholar]
- 6.Sklar EML, Post MJD, Lebwohl NH. Imaging of infection of the lumbosacral spine. Neuroimaging Clin N Am 1993;3:577-590 [Google Scholar]
- 7.Ferra C, Doebbeling BN, Hollis RJ, Pfaller MA, Lee CK, Gingrich RD. Candida tropicalis vertebral osteomyelitis: a late sequela of fungemia. Clin Infect Dis 1994;19:697-703 [DOI] [PubMed] [Google Scholar]
- 8.Seligsohn R, Rippon JW, Lerner SA. Aspergillus terreus osteomyelitis. Arch Intern Med 1977;137:918-920 [PubMed] [Google Scholar]
- 9.Post MJD, Sze G, Quencer RM, Eismont FJ, Green BA, Gahbauer H. Gadolinium-enhanced MR in spinal infection. J Comput Assist Tomogr 1990;14:721-729 [DOI] [PubMed] [Google Scholar]
- 10.Dagirmanjian A, Schils J, McHenry M, Modic MT. MR imaging of vertebral osteomyelitis revisited. AJR Am J Roentgenol 1996;167:1539-1543 [DOI] [PubMed] [Google Scholar]
- 11.Simpson MB, Merz WG, Kurlinski JP, Solomon MH. Opportunistic mycotic osteomyelitis: bone infections due to Aspergillus and Candida species. Medicine 1977;56:475-481 [PubMed] [Google Scholar]
- 12.Mirowitz SA, Reinus WR, Hammerman AM. Evaluation of fat saturation technique for T2-weighted MR imaging of the spine. Magn Reson Imaging 1994;12:599-604 [DOI] [PubMed] [Google Scholar]
- 13.Ehara S, Khurana JS, Kattapuram SV. Pyogenic vertebral osteomyelitis of the posterior elements. Skeletal Radiol 1989;18:175-178 [DOI] [PubMed] [Google Scholar]
- 14.Naim-Ur-Rahman , Atypical forms of spinal tuberculosis. J Bone Joint Surg 1980;62-B:162-165 [DOI] [PubMed] [Google Scholar]
- 15.Whelan MA, Schonfeld S, Post JD, et al. Computed tomography of nontuberculous spinal infection. J Comput Assist Tomogr 1985;9:280-287 [DOI] [PubMed] [Google Scholar]
- 16.Chapman M, Murray RO, Stoker DJ. Tuberculosis of the bones and joints. Semin Roentgenol 1979;14:266-282 [DOI] [PubMed] [Google Scholar]
- 17.Smith AS, Blaser SI. Infectious and inflammatory processes of the spine. Radiol Clin North Am 1991;29:809-827 [PubMed] [Google Scholar]
- 18.Zinreich SJ, Kennedy DW, Malat J, et al. Fungal sinusitis: Diagnosis with CT and MR imaging. Radiology 1988;169:439-444 [DOI] [PubMed] [Google Scholar]
- 19.Broner FA, Garland DE, Zigler JE. Spinal infections in the immunocompromised host. Orthop Clin North Am 1996;27:37-46 [PubMed] [Google Scholar]


