Abstract
Summary: CT, MR imaging, MR spectroscopy, and angiography were performed in two men (ages 21 and 48, respectively) with intraventricular meningioma. In both cases, CT and MR imaging showed large tumors located in the trigone of the right lateral ventricle that enhanced intensely after contrast administration. MR spectroscopy was helpful in supporting a preoperative diagnosis of meningioma in both cases.
The most useful indications for the specific diagnosis of intraventricular tumor are location of the tumor within the ventricles and age of the patient (1, 2). MR imaging and CT features other than location have been considered relatively nonspecific in identifying the type of tumor; nevertheless, some radiologic findings can suggest an unexpected diagnosis for age and location. In these cases, confirmatory presurgical diagnostic imaging may be useful in corroborating the diagnosis. MR spectroscopy has proved helpful in providing additional information in cases in which the differential diagnosis is difficult to establish by MR imaging alone. We describe two patients with intraventricular meningiomas in which MR spectroscopy revealed useful data for diagnosis.
Case Reports
Case 1
A 48-year-old man had a 2-month history of recurrent episodes of unsteady gait, visual loss, tinnitus, left arm paresthesia, and frontal headache. Neurologic examination was nonfocal. Fundoscopy revealed bilateral papilloedema. A CT scan showed a well-defined hyperdense lesion lying within the trigone of the right lateral ventricle that enhanced intensely after contrast administration. MR imaging at 1.5 T revealed a large mass with a signal intensity similar to that of gray matter on both T1- and T2-weighted images (Fig 1A) and high signal intensity on proton density–weighted images and on a fluid-attenuated inversion-recovery (FLAIR) sequence. The mass enhanced intensely after administration of contrast material (Fig 1B and C). An abundance of associated edema was present, and enlarged vessels surrounding the mass were seen originating in the choroid plexus. Angiography showed a mass supplied by the anterior and posterior choroidal arteries with persistent tumor staining.
fig 1.
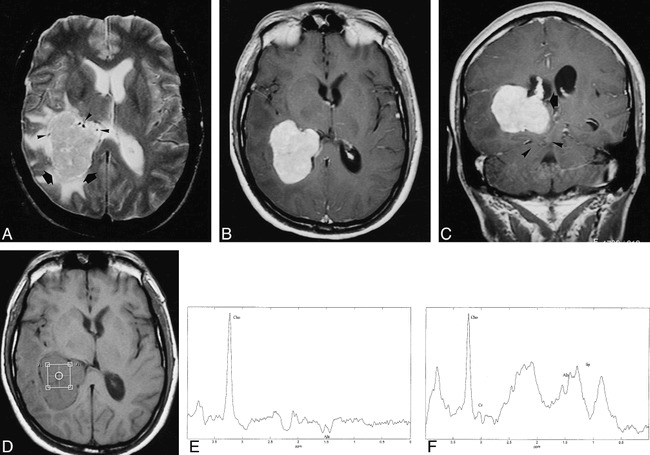
48-year-old man with a 2-month history of recurrent episodes of unsteady gait, visual loss, tinnitus, left arm paresthesia, and frontal headache.
A, Axial T2-weighted MR image shows an intraventricular meningioma in the right trigone (arrows) with heterogeneous signal, surrounded by edema. There are some enlarged vessels at the periphery of the mass (arrowheads).
B and C, Axial (B) and coronal (C) T1-weighted contrast-enhanced MR images show intense relatively homogeneous enhancement of the meningioma. Note contact with the choroid plexus (arrow, C) and posterior transtentorial herniation (arrowheads, C).
D, Axial T1-weighted MR image shows the position of the voxel for spectroscopy.
E, Localized proton MR spectrum (SE 2000/136/128) of the intraventricular meningioma shows a prominent resonance from Cho and a doublet centered at 1.47 ppm and inverted at this TE, which can be assigned to Ala.
F, Localized proton MR spectrum (SE 2000/31/128) shows a prominent resonance from Cho, a decrease in the Cr and NAA resonances, and the presence of lipids. Ala can also be assigned at this TE.
Proton MR spectroscopy was performed in a 2 × 2 × 2-cm voxel located within the mass (Fig 1D). Proton MR spectroscopic images were acquired with spin-echo (SE) pulse sequences with parameters of 2000/136/128 (TR/TE/excitations) (Fig 1E) and 2000/31/128 (Fig 1F).
Spectrum analysis was performed with the use of MRUI software (3). A long-TE spectrum (Fig 1E) showed the presence of two prominent resonances from choline-containing compounds (Cho) and from alanine (Ala), which, at this TE, appeared as a doublet centered at 1.47 ppm and inverted, owing to the J modulation. The spectrum acquired with a short TE (Fig 1F) showed the presence of Cho and reduced resonances of creatine, phosphocreatine (Cr), and N-acetylaspartate (NAA). Resonances corresponding to glutamate/glutamine and lipids, together with the doublet of Ala centered at 1.47 ppm, could also be identified at this TE. After complete tumor resection, histologic examination confirmed the diagnosis of a meningothelial meningioma.
Case 2
A 21-year-old man had a 4-month history of progressive occipital headaches associated with nausea and unsteadiness. Physical examination showed mild left-sided hemiparesis and left homonymous hemianopia. Fundoscopy revealed bilateral papilloedema. A CT scan showed a well-defined mass with its center within the atrium of the right ventricle. The mass was hyperdense relative to gray matter and enhanced homogeneously after contrast administration. MR imaging at 1.5 T confirmed the presence of a lobulated mass in the trigone of the right lateral ventricle. The lesion was isointense with gray matter on both T1- and T2 weighted images (Fig 2A) and had high signal intensity on proton density–weighted images and on the FLAIR sequence (Fig 2B). An abundance of edema was present in the surrounding white matter, and enlarged peripheral vessels were seen originating in the choroid plexus. Enhancement after contrast administration was intense and relatively homogeneous (Fig 2C and D). Angiography confirmed a hypervascular mass supplied by the anterior and posterior choroidal arteries.
fig 2.
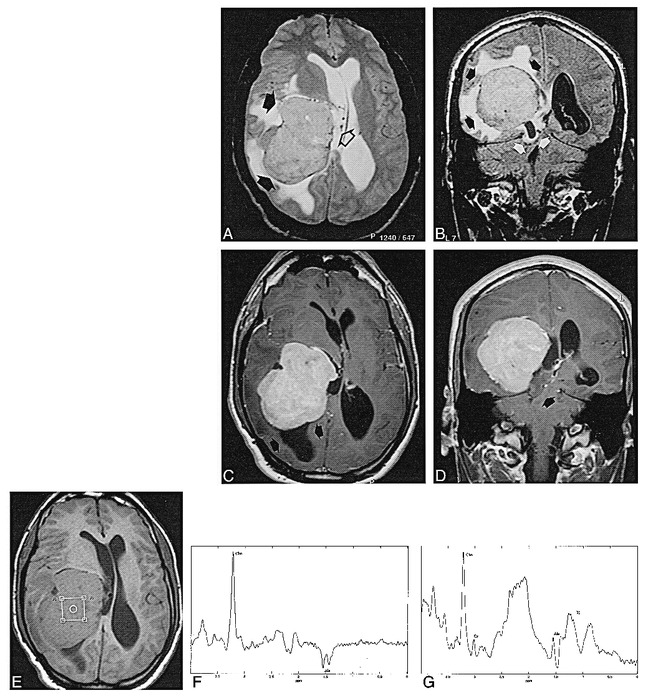
21-year-old man with a 4-month history of progressive occipital headaches associated with nausea and unsteadiness.
A, Axial T2-weighted MR image shows a large meningioma in the right trigone (closed arrows) with heterogeneous signal intensity. There are enlarged vessels at the periphery of the mass, some of them in continuity with the choroid plexus (open arrow), and an abundance of edema in the white matter.
B, Coronal FLAIR sequence shows high signal intensity of the tumor (black arrows) and extensive peritumoral edema. Note posterior transtentorial herniation of the temporal lobe and the trigone of the lateral ventricle (white arrows).
C and D, Axial (C) and coronal (D) T1-weighted contrast-enhanced MR images show intense relatively homogeneous enhancement. There is focal enlargement of the occipital horn (arrows, C) with slight hyperintensity of its content due to entrapment of the horn. Posterior transtentorial herniation is well seen in the coronal planes (arrow, D).
E, Axial T1-weighted MR image shows the position of the voxel for spectroscopy.
F, Localized proton MR spectrum (SE 2000/136/128) shows a prominent resonance from Cho and an inverted doublet centered at 1.47 ppm, corresponding to Ala.
G, Localized proton MR spectrum (SE 2000/31/128) from the same voxel shows an increase in the Cho resonance, a decrease in Cr and NAA, and the presence of lipids and Ala.
Proton MR spectroscopy was performed in a 2 × 2 × 2-cm voxel located within the mass (Fig 2E), with SE 2000/136/128 (Fig 2F) and SE 2000/31/128 (Fig 2G) sequences showing patterns similar to those obtained in case 1. A prominent resonance of Cho was found on both long- and short-TE sequences together with a doublet attributable to Ala centered at 1.47 ppm and inverted on the long-TE sequence. Reduced resonances corresponding to Cr and NAA together with the presence of lipids were seen with a short-TE sequence. Subsequent total tumor resection revealed a meningioma.
Discussion
Intraventricular tumors represent a diverse group of lesions, some of them infrequent, located in the ventricles deep within the brain, where surgery is difficult. Diagnosis of these tumors must be as accurate as possible to forewarn the surgeon of the behavior of the tumor and of possible complications. The most useful indications for the diagnosis are age at which the tumor appears and precise intraventricular location (1, 2). Radiologic findings other than location have been considered of little value. The main differential diagnosis of a tumor located in the trigone of the lateral ventricle should include choroid plexus papilloma in patients under 10 years of age; low-grade gliomas, such as ependymoma, oligodendroglioma, and low-grade astrocytoma, in patients between 10 and 40 years of age; and metastases, lymphoma, and meningioma after the fourth decade of life (1, 2).
Intraventricular meningioma is a rare but well-described tumor, most often located in the trigone. It constitutes approximately 0.5% to 2% of all intracranial meningiomas. The majority present in the fourth to the sixth decades of life and show a predominance in women of approximately 2:1. It is found only occasionally in patients under the age of 40. Neurofibromatosis should be suspected when such a tumor is found in children. The appearance on imaging studies is similar to that of other meningiomas, being sharply defined and globular. On CT scans, these tumors are usually hyperdense and may contain foci of calcification. Meningiomas are iso- or hypointense on T1-weighted MR images, and iso- or hyperintense on T2-weighted images. Contrast enhancement is strong and heterogeneous on both CT and MR studies, and areas of necrosis and cystic change may be present (4, 5). In our case 1, meningioma was the first diagnosis made from the imaging studies, but in case 2, low-grade glioma was the most likely diagnosis, considering the tumor's location and the patient's age and sex. Meningioma might have been suspected from the radiologic characteristics, although these are considered insufficient for a definitive diagnosis.
MR spectroscopy may provide additional information in cases in which the differential diagnosis of tumors by neuroimaging is difficult. Common proton MR spectroscopic findings in brain tumors include a decrease in NAA and Cr resonances, an increase in Cho, and the presence of lactate and lipid resonances in different proportions (6–9). The function of NAA is not clear; however, it appears to be present in high levels only in neuronal tissue. It is considered a neuronal marker that should not be found in extramedullary tumors unless there has been contamination of nontumoral neuronal tissue surrounding the tumor or there are normal neurons within the tumor (7, 8, 10). Total Cr content is reduced in almost all brain tumors, but it tends to be higher in neuroectodermal than in nonneuroectodermal tumors (10). Cho reflects membrane turnover, correlates with malignancy in astrocytic tumors, and forms high peaks in meningioma and pituitary adenoma. The presence of lactate in tumors is thought to be due to high anaerobic glycolysis or, alternatively, to hypoxic conditions. The most common proton spectrum found in meningiomas is a high Cho peak with low or absent NAA and Cr and variable amounts of lactate (7, 9–12). Most important, an unusually high ratio of Ala to Cr has been found in meningiomas because of the high Ala and low Cr content, and this is a relatively specific finding for meningioma (10).
Conclusion
Proton MR spectroscopy in our cases showed a high peak from Cho with very low Cr and NAA. Ala was also present in both cases. These spectra correlate well with meningioma and support the diagnosis of intraventricular meningioma, although in patient 2 it would not be the most feasible diagnosis if only age, sex, and location of tumor were considered.
Footnotes
Address reprint requests to Carlos Majós, MD.
References
- 1.Jelinek J, Smirniotopoulos JG, Parisi JE, Kanzer M. Lateral ventricular neoplasms of the brain: differential diagnosis based on clinical, CT, and MR findings. AJNR Am J Neuroradiol 1989;11:567-574 [PMC free article] [PubMed] [Google Scholar]
- 2.Tien RD. Intraventricular mass lesions of the brain: CT and MR findings. AJR Am J Roentgenol 1991;157:1283-1290 [DOI] [PubMed] [Google Scholar]
- 3.van den Boogaart A. Quantitative data analysis of in vivo MRS data sets. Magn Reson Chem 1997;35:S146-S152 [Google Scholar]
- 4.Kendall B, Reider-Grosswater I, Valentine A. Diagnosis of masses presenting within the ventricle on computed tomography. Neuroradiology 1983;25:11-22 [DOI] [PubMed] [Google Scholar]
- 5.McConachie NS, Worthington BS, Cornford EJ, Balsitis M, Kerslake RW, Jaspan T. Review article: computed tomography and magnetic resonance in the diagnosis of intraventricular cerebral masses. Br J Radiol 1994;67:223-243 [DOI] [PubMed] [Google Scholar]
- 6.Ross B, Michaelis T. Clinical applications of magnetic resonance spectroscopy. Magn Reson Q 1994;10:191-247 [PubMed] [Google Scholar]
- 7.Ott D, Henning J, Ernst T. Human brain tumors: assessment with in vitro proton MR spectroscopy. Radiology 1993;186:745-752 [DOI] [PubMed] [Google Scholar]
- 8.Ursenius JP, Kauppinen RA, Vainio PA,, et al. Quantitative metabolite patterns of human brain tumors: detection by 1H NMR spectroscopy in vivo and in vitro. J Comput Assist Tomogr 1994;18:705-713 [DOI] [PubMed] [Google Scholar]
- 9.Rand SD, Prost D, Haughton V,, et al. Accuracy of single-voxel proton MR spectroscopy in distinguishing neoplastic from nonneoplastic brain lesions. AJNR Am J Neuroradiol 1997;18:1695-1704 [PMC free article] [PubMed] [Google Scholar]
- 10.Kinoshita Y, Kajiwara H, Yokota A, Koga Y. Proton magnetic resonance spectroscopy of brain tumors: an in vitro study. Neurosurgery 1994;35:606-614 [DOI] [PubMed] [Google Scholar]
- 11.Demarael P, Johannik K, van Hecke P,, et al. Localized 1H NMR spectroscopy in fifty cases of newly diagnosed intracranial tumors. J Comput Assist Tomogr 1991;15:67-76 [DOI] [PubMed] [Google Scholar]
- 12.Castillo M, Kwock L, Mukherji SK. Clinical applications of proton MR spectroscopy. AJNR Am J Neuroradiol 1996;17:1-15 [PMC free article] [PubMed] [Google Scholar]


