Abstract
BACKGROUND AND PURPOSE: Our purpose was to describe the MR imaging findings in patients with acute coccidioidal meningitis.
METHODS: Fourteen patients (11 men, three women; 22–78 years old; mean age, 47 years) with coccidioidal meningitis underwent neuroimaging within 2 months of diagnosis. Thirteen patients had MR imaging and one had an initial CT study with a follow-up MR examination 5 months later. Initial and follow-up MR images were evaluated for the presence of ventricular dilatation, signal abnormalities, enhancement characteristics, sites of involvement, and evidence of white matter or cortical infarction. The patterns of enhancement were characterized as focal or diffuse. Pathologic specimens were reviewed in two patients.
RESULTS: Ten of the 14 images obtained at the time of initial diagnosis showed evidence of meningitis. All of the initially abnormal studies showed enhancement in the basal cisterns, sylvian fissures, or pericallosal region. Subsequent studies, which were available for three of the four patients with normal findings initially, all eventually became abnormal, with focal enhancement seen on the initial abnormal examination. Other abnormalities seen at presentation included ventricular dilatation (six patients) and deep infarcts (four patients). Pathologic specimens in two patients showed focal collections of the organism corresponding to the areas of intense enhancement on MR images.
CONCLUSION: Early in its disease course, coccidioidal meningitis may show areas of focal enhancement in the basal cisterns, which may progress to diffuse disease. Pathologically, the areas of enhancement represent focal collections of the organism. Deep infarcts and communicating hydrocephalus are associated findings.
Coccidioides immitis resides in the topsoil of the southwestern United States and northern Mexico. An estimated 3% of susceptible people annually acquire the relatively benign pulmonary form of the disease, commonly referred to as valley fever. The rate of disseminated disease is estimated to be 4% to 5% after the primary infection (1), with meningitis occurring in about one half of these cases. Improvements in imaging and treatment have taken place since the original descriptions of coccidioidal meningitis. This article describes the MR imaging features of coccidioidal meningitis in 14 patients at the time of diagnosis and on subsequent examinations.
Methods
The medical records of the Veterans Affairs Medical Center and the University of Arizona Medical Center in Tucson from 1992 to 1998 were reviewed for patients with either CSF serology positive for Coccidioides immitis or a discharge diagnosis of coccidioidal meningitis (diagnosed either by positive CSF serology or, in one case, meningeal biopsy results). A total of 41 patients were identified. MR images obtained within 2 months of diagnosis were available in 12 of these patients. One patient (case 5) included in the study had a CT examination at diagnosis and an MR imaging study 5 months later. An additional patient (case 13) was included who was sent to the University of Arizona Medical Center for consultation but had MR imaging done at an outside institution. Images were performed on a 1.5-T, a 1.0-T, or a 0.5-T superconducting magnet. Imaging protocols consisted of sagittal spin-echo (SE) T1-weighted sequences, axial proton density—and T2-weighted SE or fast SE sequences, axial SE or spoiled gradient-recalled T1-weighted sequences, and contrast-enhanced axial and coronal SE T1-weighted sequences.
Each examination was reviewed by three radiologists, two of whom were fellowship-trained neuroradiologists. The images were evaluated for signal abnormalities, sites of involvement, evidence of ventricular enlargement, ischemia, and enhancement pattern. The enhancement patterns were characterized as either focal (isolated areas of abnormal enhancement) or diffuse (confluent areas of enhancement). Pathologic specimens were available from the one meningeal biopsy as well as from a postmortem examination in another patient. The pathologic findings were compared with the imaging studies.
Results
Ten of the 13 MR images at the time of initial presentation showed evidence of meningitis. The one CT study that was performed at the time of presentation had normal findings. All the abnormal studies showed enhancement in the basal cisterns, sylvian fissures, craniocervical junction, or pericallosal regions. At presentation, four (29%) of 14 images were without evidence of meningitis, six (43%) had evidence of ventricular enlargement, four (29%) had acute deep infarcts or edema, three (21%) had focal meningeal enhancement, and six (43%) had diffuse enhancement. Follow-up examinations were available in three of the four patients whose initial imaging findings were normal. All subsequent studies in these patients eventually became abnormal, with focal enhancement on the initial abnormal examination in all three patients. In the one patient with initially normal CT findings, small infarcts developed in the internal capsule and cerebellum between the time of the initial CT study and the follow-up MR examination. The mean time between the initial normal examination and the first abnormal examination was 5.7 months (range, 2–10 months).
In most instances, the basal cisterns had normal CSF signal on the unenhanced images. However, in four patients, the involved areas were isointense with brain on the T1-weighted images. The signal of the involved areas on T2-weighted images was normal in 12 of the 14 patients. In one patient (case 14), the signal was isointense with brain, in a second (case 10), the signal was hypointense relative to brain.
Pathologic specimens in two patients showed focal collections of the organism corresponding to the areas of intense enhancement seen on MR images. The results are summarized in Tables 1, 2, and 3.
TABLE 1:
Findings in 14 patients with acute coccidioidal meningitis
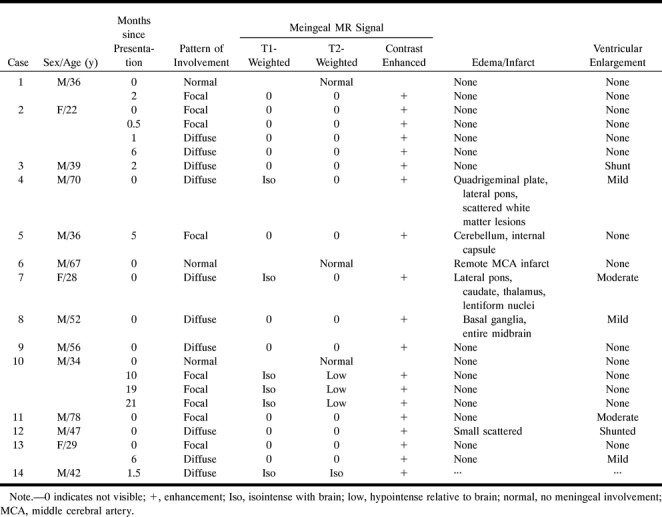
TABLE 2:
Summary of imaging findings in 14 patients at presentation (one patient had an initial CT scan)

TABLE 3:
Summary of findings in 13 patients on the first abnormal MR examination (no follow-up examination was available for one patient with an initially normal study)

Discussion
The fungus Coccidioides immitis resides in the topsoil of endemic areas of the southwestern United States and northern Mexico. Coccidioidomycosis is nearly always a result of inhalation of the arthrospore, at which time a primary pulmonary infection is established. Direct cutaneous inoculation is exceedingly rare (1). Within the host, the inhaled arthrospore develops into a globular structure known as a spherule. The spherule is 20 to 100 μm in diameter and, through a series of cytoplasmic cleavages and nuclear divisions, develops hundreds of endospores within a thick-walled capsule (2, 3) (Fig 1). When the spherule ruptures, the endospores are released, which continues the cycle of infection. If the spherule-endospore cycle is not interrupted, hematogenous spread of the endospores may occur and result in disseminated disease. Fortunately, this is a relatively rare occurrence, although there is increased risk of disseminated disease in pregnant women, in people at the extremes of age, in the immunosuppressed, and in people of Mexican, Filipino, and African ancestry.
fig 1.
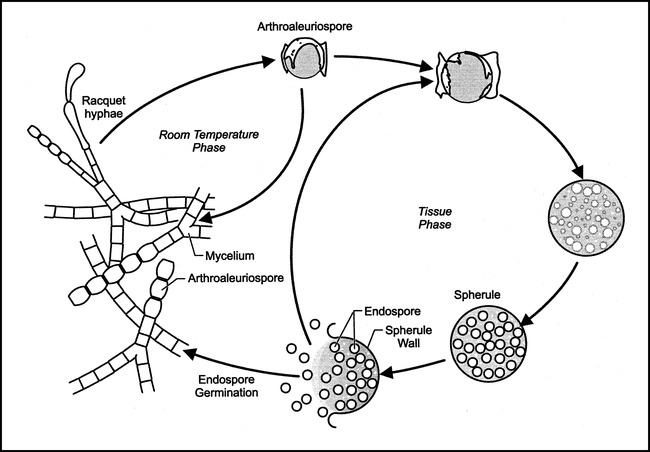
Life cycle of Coccidioides immitis.
Untreated, the clinical course of coccidioidal meningitis is uniformly fatal. Current therapies include intravenous or oral fluconazole, miconazole, and intrathecal amphotericin. The latter may be limited by the fact that sequestered areas of the CSF (eg, a trapped ventricle) will not receive the drug. Unfortunately, regardless of therapy, the likelihood of cure is small. Although the disease may be controlled with chronic antifungal therapy, relapse after withdrawal of therapy is common (1).
Previous reports of the CT and MR imaging findings in cerebral coccidioidomycosis have described dense enhancement of the basal cisterns with secondary hydrocephalus, ventriculitis, and vague white matter lesions (4–7). Focal meningeal enhancement has not been described previously, but in this series was seen in three of 14 patients at the time of presentation and in an additional three patients as the initial abnormal finding. The focal enhancement can be related to two factors. One is that our inclusion criteria differ from previous MR imaging analyses of coccidioidal meningitis in that only patients with images obtained near the time of initial presentation were included in this review. As with any pathologic process, imaging findings early in the disease course may differ from those seen at a later date. Second, improvements in treatment since the initial reports have changed the course of the disease, and may also have an influence on the imaging features. Therefore, it is not surprising that patients in this series exhibited less extensive disease than that described previously.
The initial imaging studies in four of our 14 patients showed no evidence of meningitis (Table 2). In a previous MR imaging study, which included patients with both acute and chronic coccidioidal meningitis, only 8% of examinations were normal (4). Many of these patients may have had normal examinations if they had undergone imaging acutely. Of interest is that all 13 patients in this series who had follow-up examinations either presented with or eventually developed an abnormality (Table 3).
Evidence of ischemia or infarction was present in 58% of patients in the only prior MR imaging report, which is much higher than the 29% seen at presentation in this study (4). Again, the prior study included patients with chronic rather than acute disease. Eventually, a total of five patients had evidence of acute infarction or edema involving the brain stem, cerebellum, thalamus, or basal ganglia (Fig 2). Only one cortical infarction was seen. This occurred in a 67-year-old man and appeared to be chronic at the time of diagnosis; in all probability, it was unrelated to his meningitis. Vasculitis and vascular lesions have previously been reported in association with coccidioidal meningitis (8, 9). Of some interest is the fact that all the ischemic foci in our series were located in distributions of perforating vessels, with evidence of meningitis involving the parent vessel. This phenomenon could be related either to vasospasm or direct invasion of the vessel.
fig 2.

Case 7: 28-year-old woman.
A, Coronal contrast-enhanced T1-weighted image shows diffuse abnormal meningeal enhancement (arrow) along the course of the middle and anterior cerebral arteries. A shunt catheter (arrowheads) has been placed for acute hydrocephalus.
B, Axial T2-weighted image shows edema in the deep nuclei bilaterally (arrows). This is believed to represent areas of infarction/ischemia consequent to coccidioidal vasculitis.
fig 3. Case 1: 36-year-old man. Findings on initial study were normal (not shown). Follow-up axial contrast-enhanced T1-weighted image 2 months later shows discrete nodules of abnormal enhancement (arrow) in the perimesencephalic cistern.
In three of the four patients with initially normal imaging findings, follow-up examinations were available for review. All of these studies eventually became abnormal, with the initial abnormality being a focal area of meningeal enhancement in all three (Fig 3). Subsequent images of two patients (cases 2 and 13) initially showed a focal abnormality, progressing to the more commonly described pattern of diffuse basal enhancement (Fig 4). In each of these cases, the progression was associated with a delay in diagnosis and treatment. The diffuse meningeal enhancement described in previous reports and seen in some of the cases in this series may have been a result of progressive spread of the organism throughout the CSF.
fig 4.

Case 2: 22-year-old woman with disease progression.
A, Initial coronal contrast-enhanced T1-weighted MR image with focal enhancement in the left sylvian fissure (arrow).
B, Follow-up study 2 weeks later shows a new small focus of abnormal meningeal enhancement (arrow) on the medial aspect of the uncus.
C and D, Another week later, there is marked progression of disease to diffusely involve the basilar cisterns (arrows) as well as the course of the middle cerebral arteries (arrowheads).
Ventricular enlargement (four cases) or a ventricular shunt (two cases) was present in 42% of patients in this series at presentation. Ventricular dilatation developed in an additional patient on a follow-up study. It is possible that in the older patients cerebral atrophy contributed to the imaging findings, as no periventricular interstitial edema was seen in any patient. Previous imaging reports describe hydrocephalus in 68% to 93% of patients (4–7). Again, the difference in the rates of ventricular enlargement is most probably due to differences in patient selection. Because our patients were imaged acutely, many may have been treated before the onset of hydrocephalus.
The extensive periventricular areas of diminished attenuation described in the early CT reports do not have a correlate in this series. These probably were the combination of ischemic change, hydrocephalus, and chronic intrathecal amphotericin therapy. The previous MR imaging report (4) does not mention periventricular white matter abnormalities as a consistent finding. The absence of this finding may reflect improved antifungal therapy as well a more aggressive use of ventricular shunts.
When areas of meningeal enhancement were visible on the noncontrast T1- and T2-weighted images, the material was isointense with brain on the T1-weighted studies (cases 4, 7, 10, 11) and isointense to slightly hypointense relative to brain on the T2-weighted sequences (cases 10, 14) (Figs 5 and 6). As yet, there is no clear explanation for the low signal on the T2-weighted images. This could be the result of ferromagnetic material within the fungus or perhaps dense cellularity of the focal lesion. The differential diagnosis of the diffuse form continues to include tuberculous meningitis, sarcoidosis, and leptomeningeal carcinomatosis. It is possible that the nodular form could be mistaken for a meningioma or a nerve sheath tumor in addition to mimicking neoplasm or other granulomatous meningitis.
fig 5.

Case 4: 70-year-old man.
A, Unenhanced T1-weighted image shows abnormal soft-tissue mass in the ambient cistern (arrows).
B, Dense enhancement (arrowheads) is seen after administration of gadolinium chelate.
fig 6.

Case 10: Low signal on T2-weighted images.
A, Axial T2-weighted image shows decreased signal (arrow) in the prepontine cistern.
B, Contrast-enhanced T1-weighted sequence shows marked enhancement (arrow) of the same area.
Pathologic specimens were available in two cases. One of these was from a meningeal biopsy and the other was from an autopsy. The focal areas of enhancement corresponded to collections of the organism.
Conclusion
Contrary to previous reports, which described diffuse meningeal enhancement, coccidioidal meningitis early in its disease course may show areas of focal or nodular enhancement in the basal cisterns, which may progress to confluent disease. Eventually, all the patients in whom follow-up studies were available had a focal area of enhancement as the initial abnormal finding. The differences between our findings and those of previous studies are most likely due to our inclusion of only patients who were imaged at or near the time of diagnosis, as well as to recent improvements in antifungal therapy. Pathologically, the nodular areas represent focal collections of the organism with surrounding inflammation. Deep infarcts may occur, probably subsequent to vasospasm or direct invasion of perforating vessels. Ventricular enlargement is common. The findings are similar to those described in other forms of granulomatous meningitis.
Acknowledgments
We thank Kristina Larka for manuscript preparation and the Valley Fever Center for Excellence, Tucson, AZ, for assistance.
Footnotes
Address reprint requests to William K. Erly, MD, Department of Radiology, The University of Arizona, 1501 N Campbell Ave, Tucson, AZ 85724.
References
- 1.Galgiani JN. Coccidioides immitis meningitis. In: Peterson PK, Remington JS, eds. Defense of the Brain. Malden, MA: Blackwell; 1997;227-238
- 2.Drutz DJ, Catanzaro A. Coccidioidomycosis: part I. Am Rev Respir Dis 1978;117:559-585 [DOI] [PubMed] [Google Scholar]
- 3.Druz DJ, Catanzaro A. Coccidioidomycosis: part II. Am Rev Respir Disease 1978;117:727-771 [DOI] [PubMed] [Google Scholar]
- 4.Wrobel CJ, Meyer S, Johnson RH, Hesselink J. MR findings in acute and chronic coccidioidomycosis meningitis. AJNR Am J Neuroradiol 1992;13:1241-1245 [PMC free article] [PubMed] [Google Scholar]
- 5.Dublin AB, Phillips HE. Computed tomography of disseminated cerebral coccidioidomycosis. Radiology 1980;135:361-368 [DOI] [PubMed] [Google Scholar]
- 6.McGahan PJ, Graves DS, Palmer PES, Stadalnik RC, Dublin AB. Classic and contemporary imaging of coccidioidomycosis. AJR Am J Roentgenol 1981;136:393-404 [DOI] [PubMed] [Google Scholar]
- 7.Enzmann DR, Norman D, Mani J, Newton JH. Computed tomography of granulomatous basal arachnoiditis. Radiology 1976;120:341-344 [DOI] [PubMed] [Google Scholar]
- 8.Newton TH, Cohen NH. Coccidioidal meningitis: roentgen and pathologic analyses. Acta Radiol (Diag) 1963;1:886-900 [DOI] [PubMed] [Google Scholar]
- 9.Kobayashi RM, Coel M, Niwayama G, Trauner D. Cerebral vasculitis in coccidioidal meningitis. Ann Neurol 1977;1:281-284 [DOI] [PubMed] [Google Scholar]


