Abstract
BACKGROUND AND PURPOSE: Currently available data on pituitary volume have been based on indirect methods of measurement and are mostly limited to adult populations. The purpose of this study was to determine the normal development of pituitary volume by means of direct measurements made on thin-section 3D MR acquisitions.
METHODS: The volume of the normal pituitary gland in children and adolescents was measured by using 3D MR sequences with a section thickness of 0.6 to 0.75 mm. The clinical study group consisted of 199 pediatric patients with clinically normal pituitary function and no abnormal findings on routine MR studies. The volume of the posterior pituitary was also measured in all the subjects.
RESULTS: A phantom study revealed measurement errors within 25%. In the clinical study, the measured pituitary volumes showed a growth spurt during puberty, which was more prominent in girls. Posterior pituitary volumes showed gradual growth without such a spurt. Among 5- to 9-year-olds, the posterior pituitary volumes were significantly larger for boys than for girls.
CONCLUSION: Normal development of the pituitary gland and posterior pituitary was determined by means of 3D MR volumetry. With this technique, we found a gender difference in the volume of the posterior pituitary.
MR imaging has been shown to be useful for examining pituitary gland morphology owing to its excellent contrast resolution and high spatial resolution. In addition, T1-weighted sequences permit evaluation of the hyperintense signal of the posterior pituitary (HSPP), although the origin of this signal remains controversial (1, 2).
Several authors have reported the volume of the pituitary gland as measured on MR images (3–6), although they calculated such volumes by indirect methods using 2D images and various formulas (eg, volume = ½ × length × width × height; originally used in a volumetric study based on plain radiographs of the sella) (7). More recently, some authors have reported direct measurement using thin-section 2D (8, 9) or 3D (10) MR sequences. However, most of these studies have only dealt with adult populations, except for that of Sharafuddin et al (6), which described the volumes in 17 children as a control group in a study of central precocious puberty. To our knowledge, no attempt has been made to measure the volume of the human posterior pituitary. We used thin-section 3D MR sequences to directly measure the volume of the normal pituitary gland in 199 children. The purpose of this study was to assess the normal volumetric development of the pituitary gland with special consideration to that of the posterior pituitary.
Methods
MR Acquisition
MR examinations were performed using a 1.0-T system with a quadrature head coil. Thin-section volumetric studies were obtained with 3DFT turbo field-echo sequences with a preparation inversion pulse, using the following parameters: 13/6/2 (TR/TE/excitations), inversion time of 600, 25° flip angle, 220-mm field of view, 256 × 205 matrix, and 50 partitions with a 0.9 × 1.1-mm in-plane resolution.
The images were reconstructed using a technique called overcontiguous sections (OS), based on interpolation in the direction of section selection, which produces images with a section thickness of half that in the original data.
Two different volumetric sequences with different section thicknesses were used in both the phantom and clinical studies: sequence A, with a 60-mm slab and 0.6-mm OS, and sequence B, with a 75-mm slab and 0.75-mm OS. These sequences required 4 minutes 56 seconds and 4 minutes 51 seconds, respectively.
Phantom Study/Volume Measurement
Twelve phantoms, six large (simulating a whole pituitary, volumes ranging from 157.9 to 523.7 mm3, mean 386.0 mm3) and six small (simulating a posterior pituitary, volumes ranging from 33.6 to 81.5 mm3, mean 54.9 mm3), were made from wax. Each phantom was fixed in a plastic box filled with water, and then examined using coronal thin-section 3D sequences A and B, as described above.
The MR image data were processed using an Easy Vision CT/MR workstation (Release 2, Philips Medical Systems, Eindhoven, The Netherlands). From each set of volumetric data, sagittal multiplanar reconstructed (MPR) images were created with section intervals of 1 mm and a magnification factor of 8. On these MPR images, the volume of interest was determined by manual tracing with a mouse-guided cursor. The volume of the object was then calculated by counting voxels.
All the volume measurements were performed independently by two neuroradiologists to evaluate interobserver agreement. In this way, four volume determinations were obtained for each phantom, with a total of 48 measurements.
Clinical Study
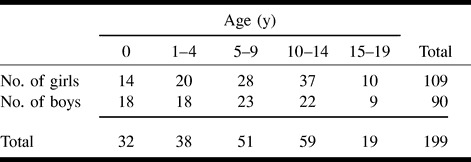
The clinical study group consisted of 199 Japanese children who ranged in age from 0 to 19 years (mean, 7.4 years; Table 1). All patients who were thought to have an endocrinologic abnormality, a history of asphyxia or shot-term delivery at less than 35 weeks, or abnormal findings on a routine MR examination were excluded from the study.
TABLE 1:
Age distribution and sex of the subjects
Thin-section 3D MR sequences were acquired with the same parameters as in the phantom study, using sequence A (0.6-mm OS) in 77 cases (39%) and sequence B (0.75-mm OS) in 122 cases (61%). In the clinical study, all the volume measurements were performed by one neuroradiologist.
In most cases (163 cases, 82%), data acquisition was performed in the coronal plane, since flow-related artifacts arising from the dural sinuses and the internal carotid arteries are the least visible in this projection. In the remaining cases, the volumes were obtained from transverse (17 cases, 9%) or sagittal (19 cases, 10%) acquisitions. In all patients, volume measurements were performed using sagittal MPR images (Fig 1), since the boundary between the anterior and posterior pituitary was most easily determined in this orientation.
fig 1.
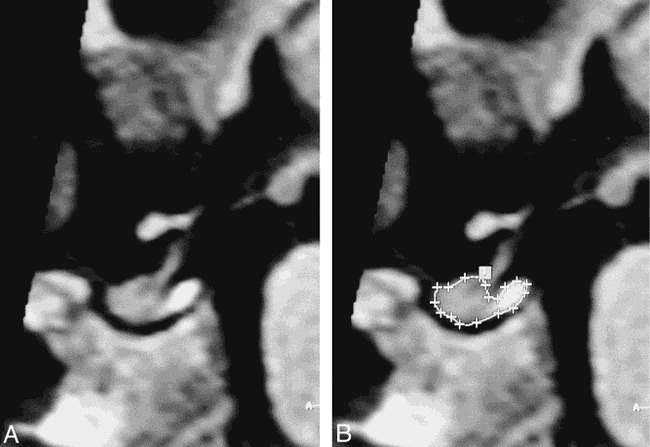
A and B, Midline sagittal reconstructed images in a 2-year-old girl. The outline of the pituitary has been traced in B.
The volumes of the intrasellar HSPP were also measured in all subjects. The Mann-Whitney U-test was used to evaluate gender differences in pituitary development. Pearson's correlation coefficient was used to assess interobserver agreement in the phantom study.
Results
Phantom Study
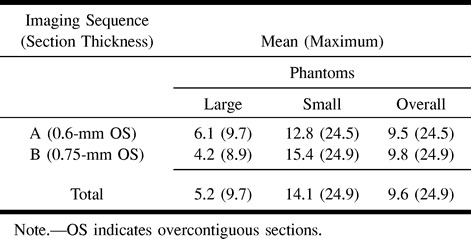
The measurement errors are summarized in Table 2. There was no statistically significant difference in errors between the two volumetric sequences obtained with different numbers and thicknesses of partitions. The interobserver correlation coefficient was .771.
TABLE 2:
Measurement error (absolute value, %) in the phantom study
Clinical Study
The boundary between the anterior and posterior pituitary was determined in all subjects. The measured volumes of the whole and posterior pituitary are shown in Table 3 and in Figures 2 and 3.
TABLE 3:
Volumes of the whole and posterior pituitary
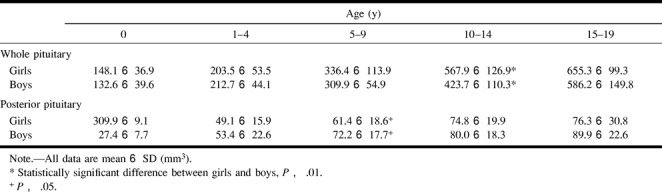
fig 2.
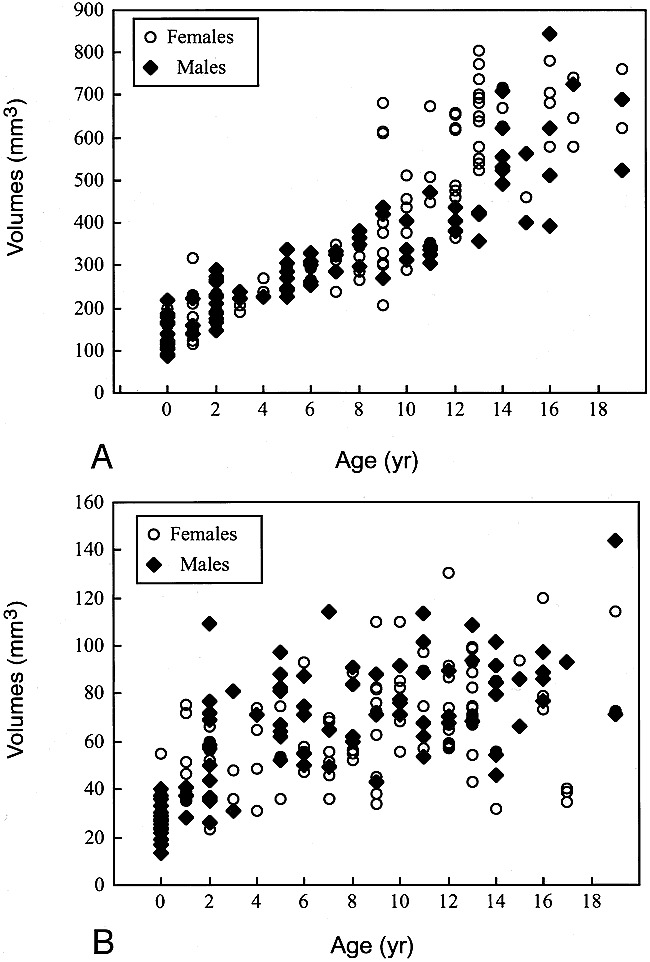
Pituitary volumes by patients' age and gender.
A, Whole pituitary.
B, Posterior pituitary.
fig 3.
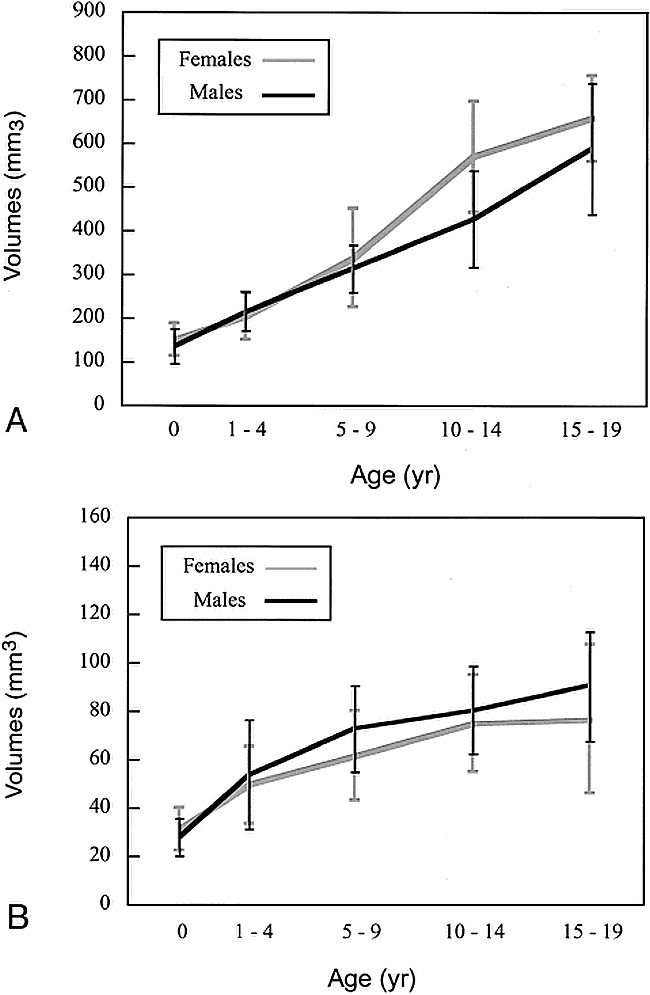
Pituitary volumes by patients' age and gender. Values are means ± SD.
A, Whole pituitary.
B, Posterior pituitary.
The whole pituitary showed a growth spurt in subjects in the early teenage years, and this change was more prominent in girls. In this age group, the pituitary volume in girls was significantly larger than that in boys (P < .01) (Table 3).
On the other hand, posterior pituitary volumes showed gradual growth without such a spurt. The volume of the posterior pituitary tended to be smaller in girls, and was significantly smaller (P < .05) in the 5- to 9-year-old age group (Table 3).
Discussion
CT and MR findings of physical hyperplasia of the pituitary gland during puberty have been reported previously (11–14). Since growth is much more prominent in girls, the pituitary gland is much larger in teenage girls than in teenage boys. This pubertal hyperplasia has been thought to reflect the physical hypersecretion of pituitary hormones at puberty (13, 15, 16). However, these earlier findings were based on linear parameters (eg, height and width of the pituitary gland) or planimetry on 2D images. Since the pituitary gland develops dynamically during puberty, a more accurate method of measurement may be needed to distinguish between a normal- and abnormal-sized pituitary. For this reason, we used thin-section 3D MR sequences, on which the pituitary volume can be measured directly. Our phantom study indicated that this 3D technique could yield acceptable results (Table 2). Growth spurts and gender differences in pituitary volume were clearly demonstrated in the present clinical study (Table 3, Figs 2 and 3). Thus, measurement of pituitary volume using this 3D technique should permit a more precise assessment of the developing pituitary gland in children and adolescents. Although the data are limited to a Japanese population and may not be extrapolated to all groups, the results can probably serve as a standard of reference for pituitary measurements in pediatric patients.
In the present study, the volumes of HSPP showed gradual growth without a spurt. In addition, these volumes were significantly larger in boys than in girls aged 5 to 9 years (Table 3, Figs 2 and 3). To our knowledge, these findings have not been reported previously. Although it is difficult to interpret this gender difference, one possible explanation is that development of the posterior lobe is mechanically inhibited by the rapidly growing anterior lobe, which develops more prominently in girls than in boys. However, this assumption is insufficient to explain the gender difference, since the difference was significant only in younger subjects (aged 5 to 9 years).
Recently, Swaab and Fliers reported a gender difference in the shape of the vasopressin-containing subnucleus of the suprachiasmatic nucleus (17). Furthermore, they also reported that the number of cells in the sexually dimorphic nucleus of the preoptic area is greater in boys than in girls, and this gender difference becomes apparent between 4 years of age and puberty (17, 18). Thus, gender differences in structures in the hypothalamus, which is part of the hypothalamoneurohypophyseal system that includes the posterior lobe, have been observed. Although further investigation is needed to assess the anatomic and physiological relationship between the hypothalamus and posterior lobe, we consider that the gender difference in HSPP volume may be related to that in the hypothalamus.
There is an apparent consensus that the HSPP reflects the functional state of the posterior pituitary, although its origin remains controversial. The HSPP is absent in patients with various endocrinologic abnormalities, such as central diabetes insipidus (1, 19). Furthermore, the volume of the HSPP in cats could be modulated by pharmacologic manipulations that influence vasopressin biosynthesis (2). Therefore, measurement of human HSPP volume may provide useful information for assessing developmental abnormalities related to the hypothalamoneurohypophyseal system.
Conclusion
Normal development of the pituitary gland and posterior pituitary was determined by using 3D MR volumetry. With this technique, we found a small but statistically significant gender difference in the volume of the posterior pituitary.
Acknowledgments
We thank Hiroyuki Hirashima and Toshiro Inoue for their technical assistance and Marc Van Cauteren for his advice on the manuscript.
Footnotes
Address reprint requests to Koichi Takano, MD, Department of Radiology, Fukuoka University Chikushi Hospital, 377–1 Oaza Zokumyoin, Chikushino 818–0067, Japan.
References
- 1.Fujisawa I, Nishimura K, Asato R, et al. Posterior lobe of the pituitary in diabetes insipidus: MR findings. J Comput Assist Tomogr 1987;11:221-225 [DOI] [PubMed] [Google Scholar]
- 2.Kucharczyk J, Kucharczyk W, Berry I, et al. Histochemical characterization and functional significance of the hyperintense signal on MR images of the posterior pituitary. AJR Am J Roentgenol 1989;152:153-157 [DOI] [PubMed] [Google Scholar]
- 3.Lurie SN, Doraiswamy PM, Husain MM, et al. In vivo assessment of pituitary gland volume with magnetic resonance imaging: the effect of age. J Clin Endocrinol Metab 1990;71:505-508 [DOI] [PubMed] [Google Scholar]
- 4.Gonzalez JG, Elizondo G, Saldivar D, Nanez H, Todd L, Villarreal JZ. Pituitary gland growth during normal pregnancy: an in vivo study using magnetic resonance imaging. Am J Med 1988;85:217-220 [DOI] [PubMed] [Google Scholar]
- 5.Krishnan KRR, Doraiswamy PM, Lurie SN, et al. Pituitary size in depression. J Clin Endocrinol Metab 1991;72:256-259 [DOI] [PubMed] [Google Scholar]
- 6.Sharafuddin MJA, Luisiri A, Garibaldi LR, et al. MR imaging diagnosis of central precocious puberty: importance of changes in the shape and size of the pituitary gland. AJR Am J Roentgenol 1994;162:1167-1173 [DOI] [PubMed] [Google Scholar]
- 7.DiChiro GD, Nelson KB. The volume of the sella turcica. AJR Am J Roentgenol 1962;87:989-1008 [PubMed] [Google Scholar]
- 8.Axelson DA, Doraiswamy PM, Boyko OB, et al. In vivo assessment of pituitary volume with magnetic resonance imaging and systematic stereology: relationship to dexamethasone suppression test results in patients. Psychiatry Res 1992;44:63-70 [DOI] [PubMed] [Google Scholar]
- 9.Teoh SK, Mendelson JH, Woods BT, et al. Pituitary volume in men with concurrent heroin and cocaine dependence. J Clin Endocrinol Metab 1993;76:1529-1532 [DOI] [PubMed] [Google Scholar]
- 10.Schwartz PJ, Loe JA, Bash CN, et al. Seasonality and pituitary volume. Psychiatry Res 1997;74:151-157 [DOI] [PubMed] [Google Scholar]
- 11.Peyster RG, Hoover ED, Viscarello RR, Moshang T, Haskin ME. CT appearance of the adolescent and preadolescent pituitary gland. AJNR Am J Neuroradiol 1983;4:411-414 [PMC free article] [PubMed] [Google Scholar]
- 12.Hayakawa K, Konishi Y, Matsuda T, et al. Development and aging of brain midline structures: assessment with MR imaging. Radiology 1989;172:171-177 [DOI] [PubMed] [Google Scholar]
- 13.Elster AD, Chen MYM, Williams DW III, Key LL. Pituitary gland: MR imaging of physiologic hypertrophy in adolescence. Radiology 1990;174:681-685 [DOI] [PubMed] [Google Scholar]
- 14.Argyropoulou M, Perignon F, Brunelle F, Brauner R, Rappaport R. Height of normal pituitary gland as a function of age evaluated by magnetic resonance imaging in children. Pediatr Radiol 1991;21:247-249 [DOI] [PubMed] [Google Scholar]
- 15.Lee PA, Xenakis T, Winer J, Matsenbaugh S. Puberty in girls: correlation of serum levels of gonadotropins, prolactin, androgens, estrogens, and progestins with physical changes. J Clin Endocrinol 1976;43:775-784 [DOI] [PubMed] [Google Scholar]
- 16.Martha PM Jr, Reiter EO. Pubertal growth and growth hormone secretion. Endocrinol Metab Clin North Am 1991;20:165-182 [PubMed] [Google Scholar]
- 17.Swaab DF, Fliers E. A sexually dimorphic nucleus in the human brain. Science 1985;228:1112-1115 [DOI] [PubMed] [Google Scholar]
- 18.Swaab DF, Hofman MA. Sexual differentiation of the human hypothalamus: ontogeny of the sexually dimorphic nucleus of the preoptic area. Dev Brain Res 1988;44:314-318 [DOI] [PubMed] [Google Scholar]
- 19.Colombo N, Berry I, Kucharczyk J, et al. Posterior pituitary gland: appearance on MR images in normal and pathologic states. Radiology 1987;165:481-485 [DOI] [PubMed] [Google Scholar]


