Abstract
BACKGROUND AND PURPOSE: Radiosurgical treatment of arteriovenous malformations (AVMs) has slow and progressive vasoocclusive effects. We sought to determine if early posttherapeutic angiography provides relevant information for the management of radiosurgically treated AVMs.
METHODS: Between 1990 and 1993, the progress of 138 of 197 cerebral AVMs treated by linear accelerator (Linac) was regularly followed by angiographic study. On each posttherapeutic angiogram (“early,” 6–18-month follow-up; “intermediate,” 19–29-month-follow-up; and “late,” >30-month follow-up), the degree of reduction across the greatest diameter of the nidus and hemodynamic modifications were analyzed. Each cerebral AVM was qualitatively classified into one of the following categories after early angiographic study: 0%-reduced, 25%-reduced, 50%-reduced, 75%-reduced, and 100%-reduced or “complete obliteration.” Vasoocclusive progress for each category was then studied over time.
RESULTS: Three (10%) of the 30 0–25%-reduced, eight (38%) of 21 50%-reduced, and 27 (84%) of 32 75%-reduced cerebral AVMs showed complete obliteration after further follow-up. The three 0–25%-reduced AVMS that went on to complete obliteration underwent very early angiography (6–7 months). Fifty-five cerebral AVMs showed complete obliteration on early angiograms (40%). In this group, more follow-up, when performed, confirmed complete obliteration in all cases (n = 17).
CONCLUSION: An early angiogram is needed to predict the effectiveness of radiosurgery. Important AVM changes seen on early angiograms are highly correlated with treatment success. Moreover, no or minor changes seen on early angiograms are highly predictive of radiosurgical failure. For these patients, further treatment should be discussed promptly.
Radiosurgery was first used to treat cerebral arteriovenous malformations (AVMs) in Sweden in the 1970s (1, 2). Different techniques have been used with similar results (obliteration rate varying between 60% to 86% after 2 years): proton beams, gamma units, heavy-charged particles, and linear accelerators (2–13). Radiosurgery is believed to result in obliteration of cerebral AVMs by endothelial cell proliferation, progressive wall thickening, and eventual luminal closure (14). As opposed to the alternative therapeutic procedures available for cerebral AVMs, surgical resection or embolization, this vasoocclusive effect develops slowly after radiosurgery, and cerebral AVMs shrink progressively (15, 16). It is generally admitted that these vasoocclusive effects peak between 1 and 2 years after radiosurgery (17, 18). Because the risk of bleeding persists as long as complete obliteration is not obtained (6, 12, 19), the time course of posttreatment changes as revealed by neuroimaing is crucial for patient management. The time course of radiosurgically induced cerebral AVM shrinkage varies considerably, because complete obliteration of cerebral AVMs can occur as early as 4 months (10) or as late as 5 years (20) after treatment, and some remain patent. At most institutions, the final result used to be documented by conventional angiography 2 years after treatment. Currently, because of the finding that some cerebral AVMs will obliterate between 2 to 3 years postoperatively, there is an increasing tendency to defer definite labeling of incomplete obliteration until 3 years after treatment (21, 22). To date, there is no consensus on the optimal follow-up protocol after treatment. Until complete obliteration is obtained, the frequency and the number of neuroimaging follow-ups vary widely between institutions. Some radiosurgical teams perform a neuroimaging follow-up every 6 months (3, 6, 8, 11, 23), whereas others perform yearly neuroimaging follow-up (20). Some radiosurgical teams use yearly angiograms, regardless of MR imaging results (6, 8, 11). Still others prefer to perform serial MR studies until there is no evidence of residual transnidal blood flow, and only use angiography to assess the final result (10, 22, 24, 25). At our institution, radiosurgically treated cerebral AVMs are followed up by serial conventional angiography, regardless of the MR results. The database resulting from this systematic approach allows a retrospective evaluation of the stages of radiosurgically induced cerebral AVM shrinkage. In this report, we focused our attention on the early angiograms (6–18 months posttreatment) to determine if they provided any relevant information on posttherapeutic response, and if they were predictive of the final outcome of treatment.
Methods
Population
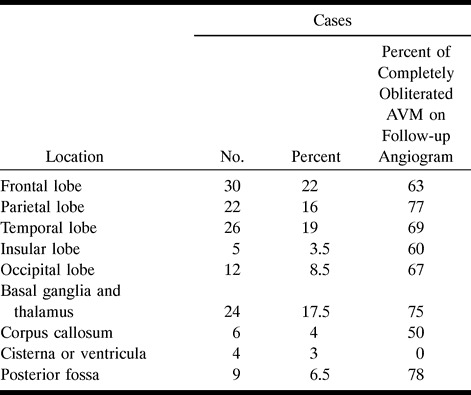
One hundred and ninety-seven patients with cerebral AVMs were treated by radiosurgery between January 1990 and December 1993. All patients were asked to undergo yearly angiographic follow-up. Early angiographic follow-up included all angiograms performed between 6 and 18 months, intermediate angiographic control included angiograms performed between 19 and 29 months, and late angiographic control included all angiograms performed 30 months after radiosurgery. The studied population was limited to patients who had early angiography with further angiographic follow-up if residual cerebral AVM was seen on the early angiogram. One hundred and thirty-eight patients, 83 men and 55 women, fulfilled these criteria. The remaining 59 patients were excluded. Twenty-six of those excluded had no angiographic follow-up (one patient died from myocardial infarction, one patient had a pulmonary neoplasm, two patients refused follow-up, and 22 patients were lost to follow-up). Most of these patients were referred from foreign institutions for radiosurgical treatment. Nineteen patients were excluded because they did not undergo an early angiographic follow-up, and 10 others who had an early angiogram showing residual nidus were not followed further. The mean age of the 138 patients studied at the time of treatment was 33.7 years (range 6 to 68 years [SD, 14.6, median, 33]). Prior surgical resection was performed in eight cases, prior embolization in 51 cases, and both treatments in five other cases. The locations of cerebral AVMs treated by radiosurgery are listed in Table 1.
TABLE 1:
Location of the 138 cerebral AVMs and the percent of completely obliterated AVMs on follow-up
Pretherapeutic Radiographic Workup
All patients underwent pretherapeutic examinations, including MR imaging and conventional angiography performed under stereotactic conditions. The angiographic technique was always performed as follows: the head was positioned by the Talairach stereotactic frame (26); films were obtained in the anteroposterior, lateral, and offset views, allowing stereotactic viewing; the distance between the radiographic source and film was 4.5 m, resulting in a constant and reproducible magnification factor of 1.05; and the exposure rate was two films per second. Target delineation was always based on angiographic and axial CT-based data.
Radiosurgery
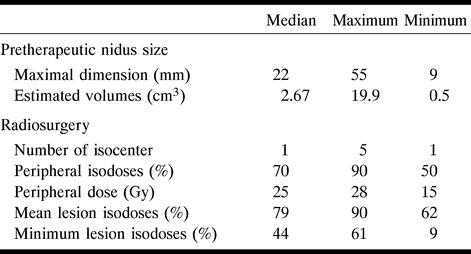
The radiosurgical technique was identical in all cases. Patients were irradiated in the O. Betti armchair (27, 28) and the head was positioned by the Talairach stereotactic frame (26). Fifteen megavolt X-ray minibeams from a Saturn 43 (General Electric; Buc, France) linear accelerator (Linac) were used along with eight additional collimators (6–20 mm). A dose of 25 Gy was delivered at the periphery of the nidus, delineated on the pretherapeutic angiogram. This dose corresponds to the 60–70% peripheral isodose range. In 73 cases, the nidus could be covered by one isocenter, in 42 by two isocenters, in 12 by three isocenters, and in 11 by four or more isocenters. Nidus shapes were spheroid in 39 cases, elliptical in 70, and irregular or complex-shaped in 29. The mean maximal length of cerebral AVMs on pretherapeutic angiograms was 23.1 mm (Standard Deviation [SD], 9.9; minimum, 9; maximum, 55; and median, 22 mm). The pretherapeutic AVM's volume was calculated according to a previously described method (29). The mean volume was 2.95 cc; the SD, 1.6; and the median, 2.67 cc (min = 0.5, max = 19.92). These data and treatment parameters are summarized in Table 2.
TABLE 2:
Treatment parameters, radiation doses, pretherapeutic volume, and maximal diameter of the nidus
Angiographic Analysis
In our follow-up protocol, digital angiographic workup was scheduled on a regular basis. All angiographic follow-ups were analyzed independently by two neuroradiologists, regardless of clinical data. Conflicting readings were resolved by consensus. Readers compared each posttreatment angiogram to the pretherapeutic angiogram, without knowledge of preceding or succeeding follow-up angiograms of each AVM studied. Readers considered the maximum-length reduction of the nidus in the anteroposterior and lateral planes, and the reduction in diameters of both feeding arteries and draining veins.
Evaluation of hemodynamic changes was based on the order of division of nonfeeding cerebral arteries visible on the first angiogram showing early draining veins. If only first-division nonfeeding arteries were seen, the AVM was considered to have a high flow rate. If second division nonfeeding arteries were already visible, the AVM was considered as having a slower flow rate.
Patients were classified subjectively in one of the five following categories: 1) 0% when no changes were seen; 2) 25% when only minimal reduction of nidus size, slight slowing of the flow, or both were observed; 3) 50% when about half of the nidus had disappeared; 4) 75% when the nidus was considerably reduced in size but still present, or whenever an isolated early draining vein persisted; and 5) 100% when “complete obliteration” was observed, as defined by Lindquist and Steiner (i.e., normal circulation time, absence of former nidus vessels, disappearance or normalization of draining veins) (30).
Results
Because of the inclusion criteria, all 138 patients had an early angiogram (6–18 months, mean, 12.03; median, 12; SD, 2.5; minimum, 6; maximum, 18). Of those who underwent an early angiogram, 83 patients (60.1%) did not achieve complete obliteration: 17 patients were categorized in the 0%-, 13 in the 25%-, 21 in the 50%-, and 32 in the 75%-reduced group. In this group of 83 patients with residual cerebral AVM revealed by early angiography, the mean follow-up duration was 31.5 months (SD, 9.1; maximum, 52 months). Sixty-four patients from this group underwent intermediate (19–29 months: mean, 24.2; median, 24; SD, 2; minimum, 19; and maximum, 29); six others late (30 months: mean,42.8; median, 45; SD, 6.9; minimum, 30; maximum, 52), and the last 13 both intermediate and late angiograms.
After further follow-up, obliteration occurred in 2 (11.8%) of 17 cases for cerebral AVMs classified as 0%-reduced by early angiographic study. Of cerebral AVMs classified as 25%-reduced by early angiography, obliteration occurred in 1 (7.7%) of 13 cases after further follow-up. Thus, three cerebral AVMs classified in the 0–25% group after early angiographic study (6–7 months posttreatment) showed favorable outcomes; angiography was performed at this time because these patients complained of headaches. Obliteration occurred in 8 (38%) of 21 cerebral AVMs classified as 50%-reduced by early angiography after further follow-up. Of cerebral AVMs classified as 75%-reduced by early angiography, obliteration occurred in 27 (84%) of 32 cases. Cerebral AVMs showed complete obliteration on the early angiograms in 55 (40%) of all 138 patients. In this 100% group, 17 patients underwent a late angiogram, whereas 38 received no further follow-up. Complete obliteration was confirmed in all cases (n = 17) of the 17 patients with angiographic follow-up. These results are summarized in Table 3.
TABLE 3:
Number of follow-up angiograms and results of the obliteration rate in each category of nidus reduction on the early follow-up angiogram
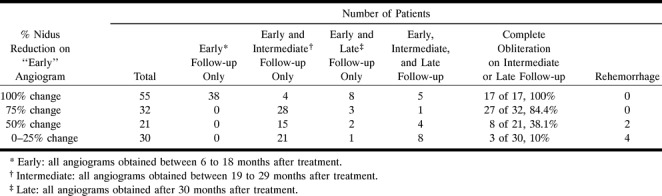
In addition to the 55 patients with complete obliteration revealed by early angiography, complete obliteration was revealed on 32 intermediate and six late angiograms (maximum, 49 months). In our selected population, the overall obliteration occurred in 93 (67.4%) of 138 cases. Among the 138 patients included in the study, six (4.3%) had a rebleeding event that led to the death of three. The mean time between treatment and rebleeding was 26.6 months. As indicated in Table 3, none of these patients belonged to the 100% group; four belonged to the 0–25% group, including the three patients who died, and the remaining two belonged to the 50% group. Of the patients with incompletely obliterated AVMs on the early angiogram, rebleeding occurred in 6 (7.2%) of 83 (see Table 3).
Discussion
Radiosurgery seems to produce cerebral AVM obliteration by inducing a disease process in the nidus, leading to gradual thickening of the vessels until occlusion occurs (31). The postradiosurgical response of a cerebral AVM appears to be highly variable, because complete obliteration can be seen as early as a few months or late as 5 years after treatment, or may never occur by the end of the follow-up period. Many authors have searched for morphologic factors for predicting posttherapeutic outcomes. In a recent study, we demonstrated that small-sized or deeply seated cerebral AVMs seem to have a greater chance of reaching complete obliteration at 2 years than large ones or those located at the brain periphery (32). Because cerebral AVMs with different characteristics seem to have variable shrinkage time courses after treatment, the same follow-up scheme should not be used for all radiosurgically treated cerebral AVMs. The frequency of neuroimaging follow-up, the length of the follow-up period, and the neuroimaging methods used (digital angiography, MR imaging, and MR angiography) vary between institutions. Radiosurgical techniques have evolved over the past decades, and much effort has been made to improve the presurgical delineation of the target, define the optimal radiation dose, and evaluate the efficiency of the procedure (6, 8, 10, 11, 25, 29, 33–41). All follow-up protocols used to monitor postradiosurgical cerebral AVM changes are issued from the following principles based on years of experience: 1) cerebral AVM changes are delayed after the time of treatment; 2) cerebral AVMs shrink slowly and progressively; 3) vasoocclusive effects peak between 1 and 2 years after radiosurgery; 4) the final results should be documented 2 or 3 years after radiosurgery; and 5) digital angiography is the only reliable method to assess the final outcome. These observations led to different protocols using serial neuroimaging follow-up performed every 6 months or every year, for 2 or 3 years. Nevertheless, these follow-up protocols have never been evaluated retrospectively. Such evaluation could allow a more rational approach to monitoring postradiosurgical changes. If a consensus about the follow-up protocol could be reached, radiosurgical series would be more homogeneous and thus easier to compare. In this report, we analyzed retrospectively the predictive value of early angiograms, comparing them to future outcomes, in a large group of 138 consecutive patients with cerebral AVMs treated by radiosurgery from 1990 to 1993. Our goal was to assess the usefulness of the early angiogram in an effort to simplify our follow-up protocol.
Assessment of Cerebral AVM Changes on Posttherapeutic Angiograms
Most authors agree that angiographically confirmed complete obliteration should be the goal of radiosurgical treatment. Because even the smallest remnant can bleed (19), the strict angiographic criteria of Lindquist and Steiner (30) are usually used to assess complete obliteration (100%). When cerebral AVM does not fulfill these criteria, it is classified as “nonobliterated” by most authors. This heterogeneous category includes various cerebral AVM responses to radiosurgery ranging from the unchanged to those with isolated early draining veins, with all possible intermediates. Partial response of cerebral AVMs to radiosurgery is difficult to assess angiographically in a reliable and reproducible manner. Angiography only provides two-dimensional delineation of the nidus. Yamamoto et al (41) calculated an approximated volume of the cerebral AVM nidus on pre- and posttherapeutic angiograms by Pasqualin's method (42). This approach produces reasonably accurate results, and offers the advantage of being reproducible and quantitative. Nonetheless, reduction of the nidus size is not the only angiographic feature of irradiation effects. Other features are prominent in follow-up angiograms of incompletely obliterated cerebral AVMs. Flow velocity is reduced through the cerebral AVM, attributed to the disappearance of arteriovenous shunts, and diameter of both feeding arteries and draining veins is reduced, an indirect sign of a decreased transnidal blood flow (5, 41). Even if flow velocity feeding artery, and draining vein diameters probably are related to nidus size, their irradiation effects are also worth considering. Taking these parameters into account, we chose to classify the radiosurgical response into five groups (100%, 75%, 50%, 25%, 0%). The first and last categories were strictly defined, whereas the remaining three were more subjective. Kemeny et al (35) proposed a similar classification of five groups based on hemodynamic and size considerations: “complete,” “almost complete,” and “partial” obliteration, “slight changes,” and “no change.” Friedman et al (8) used a four-group classification, but the authors did not mention whether this classification was based solely on nidus size reduction: “complete,” “greater than 90%,” “50 and 90%,” and “less than 50%” occlusion.
Several radiosurgical series have evaluated this process by obtaining 1-, 2- or 3-year follow-up angiograms. Obliteration rates ranged from 29% to 76% at 1 year, from 27% to 86.5% at 2 years (5, 6, 8, 10, 11, 13, 20), and from 65% to 92% at 3 years (13, 20, 11). Some care should be taken when analyzing our results, because all patients did not complete the entire follow-up protocol. Final outcomes were assessed on late angiograms, when available, or otherwise on the intermediate control. The rate of complete obliteration we observed (67.4%), although consistent with other similar studies, is therefore probably underestimated.
Predictive Value of “Early” Angiographic Data
We focused our attention on the early angiogram for the following reasons. If the follow-up protocol can be simplified, it is intuitively the early rather than the later angiograms that should be sacrificed. Some radiosurgical teams routinely control only posttherapeutic effects 2 and 3 years after treatment, whereas others choose to replace the early angiogram by a less invasive MR examination (10, 25). Given the results of the present study, the early neuroimaging follow-up contains data that can potentially modify patient management. If complete obliteration is observed on an early angiogram, which is not a rare event (40% of these patients), angiographic controls at 2 or 3 years are not necessary. If important AVM changes (75% group) are seen on an early angiogram, complete angiographic obliteration is very likely because it occurred in 84% of these patients. If only minor (25% group) or no change (0% group) is seen on an early angiogram (6–18 months after surgery), complete obliteration is only likely to occur in 10% of the cases. For those patients with only minor or no changes on the early angiogram (21.7% of the population), radiosurgical failure can be predicted confidently with early angiographic data. To be predictive of final outcomes, the early angiographic control should not be performed too early after treatment. Three patients had an angiogram performed at 6 or 7 months showing no AVM changes, but complete obliteration was revealed after further follow-up. During the first few months after treatment of some cerebral AVMs, a latency period seems to exist before visible changes of the nidus can be identified on angiograms. For those three cerebral AVMs, some changes probably existed, but may have been too subtle to be distinguished on very early angiograms. To be accurately predictive of radiosurgical failure, the early angiogram ideally should not be performed within this potential latency period.
There have been few analyses of chronological changes of radiosurgically treated nidi. Quisling et al (43) reported seven patients with cerebral AVMs with sequential postradiosurgical MR follow-up. A progressive reduction of nidus volume was observed over time. Yamamoto et al (41) considered the volume variation of the nidus after radiosurgical treatment of 17 cerebral AVMs. Based on sequential angiographic follow-up, the authors showed that most of the radiosurgical effects occurred during the first year after treatment; the volume of the nidus decreased rapidly during this interval. This nidus decrease slowed during the second year. If the kinetics of cerebral AVM changes are maximal during the first year after treatment, it is not surprising that cerebral AVMs with only minimal or no visible changes during this time will not reach complete obliteration within the follow-up period. Conversely, it is not surprising that cerebral AVMs with complete obliteration are those showing major changes on the first angiographic follow-up.
Influence of Early Angiographic Data onPatient Management
In light of our results, the data of the early angiogram can modify patient management. Patients with angiographically cured cerebral AVMs can lead a normal life 1 year after treatment, and do not need to undergo angiographic follow-up at 2 or 3 years. For all the others, two important problems need clarification: How long should a radiosurgically treated cerebral AVM be followed before definite failure can be assessed? When should complementary treatment be discussed? We are not aware of any study that has defined the timing of radiosurgical failure by angiographic criteria. Few authors have reported cases of delayed obliteration. Colombo et al (6) reported nine patients who experienced obliteration from 36 to 60 months after radiosurgery. Yamamoto et al (20, 41) cited a case of complete obliteration that occurred between 3 and 5 years after treatment. Pollock et al (38) reported a patient whose cerebral AVM was shown to be partially obliterated angiographically 48 months after treatment, and in whom complete obliteration was revealed at 65 months. These late cures, obtained well beyond the 2-year period in which most obliterations are expected, raise an important question. Because protection from bleeding is uncertain as long as complete obliteration is not obtained, should reirradiation only be considered when the cerebral AVM is still present on angiograms obtained 3 years after radiosurgery, as suggested by some radiosurgical teams (6, 22, 25, 38)?
Our results provide a partial solution to this problem because they indicate that, in selected cases, radiosurgical failure can be predicted on the basis of early angiographic results. For these patients, a long-term follow-up with sequential neuroimaging is not advisable, and could even expose the patient to the risk of bleeding (7.2% in the present study). Further treatments such as reirradiation, microsurgery, or embolization should be discussed as soon as the early angiogram shows minor or no changes, provided this angiogram is not performed too early after treatment.
Sequential neuroimaging examinations are indicated for all the other patients with incomplete obliteration on early angiogram. These patients would ideally benefit from the use of sequential noninvasive MR or CT angiography for monitoring therapeutic response (41, 43–55). If MR or CT features suggestive of complete obliteration could be delineated, an angiogram could be justified for definite assessment. MR examination offers several advantages over digital angiography. It allows three-dimensional delineation of the nidus morphology (33), which could be useful to follow-up the precise postradiosurgical volume decrease of the nidus over time. In addition to morphologic information, MR angiography provides a unique quantitative tool to monitor postradiosurgical effects on transnidus flow rates (54). Sequential MR follow-up data should provide additional data for refining our preliminary results and profiling cerebral AVM shrinkage after radiosurgery. As indicated by previous studies, cerebral AVM with different morphologic patterns have different shrinkage time courses after treatment (13, 25, 32, 41). It is our hope that a better understanding of the time course of AVM response to radiosurgery will provide individual neuroimaging follow-up schemes adapted to the variable patterns of cerebral AVMs. In practice, these personalized follow-up protocols could be scheduled before treatment and secondarily modified according to the results of the early neuroimaging data.
Footnotes
Presented in part at the 23rd Congress of the European Society of Neuroradiology, Oxford, September 1997.
Address reprint requests to: Jean François Meder, MD, PhD, Department of Neuroradiology, Centre, Hospitalier Sainte-Anne, 1, rue Cabanis 75674 Paris, France.
References
- 1.Steiner L, Leksell L, Greitz T, Foster D, Backlund E. Stereotaxic radiosurgery for cerebral arteriovenous malformation. report of a case. Acta Chir Scand 1972;138:459-464 [PubMed] [Google Scholar]
- 2.Steiner L. Treatment of arteriovenous malformations by radiosurgery. In: Wilson CB, Stein BM, eds. Intracranial Arteriovenous Malformations. Baltimore, MD: Williams & Wilkins; 1984: 295-313
- 3.Aoki Y, Nakasawa K, Tago M, Terahara A, Kurita H, Sasaki Y. Clinical evaluation of Gamma knife radiosurgery for intracranial arteriovenous malformation. . Radiation Medicine 1996;14:265-268 [PubMed] [Google Scholar]
- 4.Betti OO, Munari C. Traitement radiochirurgical avec accélérateur linéaire des “petites” malformations artérioveineuses intra-crâniennes. Neurochirurgie 1992;38:27-34 [PubMed] [Google Scholar]
- 5.Colombo F, Benedetti A, Pozza F, Marchetti C, Chierego G. Linear accelerator radiosurgery of cerebral arteriovenous malformations. Neurosurgery 1989;24:833-840 [DOI] [PubMed] [Google Scholar]
- 6.Colombo F, Pozza F, Chierego G, Casentini L, De Luca G, Francescon P. Linear accelerator radiosurgery of cerebral arteriovenous malformations: an update. Neurosurgery 1994;34:14-20 [PubMed] [Google Scholar]
- 7.Forster DE. The Sheffield ” gamma knife ” experience: results in arteriovenous malformation radiosurgery in 507 patients. In: Lunsford LD, ed. Stereotactic Radiosurgery Update. New York: Elsevier; 1992: 112-115
- 8.Friedman WA, Bova FJ. Linear accelerator radiosurgery for arteriovenous malformations. J Neurosurg 1992;77:832-841 [DOI] [PubMed] [Google Scholar]
- 9.Kondziolka D, Lunsford LD, Flickinger JC. Gamma knife stereotactic radiosurgery for cerebral vascular malformations. In: Aleksander E, Loeffler JS, Lundsford LD, eds: Stereotactic radiosurgery. New York: McGraw-Hill; 1993: 136-146
- 10.Lunsford LD, Kondziolka D, Flickinger JC, et al. Stereoactic radiosurgery for arteriovenous malformations of the brain. J Neurosurg 1991;75:512-524 [DOI] [PubMed] [Google Scholar]
- 11.Steinberg GK, Fabrikant JI, Marks MP, et al. Stereotactic heavy-charged-particle Bragg-peak radiation for intracranial arteriovenous malformations. N Engl J Med 1990;323:96-101 [DOI] [PubMed] [Google Scholar]
- 12.Steiner L, Lindquist C, Adler JR, Torner JC, Alves W, Steiner M. Clinical outcome of radiosurgery for cerebral arteriovenous malformations. J Neurosurg 1992;77:1-8 [DOI] [PubMed] [Google Scholar]
- 13.Yamamoto Y, Coffey RJ, Nichols DA, Shaw EG. Interim report on the radiosurgical treatment of cerebral arteriovenous malformations. The influence of size, dose, time and technical factors on obliteration rate. J Neurosurg 1995;83:832-837 [DOI] [PubMed] [Google Scholar]
- 14.Steiner L, Lindquist C. Radiosurgery in cerebral arteriovenous malformation. In: Tasker RR, ed. Neurosurgery: State of Art Reviews, Stereotactic Surgery. Philadelphia: Hanley and Belfus, Inc; 1987; vol 2, 329-336
- 15.Reinhold HS, Hopewell JW. Late changes in architecture of blood vessels of the rat brain after irradiation. Br J Radiol 1980;53:693-696 [DOI] [PubMed] [Google Scholar]
- 16.Yoshii Y, Philipps TL. Late vascular effects of whole brain X-irradiation in the mouse. Acta Neurochir (Wien) 1982;64:87-102 [DOI] [PubMed] [Google Scholar]
- 17.Leksell DG. Special stereotactic techniques: stereotactic radiosurgery. In: Heilbrun MP, ed. Concepts in Neurosurgery: Stereotactic Neurosurgery (vol 2).Baltimore, MD: Williams & Wilkins; 1988: 195-209
- 18.Kjellberg RN, Hanamura T, Davis KR, Lyons SL, Adams RD. Bragg peak proton-beam therapy for arteriovenous malformation of the brain. N Engl J Med 1983;309:269-274 [DOI] [PubMed] [Google Scholar]
- 19.Guo WY, Karlsson B, Ericson K, Lindqvist M. Even the smallest remnant of an AVM constitutes a risk of further bleeding. Case report. Acta Neurochir (Wien) 1993;121:212-215 [DOI] [PubMed] [Google Scholar]
- 20.Yamamoto M, Jimbo M, Kobayashi M, et al. Long-term results of radiosurgery for arteriovenous malformation: neurodiagnostic imaging and histological studies of angiographically confirmed nidus obliteration. Surg Neurol 1992;37:219-230 [DOI] [PubMed] [Google Scholar]
- 21.Kauczor HU, Engenhart R, Layer G, et al. 3D TOF MR angiography of cerebral arteriovenous malformations after radiosurgery. J Comput Assist Tomogr 1993;17:184-190 [DOI] [PubMed] [Google Scholar]
- 22.Young C, Summerfield R, Schwartz M, O'Brien P, Ramani R. Radiosurgery for arteriovenous malformations: the university of Toronto experience. . Can J Neurol Sci 1997;24:99-105 [DOI] [PubMed] [Google Scholar]
- 23.Pica A, Ayzac L, Sentenac I, et al. Stereotactic radiosurgery for arteriovenous malformations of brain using a standard linear accelerator: the Lyon experience. Radiotherapy and Oncology 1996;40:51-54 [DOI] [PubMed] [Google Scholar]
- 24.Engenhart R, Wowra B, Debus J, et al. The role of high-dose, single-fraction irradiation in small and large intracranial arteriovenous malformations. Int J Radiation Oncology Biol Phys 1994;30:521-529 [DOI] [PubMed] [Google Scholar]
- 25.Friedman WA, Bova FJ, Mendenhall WM. Linear accelerator radiosurgery for arteriovenous malformations: the relationship of size to outcome. J Neurosurg 1995;82:180-189 [DOI] [PubMed] [Google Scholar]
- 26.Talairach J, Tournoux P. Coplanar stereotaxic atlas of the human brain. Thieme G, ed. Verlag Stuttgart, New York, 1988.
- 27.Betti O, Derechinsky YE. Irradiation stéréotaxique multifaisceaux. Neurochirurgie 1983;29:295-298 [PubMed] [Google Scholar]
- 28.Betti OO, Munari C, Rosler R. Stereotactic radiosurgery with the linear accelerator: treatment of arteriovenous malformations. Neurosurgery 1989;24:311-321 [DOI] [PubMed] [Google Scholar]
- 29.Lefkopoulos D, Schlienger M, Touboul E. A 3-D dosimetric methodology for complex arteriovenous malformations. Radiother Oncol 1993;28:233-240 [DOI] [PubMed] [Google Scholar]
- 30.Lindquist C, Steiner L. Stereotactic radiosurgical treatment of malformations of the brain. In: Lundsford LD, ed. Modern Stereotactic Neurosurgery. Boston, MA: Martinus Nijhoff; 1988: 491-505
- 31.Fabrikant JI, Lyman JT, Hosobuchi Y. Stereotactic heavy-ion Bragg peak radiosurgery for intracranial vascular disorders: method for treatment of deep arterio-venous malformations. Br J Radiol 1984;57:479-490 [DOI] [PubMed] [Google Scholar]
- 32.Meder JF, Oppenheim C, Blustajn J, et al. Cerebral arteriovenous malformations: the value of radiologic parameters in predicting response to radiosurgery. AJNR Am J Neuroradiol 1997;18:1473-1483 [PMC free article] [PubMed] [Google Scholar]
- 33.Guo WY, Nordell B, Karlsson B, et al. Target delineation in radiosurgery for cerebral arteriovenous malformations. Assessment of the value of stereotaxic MR imaging and MR angiography. Acta Radiol 1993;34:457-463 [PubMed] [Google Scholar]
- 34.Blatt DR, Friedman WA, Bova FJ. Modifications based on computed tomographic imaging in planning the radiosurgical treatment of arteriovenous malformations. Neurosurgery 1993;33:588-595 [DOI] [PubMed] [Google Scholar]
- 35.Kemeny AA, Dias PS, Forster DM. Results of stereotactic radiosurgery of arteriovenous malformations: an analysis of 52 cases. J Neurol Neurosurg Psychiat 1989;52:554-558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kondziolka D, Lunsford LD, Talagala L. Stereotactic magnetic resonance angiography for targeting in arteriovenous malformation radiosurgery. Neurosurgery 1994;35:585-591 [DOI] [PubMed] [Google Scholar]
- 37.Lax I, Karlsson B. Prediction of complications in gamma knife radiosurgery of arteriovenous complication. Acta Oncol 1996;35:49-55 [DOI] [PubMed] [Google Scholar]
- 38.Pollock BE, Kondziolka D, Lundsford LD, Bissonette D, Flickinger JC. Repeat stereotactic radiosurgery of arteriovenous malformation: factors associated with incomplete obliteration. Neurosurgery 1996;38:318-324 [DOI] [PubMed] [Google Scholar]
- 39.Schlienger M, Merienne L, Lefkopoulos D, et al. Irradiation radiochirurgicale de 49 malformations artério-veineuses cérébrales au moyen d'un accélérateur linéaire. Bull Cancer/Radiother 1994;81:99-109 [PubMed] [Google Scholar]
- 40.Spiegelman R, Friedman WA, Bova FJ. Limitations of angiographic target localization in planning radiosurgical treatment. Neurosurgery 1992;30:619-624 [DOI] [PubMed] [Google Scholar]
- 41.Yamamoto M, Jimbo M, Ide M, Lindquist C, Steiner L. Postradiation volume changes in gamma unit-treated cerebral arteriovenous malformations. Surg Neurol 1993;40:485-490 [DOI] [PubMed] [Google Scholar]
- 42.Pasqualin A, Barone G, Cioffi F, Rosta L, Scinza R, Pian RD. The relevance of anatomic and hemodynamic factors to a classification of cerebral arteriovenous malformations. Neurosurgery 1987;28:370-379 [DOI] [PubMed] [Google Scholar]
- 43.Quisling RG, Peters KR, Friedman WA, Tart RP. Persistent nidus blood flow in cerebral arteriovenous malformation after stereotactic radiosurgery: MR imaging assessment. . Radiology 1991;180:785-791 [DOI] [PubMed] [Google Scholar]
- 44.Noorbehesht B, Fabrikant JI, Enzmann DR. Size determination of arteriovenous malformations by MR, CT and angio. Neuroradiol 1987;29:512-518 [DOI] [PubMed] [Google Scholar]
- 45.Abe T, Matsumoto K, Horichi Y, Hayashi T, Ikeda H, Iwata T. Magnetic resonance angiography of cerebral arteriovenous malformations. Neurol Med Chir 1995;35:580-583 [DOI] [PubMed] [Google Scholar]
- 46.Davis WL, Blatter DD, Harnsberger HR, Parker DL. Intracranial MR angiography: comparison of single-volume three dimensional time-of-flight and multiple overlapping thin slab acquisition techniques. AJR Am J Roentgenol 1994;163:915-920 [DOI] [PubMed] [Google Scholar]
- 47.Guo WY, Lindquist C, Karlsson B, Kihlström L, Steiner L. Gamma knife surgery of cerebral arteriovenous malformations: serial MR imaging studies after radiosurgery. Int J Radiat Oncol Biol Phys 1993;25:315-323 [DOI] [PubMed] [Google Scholar]
- 48.Guo WY, Pan DH, Liu RS, et al. Early irradiation effects observed on magnetic resonance imaging and angiography, and positron emission tomography for arteriovenous malformations treated by Gamma knife radiosurgery. Stereotact Funct Neurosurg 1995;64:258-269 [DOI] [PubMed] [Google Scholar]
- 49.Kauczor HU, Engenhart R, Layer G, et al. 3D TOF MR angiography of cerebral arteriovenous malformations after radiosurgery. J Comput Assist Tomogr 1993;17:184-190 [DOI] [PubMed] [Google Scholar]
- 50.Marchal G, Bosmans H, Van fraeyenhoven LV, et al. Intracranial vascular lesions: optimization and clinical evaluation of three-dimensional time-of-flight MR angiography. Radiology 1990;175:443-448 [DOI] [PubMed] [Google Scholar]
- 51.Marks MP, Delapaz RL, Fabrikant JI, Frankel KA, Phillips MH, Levy RP, Enzmann DR. Intracranial vascular malformations: imaging of charged-particle radiosurgery. Part I. Results of therapy. Radiology 1988;168:447-455 [DOI] [PubMed] [Google Scholar]
- 52.Morikawa M, Numaguchi Y, Rigamonti D, et al. Radiosurgery for cerebral arteriovenous malformations: assessment of early phase magnetic resonanace imaging and significance of gadolinium-DTPT enhancement. Int J Radiation Oncology Biol Phys 1996;34:663-675 [DOI] [PubMed] [Google Scholar]
- 53.Mukherji SK, Quisling RG, Kubilis PS, Finn JP, Friedman WA. Intracranial arteriovenous malformations: quantitative analysis of magnitude contrast MR angiography versus gradient-echo MR imaging versus conventional angiography. Radiology 1995;196:187-193 [DOI] [PubMed] [Google Scholar]
- 54.Petereit D, Mehta M, Turski P, et al. Treatment of arteriovenous malformations with stereotactic radiosurgery employing both magnetic resonance angiography and standard angiography as a database. Int J Radiat Oncol Biol Phys 1993;25:309-313 [DOI] [PubMed] [Google Scholar]
- 55.Tournade A, Krupa P, Tajahmadi T, et al. Role de l'Angio-IRM dans la surveillance des malformations artério-veineuses intra-craniennes traitées par abord endovasculaire. J Neuroradiol 1994;21:255-261 [PubMed] [Google Scholar]


