Abstract
BACKGROUND AND PURPOSE: Our purpose was to describe a variant of the carotid string sign that may be associated with a completely occluded vessel and to consider possible pathophysiological mechanisms for this observation.
METHODS: Carotid angiography was performed in three patients with suspected carotid stenosis and in a fourth with carotid dissection. Surgery was performed in one of the patients with carotid stenosis.
RESULTS: On all angiograms, instead of a single linear or curvilinear contrast “string,” either single or multiple serpiginous channels were seen. In one case, such a channel was seen emanating from below the origin of an occluded internal carotid stump, reconstituting the distal portion of the vessel. Surgery revealed a completely occluded lumen with a small intramural vessel bypassing the obstruction.
CONCLUSION: We propose that these channels are either atherosclerotically induced neovessels connecting bridging vasa vasorum or recanalized luminal thrombus. We review the literature associated with this subject.
The term carotid string sign (1), otherwise known as slim sign (2), atheromatous pseudoocclusion (3), and nearly occluded internal carotid artery (ICA) (4), has been used to describe very high grade carotid stenosis with a long, thin, barely discernible poststenotic segment. Although most cases are caused by atherosclerosis, the differential diagnosis includes carotid dissection, radiation-induced narrowing, subacute partial thrombosis, chronic subtotal thrombosis (5), narrowing associated with petrositis, ICA narrowing due to arterial spasm, ICA contrast layering, and subintimal injection (4). To the vascular surgeon, neurosurgeon, or interventional radiologist, the presence of this sign usually signifies that the ICA is not occluded, and is potentially salvageable via carotid endarterectomy or carotid stenting (personal communication, D. Rosso, S. Lownie).
We describe a variant of the string sign, associated with a completely occluded carotid artery, which currently is not treated via endarterectomy. To our knowledge, there is one previous case report and two brief comments in major textbooks that suggest that similar angiographic findings represent either collateralization through the vasa vasorum (6, 7) or recanalization of an occluded carotid artery (8). In this article we present four more cases and possibly the first surgical evidence to support collateralization (as opposed to recanalization). Comparison with previous pathologic studies done on the aorta, carotid, and coronary arteries suggests that these pathways may travel through a network of atherosclerotically induced neovessels and/or neovessels connecting bridging vasa vasorum to the lumen.
Methods
Carotid angiography was performed in all patients using ioxaglate meglumine (Hexabrix 220) contrast medium with an injection rate of 7 mL/s for a total volume of 8 mL. The patient population was as follows: a 60-year-old man who had had two prior left hemispheric cerebrovascular accidents (CVAs) (case 1, Fig 1); a 64-year-old woman who had had one right hemispheric CVA, with right carotid endarterectomy performed 1 month later (case 2, Fig 2); a 66-year-old man who had had three right hemispheric transient ischemic attacks (TIAs) and one right hemispheric CVA, in whom angiograms obtained 4 years earlier showed complete occlusion of the right internal carotid artery at its origin (case 3, Fig 3); and a 39-year-old man who had had a right hemispheric TIA and a large right hemispheric infarct 3 days later, in whom an angiogram showed a dissected and occluded right ICA with only limited collateral retrograde flow through the right ophthalmic artery (a repeat angiogram was performed 5 months later) (case 4, Fig 4).
fig 1.
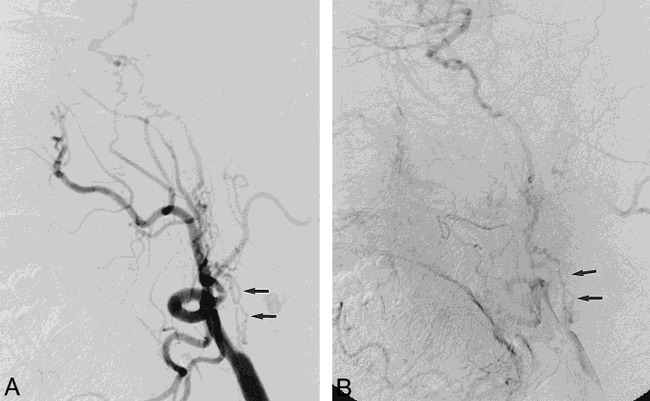
Lateral left carotid angiograms in 60-year-old man with two prior CVAs.
A, Early phase shows multiple serpiginous vessels projected over expected course of ICA (arrows).
B, Later phase of A.
fig 2.
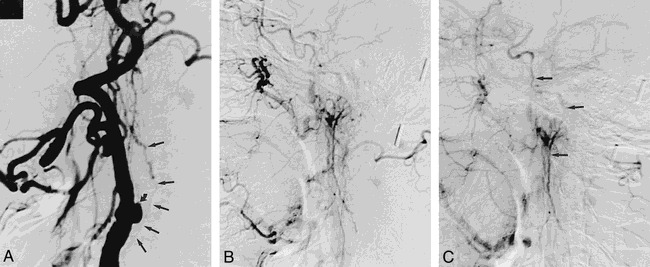
Lateral right carotid angiograms in 64-year-old woman with one prior CVA.
A, Early phase shows internal carotid stump (curved arrow) and collateral vessel originating from below ICA takeoff (straight arrows).
B and C, Later phases show continuation of collateral vessel to opacify distal ICA.
fig 3.
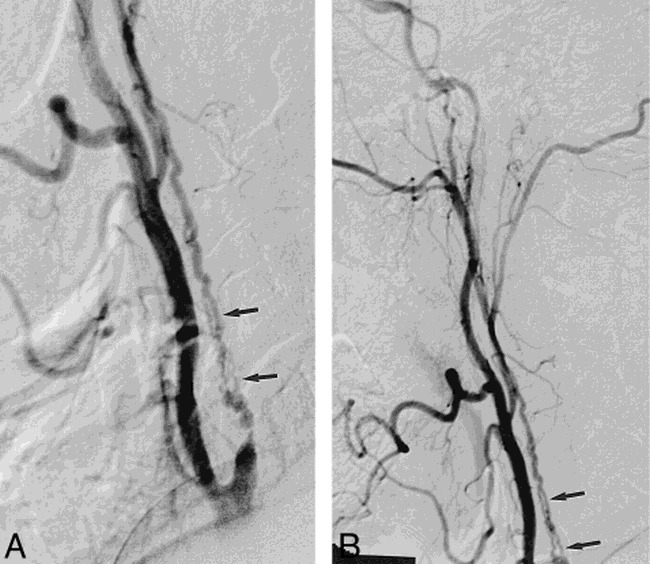
Lateral right carotid angiograms in 66-year-old man with multiple TIAs and one CVA.
A and B, Multiple serpiginous vessels project over expected course of ICA (arrows) with anterograde filling of the distal ICA.
fig 4.
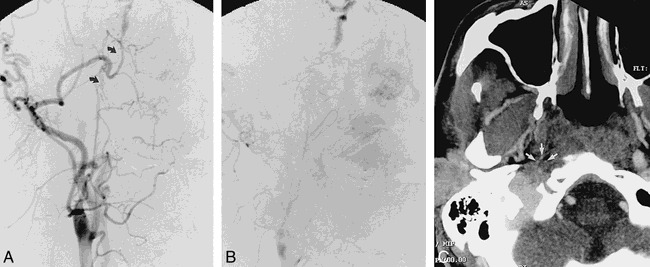
Frontal right carotid angiograms in 39-year-old man approximately 5 months after right carotid dissection with occlusion.
A and B, Short-segment serpiginous network of vessels bridge narrowed cervical ICA to petrous portion of ICA (arrows).
C, CT angiogram, transverse view, shows multiple small vessels (arrows) at perimeter of reduced-caliber thrombosed ICA.
Results
In all patients, a focal network of serpiginous vessels was seen along the expected location of the ICA with anterograde flow reconstituting the high cervical ICA. In cases 1 and 3, at least two approximately parallel, serpiginous vessels were seen in place of one carotid artery. In case 2, a small tortuous vessel was seen arising from the common carotid artery (below an occluded internal carotid stump), continuing distally to opacify a markedly thinned distal internal ICA. In this patient, the operative notes stated that “What was seen on angiography was in fact a fairly large branch within the wall of the ICA.” During surgery, no backbleeding was encountered when the ICA was opened, and the ICA was subsequently ligated. Pathologic sectioning of the excised carotid plaque revealed no evidence of luminal recanalization. In case 4, a poorly defined network of vessels projected over a gap between open (albeit narrowed) portions of the high cervical carotid artery.
Discussion
Previous reports on the carotid string sign have described a long, linear or curvilinear contrast wisp opacifying a poststenotic segment of markedly reduced caliber. This finding has been associated with high postendarterectomy patency rates (9). In our four cases, the comparable vessels were serpiginous rather than straight, and often multiple. One patient was found to have pathologically proved complete ICA occlusion at endarterectomy, with surgical visualization of a “fairly large” collateral vessel running within the arterial wall. The above findings suggest that these vascular pathways are distinct from those previously reported as the string sign. Possible explanations include a neovascular network arising in and about atheromatous plaque, neovessels connecting bridging vasa vasorum to the arterial lumen, and recanalized luminal thrombus.
The microarchitecture of the vasa vasorum has been studied in the carotid bifurcation in normal and atherosclerotic vessels in humans by preparing methylmethacrylate casts (10) and with micropaque X-ray microscopy (11). In nonatherosclerotic specimens, Clarke (11) demonstrated a relatively sparse network of vasa vasorum originating from external carotid vessels and supplying the adventitia and outer third of the media. Bo et al (10) showed that the density of this network increased dramatically adjacent to atherosclerotic plaque, and, furthermore, that additional vessels originating from the arterial lumen supplied a network of vessels in the plaque. In vivo studies with power Doppler sonography have also shown evidence of increased microvasculature adjacent to atherosclerotic plaque (1). Proliferation of vessels within atherosclerotic plaque has also been documented in the carotid arteries of monkeys (12), and a large body of literature exists describing similar findings in the coronary arteries (13–19) and the aorta (15, 20, 21). Because atherosclerotic plaques are a known angiogenic stimulus in vitro (22, 23), it is widely accepted that the vessels in and around atherosclerotic plaques are primarily a result of vascular proliferation rather than dilatation of preexisting vasa vasorum; hence, the term neovessels.
Findings in the coronary artery suggest that atherosclerotic neovessels can form collateral pathways. Depre et al (13) described small vessels forming direct communications between a diseased arterial lumen and the adventitial vasa vasorum in two of 40 specimens. Kumamoto et al (14) reported a definite yet similarly low rate of occurrence in vessels originating from the lumen in their coronary studies, with increased intimal vessel density occurring in specimens with higher grades of stenosis. Although uncommon, these neovessels could conceivably form collateral channels through their own network. However, neovascular channels in atherosclerotic plaque often exhibit complex angiomatoid morphology with anastomosing, thin-walled, dilated vascular spaces (24). This appearance is less compatible with the angiographic findings presented here. If the neovessels connected preexisting vasa vasorum to the arterial lumen, the vasa could then function as collateral pathways. Such pathways would most likely be tortuous but not angiomatoid.
Luminal recanalization of thrombosed vessels is a recognized phenomenon, found in 59% of chronic total coronary arterial occlusions (15). This should produce an angiographic appearance of either single or multiple channels within the center of the thrombosed lumen and certainly could account for the appearance in cases 1 and 3 (Figs 1 and 3). However, in both these cases, the largest, most proximal vessels traveled in a peripheral location, possibly either adjacent to or in the carotid wall; hence, they still could represent vasa vasorum. In case 2, the collateral vessel clearly traveled outside the thrombosed ICA arterial lumen, making it highly unlikely to represent recanalization. In case 4, follow-up CT angiography was performed to establish the presence of collateral vessels outside the thrombosed portion of the ICA (Fig 4C). Indeed, there was evidence of tiny vessels running along the outside of the reduced-caliber thrombosed artery. These were interpreted as the collateral bridging vessels previously seen on the angiogram.
Conclusion
The differential diagnosis for a carotid string sign should include a completely occluded artery with collateral pathways. We suggest that these pathways include vasa vasorum connected to the arterial lumen through enlarged atherosclerotically induced neovascular channels. Other possibilities include recanalized luminal thrombus and (less likely) neovascular channels coursing through atherosclerotic plaque. These collateral pathways have a morphology that differs from the classic string sign in that they are more serpiginous and sometimes multiple. It is important to recognize this variant to avoid potential surgery or stenting of completely occluded carotid arteries.
Footnotes
Address reprint requests to T. R. Marotta, MD, FRCPC
References
- 1.Belcaro G, Lauroroa G, Cearone MR, et al. Vasa vasorum visualised by power-Doppler in normal and arteriosclerotic carotid arteries. Vasa 1996;25:226-232 [PubMed] [Google Scholar]
- 2.Lippman HH, Sundt TM, Holman CB. The poststenotic carotid slim sign: spurious internal carotid hypoplasia. Mayo Clin Proc 1970;45:762-767 [PubMed] [Google Scholar]
- 3.O'Leary DH, Mattle H, Potter JE. Atheromatous pseudo-occlusion of the internal carotid artery. Stroke 1989;20:1168-1173 [DOI] [PubMed] [Google Scholar]
- 4.Gabrielsen TO, Seeger JF, Knake JE, Burke DP, Stilwill EW. The nearly occluded internal carotid artery: a diagnostic trap. Neuroradiology 1981;138:611-618 [DOI] [PubMed] [Google Scholar]
- 5.Mehigan JT, Olcott C. The carotid “string” sign: differential diagnosis and management. Am J Surg 1980;140:137-143 [DOI] [PubMed] [Google Scholar]
- 6.Kemeny V, Droste DW, Nabavi DG, Schulte-Altedorneberg G, Schuierer G, Ringestein EB. Collateralization of an occluded internal carotid artery via a vas vasorum. Stroke 1998;29:521-523 [DOI] [PubMed] [Google Scholar]
- 7.Newton TH, Potts DG. In: Radiology of the Skull and the Brain: Angiography St Louis: Mosby; 1974;1214-1215
- 8.Bradac GB, Oberson R. Angiography in Cerebro-Arterial Occlusive Diseases: Including Computer Tomography and Radionuclide Methods New York: Springer; 1998;68-69
- 9.Fredericks RK, Thomas TD, Lefkowitz DS, Troost BT. Implications of the angiographic string sign in carotid atherosclerosis. Stroke 1990;21:476-479 [DOI] [PubMed] [Google Scholar]
- 10.Bo WJ, McKinney WM, Bowden R. The origin and distribution of vasa vasorum at the bifurcation of the common carotid artery with atherosclerosis. Stroke 1989;20:1484-1487 [DOI] [PubMed] [Google Scholar]
- 11.Clarke JA. An X-ray microscopic study of the vasa vasorum of the normal human internal carotid and vertebral arteries. J Neurol Sci 1965;2:301-306 [DOI] [PubMed] [Google Scholar]
- 12.Williams JK, Orgren KI, Armstrong ML, Heistad DD. Vasa vasorum in the carotid sinus of atherosclerotic monkeys: implications for baroreceptor function. Atherosclerosis 1989;78:25-32 [DOI] [PubMed] [Google Scholar]
- 13.Depre C, Havaux X, Wijns W. Neovascularization in human coronary atherosclerotic lesions. Cathet Cardiovasc Diagn 1996;39:215-220 [DOI] [PubMed] [Google Scholar]
- 14.Kumamoto M, Nakashima Y, Sueishi K. Intimal neovascularization in human coronary atherosclerosis: its origin and pathophysiological significance. Hum Pathol 1995;26:450-456 [DOI] [PubMed] [Google Scholar]
- 15.Srivatsa SS, Edwards WD, Boos CM, et al. Histologic correlates of angiographic chronic total coronary artery occlusions. J Am Coll Cardiol 1997;29:955-963 [DOI] [PubMed] [Google Scholar]
- 16.Zamir M, Silver MD. Vasculature in the walls of human coronary arteries. Arch Pathol Lab Med 1985;109:659-662 [PubMed] [Google Scholar]
- 17.Geiringer E. Intimal vascularisation and atherosclerosis. J Pathol 1951;63:201-210 [DOI] [PubMed] [Google Scholar]
- 18.Kamat BR, Galli SJ, Barger AC, Lainey LL, Silverman KJ. Neovascularization and coronary atherosclerotic plaque: cinematographic localization and quantitative histologic analysis. Hum Pathol 1987;18:1036-1042 [DOI] [PubMed] [Google Scholar]
- 19.Barger AC, Reinier B, Lainey LL, Silverman KJ. Hypothesis: vasa vasorum and neovascularization of human coronary arteries: a possible role in the pathophysiology of atherosclerosis. Med Intell 1984;310:175-177 [DOI] [PubMed] [Google Scholar]
- 20.Okuyama K, Yaegashi H, Takahasi T, Sasaki H, Mori S. The three-dimensional architecture of vasa vasorum in the wall of the human aorta. Arch Pathol Lab Med 1988;112:726-730 [PubMed] [Google Scholar]
- 21.Heistad DD, Marcus ML. Role of vasa vasorum in nourishment of the aorta. Blood Vessels 1979;16:225-238 [DOI] [PubMed] [Google Scholar]
- 22.Alpern-Elran H, Morog N, Robert F, Hoover G, Kalant N, Brem S. Angiogenic activity of the atherosclerotic carotid artery plaque. J Neurosurg 1998;70:942-945 [DOI] [PubMed] [Google Scholar]
- 23.Kahlon R, Shapero J, Gotlieb AI. Angiogenesis in atherosclerosis. Can J Cardiol 1992;8:60-64 [PubMed] [Google Scholar]
- 24.Fryer JA, Myers PC, Appleberg M. Carotid intraplaque hemorrhage: the significance of neovascularity. J Vasc Surg 1987;6:341-349 [DOI] [PubMed] [Google Scholar]


