Abstract
Summary: We report two patients with surgically proved mucoceles involving the anterior clinoid process. One patient had a mucocele of an Onodi cell and the other had a mucocele isolated to the anterior clinoid process. The MR signal was increased on both T1- and T2-weighted images in the first patient but was isointense on both sequences in the second patient, a finding that resulted in misdiagnosis. The developmental and anatomic features, as well as the diagnostic pitfalls, are discussed.
The anterior clinoid process may be pneumatized by paranasal air spaces as part of normal development. We present two patients in whom benign inflammatory mucoceles developed at this unusual site. In one of them, the MR signal and pattern were characteristic of inflammatory sinus disease, whereas the MR appearance of the other patient's lesion mimicked a neoplasm.
Case Reports
Case 1
A 32-year-old woman had a 2-year history of bitemporal and retroorbital headaches. She had suffered from headaches since the age of 7, with increasing frequency over the past 10 years. She also reported chronic sinusitis and had had previous sinus surgery. Clinical examination was unremarkable.
An MR examination showed mild mucosal thickening of both maxillary sinuses, the left ethmoidal sinuses, and the frontal sinuses. In addition, a focal mass was noted within the anterior part of the sphenoid bone, extending medially and laterally around the left optic nerve into the anterior clinoid process. This mass displayed homogeneous high signal intensity on T1-weighted MR images and intermediate signal on T2-weighted images (Fig 1). No significant enhancement was noted after administration of intravenous contrast material. A diagnosis of mucocele of the sphenoidal sinus was made. The patient underwent successful endoscopic sphenoidectomy and marsupialization of the mucocele involving a left Onodi cell. Her headaches were lessened considerably after surgery.
fig 1.
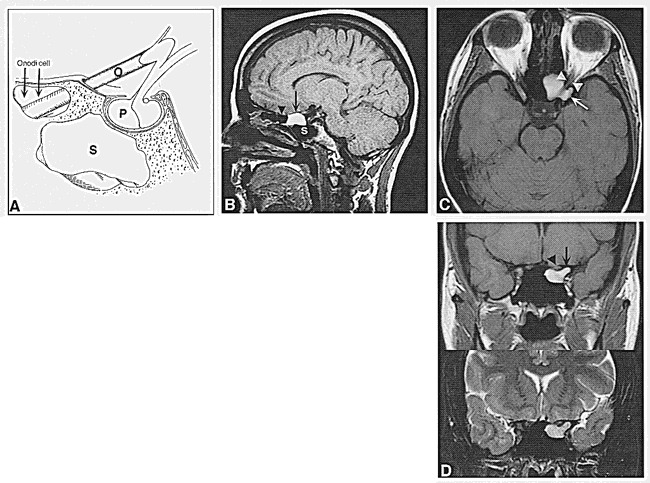
Case 1: 32-year-old woman with retroorbital headache and a long history of sinus disease.
A, Schematic representation of a sagittal section through the sphenoidal sinus (S) shows the relationship between Onodi cell (arrows), optic nerve (O), and pituitary gland (P). (Reprinted with permission from [7].)
B, T1-weighted (516/14/2 [TR/TE/excitations]) conventional spin-echo MR image shows an area of increased signal intensity (arrow) anterior and superior to the sphenoidal sinus (S), corresponding to the Onodi cell seen in A. Note that this is part of the posterior ethmoidal air cells (arrowhead).
C, Axial T1-weighted image (600/14/2) shows the mucus-filled Onodi cell extending into the left anterior clinoid process (arrow) and inferior to the optic nerve (arrowheads). Note that on axial images the optic nerve appears to course through the mucocele.
D, Coronal T1-weighted (550/14/2) (top) and T2-weighted (3700/105/2) (bottom) fast spin-echo MR images show that the mass (arrow) underlying the optic nerve (arrowhead) is of high signal intensity on the T1-weighted sequence and of intermediate intensity on the T2-weighted sequence.
Case 2
A 61-year-old man with a 1-month history of allergic rhinitis with epistaxis had sudden onset of diplopia and numbness of the forehead over the course of 1 hour. His medical history was otherwise negative except for essential hypertension. Clinical examination revealed loss of sensation of the lower left forehead and scalp in the distribution of the first division of the trigeminal nerve and a left-sided palsy of the fourth cranial nerve. His visual acuity and visual fields were normal.
MR images showed a homogeneous soft-tissue mass measuring 2 cm in maximum diameter adjacent to the left cavernous sinus and extending into the left orbital apex (Fig 2). The lesion was isointense with brain parenchyma on both T1- and T2-weighted sequences, and showed mild peripheral enhancement after administration of contrast material. Mild inflammatory sinus disease was present in the sphenoidal and maxillary antrum on the left, and in the ethmoidal air cells bilaterally. To further characterize the location of the mass, unenhanced axial and coronal CT scans (1-mm section thickness) were obtained, revealing an expansile, destructive soft-tissue mass of the left anterior clinoid process. The mass extended inferiorly and laterally through the superior orbital fissure into the orbital apex (Fig 2E). In addition, a small (5-mm) polypoid soft-tissue mass was noted along the lateral surface of the left posterior ethmoidal and sphenoidal air cells adjacent to the anterior clinoid process. The cortex of the sphenoid bone was thinned and rarefied. The contralateral anterior clinoid process was noted to be normal and not pneumatized. Findings on a chest radiograph and bone scans were normal. Investigations for chronic granulomatous diseases and primary neoplasm were also negative.
fig 2.
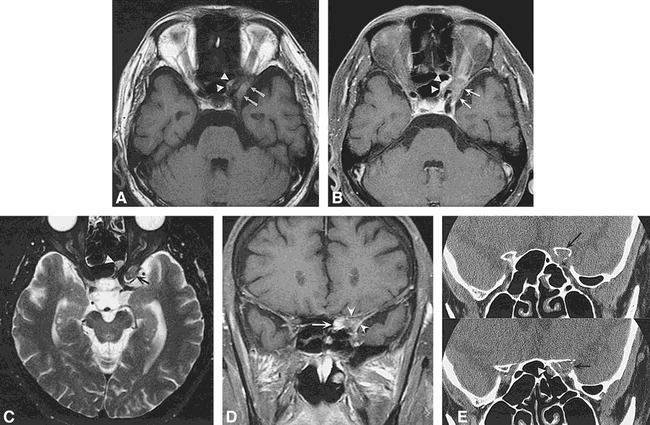
Case 2: 61-year-old man with sudden-onset diplopia and forehead numbness. Fourth cranial nerve palsy as well as V1 sensory loss was found on physical examination.
A, Axial T1-weighted image (600/14/2) shows a lesion of intermediate signal intensity extending laterally (arrows) as well as medially (arrowheads) around the left optic nerve. Note that, as in fig 1C, the optic nerve appears to course through the lesion, a clue to the sinus origin of the mass.
B, Contrast-enhanced T1-weighted (500/20/2) image with fat saturation reveals mild peripheral enhancement (arrows, arrowheads).
C, Axial T2-weighted (4000/105/2) fast spin-echo MR image shows the mass is isointense (arrow) to slightly hyperintense (arrowhead) relative to brain parenchyma. Mild inflammatory sinus disease is present in the ethmoidal air cells bilaterally.
D, Coronal contrast-enhanced T1-weighted (583/14/2) conventional spin-echo sequence with fat saturation shows the enhancing mass (arrow) involving the left anterior clinoid process (arrowheads) extending into the orbital apex.
E, Contiguous 1-mm high-resolution coronal CT scans show a soft-tissue attenuation lesion of the left anterior clinoid process (arrow) extending into the orbital apex. The cortex of the clinoid process is focally expanded and destroyed. A small polypoid soft-tissue mass (arrowhead) is also noted along the lateral surface of the left sphenoidal air cells.
The preoperative diagnosis was a primary, intraosseous, expansile, destructive mass, representing either metastasis or an inflammatory or granulomatous process. The patient underwent exploration via a left frontotemporal orbitozygomatic craniotomy and an extradural approach to the clinoid process. At surgery, a cystic lesion with mucoid greenish liquid was found and resected. The cyst wall consisted of respiratory epithelium at histopathology, but no malignant cells were found. The pathologic diagnosis was mucocele of the anterior clinoid process. The patient made a full recovery of both sensory and motor functions and has returned to work.
Discussion
These two cases represent rare occurrences of mucocele formation in the osseous structures adjacent to the orbital apex. The floor and roof of the orbital apex are formed by the greater and lesser wings of the sphenoid bone, respectively. When these bone structures become pneumatized in the course of normal development, they, like all sinuses, may become involved in obstructive inflammatory disease, such as sinusitis and mucocele formation. There have been a few reports in the literature describing mucoceles of the anterior clinoid process (1–4) but none of mucoceles involving Onodi cells.
A mucocele is a chronic, expansile process that is completely filled with mucoid secretions and lined by respiratory epithelium. Mucoceles most commonly occur in the frontal and anterior ethmoidal sinuses consequent to obstruction of the small ostia by chronic inflammatory disease. They are unusual in the posterior ethmoidal and sphenoidal sinuses. Most patients with sphenoidal and ethmoidal mucoceles present with frontal or orbital headaches or with signs and symptoms referable to cranial nerves II to VI. Optic neuropathy, proptosis, visual loss, and visual field defects can occur, as well as extraocular palsies and sensory deficits of the trigeminal nerve (1).
The MR signal characteristics of a mucocele are variable, and reflect the protein content of the mucoid material (5). The signal intensity of the contents of a mucocele on T1-weighted sequences is initially decreased owing to its high water content. With time, as water is resorbed, the protein content and viscosity are elevated, resulting in signal intensity that is at first isointense and then hyperintense relative to brain on T1-weighted images. On T2-weighted images, the signal intensity of the mucocele contents usually remains high. However, as the contents become chronically inspissated, the signal may decrease on T2-weighted images. Eventually, a signal void, mimicking a normal, aerated sinus may be seen on all sequences (6). Thus, many possible combinations of signal intensities on MR images may be seen with mucoceles, depending on their stage of development, protein content, and imaging parameters (5).
In our two patients, mucoceles of small air cells involving the anterior clinoid processes were found at surgery. In the first patient, the typical bright signal pattern of chronic sinus secretions on both T1- and T2-weighted sequences was easily appreciated within an Onodi cell. An Onodi cell is a posterior ethmoidal air cell that penetrates the sphenoid bone (7). During embryologic development, the sphenoid bone arises from two chondral ossification centers. The sphenoidal sinus develops solely within the lower ossification center, while the upper part unites with the ethmoidal labyrinth. Posterosuperior ethmoidal air cells can thus grow into the body of the upper sphenoid bone and may surround the optic canal and reach the anterior wall of the sella turcica. In our first patient, the Onodi cell extended into the left anterior clinoid process. The prevalence of Onodi cells on CT studies varies from 8% (8) to 13% (9).
In the second patient, air cells also formed around the orbital apex, but these had their origin from recesses of the sphenoidal sinus. The sphenoidal sinus is not limited to the body of the sphenoid bone. Extension of the air-filled sinus into the bony processes of the sphenoid bone occurs regularly enough to be regarded as a typical anatomic phenomenon (10). Pneumatized recesses are commonly found in the greater wing and base of the pterygoid process. Occasionally, there are extensions superiorly into the lesser wing (including the anterior clinoid process), and there may also be anterior and posterior extension into the basilar occipital bone. The sphenoidal diverticulum may grow superiorly into the lesser wing of the sphenoid bone and involve the anterior clinoid process, as well as inferiorly to encompass the optic canal (11). In this patient, the lateral superior air cell recesses were asymmetrical, being present in the lesser wing and anterior clinoid process on the left, and absent on the right side. Subsequent involvement by inflammatory disease resulted in mucocele formation of the left anterior clinoid process. The expansile lesion compressed the orbital apex from a superior direction, accounting for the palsy in cranial nerves V1 and IV, as these nerves are location in the superior aspect of the superior orbital fissure.
The MR signal of the mucocele contents in the second patient was not typical, as in the first patient, since it was almost isointense with gray matter on both T1- and T2-weighted sequences. Furthermore, mild diffuse contrast enhancement was present. This combination of nonspecific MR signal patterns and a contrast-enhancing expansile mass in the anterior clinoid process made it imperative to consider malignant disorders, such as metastasis, myeloma, plasmacytoma, and lymphoma. Meningioma and schwannoma were also considered before CT documented the osseus origin of the mass. In hindsight, this anatomic variant was difficult to identify because of its unilateral position and lack of contralateral aeration of the right anterior clinoid process. A clue to the diagnosis of inflammatory disease in both patients was the presence of mucosal thickening medial to the optic nerve, as seen on axial views (Figs 1C and 2A and C).
By contrast, the mucocele seen in the first patient had the characteristic signal pattern of retained mucous secretions; namely, high signal on T1-weighted images. This made the classic anatomy of an Onodi cell readily apparent. Sagittal images showed an ethmoidal cell occupying the anterosuperior portion of the sphenoid bone (Fig 1B). These features, and concomitant inflammatory sinus disease elsewhere, made the diagnosis relatively straightforward. In both patients, associated sinus inflammatory disease was present, providing supporting evidence for the diagnosis of mucocele.
Conclusion
The bones forming the orbital apex and anterior clinoid process are often aerated either by an Onodi cell or a lateral recess of the sphenoidal sinus. These may in turn become obstructed, leading to expansile mucoceles. It is important to understand these developmental variants, particularly if a mass is detected in these positions. In a patient with signs and symptoms referable to the orbital apex, a mucocele of the paranasal sinus should be considered in the differential diagnosis.
Acknowledgments
We gratefully acknowledge Andrew Murr for his assistance.
Footnotes
Address reprint requests to William P. Dillon, MD, Department of Radiology, Neuroradiology Section, University of California San Francisco, 505 Parnassus Ave, L-371, San Francisco, CA 94143.
References
- 1.Johnson LN, Hepler RS, Yee RD, Batzdorf U. Case reports: sphenoid sinus mucocele (anterior clinoid variant) mimicking diabetic ophthalmoplegia and retrobulbar neuritis. Am J Ophthalmol 1986;102:111-115 [DOI] [PubMed] [Google Scholar]
- 2.Schwaighofer BW, Sobel DF, Klein MV, Zyroff J, Hesselink JR. Mucocele of the anterior clinoid process: CT and MR findings. J Comput Assist Tomogr 1989;13:501-503 [DOI] [PubMed] [Google Scholar]
- 3.Dunya IM, Frangieh GT, Heilman CB, Miranda MR, Rand LI, Hedges TR. Anterior clinoid mucocele masquerading as retrobulbar neuritis. Ophthalmol Plast Reconstr Surg 1996;12:171-173 [DOI] [PubMed] [Google Scholar]
- 4.Matsuoka S, Nishimura H, Kitamura K, Numaguchi Y. Circular enlargement of the optic canal caused by paranasal sinus mucocele. Surg Neurol 1983;19:544-547 [DOI] [PubMed] [Google Scholar]
- 5.Som PM, Dillon WP, Fullerton GD, Zimmerman RA, Rajagopalan B, Marom Z. Chronically obstructed sinonasal secretions: observations on T1 and T2 shortening. Radiology 1989;172:515-520 [DOI] [PubMed] [Google Scholar]
- 6.Dillon WP, Som PM, Fullerton GD. Hypointense MR signal in chronically inspissated sinonasal secretions. Radiology 1990;174:73-78 [DOI] [PubMed] [Google Scholar]
- 7.Lang J. Clinical Anatomy of the Nose, Nasal Cavity and Paranasal Sinuses New York: Thieme; 1989;88-89
- 8.Jones NS, Strobl A, Holland I. A study of the CT findings in 100 patients with rhinosinusitis and 100 controls. Clin Otolaryngol 1997;22:47-51 [DOI] [PubMed] [Google Scholar]
- 9.Meloni F, Mini R, Rovasio S, Stomeo F, Teatini GP. Anatomic variations of surgical importance in ethmoid labyrinth and sphenoid sinus: a study of radiological anatomy. Surg Radiol Anat 1992;14:65-70 [DOI] [PubMed] [Google Scholar]
- 10.Van Alyea OE. Ethmoid labyrinth: anatomic study, with consideration of the clinical significance of its structural characteristics. Arch Otolaryngol 1941;29:891-902 [Google Scholar]
- 11.Lang J. Clinical Anatomy of the Nose, Nasal Cavity and Paranasal Sinuses New York: Thieme; 1989;90-91


