Abstract
Summary: Spontaneous thrombosis of a posterior fossa developmental venous anomaly (DVA) caused a nonhemorrhagic cerebellar infarct in a 31-year-old man who also harbored a midbrain cavernous angioma. DVA thrombosis was well depicted on CT and MR studies and was proved at angiography by the demonstration of an endoluminal clot.
Developmental venous anomaly (DVA), formally known as venous angioma, is a congenital anatomic variant of the venous drainage of the brain (1, 2). Its clinical relevance and relationship to other vascular lesions, mainly cavernous angiomas, is still debated (1–7).
Case Report
A 31-year-old man was first seen in 1989 at the age of 24 years, when he sought treatment for a headache of acute onset, diplopia, and numbness of the left side of the face and left arm. A right sixth nerve palsy was noted on physical examination. MR examination showed a lesion of mixed signal intensity on both T1- and T2-weighed images that was consistent with a right-sided lower midbrain cavernous angioma. The venous phase of the left vertebral angiogram showed bilateral cerebellar DVAs with deep collectors joining the vein of Galen via the precentral veins. The symptomatology was attributed to the cavernous angioma and no specific treatment was proposed.
The patient recovered and was well until November 1996, when he presented with severe headache associated with vomiting, ataxia, and right-sided facial palsy. His level of consciousness deteriorated rapidly, and he was intubated upon arrival at the hospital. His Glasgow coma score was 8/15. A left-sided hemiparesis was noted. A noncontrast CT scan showed tubular hyperdensities in the posterior fossa associated with right-sided cerebellar edema and acute hydrocephalus (Fig 1A and B). A ventricular shunt was inserted. Bilateral vertebral angiography showed delayed opacification of the right DVA as compared with the left DVA. A filling defect was clearly seen in the venous collector of the right DVA (Fig 1C). MR imaging performed 1 week later revealed an acute subcortical cerebellar infarct in the edematous state involving the right hemisphere as well as ischemic lesions in the pons (Fig 1D). High signal intensity on both noncontrast T1- and T2-weighed images was present in the collector of the right DVA (Fig 1E). The midbrain cavernous angioma was unchanged and without surrounding edema.
fig 1.
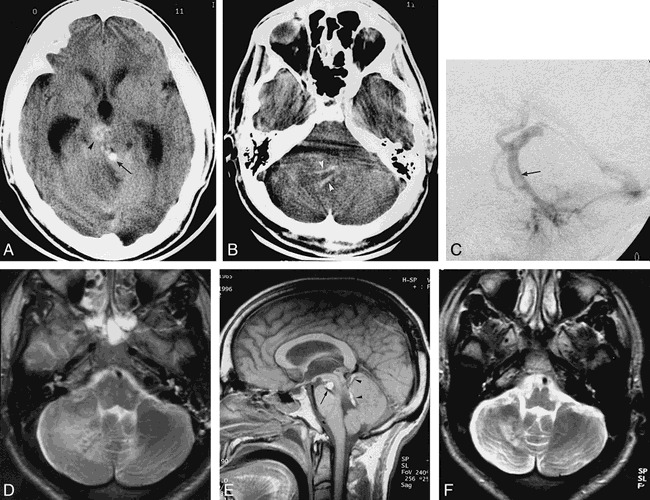
31-year-old man with severe headache, vomiting, ataxia, and right-sided facial palsy.
A, Unenhanced CT scan at the level of the midbrain shows a spontaneously dense tubular structure (arrow) in front of the vermis, later proved to be the thrombus in the DVA collector. Arrowhead points to the cavernous angioma. There is edema of the right cerebellum and secondary obstructive hydrocephalus.
B, Unenhanced CT scan at the level of the pons shows tubular high-density structures (arrowheads) corresponding to the thrombus in the branches of the DVA.
C, Late venous phase of left vertebral arteriogram proves the presence of a clot inside the DVA collector (arrow).
D, Axial T2-weighed MR image at the level of the pontomedullary junction reveals nonhemorrhagic subcortical infarction of the right cerebellum.
E, On unenhanced sagittal T1-weighed MR image, high signal intensity corresponds to subacute clot inside the DVA collector (arrowheads) and correlates well with angiographic findings (C). Arrow points to the midbrain cavernous angioma.
F, Axial T2-weighed MR image obtained in April 1998 confirms the subcortical infarction.
The patient slowly recovered to become independent at 1 year, but was left with a right sixth nerve palsy, slight ataxia, right-sided dysmetria, and right vocal cord hypomotility. Late follow-up MR imaging (April 1998) confirmed the cerebellar infarct (Fig 1F).
Discussion
DVA represents a dilatation of a transcerebral vein that acts as a collector for small venules; it is thought to develop in response to the absence of one of the usual venous drainage routes (1). The appearance of a DVA on angiography is synchronous with the normal venous phase. It drains normal brain and the underdevelopment of the usual pattern is clearly visible in most cases (1, 2). A DVA may drain a cortical area toward the deep venous system or a deep area toward the superficial venous system (1, 2). In the posterior fossa, most DVAs drain centrally to exit through the veins of the lateral recess of the fourth ventricle, a transpontine vein, the precentral veins, or the longitudinal intrategmental vein (8). DVAs have a characteristic appearance on CT and MR studies, and angiography is seldom needed (9).
There is still controversy regarding the clinical significance of DVAs. Once thought to be a rare lesion with a high propensity for hemorrhage (5, 6), DVAs are now recognized as the most frequent cerebral vascular anomaly and are rarely symptomatic (1–4, 7). Hemorrhagic complications have been reported (6, 10, 11), but the frequent association with cavernous angiomas (4, 7) creates confusion as to the origin of the hemorrhage. Many authors (4, 7) suggest looking for cavernous angiomas with MR imaging before attributing hemorrhage to the DVA, which may be more evident at first on imaging studies. Other authors (5, 6) are convinced that DVAs do hemorrhage and that they should be removed surgically when accessible. Another recent hypothesis suggests that most cavernous angiomas represent old hematomas secondary to a DVA that could show autonomous growth by way of some angiogenetic factors or repeated hemorrhages (4, 7). In our patient, the associated midbrain cavernous angioma was not located within the territory of the DVA, and all the venules contributing to the caput medusae were located in the cerebellum, which does not support this hypothesis.
Seizures and other nonhemorrhagic events have also been associated with DVAs (2, 12–14). Spontaneous thrombosis of DVAs have been reported (10, 11, 14, 15), but, to our knowledge, this is the first demonstration of the clot as a filling defect within the collector of the DVA on angiography and the first presentation with a pure nonhemorrhagic infarct. Thrombosis led to nonhemorrhagic infarction probably because of early partial recanalization of the vein. Infarction of the territory drained by the DVA supports the fact that the DVA is essential for the venous drainage of the brain and that surgical resection should generally be avoided (1, 3, 7).
Thrombosis of the DVA was clearly responsible for the ataxia and acute hydrocephalus related to cerebellar swelling that occurred in the second clinical episode, although the appearance of the cavernous angioma on both CT and MR studies may suggest acute hemorrhage. We doubt that two events related to two different lesions would become symptomatic at the same time. We did not find any coagulation disorder in this patient. DVAs may represent a vulnerable anatomic situation prone to hemorrhage or thrombosis, even if they are essential to the venous drainage (1).
Stenosis at the dural opening of a DVA has been reported (2) and may cause venous hypertension with secondary rupture or stagnation leading to thrombosis. Hemorrhagic events may be preceded by thrombosis and, as such, represent hemorrhagic infarctions (10, 11). If this hypothesis is true, anticoagulation could be suggested for symptomatic DVAs (11), as it is recommended for the management of cerebral thrombophlebitis even in the presence of hemorrhage. We were not able to document a structural venous stenosis in our patient. Considering the rarity of this clinical presentation and the presence of another lesion potentially at risk for brain stem hemorrhage, anticoagulation therapy was not considered appropriate in this patient.
Conclusion
Spontaneous thrombosis of a large DVA, although rare, may lead to nonhemorrhagic infarction. DVAs are essential for venous drainage of the brain and surgical resection is contraindicated. Even though they are most frequently considered insignificant anatomic variants, DVAs are rarely the cause of ischemic or hemorrhagic complications.
Footnotes
Address reprint requests to Daniel Roy, MD.
References
- 1.Lasjaunias P, Burrows P, Planet C. Developmental venous anomalies (DVA): the so-called venous angioma. Neurosurg Rev 1986;9:233-242 [DOI] [PubMed] [Google Scholar]
- 2.Truwit CL. Venous angioma of the brain: history, significance, and imaging findings. AJR Am J Roentgenol 1992;159:1299-1307 [DOI] [PubMed] [Google Scholar]
- 3.Garner TB, Del Curling O, Kelly DL, Laster DW. The natural history of intracranial venous angiomas. J Neurosurg 1991;75:715-722 [DOI] [PubMed] [Google Scholar]
- 4.Wilms G, Bleus E, Demaerel P, et al. Simultaneous occurrence of developmental venous anomalies and cavernous angiomas. AJNR Am J Neuroradiol 1994;15:1247-1254 [PMC free article] [PubMed] [Google Scholar]
- 5.Lupret V, Negovetic L, Smiljanic D, Klanfar Z, Lambasa S. Cerebral venous angiomas surgery as a mode of treatment for selected cases. Acta Neurochir (Wien) 1993;120:33-39 [DOI] [PubMed] [Google Scholar]
- 6.Malik GM, Morgan JK, Boulos RS, Ausman JI. Venous angiomas: an underestimated cause of intracranial hemorrhage. Surg Neurol 1988;30:350-358 [DOI] [PubMed] [Google Scholar]
- 7.McCormick PW, Spetzler RF, Johnson PC, Drayer BP. Cerebellar hemorrhage associated with capillary telangiectasia and venous angioma: a case report. Surg Neurol 1993;39:451-457 [DOI] [PubMed] [Google Scholar]
- 8.Damiano TR, Truwit CL, Dowd CF, Symonds DL. Posterior fossa venous angiomas with drainage through the brain stem. AJNR Am J Neuroradiol 1994;15:643-652 [PMC free article] [PubMed] [Google Scholar]
- 9.Zouaoui A, Maillard JC, Ganthier V, Chedid G, Dangeard S. L'imagerie moderne dans les angiomes veineux cérébraux. J Neuroradiol 1995;22:86-102 [PubMed] [Google Scholar]
- 10.Field LR, Russell EJ. Spontaneous hemorrhage from a cerebral venous malformation related to thrombosis of the central draining vein: demonstration with angiography and serial MR. AJNR Am J Neuroradiol 1994;16:1885-1888 [PMC free article] [PubMed] [Google Scholar]
- 11.Merten CL, Knitelius HO, Hedde JP, Assheuer J, Bewermeyer H. Intracerebral haemorrhage from a venous angioma following thrombosis of a draining vein. Neuroradiology 1998;40:15-18 [DOI] [PubMed] [Google Scholar]
- 12.Pelz DM, Vinuela F, Fox AJ. Unusual radiologic and clinical presentations of posterior fossa venous angiomas. AJNR Am J Neuroradiol 1983;4:81-84 [PMC free article] [PubMed] [Google Scholar]
- 13.Burke L, Berenberg RA, Kim KS. Choreoballismus: a nonhemorrhagic complication of venous angiomas. Surg Neurol 1984;14:245-248 [DOI] [PubMed] [Google Scholar]
- 14.Kim P, Castellani R, Tresser N. Cerebral venous malformation complicated by spontaneous thrombosis. Childs Nerv Syst 1996;12:172-175 [DOI] [PubMed] [Google Scholar]
- 15.Bouchacourt E, Carpena JP, Bories J, Koussa A, Chiras J. Accident ischémique par thrombose d'un angiome veineux: a propos d'un cas. J Radiol 1986;67:631-635 [PubMed] [Google Scholar]


