Abstract
BACKGROUND AND PURPOSE: Our purpose was to determine the brain MR imaging characteristics of merosin-deficient congenital muscular dystrophy in children.
METHODS: We reviewed the MR imaging findings of the brain in three children with known merosin-deficient congenital muscular dystrophy to determine the presence of any cerebral or cerebellar abnormalities of development or abnormalities of the white matter.
RESULTS: In all three patients, there was normal formation of the cerebrum, the cerebellum, and no evidence of neuronal migration anomalies. All three patients had abnormal white matter in the cerebrum, with sparing of the corpus callosum, internal capsule, cerebellum, and brain stem.
CONCLUSION: MR imaging of the brain in children with merosin-deficient congenital muscular dystrophy reveals a consistent pattern of white matter abnormality. We postulate that disruption of the blood-brain barrier associated with merosin deficiency leads to increased water content, resulting in abnormal white matter signal intensity.
Congenital muscular dystrophy (CMD) comprises a heterogeneous group of disorders that present at birth with muscle weakness, hypotonia, and contractures. A dystrophic pattern is present on muscle biopsy specimens (1, 2). There are two well-recognized forms of the disease. The classic or pure form of CMD occurs in patients with normal or near-normal intelligence. The second form differs from the pure form in that severe mental retardation and brain anomalies are present (2). This second group includes the Japanese variant known as Fukuyama CMD, the Walker-Warburg syndrome, and the Santavuori syndrome (muscle-eye-brain disease) (3).
Fukuyama, Walker-Warburg, and Santavuori syndromes have a variety of neuropathologic abnormalities that include abnormal cerebral and cerebellar gyral patterns, cerebellar cysts, and white matter changes on MR images (2–9). The white matter findings are observed in late infancy and decrease in severity with age (2–9). The pathogenesis of these changes remains uncertain at this time. Imaging findings in the pure form of CMD have recently been reported, along with their association with abnormal sensory and visual evoked potentials (6, 7, 10). In this article, we outline the specific MR imaging findings seen in patients with documented merosin-deficient CMD and suggest the causes.
Methods
Three children with the classical or pure form of CMD found to be negative for merosin (laminin α2) immunostaining on muscle biopsy specimens were studied with MR imaging and CT of the brain. Immunocytochemical analysis was used to test skeletal muscle for merosin. A monoclonal antimerosin (M chain) antibody (Chemicon International, Temecula, CA) was used to detect the presence or absence of merosin around each muscle fiber.
MR images were obtained on a 1.5-T imager. T1-weighted sagittal images (600/15 [TR/TE]), axial variable-echo T2-weighted images (3000/90–30), and coronal T2-weighted fast spin-echo images were obtained in all three patients. The CT scans were obtained in 8-mm-thick sections in the axial plane. The MR imaging studies were analyzed for structural abnormalities of the cerebrum and cerebellum, cortical migration anomalies, and white matter disorders.
Results
Case summaries for all three patients are presented in Tables 1 and 2 (see Figs 1–3).
TABLE 1:
Case summaries I
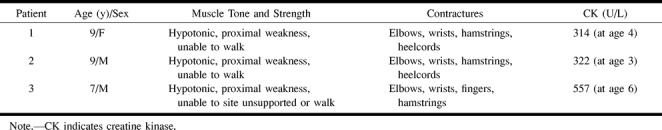
TABLE 2:
Case summaries (II)
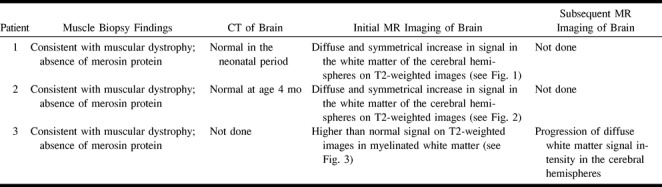
fig 1.
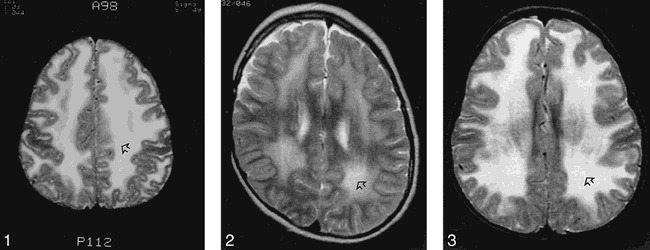
Patient 1: T2-weighted MR image of the brain at age 9 years shows diffuse and symmetrical increase in signal in the white matter of the cerebral hemispheres (arrow).
fig 2. Patient 2: T2-weighted MR image of the brain at age 8 years shows diffuse and symmetrical increase in signal in the white matter of the cerebral hemispheres (arrow).
fig 3. Patient 3: T2-weighted MR image of the brain at age 3 years shows diffuse increased signal in the white matter (arrow).
Discussion
Merosin itself is an isoform of laminin that is expressed in skeletal muscle, Schwann cells, cardiac muscle, and placental villi. Laminins are a family of basement membrane proteins, and the predominant laminin variant in adult striated muscle basement membrane is merosin (laminin α2). Merosin binds α-dystroglycan and in turn is linked to the subsacrolemmal cytoskeleton via the dystrophin-glycoprotein complex. Researchers maintain that a deficiency of merosin could disrupt the link between the extracellular matrix and the subsacrolemmal cytoskeleton, thus causing muscle degeneration (11, 12). Recent findings have suggested that myofiber injury may be the result of an inflammatory attack on muscle, followed by poor regeneration of myofibers caused by a defective basal lamina (13).
Clinically, patients with merosin-deficient CMD have congenital hypotonia and weakness, and, in late infancy, have contractures and delayed motor milestones. If they survive past the neonatal period, their neuromuscular condition stabilizes. They also have normal or near-normal intelligence. All three patients in this study had normal intelligence. Our experience is similar to that found in the literature in that CNS involvement is rarely clinically evident, but MR white matter abnormalities are always present (4, 6).
MR imaging findings were strikingly similar for all three patients. On T2-weighted images, all three patients had a diffuse and symmetrical increase in signal in the white matter of the cerebral hemispheres, normal white matter signal in the cerebellum, and no evidence of structural abnormalities or neuronal migration anomalies, such as focal occipital agyria, white matter cysts, enlarged lateral ventricles, or hypoplasia of the cerebellar vermis and pons. The signal characteristic of the white matter within the corpus callosum, internal capsule, and brain stem was normal in all three children. The brain stem and cerebellum were structurally normal. Patient 2 was the only one who had an available CT scan, which showed a normal brain and cerebellum at 4 months of age.
The changes seen in the white matter in our patients were similar to those described in the literature in that there was progression of white matter disease (10). Trevisan et al describe one patient with merosin-deficient CMD whose imaging studies from age 2 months to 5 years showed progression of leukoencephalopathy (10). This finding indicates that if MR findings in early infancy (ie, before 4 months of age) are normal in a child suspected of having CMD, the diagnosis of merosin-deficient CMD should not be discarded. Instead, the study should be repeated later (ie, at least after age 1 year) to look for changes in the white matter.
Why there is abnormal white matter in patients who do not clinically have CNS involvement is unknown. It is known, however, that merosin promotes neurite outgrowth and Schwann cell migration (11, 14, 15). In the brain, merosin has been found in the basement membrane of blood vessels (16). As postulated by Villanova et al, this may result in an alteration in the blood-brain barrier, leading to vascular hyperpermeability and the penetration of substances into the CNS (16). We propose that the increased T2 prolongation time on an MR image may be attributable to increased water content in the white matter owing to an abnormal blood-brain barrier rather than to decreased or abnormal myelination. The literature supports this hypothesis, and findings report normal brain stem auditory evoked responses in patients with merosin-deficient CMD (6, 17). Brain stem auditory evoked responses are typically abnormal in patients with leukodystrophies (18). However, further studies are needed to prove this hypothesis.
Conclusion
These three cases have demonstrated the manifestations of merosin deficiency through clinical presentations, muscle biopsy specimens, and MR imaging findings. Imaging studies play an important role in the diagnosis of merosin-deficient CMD. Subtle changes in the white matter may be seen in early infancy and aid in the diagnosis of this form of CMD. However, if white matter changes are not seen in infancy, a repeat study in early childhood could be revealing and help to confirm the diagnosis in a child with features characteristic of merosin-deficient CMD.
Footnotes
Address reprint requests to Mena Scavina, DO, c/o Editorial Services, Alfred I. duPont Hospital for Children, P.O. Box 269, Wilmington, DE 19899.
References
- 1.Tome FM, Evangelista T, Leclerc A, et al. Congenital muscular dystrophy with merosin deficiency. C R Acad Sci III 1994;317:351-357 [PubMed] [Google Scholar]
- 2.Leyten QH, Gabreels FJ, Renier WO, et al. White matter abnormalities in congenital muscular dystrophy. J Neurol Sci 1995;129:162-169 [DOI] [PubMed] [Google Scholar]
- 3.Trevisan CP, Martinello F, Ferruzza E, Angelini C. Divergence of central nervous system involvement in 2 Italian sisters with congenital muscular dystrophy: a clinical and neuroradiological follow-up. Eur Neurol 1995;35:230-235 [DOI] [PubMed] [Google Scholar]
- 4.Trevisan CP, Carollo C, Segalla P, Angelini C, Drigo P, Giordano R. Congenital muscular dystrophy: brain alterations in an unselected series of Western patients. J Neurol Neurosurg Psychiatry 1991;54:330-334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Aida N, Tamagawa K, Takada K, et al. Brain MRI in Fukuyama congenital muscular dystrophy. AJNR Am J Neuroradiol 1996;17:605-613 [PMC free article] [PubMed] [Google Scholar]
- 6.Mercuri E, Muntoni F, Berardinelli A, et al. Somatosensory and visual evoked potentials in congenital muscular dystrophy: correlation with MRI changes and muscle merosin status. Neuropediatrics 1995;26:3-7 [DOI] [PubMed] [Google Scholar]
- 7.Reed UC, Marie SK, Vainzof M, et al. Congenital muscular dystrophy with cerebral white matter hypodensity: correlation of clinical features and merosin deficiency. Brain Dev 1996;18:53-58 [DOI] [PubMed] [Google Scholar]
- 8.Nogen AG. Congenital muscle disease and abnormal findings on computerized tomography. Dev Med Child Neurol 1980;22:658-663 [DOI] [PubMed] [Google Scholar]
- 9.Yoshioka M, Kuroki S, Mizue H. Congenital muscular dystrophy of non-Fukuyama type with characteristic CT images. Brain Dev 1987;9:316-318 [DOI] [PubMed] [Google Scholar]
- 10.Trevisan CP, Martinello F, Ferruzza E, Fanin M, Chevallay M, Tome FM. Brain alterations in the classical form of congenital muscular dystrophy: clinical and neuroimaging follow-up of 12 cases and correlation with the expression of merosin in muscle. Childs Nerv Syst 1996;12:604-610 [DOI] [PubMed] [Google Scholar]
- 11.Engvall E, Davis GE, Dickerson K, Ruoslahti E, Varon S, Manthorpe M. Mapping of domains in human laminin using monoclonal antibodies: localization of the neurite-promoting site. J Cell Biol 1986;103:2457-2465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vuolteenaho R, Nissinen M, Sainio K, et al. Human laminin M chain (merosin): complete primary structure, chromosomal assignment, and expression of the M and A chain in human fetal tissues. J Cell Biol 1994;124:381-394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pegoraro E, Mancias P, Swerdlow SH, et al. Congenital muscular dystrophy with primary laminin alpha2 (merosin) deficiency presenting as inflammatory myopathy. Ann Neurol 1996;40:782-791 [DOI] [PubMed] [Google Scholar]
- 14.Engvall E, Earwicker D, Day A, Muir D, Manthorpe M, Paulsson M. Merosin promotes cell attachment and neurite outgrowth and is a component of the neurite-promoting factor of RN22 schwannoma cells. Exp Cell Res 1992;198:115-123 [DOI] [PubMed] [Google Scholar]
- 15.Tan E, Topaloglu H, Sewry C, et al. Late onset muscular dystrophy with cerebral white matter changes due to partial merosin deficiency. Neuromuscul Disord 1997;7:85-89 [DOI] [PubMed] [Google Scholar]
- 16.Villanova M, Malandrini A, Toti PE, et al. Localization of merosin in the normal human brain: implications for congenital muscular dystrophy with merosin deficiency. J Submicrosc Cytol Pathol 1996;28:1-4 [PubMed] [Google Scholar]
- 17.Leyten QH, Gabreels FJM, Renier WO, et al. White matter abnormalities in congenital muscular dystrophy. J Neurol Sci 1995;129:162-169 [DOI] [PubMed] [Google Scholar]
- 18.Hall JA. Neurodiagnosis: central nervous system. Handbook of Auditory Evoked Responses Boston: Allyn & Bacon; 1992;419-472


