Abstract
Summary: In two patients with surgically proved CSF fistula through the facial nerve canal, MR and CT examinations showed smooth enlargement of the geniculate fossa with CSF signal. In the clinical setting of CSF otorrhea or rhinorrhea, the presence of an enlarged labyrinthine facial nerve canal and enlarged geniculate fossa on CT scans and CSF intensity on MR images strongly suggests a CSF fistula through the facial nerve canal.
Spontaneous CSF fistulas from the subarachnoid space into the middle ear cavity rarely occur via the proximal facial nerve canal (1, 2). Gacek and Leipzig detailed such a case in 1979, accompanied by surgical findings, illustrative drawings, and histopathologic sections (1). In 1983, Phelps briefly described a similar case in which he filled an arachnoid sac in the region of the geniculate fossa with intrathecal iophendylate (2). However, this route of CSF fistula is not widely known, because few, if any, additional cases have been reported subsequently (3). We report two such recent cases, both diagnosed on high-resolution CT scans and confirmed at surgery.
Case Reports
Case 1
A 34-year-old man was referred to his otologist with a history of three episodes of meningitis over the course of 1 year. Earlier, he was thought to have chronic otitis and in retrospect had had one episode of CSF otorrhea from his right ear and one episode of CSF rhinorrhea from his right nostril. Physical examination revealed a polyethylene tube through the right tympanic membrane. Audiographic findings and neurologic status were normal. High-resolution CT of the temporal bone revealed opacification of most of the right mastoid air cells. In addition, the right labyrinthine facial nerve canal was slightly widened and led to a smoothly enlarged geniculate fossa (Fig 1). The osseous inner ear structures were normal. No positive contrast cisternography was carried out.
fig 1.
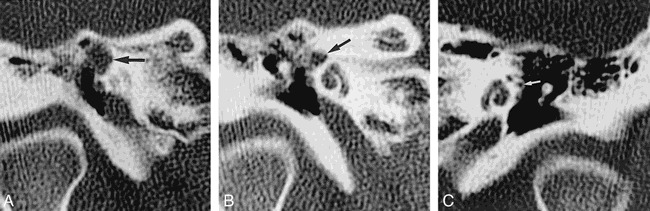
A–C, 34-year-old man with three episodes of meningitis over the course of 1 year. High-resolution coronal temporal bone CT scans of the right ear show smooth enlargement of the right geniculate fossa (arrow, A) and of the labyrinthine facial nerve canal (arrow, B), and a normal labyrinthine facial nerve canal of the left ear (arrow, C)
Through a middle fossa craniotomy, a bulge in the area of the geniculate ganglion was observed. The overlying bone was intact, and upon removal of just a little bit of the bone, CSF was released. Further dissection and inspection of the geniculate fossa revealed that the facial nerve fibers lay free in the geniculate fossa and were separated into multiple filaments. A CSF leak from the cerebellopontine angle via the meatal foramen of the fallopian canal into the geniculate fossa was found and repaired by filling the entire geniculate fossa with a temporalis muscle graft. The patient did well postoperatively, with no facial nerve dysfunction and with resolution of the CSF leak.
Case 2
A 5-year-old boy underwent myringotomy at another institution for chronic left-sided otitis media. At the time of surgery, spontaneous CSF otorrhea was encountered. A CT scan suggested a small defect in the roof of the temporal bone that was surgically sealed with temporalis fascia. Eleven months after the initial repair, the patient had a recurrence of the spontaneous CSF leak from the left ear. High-resolution CT of the temporal bone without intrathecal contrast material revealed smooth enlargement of the pre- and postgeniculate facial nerve canal, including the geniculate fossa (Fig 2A and B). Abnormal soft tissue and/or liquid was also present within the middle ear cavity. A coronal T2-weighted MR image revealed a focus of high signal intensity in the region of the geniculate fossa (Fig 2C). Through a middle fossa craniotomy, upon elevating the dura from the greater superficial petrosal nerve, CSF was seen emanating from the facial hiatus along the greater superficial petrosal nerve. The bony covering of the geniculate fossa was intact, and upon removal of this bone, pulsation of the sheath over the geniculate ganglion was apparent. Removal of the bone from over the enlarged proximal tympanic segment of the facial nerve revealed it to be filled with CSF. The labyrinthine segment of the facial nerve canal was also expanded and filled with CSF. The area of the labyrinthine segment of the facial nerve and geniculate ganglion was successfully tamponaded with temporalis muscle with no loss of facial nerve function postoperatively. The CSF leak resolved after surgery.
fig 2.
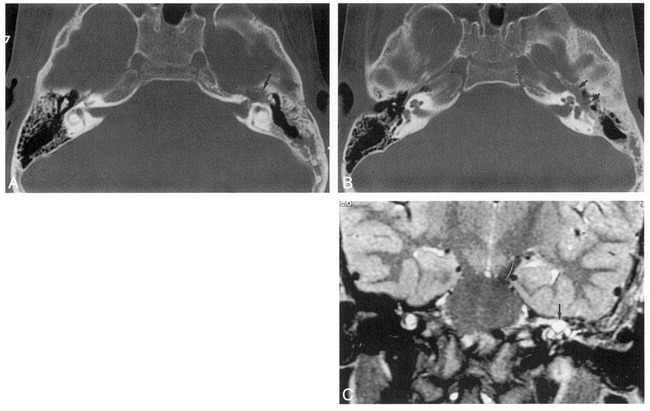
5-year-old boy in whom myringotomy had been performed to correct left-sided otitis media.
A and B, Axial CT scans of the temporal bones show enlarged left geniculate fossa (arrow, A) and enlarged tympanic facial nerve canal (arrows, B).
C, Coronal T2-weighted MR image shows an area of high signal intensity, consistent with CSF superior to the cochlea in the region of the left geniculate fossa (arrow).
Discussion
In patients with recurrent meningitis, one of the possible routes of infection is via an abnormal pathway connecting the CSF space with the middle ear or mastoid air cells. These patients may also have CSF rhinorrhea or CSF otorrhea, depending on the integrity of the ear drum.
The commonly recognized causes of CSF leakage into the middle ear include trauma to the temporal bone and prior surgery with subsequent temporal bone defects and chronic infection (4, 5). Spontaneous CSF fistulas from the subarachnoid space into the middle ear cavity are uncommon and may be classified into two types: those that extend to the middle ear or external canal (perilabyrinthine) and those associated with a developmental anomaly of the cochlea (translabyrinthine) (2, 6).
The more common translabyrinthine variety is nearly always associated with anacusis and a severe labyrinthine dysplasia. The involved cochlea completely lacks a modiolus and thus permits ready communication between the CSF and the perilymphatic spaces and leakage through the oval window or a hole in the stapedial footplate (2, 6, 7). Contrary to popular belief, however, a true Mondini malformation, which consists of an incompletely partitioned cochlea with an intact base of the modiolus, is not a risk factor for spontaneous fistulas (2, 8).
The rarer spontaneous perilabyrinthine fistulas may be congenital or acquired (2). Four potential congenital pathways were proposed by Gacek and Leipzig (1). Two of the four, both rare, have been well documented; namely, those through the proximal facial nerve canal, such as our two cases, and those through the Hyrtl fissure, which is located inferior to the round window niche and extends along the inferior aspect of the cochlea connecting the hypotympanum with the posterior fossa (2). The petromastoid canal, which carries the subarcuate vessels beneath the superior semicircular canal to the periantral mastoid air cells, has been proposed but not proved to be a route for fistulas or meningitis (2, 9).
Fistulization through a dilated cochlear aqueduct has been the subject of considerable controversy (10, 11). The cochlea aqueduct has been considered as a route for an “ooze,” a gentle flow of perilymph and/or CSF that can occur at stapedial surgery, but is never wide enough for a “gush” (12). For years, a wide cochlear aqueduct has been frequently cited as a cause of spontaneous CSF fistula, but under close scrutiny, never satisfactorily documented (2, 11, 13). Recently, Veillon published CT scans quite suggestive of such a case (14). If the fistula enters the middle ear cavity through the cochlear aqueduct and exits through the round or oval window, it should be classified as translabyrinthine.
Spontaneous perilabyrinthine fistulas may also be acquired. These are most likely caused by arachnoid granulations remote from dural sinuses pitting and eroding the petromastoid temporal bone (15, 16). Fistulization through the tegmen or the posterior petromastoid wall may enter petrous apical or mastoid air cells (17–19).
Our two cases of spontaneous fistulas were due to lateral extension of the subarachnoid space through the labyrinthine segments of the facial canal. Normally, the subarachnoid space extends a short distance along the labyrinthine facial nerve, which courses superior to the cochlea, just anterior to the ampullate ends of the lateral and superior semicircular canals (20). Beyond that, the facial nerve becomes tightly ensheathed by the dural extension that accompanies it through the temporal bone. In both our cases, the proximal facial nerve canal was widened by lateral extension of a dilated subarachnoid space as far as the geniculate fossa in case 1 and farther into the proximal tympanic facial nerve canal in case 2. Over time, areas of bony dehiscence may occur and result in a fistula into the epitympanic space. The lack of facial nerve paralysis in either of our patients may be attributed to the gradual stretching of the facial nerve fibers within this subarachnoid space extension.
High-resolution temporal bone CT is the initial examination of choice in the investigation of recurrent meningitis or episodes of otorhinorrhea. The use of intrathecally injected contrast material followed by CT has been advocated as the imaging standard of reference to observe the communication between the basal cisterns and the perilymphatic space or middle ear cavity (21). Preliminary work by Levy et al (22) suggests that newer MR imaging techniques that impart great sensitivity to the motion of fluids may have a role in the evaluation of CSF leaks involving the skull base and temporal bone.
Once the site of CSF fistula has been identified, it must be sealed. The surgical repair technique relies on the insertion of a generous soft-tissue graft (fat, muscle, or fibrous tissue) through the bony defect to fill the enlarged facial nerve canal. Back pressure of the CSF helps to close the bony aperture.
Conclusion
By themselves, the CT findings in our two patients would have raised the possibility of a facial nerve schwannoma, epidermoidoma, or hemangioma (23). However, in the appropriate clinical scenario of recurrent meningitis or CSF otorhinorrhea, the presence of an enlarged labyrinthine facial nerve canal and enlarged geniculate fossa strongly suggests a CSF fistula through the proximal facial nerve canal. The diagnosis may be further strengthened by the MR imaging finding of CSF intensity in the enlarged geniculate fossa, as in case 2. Administration of intrathecal contrast material can be obviated in such situations.
Footnotes
Address reprint requests to Leonard V. Petrus, MD, Department of Radiological Sciences, Olive View-UCLA Medical Center, 14445 Olive View Dr, 2D139, Sylmar, CA 91342.
References
- 1.Gacek RR, Leipzig B. Congenital cerebrospinal otorrhea. Ann Otol 1979;88:358-365 [DOI] [PubMed] [Google Scholar]
- 2.Phelps PD. Congenital cerebrospinal fluid fistulae of the petrous temporal bone. Clin Otolaryngol 1986;11:79-92 [DOI] [PubMed] [Google Scholar]
- 3.May JS, Mikus JL, Matthews BL, Browne JD. Spontaneous cerebrospinal fluid otorrhea from defects of the temporal bone: a rare entity? Am J Otol 1995;16:765-771 [PubMed] [Google Scholar]
- 4.Gundersen T, Haye R. Cerebrospinal otorrhea. Arch Otolaryngol 1970;91:19-23 [DOI] [PubMed] [Google Scholar]
- 5.Harrington JW Jr, Birck HG. Recurrent meningitis due to congenital petrous fistula: a case report. Arch Otolaryngol 1967;85:572-575 [DOI] [PubMed] [Google Scholar]
- 6.Carter BL, Wolpert JW, Karmody CS. Recurrent meningitis associated with an anomaly of the inner ear. Neuroradiology 1975;9:55-61 [Google Scholar]
- 7.Jensen J. Congenital anomalies of the inner ear. Radiol Clin North Am 1974;12:473-482 [PubMed] [Google Scholar]
- 8.Phelps PD, Lloyd GAS. Mondini dysplasia is not associated with meningitis and cerebrospinal fluid fistula (letter). . Arch Otolaryngol Head Neck Surg 1991;117:931. [DOI] [PubMed] [Google Scholar]
- 9.Schuknecht HF. Pathology of the Ear Cambridge, MA: Harvard University Press; 1974
- 10.Phelps PD. Dilation of the cochlear aqueduct: another otological myth? Clin Otolaryngol 1993;18:339-340 [DOI] [PubMed] [Google Scholar]
- 11.Jackler RK, Luxford WM, House WF. Congenial malformation of the inner ear: a classification based on embryogenesis. Laryngoscope 1987;97:2-14 [DOI] [PubMed] [Google Scholar]
- 12.Schuknecht HF, Reisser C. The morphologic basis for perilymphatic gushers and oozers. Adv Otorhinolaryngol 1988;39:1-12 [DOI] [PubMed] [Google Scholar]
- 13.Jackler RK, Hwang PH. Enlargement of the cochlear aqueduct: fact or fiction? Otolaryngol Head Neck Surg 1993;109:14-25 [DOI] [PubMed] [Google Scholar]
- 14.Veillon F. Imagerie de l'Oreille. Medecine-Sciences Paris: Hammarion; 1991;233
- 15.Gacek RR. Arachnoid granulation cerebrospinal fluid otorrhea. Ann Otol Rhinol Laryngol 1990;99:854-862 [DOI] [PubMed] [Google Scholar]
- 16.Gacek RR. Evaluation and management of temporal bone arachnoid granulations. Arch Otolaryngol Head Neck Surg 1992;118:327-332 [DOI] [PubMed] [Google Scholar]
- 17.Kaufman B, Nulsen FE, Weiss MH, Brodkey JS, White RJ, Sykora CF. Acquired spontaneous, nontraumatic normal-pressure cerebrospinal fluid fistulas originating from the middle fossa. Radiology 1977;122:379-387 [DOI] [PubMed] [Google Scholar]
- 18.Gavilan J, Trujillo M, Gavilan C. Spontaneous encephalocele of the middle ear. Arch Otolaryngol 1984;110:206-207 [DOI] [PubMed] [Google Scholar]
- 19.Kraus EM, McCabe BF. The giant apical air cell syndrome: a new entity. Ann Otol Rhinol Laryngol 1982;91:237-239 [DOI] [PubMed] [Google Scholar]
- 20.Gacek RR. Anatomy and significance of the subarachnoid space in the fallopian canal. Am J Otol 1998;19:358-365 [PubMed] [Google Scholar]
- 21.Lovblad KO, Ozdoba C, Negri S, et al. CT cisternography in congenital perilymphatic fistula of the inner ear. J Comput Assist Tomogr 1995;19:797-799 [DOI] [PubMed] [Google Scholar]
- 22.Levy LM, Gulya J, Davis SW, LeBihan D, Rajan SS, Schellinger D. Flow sensitive magnetic resonance imaging in the evaluation of cerebrospinal fluid leaks. Am J Otol 1995;16:591-596 [PubMed] [Google Scholar]
- 23.Fisch U, Ruttner J. Pathology of infratemporal tumor involving the facial nerve. In: Fisch U, ed. Facial Nerve Surgery Birmingham, AL: Aesculapius; 1977;448-456


