Abstract
BACKGROUND AND PURPOSE: Patients with mild traumatic brain injury (TBI) often show significant neuropsychological dysfunction despite the absence of abnormalities on traditional neuroradiologic examinations or EEG. Our objective was to determine if magnetic source imaging (MSI), using a combination of MR imaging and magnetoencephalography (MEG), is more sensitive than EEG and MR imaging in providing objective evidence of minor brain injury.
METHODS: Four subject groups were evaluated with MR, MSI, and EEG. Group A consisted of 20 neurologically normal control subjects without histories of head trauma. Group B consisted of 10 subjects with histories of mild head trauma but complete recovery. Group C consisted of 20 subjects with histories of mild head injury and persistent postconcussive symptoms. The 15 subjects included in group D underwent repeat examinations at an interval of 2 to 4 months.
RESULTS: No MR abnormalities were seen in the normal control group or the asymptomatic group, but five (20%) of the patients with persistent postconcussive symptoms had abnormal MR findings. EEG was abnormal for one subject (5%) from the normal control group, one (10%) from the asymptomatic group, and five (20%) from the group with persistent postconcussive symptoms. MSI was abnormal for one subject (5%) from the normal control group, one (10%) from the asymptomatic group, and 13 (65%) from the group with persistent postconcussive symptoms. There was a direct correlation between symptom resolution and MSI findings for the symptomatic head trauma group.
CONCLUSION: MSI indicated brain dysfunction in significantly more patients with postconcussive symptoms than either EEG or MR imaging (P < .01). The presence of excessive abnormal low-frequency magnetic activity provides objective evidence of brain injury in patients with postconcussive syndromes and correlates well with the degree of symptomatic recovery.
Patients with mild traumatic brain injury (TBI) often show significant neuropsychological dysfunction despite the absence of any abnormalities on traditional neuroradiologic examinations or EEG. It is well established that severe blunt trauma can cause brain atrophy, but the neural consequences of relatively minor head injury remain poorly understood (1–3). Symptoms such as persistent headaches, nausea, cognitive decline, and personality changes may be identified clinically, but traditional neuroimaging studies rarely reveal consistent brain changes to explain these problems (4–6). The significant structural changes that CT and MR imaging show in cases of severe TBI tend to be absent in cases of mild TBI (7–9), and EEG generally shows normal or only mild, diffuse pathophysiology, even in patients with very specific cognitive and psychological deficits (10, 11).
Because the sensitivity of traditional methods to trauma-induced brain dysfunction is so low, many patients with neurologic bases for their posttraumatic psychological deficits fail to be diagnosed with TBI or are misdiagnosed as psychiatric problems (12). As a result, the conditions of many of these patients are not treated or the patients are referred for inappropriate psychotherapy. Especially in cases of mild trauma, there is a general reluctance to medically treat specific postconcussive problems, such as attentional deficits, in the absence of a clear neurobiological basis for the problem. Clearly, availability of reliable, objective evidence that a particular brain region has been rendered dysfunctional by mild TBI would augment patient care by allowing clinicians to better develop cost-effective, individually tailored treatment and therapy programs (13).
Considering the above, the need to develop neuroimaging techniques responsive to mild TBI is all too apparent. Magnetic source imaging (MSI) offers promise because it provides a high degree of resolution of normal and abnormal brain physiology in both spatial and temporal domains (14–19). MSI involves the integration of anatomic data obtained by the familiar method of MR imaging with electrophysiological data obtained by magnetoencephalography (MEG). Electrical currents flowing within dendrites give rise to extracellular current sources and sinks, which establish the scalp potential gradients measured by EEG. These currents also give rise to a surrounding neuromagnetic field that is measured by MEG. The biophysics of EEG and MEG are complementary. MEG provides a selective reflection of activity in dendrites oriented parallel to the skull surface, whereas EEG reflects mostly activity arising in dendrites perpendicular to the skull. It is noteworthy that electrical conductivity differences between the brain, CSF, skull, and scalp smear and distort the scalp-EEG view of the brain's electrical activity but have minimal impact on MEG (14–19). In many instances, MEG data can be evaluated using straightforward mathematical models that allow localization of the neuronal generators of particular signal components.
Normal MEG data, like normal EEG data, are dominated by frequencies above 8 Hz. Severe TBI usually causes a shift in the background EEG power spectra toward lower-frequency signals, but similar changes are rarely apparent in cases of minor TBI (10). Several investigators have observed that MEG more often reveals low-frequency signals than does simultaneously conducted EEG (16, 20). We therefore hypothesized that MEG might reveal trauma-induced changes in the spectral content of spontaneous brain activity, even in cases of relatively minor trauma. MEG studies of patients with stroke and of patients with tumors frequently reveal the presence of abnormal low-frequency magnetic activity (ALFMA) with a dipolar magnetic field configuration. Source modeling of ALFMA shows that individual dipolar slow-wave events are generated by compromised tissues at the margins of lesions (15, 16, 21–23). In patients with epilepsy, MSI reveals that sources of focal ALFMA co-localize with sources of spikes, even when there is an absence of gross structural pathologic findings. Taken together, these data indicate that dipole modeling is useful in the identification of compromised brain tissue, and they suggest that source analyses of MEG data may aid in the identification of TBI-induced regional brain dysfunction. To test these hypotheses, we used MEG, MR imaging, and EEG to examine four groups. Group A consisted of neurologically normal control subjects without a history of trauma. Group B consisted of subjects with a history of mild head trauma who had achieved complete recovery. Group C consisted of subjects with postconcussive symptoms subsequent to mild head trauma. The subjects included in group D were a subset of control subjects and symptomatic patients who underwent repeat examinations.
Methods
The study population consisted of four subject groups. Group A was composed of 20 healthy volunteers (age range, 18–57 years; 10 men and 10 women) with no history of closed head injury or other neurologic or psychiatric problems. These volunteers were studied as a normal control population.
Group B consisted of 10 subjects (age range, 14–60 years; six male and four female subjects) with a history of mild closed head injury with brief loss of consciousness (<20 minutes). At the time of the neuroimaging evaluation (2–16 months after the initial trauma), no postconcussive symptoms were present, as indicated by self-report, mini-mental status screening, clinical evaluation, and a symptom questionnaire modified from the Structured Clinical Interview DSM-IIIR nonpatient version interview schedule. All the subjects in group B were free of diagnosed neurologic or psychiatric dysfunction. All had Glasgow Coma Scale scores of 13+ at the time of initial hospital admission, and all were discharged within 24 hours.
Group C consisted of 20 subjects (age range, 17–62 years; 12 male and eight female subjects) with a history of mild closed head injury with brief loss of consciousness (<20 minutes). In all cases, trauma was blunt and associated with a motor vehicle accident, a blow to the head, or a fall. The results of the CT study were interpreted as normal for all patients except two, one of whom had right frontal and parietal subdural hematomas and one of whom had a right temporal tip contusion. (Additional information regarding these patients, subjects 3 and 6, respectively, is provided in Table 1.) At the time of neuroimaging evaluation (2–38 months after the initial trauma), significant postconcussive symptoms were present, as indicated by self-report, mini-mental status screening, and a symptom questionnaire modified from the Structured Clinical Interview DSM-IIIR interview schedule. All the subjects in group C were free of premorbid diagnosed neurologic or psychiatric dysfunction. All had Glasgow Coma Scale scores of 13+ at the time of initial hospital admission, and all were discharged within 24 hours. Sixteen subjects showed some degree of cognitive dysfunction (memory or attentional problems), two reported only pain (frequent headaches and body aches) as a persistent symptom, and two reported pain and depression without cognitive changes.
TABLE 1:
Traumatic brain injury patient data
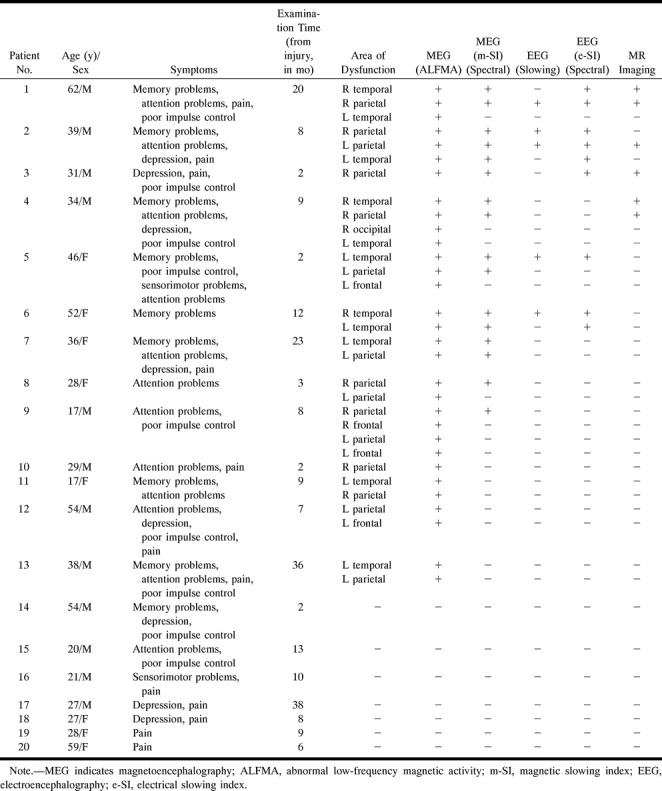
Group D consisted of 15 subjects (10 from the normal control group and five from the symptomatic head trauma group) who underwent complete follow-up examinations during a 2- to 4-month interval after the initial study. An attempt was made to follow up all the subjects, but only 50% of the control subjects and 25% of the trauma subjects were available.
MR Imaging
Imaging examinations were performed with a 1.5-T whole-body MR system. Gradient-echo and conventional spin-echo sequences included sagittal 1.5-mm contiguous (40/6 [TR/TE]) sections, coronal 5.0-mm (800/15) sections with a 1-mm gap, axial 5.0-mm (2500/90) sections with a 1-mm gap, and axial 5.0-mm (2500/22) sections with a 1-mm gap. The images were examined for pathologic abnormalities by two neuroradiologists who were blinded to the clinical status of the subjects.
MEG Examination
Neuromagnetic data were collected in a magnetically shielded room using a 37-channel, axial-gradiometer system (Magnes; Biomagnetic Technologies, San Diego, CA). Three minutes of continuous data were collected at each of eight placements of the sensor array. The placements were over the following cortical regions: midline frontal, left temporal/inferior frontal, right temporal/inferior frontal, left temporal-occipital-parietal junction, left parietal, right parietal, right temporal-occipital-parietal junction, and midline occipital regions.
The data were recorded with the subject in a prone, supine, or lateral position, as needed for appropriate positioning of the sensor. The subjects were instructed to rest quietly with their eyes closed during the data collection period. Data were collected at a digitization rate of 300 Hz, with on-line filtering from 1 to 100 Hz. The data set from each probe position was analyzed separately. Two analysis procedures were applied to each data set. One procedure involved spectral analysis, whereas the other involved dipole modeling of ALFMA (Fig 1). All data processing was computer-automated, and the interpretation of the data was performed in a blinded fashion with respect to trauma history.
fig 1.
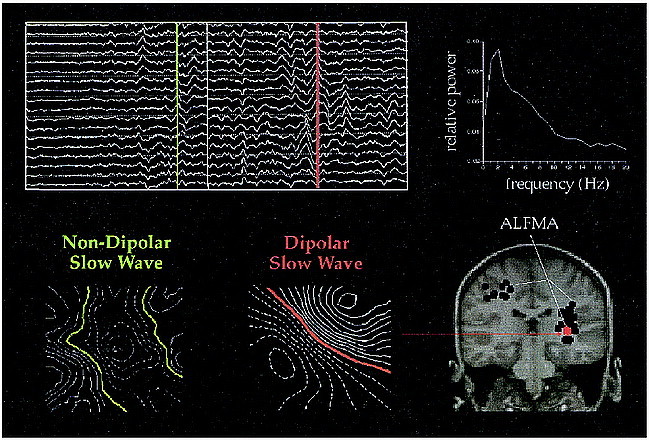
Spontaneous MEG data are waveforms showing how magnetic flux changes with time. Data regarding the trauma patients often show generalized slowing of the background activity and focal slow waves. Ten seconds of data are shown from several channels. Two examples of slow waves are marked by the green and red lines. One way to express the data is as a power spectrum, shown on the right. Most slow waves have complicated magnetic field patterns, as shown by the leftmost iso-field contour map. Some slow waves have very dipolar patterns, and the source can be localized and plotted on spatially aligned MR images. The red dot shows the location of the indicated slow wave, with black dots showing slow-wave sources from other points in time
For spectral analysis, the 180 seconds of the continuous data stream recorded at each sensor position was broken into 90 2-second sequential epochs. A fast-Fourier transform was applied to the data of each epoch, and the average power spectrum was calculated. The relative power in the 1- to 6-Hz (delta and theta) and 8- to 13-Hz (alpha) bands was determined and a ratio calculated at each sensor. An average of the ratios across the 37 sensors of a single probe placement was then calculated to provide a magnetic slowing index (m-SI) for each probe placement. Each m-SI value was subjected to a log transform that provided a new index, L(m-SI), that was normally distributed.
The L(m-SI) values at each probe position, for each subject in this study, were transformed into z scores relative to mean and standard deviation values in a normative database previously derived from 36 neurologically normal subjects (studied and analyzed in an identical fashion). The database contained information derived from 22 male and 14 female subjects (age range, 17–55 years). The newly derived z scores indicated the likelihood that a particular L(m-SI) value was not from the distribution of L(m-SI) values derived for the normal database population. For this study, a z score greater than 2.5 was considered to be indicative of an abnormality. A z score greater than 2.5 implies 95% confidence against false-positive identification.
The dipole analysis of ALFMA involved four steps: 1) filtering and peak selection, 2) dipole modeling, 3) cluster analysis, and 4) generation of magnetic source localization images. First, MEG data were digitally filtered with a bandpass of 1 to 6 Hz to isolate ALFMA in the delta and theta bands. Independent analyses were performed on the data collected at each dewar position. After identification of the latencies of local amplitude peaks for which the magnetic field strength was greater than 200 femtoTesla, single dipole modeling was performed every 8 milliseconds during a 40-millisecond window spanning each amplitude peak. In most cases, more than 5000 dipole fits were calculated for the data stream collected at each dewar position. Only dipole fits for which the correlation coefficient between the model and empirical field pattern exceeded 0.97 were considered in subsequent analyses. As a consequence of this strict criterion for dipolarity, more than 99% of the total amount of data collected was rejected from further consideration. A spatial clustering algorithm was used to determine whether a particular cortical region was responsible for the generation of multiple dipolar low-frequency events. A 1-cm radius volume element was considered to be pathologic only if it was responsible for the generation of 15 distinct low-frequency events. Magnetic source localization images were generated by identifying the fiducials that defined the MEG coordinate space on the appropriate MR images. Through a series of axis translations and rotations, it was then possible to plot MEG dipoles on appropriate sections. For this study, we plotted only dipole sources of ALFMA that were members of a cluster of 15 events (see Fig 1).
EEG Examination
Neuroelectric data were collected simultaneously with MEG data using an electrode cap with 19 contacts positioned at standard 10 to 20 locations referenced to Cz. Eight 3-minute continuous records were obtained (each simultaneous with data collection at one of the eight MEG sensor placements). EEG signals were digitized at a rate of 300 Hz by the same system that digitized the MEG data. The data were examined for evidence of abnormal slow waves by a trained neurophysiologist who was blinded to the patients' histories.
The data were then re-referenced using an average-reference strategy, and the data stream collected at each electrode (combined across the eight MEG recording blocks; total time, 24 minutes) was divided into a sequence of 2-second epochs. In a fashion similar to that used for the analysis of MEG data, the ratio of 1 to 6 Hz power to alpha power was calculated at each electrode. Average, regional electrical slowing indexes (e-SIs) were then calculated for eight electrode groupings that approximately corresponded to the eight MEG probe positions: midline frontal (Fp1, Fp2, Fz, F3, F4); left temporal/inferior frontal (F7, F3, C3, T3); right temporal/inferior frontal (F8, F4, C4, T4); left temporal-occipital-parietal junction (C3, P3, T3, T5); left parietal (Cz, Pz, C3, P3); right parietal (Cz, Pz, C4, P4); right temporal-occipital-parietal junction (C4, P4, T4, T6); and midline occipital (Pz, P3, P4, O1, O2). The data regarding the subjects in the normative database were used to provide for a z score analysis of log-transformed e-SI data identical to that described for the log-transformed m-SI data in the MEG analysis.
Repeat Examinations
The repeat examinations were performed in the exact same manner as the initial studies in each case. The follow-up evaluations were performed at a 2- to 4-month interval from the initial study, based on the availability of the subject.
Results
The results for the first three groups of subjects are summarized in Table 2.
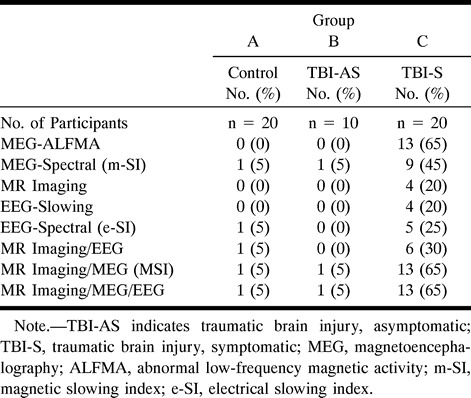
TABLE 2:
Magnetic source imaging (MSI), MR imaging, and electroencephalography (EEG) in cases of minor head trauma
MR Imaging
Structural changes revealed by MR imaging were not found for any of the control subjects. None of the patients who had suffered head trauma without postconcussive symptoms showed MR abnormalities. Four (20%) of the 20 patients who had suffered head trauma with postconcussive symptoms showed structural MR abnormalities. These included right hemisphere atrophy (case 1), left parietal white matter disease (case 2), right parietal hematoma (case 3), and right temporal and parietal white matter disease (case 4). The findings in the symptomatic patient group are summarized in Table 1.
EEG
None of the EEG examinations of the control subjects showed gross delta or theta activity in the continuous polygraph record, although an abnormal right parietal e-SI was found for one of the patients. This patient also had an abnormal m-SI value over the right parietal area. The clinical EEG results and e-SI values were normal for all asymptomatic patients. Of the 20 symptomatic patients, EEG showed gross delta and theta activity in four (20%). All symptomatic patients with obvious theta and delta activity revealed by clinical EEG, plus one other (total, five patients; 25%) showed abnormal e-SI values indicative of low-frequency dominance of the EEG power spectra at one or more regions. In patients with MR findings, the regions that showed the most abnormal values were near the site of structural compromise.
MEG
The magnitude of the m-SI varied across probe positions. Data from the control subjects agreed well with data in the previously established normative database. For the control subjects, posterior placements demonstrated m-SI values below 0.75 (indicating dominance of posterior rhythms by alpha activity), whereas m-SI values from the midline frontal placement were close to 1.0. At no placement did normative values exceed 1.2. One of the 20 normal control subjects from this study had an “abnormal” m-SI value (1.4, z = 2.6) at the right parietal probe positions. (This single false-positive finding is not surprising considering the total number of statistical evaluations [8 × 30 = 240], and this area was also abnormal on the EEG e-SI.) Only one subject from the asymptomatic head trauma group had an abnormality shown by MEG, a result indicating a general lack of persistent brain injury in trauma victims who achieve complete recovery from their accidents. In marked contrast, nine (45%) of the patients who suffered head trauma with postconcussive symptoms had one or more abnormal m-SI values shown by MEG. There was no significant correlation between the presence of abnormal slowing and time since injury (P > .1).
Spatial clustering of 15 or more dipolar sources of ALFMA was observed in two (10%) of the control subjects. In each case, only a single cluster was observed, and it was invariably located in a midline occipital region. Careful visual inspection of the waveform morphology at the time points at which the automatic processing routines identified these dipoles revealed that the relevant activity was actually high-amplitude alpha activity that was not adequately suppressed by the digital 1- to 6-Hz filtering procedure. That is, these dipole clusters were not associated with pathologic abnormalities but rather with a normal spontaneous rhythm. The results were, therefore, not coded as indicating abnormal activity (see Table 2).
By automatic processing, one subject from the asymptomatic head trauma group also had an initial occipital ALFMA cluster that reflected alpha activity. Careful visual inspection subsequently determined this to be within normal limits, and it was not included as an abnormal finding (see Table 2).
Each of 13 patients (65%) with posttraumatic symptoms had one or more clusters of ALFMA that could not be traced to normal spontaneous rhythms, although two of these patients had “false alpha clusters” in addition to the clearly abnormal clusters. Eight of the 13 trauma patients with postconcussive symptoms, clusters of ALFMA, and abnormal m-SI values had been hit by blunt objects. In each of these cases, the most abnormal m-SI value was for the probe position nearest the site of impact or at a contrecoup location. Clusters of ALFMA localized to the underlying brain region or at a contrecoup site (see Table 1).
All patients with MR abnormalities had abnormal MEG findings. Abnormal m-SI values and dipole clusters tended to be located in the vicinity of the structural damage (within 2 cm) or at a contrecoup location.
Comparison of Methods
Figure 2 is a set diagram summarizing the interrelationship among findings from the procedures for all the subjects. It is noteworthy that all of the patients' data that were characterized as abnormal by either MR imaging or EEG were also characterized as abnormal by MEG. For seven patients with postconcussive symptoms, MEG was the only examination to reveal abnormalities. For example, the patient data shown in Figure 3 was characterized as abnormal by MEG but as normal by MR imaging and EEG. The superior diagnostic sensitivity of MEG relative to either EEG or MR imaging was highly significant (P < .01); separate tests for significance of the difference between two correlated proportions, as developed by McNemar (6), were used.
fig 2.
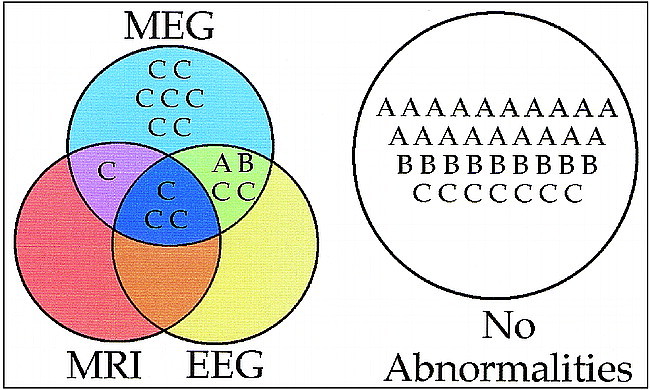
Summary of initial neuroimaging data from all subjects. Group A represents the normal control subjects, group B is the asymptomatic head trauma subjects, and group C is the symptomatic head trauma patients. The set diagram shows what diagnostic tests, if any, provided abnormal findings for each subject. Of particular note are the low false-positive rates for group A and B subjects (all without symptoms), the high sensitivity of MEG to abnormalities in patients with postconcussive symptoms, and the finding that MEG identified abnormalities in all group C subjects who had MR imaging or EEG abnormalities plus seven additional group C subjects who had normal MR imaging and EEG findings
fig 3.
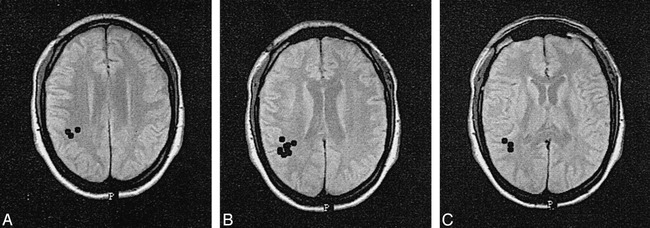
A–C, Magnetic source localization images of a 29-year-old man who, 2 months before the examinations, hit his head after falling from a ladder. At the time of the study, attentional problems and neck pain had precluded his return to work. The results of MR imaging and clinical EEG were normal, but MEG showed focal right parietal ALFMA.
Repeat Examinations
Ten control subjects underwent follow-up MSI examinations (2- to 4-month interval). Intersession m-SI values were highly stable for the control subjects, never changing by more than 0.2 at any probe position and never reaching “abnormal” values. Five patients with postconcussive symptoms and abnormal MSI findings during their initial examinations also participated in 2-month follow-up examinations. Two patients indicated complete alleviation of their previous symptoms at the time of reexamination. In both cases, all m-SI values were normal at the time of follow-up, and previously observed dipole clusters of ALFMA were no longer present. One patient presented with partial alleviation of symptoms at the 2-month follow-up examination, with a reduction but incomplete normalization of initially abnormal m-SI values. There was also a reduction of dipolar ALFMA, but it did not disappear completely (Fig 4). The remaining two patients had stable postconcussive symptoms (attentional and mnemonic deficits), and follow-up MEG examinations indicated stable m-SI values (that remained abnormal) and stable dipolar ALFMA (see Table 3).
fig 4.
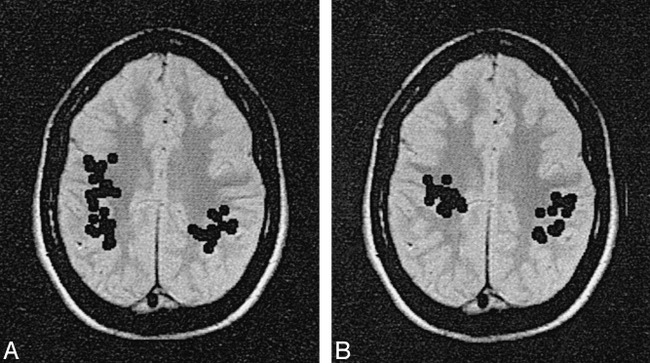
A and B, Magnetic source localization images of a 17-year-old patient who suffered diffuse blows to the head during a fight at school. The patient showed a significant cognitive decline and manifestation of an attentional deficit syndrome subsequent to the trauma. An initial examination was performed 4 months after the trauma occurred (A). The results of MR imaging and EEG were normal. MEG revealed abnormal m-SI values bilaterally at temporal and parietal sites. Multiple dipole clusters of low-frequency activity were found in each hemisphere. Sources spread throughout the right and left parietal and frontal regions, with more activity on the right. At the 9-month follow-up examination (B), the patient continued to show an impaired cognitive profile, and similar MEG results were found. There was some minor lessening of the right hemisphere MEG abnormalities, but the residual ALFMA was still significant and widespread.
TABLE 3:
Repeat magnetic source imaging (MSI) in control participants and patients with traumatic brain injury (TBI)

Discussion
The presented data confirm previous observations that posttraumatic syndromes are not necessarily limited to cases with gross structural pathologic abnormalities (7). The best neuromagnetic index of pathophysiology was the ALFMA evaluation, which indicated abnormalities in 13 of the 20 patients with postconcussive symptoms. The diagnostic sensitivity of MSI (combined ALFMA and m-SI data) for the identification of brain abnormalities as a consequence of trauma was more than three times that for MR imaging alone (0.65 versus 0.20). MSI was also more sensitive than EEG (0.65 versus 0.25). It is equally important that the false-positive rate associated with MEG was low (<10%) for both the normal control group and those post-trauma patients without postconcussive symptoms. That is, when there are no postconcussive symptoms, there are no MSI abnormalities.
Dipole cluster analysis of ALFMA in the 1- to 6-Hz band was a more sensitive index of dysfunction than were m-SI values. The increased sensitivity of the dipole analysis method reflects the paroxysmal nature of slow waves for most patients. Although single dipole modeling of spontaneous brain activity is generally considered less than ideal (15, 16), the present results indicate that this approach can be useful in identifying focal pathophysiology. The fundamental rationale for the dipole strategy is that abnormal signals from dysfunctional regions are occasionally so large that they dominate the recorded magnetic field pattern, with the simple dipole model being only minimally perturbed by the lower amplitude activity from normal tissue. Support for the usefulness of the dipole analysis strategy comes from work in stroke and brain tumor, where sources for ALFMA localize selectively to structurally compromised regions (15, 16, 21–23).
In evoked response studies in which MSI is used to identify the location of the primary somatosensory cortex, source locations have been shown to be accurate to within a few millimeters (23–25). This is probably not the case in ALFMA studies, in which there is no signal averaging and the magnitude of the extraneous background brain activity is high. Dipole sources of ALFMA occasionally localized in the white matter, rather than at the cortical mantle as expected on the basis of biophysical considerations. This most likely indicates that an extended region of the overlying cortex was actually responsible for ALFMA generation, with dipole modeling of this extended activity giving an erroneously deep source location (the magnetic field pattern of an extended cortical sheet and a deeper dipole source are often indistinguishable). Nevertheless, ALFMA source locations are probably an accurate reflection of the physiology to within 1 to 2 cm. Whereas this could be an inadequate resolution for some neurosurgical planning, it is more than sufficient for identifying which gross brain regions are compromised by a traumatic injury.
For seven of the patients with postconcussive symptoms, MEG was the only technique to provide objective evidence that the nervous system was compromised. Admittedly, there were significant differences between EEG and MEG examinations with respect to both data collection and analyses. Whereas spatial sampling in the MEG data was higher (eight positions × 37 sensors versus 20 electrode pairs), data were collected for a longer time with EEG (24 minutes total for EEG versus only 3 minutes at each probe placement for MEG). These differences were mandated by technical considerations. Spectral analyses of the two data sets were similar, but only MEG data were subjected to extensive source modeling. Considering these differences in the methods, it would be premature to conclude that EEG cannot attain the same level of diagnostic sensitivity as MEG; on the other hand, the presented comparison is totally valid with respect to MEG and EEG evaluations as they are routinely applied in clinical practice.
In considering the usefulness of MSI, it is noteworthy that the locations of dipole clusters for ALFMA and m-SI abnormalities made “clinical sense” with respect to patient symptomatology (see Table 1). For example, the dominant complaint for nine patients was short-term memory problems. Eight showed ALFMA, and in each case, the dominant zone of abnormality was the left or right temporal lobe. In contrast, five subjects reported mainly attentional problems, and among the four with ALFMA, each had parietal activity as the dominant finding. However, only three subjects showed frontal ALFMA, even though nine showed significant impairments in decision making and impulse control, which are deficits that are typically associated with frontal lobe dysfunction. On the one hand, the brain is a highly integrated organ, and frontal lobe dysfunction on neuropsychological examination might reflect primary pathologic abnormalities in other regions. On the other hand, it is also possible that a lack of frontal lobe MEG findings reflects a limitation of the method, perhaps associated with the technique's lack of sensitivity to currents oriented radial to the skull surface, which is the situation for pyramidal cell currents for much of the frontal cortex. Overall, the latter explanation seems more likely considering that in MR observations of moderate and severe trauma, structural damage to the frontal lobes and temporal poles is more common than is damage to parietal regions.
At present, it is unclear why some trauma patients have abnormal m-SI values and dipole clustering of ALFMA whereas others do not. In part, this probably reflects the limited responsivity of MEG to radial sources and subcortical activity and the limitations of dipole analysis strategies (14–19). However, it also may be a consequence of as yet unspecified differences in pathophysiological mechanisms among patients with different symptoms. For example, 16 of the subjects with postconcussive symptoms showed some cognitive dysfunction, and ALFMA was seen in 13 (81%) of these. In contrast, no ALFMA was seen in any of the four patients with pain and/or depression as isolated symptoms.
Although abnormal neuroimaging findings are strong evidence of pathophysiology, the absence of such findings does not necessarily indicate that the brain is normal. On the other hand, it may have been that some of the patients with normal MSI were without brain dysfunction and were simply malingering. However, a review of the previous neuropsychological evaluation of these patients suggests this to be unlikely.
Throughout the studies, the state of each subject was monitored, although not explicitly controlled. It is well known that low-frequency activity increases as subjects pass through drowsiness to deeper sleep stages. In these studies, data were not collected if the subjects fell asleep, and there is no evidence that trauma patients showed greater drowsiness than control subjects. Also, if differential drowsiness was the source of the observed differences between control subjects and trauma patients, such an effect would most likely be equal in both EEG and MEG data sets.
A final consideration in any study relating neuroimaging findings to cognitive symptoms is the stability of the measure. The available follow-up data suggest that MSI results closely track clinical presentation. For 10 control subjects without trauma and two trauma patients with stable cognitive symptoms (Table 3), MSI findings did not change with time. In contrast, for the three patients who underwent follow-up examinations and who showed complete or partial remission of their cognitive symptoms, there was a concomitant normalization of MSI data. This suggests a causative link between MSI abnormalities and cognitive dysfunction, and it strengthens the supposition that the observed MSI abnormalities were the result of trauma and not of some other preexisting condition.
In considering the ultimate usefulness of MSI or any other method in the evaluation of mild TBI, it is important to consider the sensitivity and specificity of the method. The presented data indicate a moderately high sensitivity of MSI for providing objective evidence of brain dysfunction in patients with postconcussive symptoms. When considering all the patients with symptoms (group C), the sensitivity was 0.65 (13 of 20 patients). If one considers just the subset of this group with neurocognitive symptoms (ie, those patients with memory, attention, and impulse control problems but not those with only pain, depression, or sensorimotor findings), then the sensitivity was 0.81 (13 of 16 patients). The diagnostic specificity of the technique was also high, at least with respect to not finding abnormalities in control subjects, with only one group A subject showing abnormal findings for a specificity value of 0.95. Specificity was also high (0.90) with respect to patients with a history of closed head injury but no postconcussive symptoms. However, some caution must be made in the application of MSI, because ALFMA seems to be a relatively nonspecific measure of brain dysfunction. Many patients without head trauma but with other conditions, such as stroke or epilepsy, also have ALFMA (15, 16, 21–23). Hence, the clinical interpretation of the MSI data and the linkage of ALFMA to a TBI must be done within the context of the patient's clinical history. Investigations are presently under way to determine whether there are subtle, as yet unspecified characteristics (amplitude, periodicity) of the ALFMA in head trauma that are different from those characteristics observed in other types of neurobiological dysfunction. If such differences can be identified, it should be possible to develop a universally specific MSI test for TBI that will be viable even in the presence of other types of brain abnormalities.
Conclusion
MEG indicated brain dysfunction in significantly more patients with postconcussive symptoms than did either EEG or MR imaging. The presence of excessive abnormal low-frequency magnetic activity provides objective evidence of brain injury in patients with postconcussive syndromes and correlates well with the degree of symptomatic recovery.
Footnotes
Address reprint requests to Jeffrey David Lewine, PhD, Department of Radiology, University of Utah School of Medicine, 1A71 Medical Center, 50 N Medical Dr, Salt Lake City, UT 84132.
References
- 1.Baker SP. Editorial: injury science comes of age. JAMA 1989;262:2248-2258 [PubMed] [Google Scholar]
- 2.Gentry LR, Godersky JC, Thompson BH. MR imaging of head trauma: review of the distribution and radiopathologic features of traumatic lesions. AJNR Am J Neuroradiol 1988;9:101-110 [DOI] [PubMed] [Google Scholar]
- 3.Hesselink JR, Dowd CF, Healy ME, et al. MR imaging of brain contusions: a comparative study with CT. AJR Am J Roentgenol 1988;150:1133-1142 [DOI] [PubMed] [Google Scholar]
- 4.Rutherford WH, Merrett JD, McDonald JR. Sequelae of concussion caused by minor head injuries. Lancet 1977;1:1-4 [DOI] [PubMed] [Google Scholar]
- 5.Leninger BE, Gramling SE, Farrell ED, et al. Neuropsychological deficits in symptomatic minor head injury patients after concussion and mild concussion. J Neurol Neurosurg Psychiatry 1990;53:293-296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McNemar Q. Note on the sampling error of the differences between correlated proportions or percentages. . Psychometrika 1947;12:153-157 [DOI] [PubMed] [Google Scholar]
- 7.Levin HS, Benton AL, Grossman RG. Neurobehavioral Consequences of Closed Head Injury.. New York: Oxford University Press; 1982
- 8.Ruff RM, Buchsbaum MS, Troster AI, et al. Computerized tomography, neuro-psychology, and positron emission tomography in the evaluation of head injury. Neuropsychiatry Neuropsychol Behav Neurol 1989;2:103-123 [Google Scholar]
- 9.Orrison WW, Gentry LR, Stimac GK, et al. Blinded comparison of cranial CT and MR in closed head injury evaluation. AJNR Am J Neuroradiol 1994;15:351-356 [PMC free article] [PubMed] [Google Scholar]
- 10.Courjon J. Traumatic disorders. In: Remond A, ed. Handbook of Electroencephalography and Clinical Neurophysiology. Amsterdam: Elsevier; 14(Part B):1972
- 11.Shoenhuber R, Gentilini M. Neurophysiological findings in closed head injury. In: Levin HS, Benton AL, Grossman RG, eds. Neurobehavioral Consequences of Closed Head Injury. London: Oxford University Press; 1982:142–152
- 12.Hugenholtz H, Stuss DT, Stethem LL, et al. How long does it take to recover from a mild concussion? Neurosurgery 1988;22:853-858 [PubMed] [Google Scholar]
- 13. The United States Office of Science and Technology Policy. Maximizing Human Potential: Decade of the Brain 1990–2000.. Washington: US Government Printing Office; 1991
- 14.Lewine JD. Neuromagnetic techniques for the noninvasive analysis of brain function. In: Freeman SE, Fukishima E, Greene ER, eds. Noninvasive Techniques in Biology and Medicine. San Francisco: San Francisco Press; 1990
- 15.Lewine JD, Orrison WW. Magnetoencephalography and magnetic source imaging. In: Orrison WW, Lewine JD, Sanders JA, Hartshorne MF, eds. Functional Brain Imaging. St Louis: Mosby; 1995:369–418
- 16.Lewine JD, Orrison WW, Astur RS, et al. Explorations of pathophysiological spontaneous activity by magnetic source imaging. In: Baumgartner C, Deecke L, Stroink G, Williamson SJ, eds. Biomagnetism: Fundamental Research and Clinical Applications, Studies in Applied Electromagnetics and Mechanics 7. Amsterdam: IOS Press, Elsevier; 1995:55–59
- 17.Schwartz BJ, Gallen CC, Aung M, et al. Magnetoencephalographic detection of focal slowing associated with head trauma. In: Baumgartner C, Deecke L, Stroink G, Williamson SJ, eds. Biomagnetism: Fundamental Research and Clinical Applications, Studies in Applied Electromagnetics and Mechanics 7. Amsterdam: IOS Press, Elsevier; 1995:66–69
- 18.Hari R, Ilmoniemi RJ. Cerebral magnetic fields. Crit Rev Biomed Eng 1986;14:93-126 [PubMed] [Google Scholar]
- 19.Williamson SJ, Kaufman L. Analysis of neuromagnetic signals. In: Gevins AS, Remond J, eds. Handbook of Electroencephalography and Clinical Neurophysiology, Volume 1: Methods and Analysis of Brain Electrical Signals. Amsterdam: Elsevier; 1987
- 20.Hughes JR, Cohen J, Mayman CI, et al. Relationship of the magnetoencephalogram to abnormal activity in the electroencephalogram. . J Neurol 1977;217:79-93 [DOI] [PubMed] [Google Scholar]
- 21.Gallen CC, Tecoma E, Iraqui U, et al. Magnetic source imaging of abnormal low frequency magnetic activity in presurgical evaluation of epilepsy. . Epilepsia 1997;38:452-460 [DOI] [PubMed] [Google Scholar]
- 22.Vieth J. Magnetoencephalography in the study of stroke (cerebrovascular accident). Adv Neurol 1990;54:261-269 [PubMed] [Google Scholar]
- 23.Benzel EC, Lewine JD, Bucholz R, et al. Magnetic source imaging: a review of the Magnes system by Biomagnetic Technologies, Inc. Neurosurgery 1993;33:252-259 [DOI] [PubMed] [Google Scholar]
- 24.Lewine JD, Baldwin NG, Bucholz RD, et al. Preoperative localization of sensorimotor cortex by combined magnetoencephalography and magnetic resonance imaging: intraoperative validation. J Neurosurg 1997;
- 25.Lewine JD, Orrison WW, Halliday A, et al. MEG functional mapping in epilepsy surgery. In: Cascino GD, Jack CR, eds. Neuroimaging in Epilepsy: Principles and Practice. Boston: Butterworth and Heinemann; 1996:193–208


