Abstract
BACKGROUND AND PURPOSE: The likelihood that carotid plaque will give rise to cerebral ischemia probably relates to the degree of arterial stenosis and to plaque morphology. The aim of this study was to assess whether features seen at CT angiography might be used to predict carotid plaque stability by comparing CT angiograms with histopathologic examinations of the carotid artery bifurcation.
METHODS: Nine patients with symptomatic severe carotid stenosis at intraarterial angiography had CT angiography of the carotid bifurcation before carotid endarterectomy. After endarterectomy, multiple sections of the specimens through the carotid bifurcation were examined histologically. Plaque characteristics recorded included the proportion of necrotic/lipid core, presence of hemorrhage, extent of fibrosis, ulceration, calcification, inflammatory cell infiltrate, and fibrous cap thickness. Corresponding CT angiograms were assessed for plaque size, distribution, and radiodensity as well as presence of calcific density and ulceration. Histologic findings and CT angiograms were compared.
RESULTS: Plaque with a large necrotic/lipid core, which was often hemorrhagic, was found in 16 of 23 sections, and in 15 of these this histologic appearance corresponded with patchy or homogeneous low density on CT angiograms. Six of seven predominantly fibrous plaques were of soft-tissue density on CT angiograms. High density consistent with calcification was seen more frequently on CT angiograms than it was detected histologically, but CT angiography depicted plaque ulceration poorly (four ulcers at histology; two false-positive and two false-negative findings at CT angiography).
CONCLUSION: CT angiography is a promising method for assessing the lumen and wall of the carotid artery. The apparent correlation between histologic appearance and plaque density on CT angiograms has important implications for the prediction of plaque stability, even though ulceration is shown inconsistently.
It is likely that the frequency of symptomatic cerebral ischemic episodes is related not only to the degree of carotid artery stenosis but also to the histopathologic composition of the atheromatous plaque causing the stenosis. Necropsy studies of coronary arteries in patients suffering crescendo angina, myocardial infarction, and sudden cardiac death have documented features that delineate an “unstable” atheromatous plaque (1–3), characterized histologically by a large lipid/necrotic core with a thin or ruptured fibrous cap and a dense inflammatory cellular infiltrate. Histologic studies of carotid endarterectomy specimens have also shown a correlation between recent symptoms of cerebral ischemia and plaques with high lipid content, intraplaque hemorrhage, low levels of collagen, and ulceration (4, 5).
Until recently, digital subtraction angiography (DSA) has been the standard of reference for evaluation of symptomatic carotid stenosis. However, DSA shows arterial calcification poorly, is an insensitive method for depicting plaque ulceration (6), and provides minimal information on the composition of the arterial wall. CT angiography is a relatively recent development and correlates well with DSA in the assessment of carotid artery stenosis (7–9). However, it has not been used to assess atherosclerotic plaque characteristics. The aim of this study was to compare the histologic and CT angiographic appearances of atheromatous plaque at the carotid bifurcation and to assess whether features seen on CT angiograms might be used to predict plaque stability.
Methods
The study formed part of a larger prospective series comparing DSA, Doppler sonography, MR angiography, and CT angiography in the evaluation of symptomatically severe (>70%) carotid stenosis. Of 13 patients examined by CT angiography who proceeded to carotid endarterectomy, three were found to have pathologic specimens unsuitable for assessment and one had a CT angiographic examination that was technologically inadequate. This left nine patients whose examinations were suitable for analysis.
The nine endarterectomy specimens were resected en bloc, orientated, fixed in buffered formalin, decalcified if necessary in 8% formic acid, examined macroscopically, and sectioned serially at 5-mm intervals. Paraffin sections (5 μm thick) were stained with hematoxylin-eosin and Martin's scarlet blue. The histologic appearances were assessed independently by two observers, and 2D axial plaque sections were used to draw longitudinal plaque profiles. Suitable representative sections through the distal common carotid artery, carotid bulb, and proximal internal carotid artery were selected by an experienced neuropathologist from each specimen. The exact level of the sections was defined by reference to the level of the bifurcation. Each section was examined for the following plaque characteristics: size of necrotic/lipid core, hemorrhage, fibrosis, ulceration or fissuring, calcification, inflammatory cell infiltrate, and fibrous cap thickness.
CT angiography was performed using a Somatom Plus scanner (Siemens, Erlangen, Germany). After a planning scan to identify the bifurcation level, a test dose of 18 mL of contrast material (300 mg/mL, 3 mL/s by pump injector) was given to determine the optimal scan delay. A spiral scan of the carotid bifurcation was then performed (3-mm section thickness, 1.0 pitch, single 24-second breath-hold, 120 kV, 180 mA, and 90 mL of contrast material at 3 mL/s) and images were reconstructed at 1-mm increment intervals.
Hard-copy images corresponding to the same level as the histologic sections (defined by distance from the bifurcation in millimeters), along with 3D maximum intensity projection (MIP) images, were reviewed by two radiologists who were unaware of the pathologic findings. Features assessed included size, distribution, and radiodensity of plaque (relative to muscle and fat density), plaque surface irregularity suggestive of ulceration, and presence and extent of high plaque density consistent with calcification.
CT angiograms and histologic findings were compared.
Results
Twenty-three sites covering the carotid bifurcation of nine arteries were compared. Atheromatous plaque that was uniformly isodense with muscle was associated with fibrosis at histology in all but one case. Conversely, the CT appearance of plaque that was hypodense relative to muscle or that contained any elements of low density was associated histologically with plaque that was composed predominantly of necrotic lipid debris, often with hemorrhage. This association was found in 15 of 16 histologic sections (Figs 1 and 2). CT failed to depict significant plaque necrosis on one section (false-negative finding). On one other section, a false-positive diagnosis of necrosis was made on CT scans, the corresponding histologic section showing fibrosis. However, histologic analysis of this specimen suggested that the fibrous tissue had only recently developed.
fig 1.

Low-density plaque.
A, CT angiogram of internal carotid artery 1 cm above the bifurcation shows a severe stenosis (black arrow indicates contrast in lumen) caused by a large eccentric hypodense plaque (white arrowheads). The small area of calcification (open arrow) was not detected on the histologic section.
B, Corresponding histologic section shows a large necrotic core (N). The fibrous cap is intact at this level but of variable thickness; at O, it is thinned, and higher power showed superficial inflammation. S indicates the site of surgical incision.
fig 2.

Mixed-density plaque.
A, CT angiogram of the proximal internal carotid artery shows a predominantly intermediate-density plaque with foci of hypodensity (arrowheads). Curved arrow indicates internal jugular vein.
B, Corresponding histologic section shows an irregular slitlike lumen (Lu) separated by a reasonably thick fibrous cap (C) from a large necrotic core (N) comprising necrotic and lipid debris as well as thrombus. S indicates surgical incision.
CT angiography was a poor predictor of plaque ulceration. Two histologically verified ulcers were correctly identified (Fig 3); however, in two other cases, a diagnosis of ulceration was made on CT angiograms but not confirmed histologically (false-positive findings) and two other ulcers were missed by CT angiography. Thickness of the fibrous cap and presence of plaque inflammation, two other features of an unstable plaque, could not be assessed by CT angiography.
fig 3.
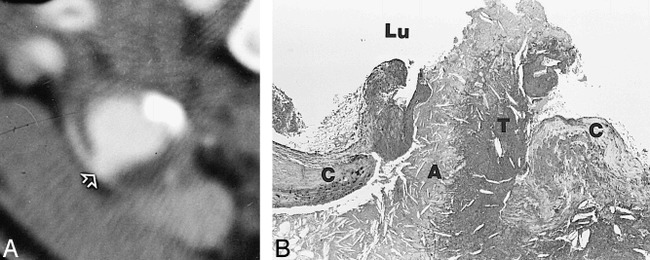
Plaque ulceration.
A, CT angiogram of the common carotid artery 1 cm below the bifurcation shows circumferential arterial wall thickening, with a focal contrast-containing defect interpreted as an ulcer (arrow).
B, Corresponding histologic section shows a ruptured fibrous cap (C) with a mixture of necrotic debris (A) and thrombus (T) projecting into the lumen (Lu).
Calcification was depicted by CT angiography in 16 sections. In nine of these sections, this was not seen on the corresponding pathologic section. In two other sections, microcalcification was missed on CT angiograms.
The accuracy of CT angiography in depicting individual histologic features is documented in the Table.
Sensitivity, specificity, and accuracy of CT angiography in the identification of histologic characteristics of carotid atheromatous plaque

Discussion
Carotid bifurcation atheroma is an important cause of stroke, which may be mediated by embolic, occlusive, or low-flow–related mechanisms (10, 11). A relationship between the degree of carotid stenosis and risk of stroke has been established (12, 13), and two studies have clearly shown improved outcomes in patients with severe stenosis (>70%) after surgical intervention, as compared with the best medical treatment (14, 15).
Observations that a small proportion of stroke patients have severe carotid stenosis (16, 17) and that many elderly people have severe carotid stenosis but no symptoms (18) suggest that the degree of stenosis is not the sole variable in predicting stroke risk. It has been argued that the nature of the plaque may be at least as important, and histologic studies have identified patterns of carotid artery plaque composition that correlate with symptoms (4, 5). If unstable carotid plaque composition could be predicted by imaging, stroke risk assessment might be refined, allowing better selection of patients for surgery.
The concept of atheromatous plaque instability was developed initially through clinicopathologic study of postmortem coronary arteries (1, 3, 19, 20), although the relevance of this concept for the extracranial carotid circulation remains undetermined. The resistance of coronary and carotid arteries differs significantly, largely because of differences in vessel diameter, and the effect of these differences on hemodynamics and plaque stability is as yet unknown.
The presence of plaque irregularity at DSA has been shown to be associated with an increased risk of early stroke (21). However, the ability to detect plaque ulceration accurately using DSA has been questioned (6): arterial calcification is poorly depicted, the morphology of atheromatous plaque cannot be defined, and the technique is associated with a significant periprocedural morbidity risk of 0.5% to 4%, the highest risk occurring in patients with atheromatous bifurcations.
Doppler sonography has been studied as a method of predicting carotid artery plaque stability, and reports suggest that plaques that are echolucent, heterogeneous, or irregular are more likely to be associated with current or future symptoms (5, 22, 23), but no consensus exists as to which features are most important, how they should be graded (24, 25), or what these changes represent histologically (26, 27). In addition, calcification at the level of stenosis may obscure the underlying noncalcified plaque, thus preventing adequate assessment.
Features of carotid artery plaque morphology have recently been observed with MR imaging, although several sequences may be required, and difficulties in characterizing plaque may be encountered owing to the wide variation in signal characteristics within thrombus and fibrous tissue of different ages (28).
The number of patients in our preliminary descriptive study of CT angiographic plaque morphology is small, and this prevents statistical analysis of the results. Case selection was limited to those patients in whom the histologic appearance of plaque was sufficiently well defined to allow accurate characterization. As a result, some damaged specimens were excluded from the study.
We found that plaque with elements of fat density on CT angiograms was usually composed predominantly of necrotic lipid, and this was often associated with varying degrees of intraplaque hemorrhage. The presence of a large lipid/necrotic core is a key feature of plaque instability. Conversely, plaques that appeared solely to be of soft-tissue density on CT angiograms were found histologically to be composed of “stable” fibrous tissue.
Further studies are required to establish the true significance of plaque density, but these preliminary results suggest that the presence of low density on CT angiograms may predict plaque that is histologically unstable. The potential to assess the degree of carotid stenosis and to predict the histopathologic constitution of stenotic carotid plaque, using CT angiography as a single examination, has important implications for the cost-effective, noninvasive assessment of stroke risk.
The inability of CT angiography to identify accurately a discrete fibrous cap or plaque ulceration is disappointing. However, this may be partly due to technique, as the two missed ulcers were small and may have been obscured by partial volume effects in the relatively thick CT sections. At the time of our study, the maximum spiral scan time allowed by tube heating capacity was 24 seconds, and a 3-mm section thickness was selected to ensure adequate coverage of the carotid bifurcation. By reducing section collimation to 2 mm while maintaining a 1-mm section overlap, pitch, and tube current, it is possible to achieve a reduction in the contribution from partial-volume effects and consequent improvement in z-axis resolution, at the expense of increased image noise and reduced z-axis coverage. The z-axis coverage of the scan can be increased by using a larger section thickness, an increased scan pitch (both of which will decrease the z-axis resolution), or a longer scan duration. Improvements in scanner technology now permit spiral scans of 60 seconds or greater. The required coverage of the scan depends on the preferences of the radiologist and clinician.
Cumming and Morrow (7) used multiplanar reformatted CT angiograms to assess the carotid bifurcation and found good correlation with conventional angiograms in the demonstration of plaque irregularity suggestive of ulceration. No histologic analysis was performed. In our study, MIP reconstructions were useful in assessing the site and degree of stenosis. However, in all other respects, we feel that plaque morphology was better depicted on source axial images.
The contrast resolution provided by CT angiography allowed excellent analysis of calcification, with considerably more calcified plaque identified than at histologic examination. Cases in which calcification was evident on CT scans but was not seen at histology probably represent false-negative histologic examinations and reflect the greater sensitivity of CT. The disparity between histology and CT may be partly due to the volume incorporated within the CT sections (3-mm thickness), which was considerably greater than that of the pathologic sections (5-μm thickness). It is also possible that larger calcified foci were displaced from the section during slide preparation and that early stages of mineralization, which closely resemble mature sclerotic tissue, were not detected microscopically. CT has an advantage over sonography in that heavy calcification does not obscure the rest of the arterial wall. Plaque calcification is not a feature of the unstable plaque, but its accurate depiction is of practical use in planning the site of arteriotomy or extent of endarterectomy.
Conclusion
CT angiography of the carotid bifurcation is a fast, noninvasive technique that provides information on both the vessel lumen and the atheromatous vessel wall. It allows assessment of plaque density, which reflects histologic findings related to plaque stability and provides excellent analysis of plaque calcification. Owing to limitations in spatial resolution, it does not show plaque ulceration accurately. Further studies with larger numbers of patients, higher-resolution scanning, and clinical follow-up are needed to assess the potential of CT angiography to predict outcome based on the combination of degree of carotid artery stenosis and plaque morphology.
Footnotes
Presented in part at the International Congress of Head and Neck Radiology, Strasbourg, France, November 1997.
Address reprint requests to Dr. D. A. Collie, Department of Neuroradiology, Western General Hospital, Crewe Rd South, Edinburgh EH4 2XU, Scotland, U.K.
References
- 1.Davies MJ, Richardson PD, Woolf N, Katz DR, Mann J. Risk of thrombosis in human atherosclerotic plaques: role of extracellular lipid, macrophage and smooth muscle content. Br Heart J 1993;69:377-381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rosenschein U, Ellis SG, Haudenschild CC, et al. Comparison of histopathologic coronary lesions obtained from directional atherectomy in stable angina versus acute coronary syndromes. Am J Cardiol 1994;73:508-510 [DOI] [PubMed] [Google Scholar]
- 3.Davies MJ, Thomas A. Thrombosis and acute coronary-artery lesions in sudden cardiac ischaemic death. N Engl J Med 1984;310:1137-1140 [DOI] [PubMed] [Google Scholar]
- 4.Seeger JM, Barratt E, Lawson GA, Klingman N. The relationship between carotid plaque composition, plaque morphology and neurologic symptoms. J Surg Res 1995;58:330-336 [DOI] [PubMed] [Google Scholar]
- 5. European Carotid Plaque Study Group. Carotid artery plaque composition: relationship to clinical presentation and sonography B-mode imaging. Eur J Vasc Endovasc Surg 1995;10:23-30 [DOI] [PubMed] [Google Scholar]
- 6.Streifler JY, Eliasziw M, Fox AJ, et al. Angiographic detection of carotid plaque ulceration: comparison with surgical observations in a multicenter study, North American Symptomatic Carotid Endarterectomy Trial. Stroke 1994;25:1130-1132 [DOI] [PubMed] [Google Scholar]
- 7.Cumming MJ, Morrow IM. Carotid artery stenosis: a prospective comparison of CT angiography and conventional angiography. AJR Am J Roentgenol 1994;163:517-523 [DOI] [PubMed] [Google Scholar]
- 8.Collie DA, Wardlaw JM, Wright AR, Gibson RJ, Sellar RJ. Comparison of Doppler ultrasound, CT angiography, MR angiography and transfemoral angiography in the assessment of carotid stenosis: a prospective comparative trial (abstr). Neuroradiology 1996;38:191 [Google Scholar]
- 9.Link J, Brossmann J, Grabener M, et al. Spiral CT angiography and selective digital subtraction angiography of internal carotid artery stenosis. AJNR Am J Neuroradiol 1996;17:89-94 [PMC free article] [PubMed] [Google Scholar]
- 10.Ogata J, Masuda J, Yutani C, Yamaguchi T. Rupture of atheromatous plaque as a cause of thrombotic occlusion of stenotic internal carotid artery. Stroke 1990;21:1740-1745 [DOI] [PubMed] [Google Scholar]
- 11.Pessin MS, Hinton RC, Davis KR, et al. Mechanisms of acute carotid stroke. Ann Neurol 1979;6:245-252 [DOI] [PubMed] [Google Scholar]
- 12.Norris JW, Zhu CZ, Bornstein NM, Chambers BR. Vascular risks of asymptomatic carotid stenosis. Stroke 1991;22:1485-1490 [DOI] [PubMed] [Google Scholar]
- 13.Roederer GO, Langlois YE, Jager KA, et al. The natural history of carotid arterial diseases in asymptomatic patients with cervical bruits. Stroke 1984;15:605-613 [DOI] [PubMed] [Google Scholar]
- 14. European Carotid Surgery Trialists Collaboration Group. MRC European Carotid Surgery Trial: interim results for symptomatic patients with severe (70–99%) or with mild (0–29%) carotid stenosis. Lancet 1991;337:1235-1243 [PubMed] [Google Scholar]
- 15. North American Symptomatic Carotid Endarterectomy Trial Collaborators. Beneficial effect of carotid endarterectomy in symptomatic patients with high-grade carotid stenosis. N Engl J Med 1991;325:445-453 [DOI] [PubMed] [Google Scholar]
- 16.Brown PB, Zwiebel WJ, Call GK. Degree of cervical carotid artery stenosis and hemispheric stroke: duplex US findings. Radiology 1989;170:541-543 [DOI] [PubMed] [Google Scholar]
- 17.Weinstein R. Noninvasive carotid duplex ultrasound imaging for the evaluation and management of carotid atherosclerotic disease. Hematol Oncol Clin North Am 1992;6:1131-1139 [PubMed] [Google Scholar]
- 18.Salonen R, Seppanen K, Rauramaa R, Salonen JT. Prevalence of carotid atherosclerosis and serum cholesterol levels in eastern Finland. Arteriosclerosis 1988;8:788-792 [DOI] [PubMed] [Google Scholar]
- 19.Davies MJ, Thomas AC. Plaque fissuring: the cause of acute myocardial infarction, sudden ischaemic death and crescendo angina. Br Heart J 1985;53:363-373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Falk E. Plaque rupture with severe pre-existing stenosis precipitating coronary thrombosis: characteristics of coronary atherosclerotic plaques underlying fatal occlusive thrombi. Br Heart J 1983;50:127-134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rothwell PM, Villagra JY, Fox AJ, et al. The role of carotid atherosclerosis in the etiology of ischemic stroke. Cerebrovasc Dis 1996;6(Suppl 2):1 [Google Scholar]
- 22.Cave EM, Pugh ND, Wilson RJ, Sissons GR, Woodcock JP. Carotid artery duplex scanning: does plaque echogenicity correlate with patient symptoms? Eur J Vasc Endovasc Surg 1995;10:77-81 [DOI] [PubMed] [Google Scholar]
- 23.Belcaro G, Laurora G, Cesarone MR, et al. Ultrasonic classification of carotid plaques causing less than 60% stenosis according to ultrasound morphology and events. J Cardiovasc Surg 1993;34:287-294 [PubMed] [Google Scholar]
- 24.Steffen CM, Gray-Weale AC, Byrne KE, Lusby RJ. Carotid atheroma: ultrasound appearance in symptomatic and asymptomatic vessels. Aust N Z J Surg 1989;59:529-534 [DOI] [PubMed] [Google Scholar]
- 25.Theile BL, Jones AM, Hobson RW, et al. Standards in noninvasive cerebrovascular testing. J Vasc Surg 1992;15:495-503 [PubMed] [Google Scholar]
- 26.Barry R, Pienaar C, Nel CJ. Accuracy of B-mode ultrasonography in detecting carotid plaque hemorrhage and ulceration. Ann Vasc Surg 1990;4:466-470 [DOI] [PubMed] [Google Scholar]
- 27.O'Donnell TF, Erodes L, Mackey WC, et al. Correlation of B-mode ultrasound imaging and arteriography with pathologic findings of carotid endarterectomy. Arch Surg 1985;120:443-449 [DOI] [PubMed] [Google Scholar]
- 28.von Ingersleben G, Schmiedl UP, Hatsukami TS, et al. Characterization of atherosclerotic plaques at the carotid bifurcation: correlation of high-resolution MR imaging with histologic analysis: preliminary study. Radiographics 1997;17:1417-1423 [DOI] [PubMed] [Google Scholar]


