Abstract
BACKGROUND AND PURPOSE: For cases of multiple sclerosis (MS), magnetization transfer (MT) imaging may provide more pathologically specific and accurate estimates of the disease process than does conventional imaging. In this study, we evaluated changes of the MT ratio (MTR) of newly enhancing lesions, the MTR of normal-appearing white matter (NAWM), the average lesion MTR, and the MT histogram-derived metrics during a 3-year follow-up period for patients with relapsing-remitting or secondary progressive MS.
METHODS: Dual-echo, conventional spin-echo, and MT images were obtained from seven patients with relapsing-remitting MS, seven patients with secondary progressive MS, and five age- and sex-matched control subjects at the time of study entry and 1, 13, and 37 months later.
RESULTS: Newly enhancing lesions in the patients with secondary progressive MS presented a more severe and significant MTR reduction during the follow-up period as compared with those in the relapsing-remitting group. In cases of secondary progressive MS, we also observed a significant reduction of the MTR values of the NAWM and a trend toward reduction of average lesion MTR values. The patients with MS had mean percentage changes of MT histogram-derived measures that were approximately two to 10 times higher than those of the control subjects.
CONCLUSION: This preliminary 3-year follow-up study shows that newly enhancing lesions and NAWM in patients with secondary progressive MS have significantly lower MTR values than do those in patients with relapsing-remitting MS. It also shows that the tissue damage that remains after enhancement ceases is more severe in secondary progressive disease.
In cases of multiple sclerosis (MS), conventional MR imaging is quite sensitive in detecting new lesion formation and lesion changes over time (1–3). Nonetheless, conventional MR imaging is not without limitations. These include the lack of specificity to the heterogeneous pathologic substrates of the MS lesions, which range from edema and inflammation to severe demyelination and axonal loss (4), and the inability to detect the full extent of the disease process (5–8). New lesion formation, progressive damage in preexisting lesions, and progressive damage in normal-appearing white matter (NAWM) may all contribute to determining MS evolution. The two latter factors cannot be evaluated using conventional MR imaging, and this might explain the paucity of correlations found between clinical and MR findings in previous studies (1–3, 9).
Magnetization transfer (MT) imaging is promising for more accurate monitoring of the evolution of MS. MT imaging is based on the interactions between protons in a relatively free environment and in cases in which motion is restricted. In the brain, these two states correspond to the protons in tissue water versus those related to the macromolecules of myelin and other cell membranes. Off-resonance irradiation is applied, which saturates the magnetization of the less mobile protons, but this is transferred to the mobile protons, thus reducing the signal intensity from the observable magnetization. The degree of signal loss depends on the concentration and biophysics of the macromolecules in a particular tissue. Therefore, a low MT ratio (MTR) indicates a reduced capacity of the macromolecules in brain tissue to exchange magnetization with the surrounding water molecules, thus possibly reflecting matrix damage (10). The analysis of MT changes can be performed on a regional basis, thus providing information regarding individual lesions or discrete areas of the NAWM or, on a more global basis, using MTR histograms (8), thus giving a quantitative estimate of the overall disease burden in MS.
Previous studies of MS have shown that MS lesions have a dramatic MTR drop when they start to enhance (11–14) and that in these lesions, the short-term changes (3–12 months) of MTR are highly variable, ranging from a persistent and severe reduction to an almost complete recovery (11–14). Previous studies have also shown that chronic MS lesions are characterized by highly variable MTR values (5), that NAWM has lower MTR values than does the white matter in healthy control subjects (5–7, 15), and that MT-derived measures are better correlated to the physical disability (16) and the cognitive impairment (17, 18) of patients with MS than are conventional MR measures. All of these studies, however, were either cross-sectional (5–7, 16–18) or had short follow-up durations (11–15). In this 3-year follow-up study, we measured and compared MTR changes of newly enhancing MS lesions, NAWM, the entire lesion population, and the entire brain tissue in patients with relapsing-remitting and secondary progressive MS to obtain new insights into the factors underlying MS evolution.
Methods
Subjects
We studied 14 patients with clinically definite MS (19) (eight women and six men) for 3 years, with MR images obtained on four different occasions. Seven patients had relapsing-remitting disease and seven had secondary progressive disease (20). Their mean age was 35 years (standard deviation [SD] = 10 years), the median duration of the disease was 6.5 years (range, 2–16 years), and the median Expanded Disability Status Scale (EDSS) score (21) was 3.5 (range, 1–6) at the time of entry into the study and 5 (range, 1–8) at the end of the follow-up period. To be included in the study, patients could not have received immunosuppressive or immunomodulatory drugs for at least 1 year before the study or during the study period. They also could not have had relapses or steroid treatment during the 3 months preceding study initiation. In case of relapses during the study, only treatment with intravenous methylprednisolone (1 g administered daily for 3 days) was allowed. When the steroid treatment was needed near the time of a prescheduled MR examination, MR imaging was performed before treatment initiation or obtained at least 15 days after the treatment ended. No other immunomodulating or immunosuppressive treatment was allowed during the study period. In Table 1, the main demographic and clinical characteristics of the two groups of patients are reported. The patients with secondary progressive MS were older, had longer disease duration, and were more disabled than the patients with relapsing-remitting MS. The EDSS changes observed during the follow-up period, however, were similar in the two groups of patients. This might be explained by the high EDSS scores that the patients with secondary progressive MS had at the time of study entry. Five healthy volunteers (three women and two men; mean age, 35 years; SD, 8 years) served as control subjects. Local ethical committee approval and written informed consent from all subjects were obtained before study initiation.
TABLE 1:
Main demographic and clinical characteristics of the patients with relapsing-remitting and secondary progressive multiple sclerosis

MR Imaging
MR images of the brain were obtained from all subjects using a 1.5-T unit at the time of study entry (entry images) and 1 month (±7 days) (baseline images), 13 months (±28 days) (1-year images), and 37 months (±28 days) (3-year images) later. On each occasion, the machine was on a regular course of maintenance and no major upgrades occurred during the study period. The acquisition of images from the control subjects was interleaved with the acquisition of images from the patients. In each session, the following images were obtained: 1) dual-echo conventional spin-echo (CSE) (2400/30,80/1 [TR/TE/excitations]); 2) 2D gradient echo (600/12, α = 20°), with and without a saturation pulse (the saturation pulse was an off-resonance RF pulse centered 1.5 kHz below the water frequency and with a gaussian envelope of duration of 16.4 milliseconds, a bandwidth of 250 Hz, and an amplitude of 3.4 × 10−6 T); 3) unenhanced T1-weighted CSE (768/15/2); and 4) contrast-enhanced T1-weighted CSE, with the same acquisition parameters as before contrast injection, 5 minutes after injection of contrast medium (0.1 mmol/kg). For all images, 24 contiguous interleaved axial sections were acquired with a 5-mm section thickness, 256 × 256 matrix, and 250-mm field of view, giving an in-plane resolution of approximately 1 × 1 mm. The same acquisition parameters were used for the MT images, except for the number of sections, which was 20. The set of sections for the MT images was positioned to obtain the same central 20 sections as for the dual-echo and T1-weighted images. For follow-up studies, the patients were carefully repositioned according to published guidelines (22).
From the two images, one without (Mo) and one with (Ms) saturation pulse, quantitative MTR images were derived pixel by pixel according to the following equation: MTR = (Mo − Ms)/Mo × 100, in which Mo is the mean signal intensity for a particular pixel without the saturation pulse and Ms is the mean signal intensity for the same pixel when the saturation pulse is applied. Signal intensities in the calculated images are represented by the MTR value.
Image Review
Newly enhancing lesions were those that enhanced on follow-up images but were not enhancing on the entry images. They were identified and marked on the hard copies, by agreement, by two observers who were unaware of the patients' clinical status. Then, a single observer displayed the baseline enhanced images on a computer screen and, using marked hard copies as a reference, outlined these lesions, applying a semiautomated segmentation technique based on local thresholding and characterized by high intra- and interrater reproducibility (23). The regions of interest (ROIs) outlined on the enhanced images were then mapped onto the coregistered MT images, and the areas and MTR (on the corresponding images and on two follow-up images) of the regions were measured. Coregistration of images was performed using the realignment method implemented in the SPM96 software package (Wellcome Department of Cognitive Neurology, Institute of Neurology, London, England) for intratechnique image registration (24). This technique estimates the spatial transformation needed to remap images from different MR studies into the same spatial reference system, thus enabling a voxel-by-voxel correspondence between different MR studies to be obtained and ensuring the correct positioning of ROIs across multiple images. On the same occasions, using the same method and square ROIs of 8.6 mm2, MTR values of two areas in the CSF of both lateral ventricles were measured in the patients with MS. MTR values of the NAWM in different brain regions were also studied on the baseline, 1-year, and 3-year images, using square ROIs of 8.6 mm2. The NAWM areas selected had no adjacent visible MS lesions on the coregistered dual-echo images, either in the same section or the sections above and below. For each MTR examination, ROIs were placed in 11 different brain areas with NAWM (anterior part of the pons, left and right cerebellar hemispheres, left and right internal capsules, white matter close to the anterior and posterior parts of the left and right lateral ventricles, and white matter areas close to the cortical gray matter of the right and left central fissures). The MTR of NAWM from the patients were compared with the MTR of white matter from the healthy control subjects, obtained using the same approach.
Using the dual-echo images as a reference, a single observer who was unaware of the patients' clinical status and the order of acquisition of the images, outlined lesions on the MTR maps. Average lesion MTR was calculated for each patient according to the following formula:
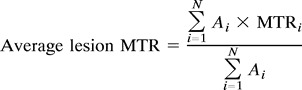 |
where N is the number of lesions in that patient, Ai is the area of lesion i, and MTRi is the average MTR in lesion i.
Histograms of the brain were obtained from all subjects at baseline and at the end of the follow-up period after the image postprocessing method previously described (8, 17, 18). The entire brain was first manually segmented from the MTR maps by a single observer who was unaware of the patients' clinical status and the order of acquisition of the images, and then MTR histograms (with bins 1% in width) were created. We excluded from analysis all pixels with MTR values lower than 10% to eliminate CSF and points corresponding to noise alone. To correct for the between-participant variability of brain volume, each histogram was normalized by dividing it by the total number of pixels included. For each histogram, the following measures were derived: 1) the relative peak height (ie, the number of pixels at the most common MTR values); 2) the peak position (ie, the most common MTR); 3) the mean brain MTR; 4) MTR25, MTR50, and MTR75, which indicate the MTR at which the respective integrals of the histogram are 25%, 50%, and 75% of the total area under the curve; and 5) the number of segmented pixels, which is a measure of the overall brain size. All histogram-derived measures are representative of the tissue studied as a whole, thus including MS lesions, NAWM, and normal-appearing gray matter.
Statistical Analysis
Differences in clinical measures between the two groups of patients were studied using Student's t-test for unpaired data when the data were normally distributed or using the Mann-Whitney test when the data were not normally distributed. One-way analysis of variance was used to evaluate changes over time of MT-derived measures (MTR of newly enhancing lesions, MTR of NAWM, MTR of CSF, and average lesion MTR) from the patients and control subjects and to compare MT-derived measures at each time point for the control subjects, patients with relapsing-remitting MS, and patients with secondary progressive MS. Post hoc analysis was performed using Student's t-test for unpaired data (lesions) and for paired data (NAWM and CSF). MT histogram-derived measures were compared using the Mann-Whitney test.
Results
No abnormalities were found on the images obtained from the control subjects. Fifty (40 in the relapsing-remitting group and 10 in the secondary progressive group) newly enhancing lesions were found on the baseline images. The mean area of these lesions was 0.13 mL (SD = 0.08 mL) for the patients with relapsing-remitting MS and 0.17 mL (SD = 0.05 mL) for the patients with secondary progressive MS (this difference was not statistically significant). In Table 2, the MTR of these lesions at the time of their appearance and their changes over time are reported. In the whole sample size, there was no significant change of MTR values over time. Nonetheless, newly enhancing lesions in the patients with secondary progressive MS compared with those in the patients with relapsing-remitting MS had lower MTR at the time of their appearance and presented a more severe and significant MTR reduction during the follow-up period (Table 2). The mean MTR reduction of newly enhancing lesions between the baseline and 3-year images was 6.05% (SD = 6.06%) in the patients with secondary progressive disease and 0.33% (SD = 4.66%) in the patients with relapsing-remitting disease (P = .01). The MTR values from the CSF ROI were within the expected range, were similar for the two groups of patients, and remained stable over time (Table 3).
TABLE 2:
Magnetization transfer ratio values of newly enhancing lesions from patients with relapsing-remitting and secondary progressive multiple sclerosis on the baseline and follow-up images
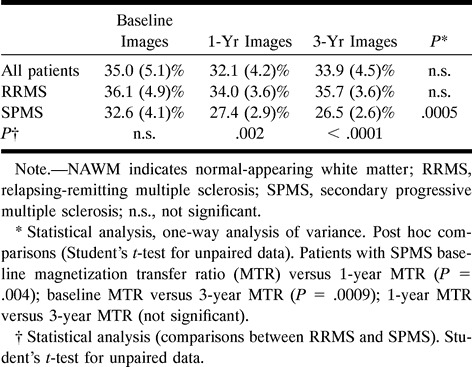
TABLE 3:
Magnetization transfer ratio values of CSF areas from patients with relapsing-remitting and secondary progressive multiple sclerosis

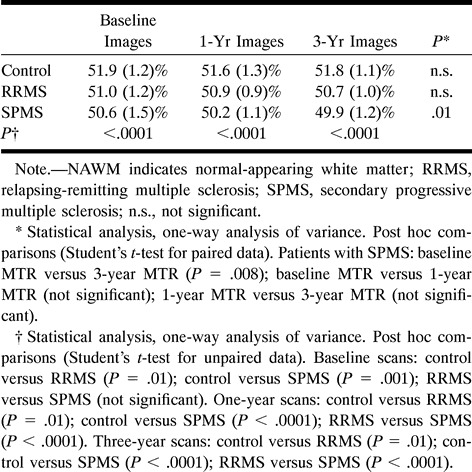
The average MTR values obtained for the NAWM areas studied were higher for the control subjects than for the patients at any time point (Table 4). During the follow-up period, the MTR values of the NAWM from the control subjects and from the patients with relapsing-remitting MS remained stable, whereas the MTR values of the NAWM from the patients with secondary progressive MS showed a significant reduction (Table 4).
TABLE 4:
Magnetization transfer ratio values of normal-appearing white matter areas from control participants and from patients with relapsing-remitting and secondary progressive multiple sclerosis
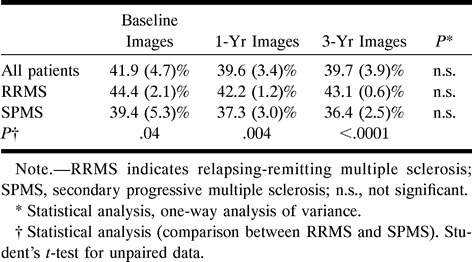
In Table 5, the average lesion MTR at each time point is reported for the whole sample size and for the patients with relapsing-remitting and secondary progressive MS. At each time point, the patients with secondary progressive MS had significantly lower MTR values than did the patients with relapsing-remitting MS. Although no significant changes were observed in either of the groups (perhaps because of the relatively small sample sizes), the average lesion MTR appeared to remain stable through the 3-year follow-up period in the patients with relapsing-remitting disease whereas it showed a trend toward progressive reduction in the patients with secondary progressive disease (this is reflected by the increased significance of the MTR differences between the two groups during the follow-up period).
TABLE 5:
Average lesion magnetization transfer ratio from patients with relapsing-remitting and secondary progressive multiple sclerosis
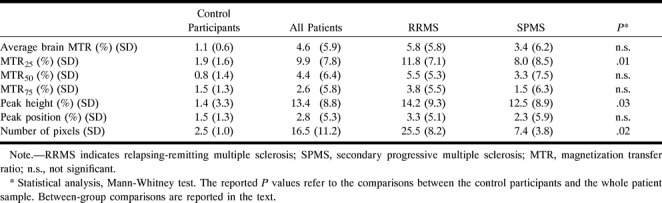
In Table 6, the MT histogram-derived metrics are reported at baseline and 3-year follow-up for the control subjects and patients. In Figure 1, the MT histograms from patients with relapsing-remitting and secondary progressive MS at baseline and at 3-year follow-up are presented. In the control subjects, there was no significant change in any of the parameters, whereas in the patients, we observed a significant reduction of average brain MTR, MTR25, MTR50, and peak height. A significant reduction in brain size was also noticed in the patients. Considering patients with relapsing-remitting and secondary progressive MS separately, no significant change was observed in secondary progressive MS whereas MTR25 (P = .02) and the number of segmented pixels (P = .001) decreased significantly during the follow-up period in the patients with relapsing-remitting MS. The percentage of changes during the follow-up period of the MT histogram-derived parameters from the control subjects and patients are reported in Table 7. The patients had mean percentage changes that were approximately two to 10 times higher than those in the control subjects and reached significance for MTR25, peak height, and number of segmented pixels. When considering the two patient groups separately, those with relapsing-remitting disease showed the highest mean percentage of change. Compared with the healthy control subjects, they had significantly greater changes for MTR25 (P = .01) and number of segmented pixels (P = .002). Compared with the patients with secondary progressive MS, they had a greater reduction in the number of segmented pixels (P = .03).
TABLE 6:
Mean magnetization transfer metrics at baseline and at 3-year follow-up in control participants and in patients with multiple sclerosis
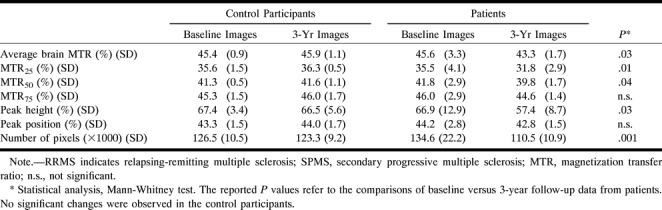
fig 1.
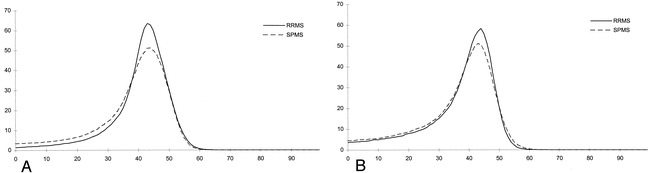
MTR histograms from patients with relapsing-remitting (solid lines) and secondary progressive (dotted lines) MS at baseline (A) and at 3-year follow-up (B).
TABLE 7:
Mean percentage changes over time of the magnetization transfer histogram-derived metrics from control participants and from patients with multiple sclerosis
Discussion
This 3-year follow-up study showed that newly enhancing lesions in patients with secondary progressive MS have significantly lower MTR values than those in patients with relapsing-remitting MS. It also showed that in these lesions, the tissue damage that remains after enhancement has ceased is more severe. The lower MTR of newly enhancing lesions in patients with secondary progressive MS might result from an unfavorable balance between damaging and reparative mechanisms active from the very early stages of MS lesion formation (25) or might be due to new damage being superimposed on older lesions, where relevant damage has already occurred. The further reduction in MTR values of these lesions during the follow-up period might be caused by multiple disease reactivation within lesions in patients with secondary progressive MS or might be the result of a more severe intrinsic pathologic process once the lesions of such patients are formed. Because of the imaging schedule we used, the present study cannot solve this issue. Nevertheless, whatever the mechanisms underlying the different evolution of newly enhancing lesions in patients with relapsing-remitting MS and secondary progressive MS are, these results fit well with the more severe clinical evolution of patients with secondary progressive disease. Although MTR reduction is more evident in newly enhancing lesions, a similar trend was also observed in the overall lesion population of the patients with secondary progressive MS. This finding strengthens previous cross-sectional observations (16) that patients with secondary progressive MS have the lowest average lesion MTR values. The lower sensitivity of average lesion MTR compared with individual lesion assessment in detecting changes over time might be a consequence of the less dramatic changes occurring in the population of chronic lesions and to the concomitant presence of various degrees of damaging and reparative mechanisms in the different lesions in the same patients.
Although the pathologic substrates of marked MTR reductions in MS lesions are still unclear, profound MTR reductions might be associated with severe loss of tissue structural integrity. A recent preliminary postmortem report found a correlation between MTR and percentage of residual axons in MS lesions (26). Animal studies also have showed that low MTR values correlated with histopathologic findings of myelin loss and axon destruction (27–29), whereas edematous lesions resulted in slightly increased MTR values (5). Finally, dramatically reduced MTR values are found in the “pure” demyelinating lesions of patients with progressive multifocal leukoencephalopathy (30) or central pontine myelinolysis (31).
In patients with secondary progressive MS, but not in patients with relapsing-remitting MS, MTR of the NAWM also tends to decrease over time. This reduction is less than that observed in newly enhancing lesions, but it might nevertheless be clinically relevant. We used very accurate repositioning and coregistration techniques, and systematic changes of the MTR were not observed in the white matter of the control subjects or in the CSF of the patients. There are possible pathologic substrates that may contribute to our observation. MTR reduction reflects increased unbound water content in diseased brain tissue. In the macroscopically examined NAWM of patients with MS, marked astrocytic proliferation, perivascular inflammation, and, occasionally, demyelination and axonal loss have been detected (32–34). Any of these processes may account for an increased amount of unbound water in the areas that we studied.
Our study also shows that MT histograms are able to depict changes over time and that the degree of such changes might be related to the different phases of disease evolution. Previous studies have found that patients with MS have reduced MT histogram-derived metrics (8, 17) and brain size (35, 36) as compared with those of control subjects. Our study suggests that overall brain changes are not prominent in cases of secondary progressive MS; possibly, it is more difficult to detect changes because of the severe brain involvement already present in such patients. These findings highlight the importance of individual lesion monitoring in the most advanced phases of the disease in which such an approach, as suggested by the present study, might provide more sensitive measures for assessing disease evolution, either natural or modified by treatment.
Conclusion
This preliminary study confirms that MT-derived measures are promising for the assessment of MS disease evolution and that the factors underlying the development of disability in MS may change in the different phases of the disease. As a consequence, it also suggests that MT measurement strategies should be tailored to the patient group studied.
Acknowledgments
We thank Clodoaldo Pereira and Arianna Pratesi for their skillful technical assistance in collecting and postprocessing the MR data.
Footnotes
Address reprint requests to Massimo Filippi, MD, Neuroimaging Research Unit, Department of Neuroscience, Scientific Institute Ospedale San Raffaele, Via Olgettina, 60, 20132 Milan, Italy.
References
- 1.Miller DH, Albert PS, Barkhof F,, et al. Guidelines for the use of magnetic resonance techniques in monitoring the treatment of multiple sclerosis. Ann Neurol 1996;39:6-16 [DOI] [PubMed] [Google Scholar]
- 2.Filippi M, Miller DH. MRI in the differential diagnosis and monitoring the treatment of multiple sclerosis. Curr Opin Neurol 1996;9:176-186 [DOI] [PubMed] [Google Scholar]
- 3.Filippi M, Horsfield MA, Ader HI,, et al. Guidelines for using quantitative measures of brain magnetic resonance imaging abnormalities in monitoring the treatment of multiple sclerosis. Ann Neurol 1998;43:449-506 [DOI] [PubMed] [Google Scholar]
- 4.Filippi M. The role of non-conventional magnetic resonance techniques in monitoring evolution of multiple sclerosis. J Neurol Neurosurg Psychiatry 1998;64(Suppl):S52-S58 [PubMed] [Google Scholar]
- 5.Dousset V, Grossman RI, Ramer KN,, et al. Experimental allergic encephalomyelitis and multiple sclerosis: lesion characterization with magnetization transfer imaging. Radiology 1992;182:483-491 [DOI] [PubMed] [Google Scholar]
- 6.Filippi M, Campi A, Dousset V,, et al. A magnetization transfer imaging study of normal-appearing white matter in multiple sclerosis. Neurology 1995;45:478-482 [DOI] [PubMed] [Google Scholar]
- 7.Loevner LA, Grossman RI, Cohen JA, Lexa FJ, Kessler D, Kolson DL. Microscopic disease in normal-appearing white matter on conventional MR images in patients with multiple sclerosis: assessment with magnetization-transfer measurements. Radiology 1995;96:511-515 [DOI] [PubMed] [Google Scholar]
- 8.van Buchem MA, McGowan JC, Kolson DL, Polansky M, Grossman RI. Quantitative volumetric magnetization transfer analysis in multiple sclerosis: estimation of macroscopic and microscopic disease burden. Magn Reson Med 1996;36:632-636 [DOI] [PubMed] [Google Scholar]
- 9. The IFNB Multiple Sclerosis Study Group and the University of British Columbia MS/MRI Analysis Group. Interferon beta-1b in the treatment of multiple sclerosis: final outcome of the randomized controlled trial. Neurology 1995;45:1277-1285 [PubMed] [Google Scholar]
- 10.Grossman RI. Magnetization transfer in multiple sclerosis. Ann Neurol 1994;36(Suppl):S97-S99 [DOI] [PubMed] [Google Scholar]
- 11.Filippi M, Rocca MA, Rizzo G,, et al. Magnetization transfer ratios in multiple sclerosis lesions enhancing after different doses of gadolinium. Neurology 1998;50:1289-1293 [DOI] [PubMed] [Google Scholar]
- 12.Silver NC, Lai M, Symms MR, Barker GJ, McDonald WI, Miller DH. Serial magnetization transfer imaging to characterize the early evolution of new MS lesions. Neurology 1998;51:758-764 [DOI] [PubMed] [Google Scholar]
- 13.van Waesberghe JH, van Walderveen MA, Castelijns JA,, et al. Patterns of lesion development in multiple sclerosis: longitudinal observations with T1-weighted spin-echo and magnetization transfer MR. AJNR Am J Neuroradiol 1998;19:675-683 [PMC free article] [PubMed] [Google Scholar]
- 14.Lai HM, Davie CA, Gass A,, et al. Serial magnetization transfer ratios in gadolinium-enhancing lesions in multiple sclerosis. J Neurol 1997;244:308-311 [DOI] [PubMed] [Google Scholar]
- 15.Filippi M, Rocca MA, Martino G, Horsfield MA, Comi G. Magnetization transfer changes in the normal appearing white matter precede the appearance of enhancing lesions in patients with multiple sclerosis. Ann Neurol 1998;43:809-814 [DOI] [PubMed] [Google Scholar]
- 16.Gass A, Barker GJ, Kidd D,, et al. Correlation of magnetization transfer ratio with disability in multiple sclerosis. Ann Neurol 1994;36:62-67 [DOI] [PubMed] [Google Scholar]
- 17.Rovaris M, Filippi M, Falautano M,, et al. Relation between MR abnormalities and patterns of cognitive impairment in multiple sclerosis. Neurology 1998;50:1601-1608 [DOI] [PubMed] [Google Scholar]
- 18.van Buchem MA, Grossman RI, Armstrong C,, et al. Correlation of volumetric magnetization transfer imaging with clinical data in MS. Neurology 1998;50:1609-1617 [DOI] [PubMed] [Google Scholar]
- 19.Poser CM, Paty DW, Scheinberg L,, et al. New diagnostic criteria for multiple sclerosis: guidelines for research protocols. Ann Neurol 1983;13:227-231 [DOI] [PubMed] [Google Scholar]
- 20.Lublin FD, Reingold SC, the National Multiple Sclerosis Society (USA) Advisory Committee on Clinical Trials of New Agents in Multiple Sclerosis. Defining the clinical course of multiple sclerosis: results of an international survey. Neurology 1996;46:907-911 [DOI] [PubMed] [Google Scholar]
- 21.Kurtzke JF. Rating neurological impairment in multiple sclerosis: an expanded disability status scale (EDSS). Neurology 1983;33:1444-1452 [DOI] [PubMed] [Google Scholar]
- 22.Miller DH, Barkhof F, Berry I, Kappos L, Scotti G, Thompson AJ. Magnetic resonance imaging in monitoring the treatment of multiple sclerosis: concerted action guidelines. J Neurol Neurosurg Psychiatry 1991;54:683-688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rovaris M, Filippi M, Calori G,, et al. Intra-observer reproducibility in measuring new putative MR markers of demyelination and axonal loss in multiple sclerosis: a comparison with conventional T2-weighted images. J Neurol 1997;244:266-270 [DOI] [PubMed] [Google Scholar]
- 24.Friston KJ, Ashburner J, Poline JB,, et al. Spatial realignment and normalization of images. Hum Brain Mapping 1995;3:165-189 [Google Scholar]
- 25.Prineas JW, Suchanek G, Ozawa K. Histopathology and the blood-cerebrospinal fluid barrier in multiple sclerosis. Ann Neurol 1994;36(Suppl):S42-S46 [DOI] [PubMed] [Google Scholar]
- 26.van Waesberghe JHTM, van Walderveen MAA, de Groot C, et al. Postmortem correlation between axonal loss, MTR, and hypointensity on T1 SE in MS. Proceedings of the International Society for Magnetic Resonance in Medicine 1998;1334: [Google Scholar]
- 27.Lexa FJ, Grossman RI, Rosenquist AC. MR of wallerian degeneration in the feline visual system: characterization by magnetization transfer rate with histopathologic correlation. AJNR Am J Neuroradiol 1994;15:201-212 [PMC free article] [PubMed] [Google Scholar]
- 28.Dousset V, Brochet B, Vital A,, et al. Lysolecithin-induced demyelination in primates: preliminary in vivo study with MR and magnetization transfer. AJNR Am J Neuroradiol 1995;16:225-231 [PMC free article] [PubMed] [Google Scholar]
- 29.Kimura H, Meaney DF, McGowan JC,, et al. Magnetization transfer imaging of diffuse axonal injury following experimental brain injury in the pig: characterization by magnetization transfer ratio with histopathologic correlation. J Comput Assist Tomogr 1996;20:540-546 [DOI] [PubMed] [Google Scholar]
- 30.Dousset V, Armand JP, Lacost D,, et al. Magnetization transfer study of HIV encephalitis and progressive multifocal leukoencephalopathy. AJNR Am J Neuroradiol 1997;18:895-901 [PMC free article] [PubMed] [Google Scholar]
- 31.Silver NC, Barker GJ, MacManus DG,, et al. Decreased magnetization transfer ratio due to demyelination: a case of central pontine myelinolysis. J Neurol Neurosurg Psychiatry 1996;61:208-209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Adams CWM. Pathology of multiple sclerosis: progression of the lesion. Br Med Bull 1977;33:15-20 [DOI] [PubMed] [Google Scholar]
- 33.Allen IV, McKeown SR. A histological, histochemical and biochemical study of the macroscopically normal white matter in multiple sclerosis. J Neurol Sci 1979;41:81-91 [DOI] [PubMed] [Google Scholar]
- 34.Trapp BD, Peterson J, Ransohoff RM, Rudik R, Mork S, Bo L. Axonal transection in the lesions of multiple sclerosis. N Engl J Med 1998;338:278-285 [DOI] [PubMed] [Google Scholar]
- 35.Filippi M, Mastronardo G, Rocca MA, Pereira C, Comi G. Quantitative volumetric analysis of brain magnetic resonance imaging from patients with multiple sclerosis. J Neurol Sci 1998;158:148-153 [DOI] [PubMed] [Google Scholar]
- 36.Losseff NA, Wang L, Lai HM,, et al. Progressive cerebral atrophy in multiple sclerosis: a serial MRI study. Brain 1996;119:2009-2019 [DOI] [PubMed] [Google Scholar]


