Abstract
Summary: Intracranial percutaneous transluminal balloon angioplasty (PTA) has been used as a technique of last resort in the treatment of intracranial atherosclerotic disease when medical and surgical alternatives have failed or cannot be applied. The major risks associated with PTA include intracranial vessel rupture and abrupt vessel dissection causing occlusion. Angioplasty techniques in the extracranial circulation have been improved by the development of safe stent technology in combination with potent antiplatelet agents. We report three successful cases of symptomatic intracranial atherosclerotic disease in middle-aged adults treated by endovascular PTA followed by deployment of coronary stents.
Endovascular balloon angioplasty has attracted attention in recent years as an alternative technique for treating critical stenotic intracranial disease (1–3), although complication rates as high as 20% to 30% have been reported. Stent deployment, as compared with percutaneous transluminal balloon angioplasty (PTA) alone, has become the established and safer mode of interventional revascularization in the peripheral and coronary fields (4–11). We report our experience with three middle-aged patients with symptomatic intracranial atherosclerotic disease who were successfully treated with PTA and stent deployment.
Case Reports
Case 1
A 42-year-old right-handed man with a long history of heavy cigarette smoking presented with acute complaints of fluctuating left-sided weakness and sensory deficits. Investigation with CT, MR imaging, and Doppler sonography revealed that the patient had a complete occlusion of the right internal carotid artery of uncertain duration, an old moderately sized infarction of the left parietal area, and acute infarctions in the territory of the left anterior cerebral artery and in the watershed territory of the right hemisphere. There was a small posterior communicating artery on the right side and collateral flow via the right ophthalmic artery, but most of the flow to the right hemisphere was dependent on the left carotid artery. The cavernous segment of the left internal carotid artery had a focal atherosclerotic stenosis (Fig 1A–B). A stenosis of the A1 segment of the left anterior cerebral artery, the major collateral route to the right hemisphere, was noted also. Despite heparinization to an activated partial thromboplastin time (PTT) of 60 to 80 seconds in the hospital, the patient's symptoms continued and clinical evidence of extension of the infarctions was suspected. Consideration was given to performing a right external carotid artery to middle cerebral artery bypass, but the risks of reperfusion injury in the setting of acute hemispheric infarctions suggested that this procedure might be safer at a later time when the infarctions were less acute. However, ongoing symptoms on a daily basis forced the decision to use balloon angioplasty of the left internal carotid artery as a reasonable temporizing measure to improve perfusion.
fig 1.
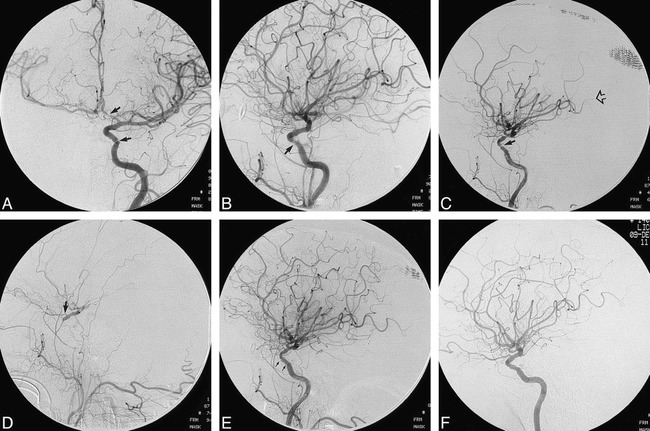
Case 1.
A and B, Pretreatment anteroposterior (A) and lateral (B) views of the left internal carotid artery show stenoses of the cavernous segment and of the A1 segment of the left anterior cerebral artery (arrows).
C and D, After unprotected PTA, angiograms show a dissection of the cavernous carotid artery (closed arrow, C) and compromise of distal perfusion (open arrow, C). This progressed quickly to complete occlusion of the left internal carotid artery (D). Prompt reconstitution of the supraclinoid internal carotid artery via the ophthalmic artery (arrow, D) helped to mitigate the effects of the temporary occlusion.
E, Immediate reopening of the internal carotid artery is evident after placement of an AVE 3.5 × 8-mm GFX stent. Arrows indicate the stent placement.
F, Follow-up angiogram 6 months later shows healing of the stent site, and a smooth luminal contour.
The patient was premedicated with ticlodipine hydrochloride 250 mg by mouth for three doses in advance of the procedure, which was performed under general anesthesia. Access to the left internal carotid artery was gained using a 90-cm 7F sheath (Cook, Bloomington, IN) over an exchange wire. The patient underwent heparinization during the procedure to an activated clotting time (ACT) of 250 to 300 seconds. Under normotensive conditions, the stenotic lesion was crossed with a 300-cm ACS Hi-Torque Traverse microwire (Advanced Cardiovascular Systems, Temecula, CA), and the tip was advanced into the left middle cerebral artery. The stenotic lesion was dilated initially by using a 2.5-mm Surpass angioplasty balloon catheter (SciMed Life Systems, Boston Scientific, Maple Grove, MN). The balloon was advanced with minor difficulty and inflated for less than 1 minute at 6 atm. The balloon was deflated and withdrawn. Roadmap imaging revealed satisfactory anterograde flow across the angioplasty site, although a clear improvement in diameter could not be discerned. The wire was maintained across the lesion and used to advance a 3-mm NC Ranger balloon catheter (SciMed Life Systems), which tracked easily up the carotid artery. This was inflated fully with obliteration of the balloon waist on the roadmap, using time and pressure parameters similar to the first balloon. The balloon was deflated and removed, and the wire was maintained across the lesion. After PTA, the angiographic appearance of the irregular, narrowed cavernous segment of the left ICA was unsatisfactory, with a prominent dissection flap (Fig 1C). Furthermore, there was evidence of hemodynamic compromise of intracranial flow with diminishing peripheral runoff over the following 10 to 15 minutes. In the hope that this represented vasospasm at the angioplasty site, 50 mg of papaverine hydrochloride and 300 mcg of nitroglycerine were infused into the precavernous internal carotid artery. This was done without moving the exchange wire. A microcatheter was introduced over a standard-length microwire parallel to the ACS microwire, with minimal leakage at the Tuohy-Borst valve. Despite the infusion of vasodilators, flow deteriorated rapidly, and a hand injection of contrast material showed complete occlusion of the left internal carotid artery, confirmed with biplane angiography (Fig 1D). While a coronary stent was being prepared, 300,000 U of urokinase was infused into the occluded internal carotid artery via the microcatheter to dissolve any clot that might have formed as a result of stasis. Administration of abciximab (ReoPro, Lilly, Indianapolis, IN) in preparation for the stent placement was considered but decided against as too hazardous in view of the extent of the patient's infarcts, the degree of heparinization, and concurrent administration of urokinase. With the microwire still across the lesion, the angioplasty site was crossed with an AVE 3.5 × 8-mm GFX coronary stent (Arterial Vascular Engineering, Santa Rosa, CA), which was deployed easily with 6 atm of pressure. This resulted in complete and immediate reopening of the internal carotid artery, which had been occluded for approximately 20 minutes (Fig 1E). Biplane angiography of the head after stent placement showed prompt peripheral runoff and no evidence of emboli. The groin site was closed without reversal of heparin using an 8F Pro Star XL device (Perclose, Menlo Park, CA). The patient awoke from general anesthesia with neurologic status at preprocedure baseline. A CT study showed contrast staining at the sites of previous infarction, which cleared by the next day, and no new infarcts were detected. The patient subsequently had a successful bypass procedure to the right hemisphere. He was maintained on ticlodipine for 1 month after discharge and then switched to clopidogrel and aspirin. An angiogram performed 6 months later (Fig 1F) showed complete patency of the stent site on the left and of the bypass on the right. The patient continues to do well neurologically, and has returned to full-time work with no new ischemic episodes.
Case 2
A 44-year-old left-handed man presented with transient complaints of left-sided sensory deficits and weakness lasting some minutes, perioral tingling, and complaints of episodic diplopia over a 3-month period. MR imaging and MR angiography showed no evidence of infarction, but a tight stenosis of the intradural right vertebral artery was confirmed by angiography. A long-segment stenosis of this vessel (Fig 2A) with an irregular contour was identified proximal to and involving the site of origin of the hypoplastic right posterior inferior cerebellar artery (PICA) (Fig 2B). The left vertebral artery was occluded distal to the left PICA, and the posterior communicating arteries were small. Despite heparinization, the patient's symptoms persisted. Consideration was given to performing a surgical bypass procedure, but the likelihood of success was not thought to be good. Therefore, after a thorough discussion of the risks of balloon angioplasty with the patient, as well as the benefits, risks, and consequences of stent placement, it was decided to revascularize the stenotic lesion by endovascular means.
fig 2.
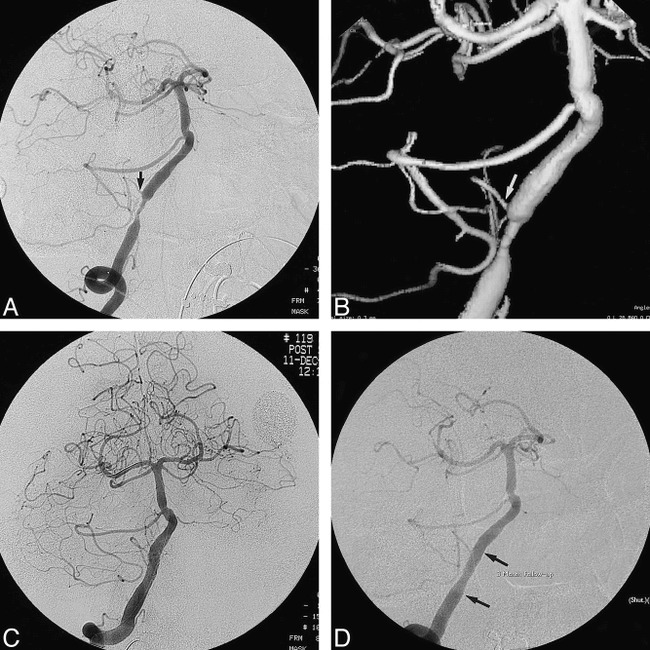
Case 2.
A, Oblique view of the right vertebral artery shows a relatively long segment of tight stenosis from the end of which arises the right PICA (arrow). A second area of atherosclerotic narrowing in the basilar artery was not treated on this occasion. The left vertebral artery was occluded distal to the left PICA.
B, 3D surface-rendered image of the right vertebral artery before treatment shows the circumferential “apple-core” configuration of the lesion and served as a baseline for evaluation of the hypoplastic PICA (arrow).
C, After predilatation, as described in the text, and placement of an AVE 4 × 18-mm GFX stent expanded with low pressures, a satisfactory lumen is observed.
D, 3-month follow-up angiogram shows no evidence of restenosis at the stent site (arrows). The right PICA remains patent.
The patient was premedicated with clopidogrel 75 mg by mouth for two doses before the procedure, which was performed under general anesthesia. Topical nitropaste was applied to the skin to help reduce vasospasm. The right vertebral artery was selected using a 7F Brite-Tip catheter (Cordis, Miami, FL) and the patient underwent heparinization to an ACT of 250 to 300 seconds. A bolus injection calculated by body weight of abciximab (ReoPro) was administered, followed by a 12-hour infusion. An infusion of papaverine 90 mg was given in the right vertebral artery to reduce the likelihood of vasospasm. The stenotic lesion was crossed with considerable difficulty, owing to its irregularity and severe narrowing, using an ACS Hi-Torque Balance 300-cm 0.014 wire (Advanced Cardiovascular Systems), which was advanced distally in the right posterior cerebral artery. This wire was chosen because of its combination of an atraumatic distal end with a favorable profile for steerability.
The stenotic lesion was crossed with a 2 × 20-mm Ranger angioplasty balloon catheter (SciMed Life Systems) and dilated slowly in 1-minute increments to a final maximum of 6 atm. Occlusion times were confined to 1 minute, punctuated by deflation to allow reperfusion. This balloon was exchanged for a 3 × 20-mm Quantum Ranger balloon (SciMed Life Systems), which was similarly dilated slowly and intermittently to approximately 6 atm. Total occlusion times in the vertebral artery did not exceed 1 minute during use of these balloons. An AVE 4 × 18-mm GFX stent (Arterial Vascular Engineering) was prepared and advanced across the lesion and deployed using up to 6 atm of pressure. A considerable waist on the stent at the site of maximal stenosis was expanded very slowly by repeated slow inflations of the balloon, which was pulled back slightly within the stent. With a satisfactory reduction of the waist, a 4.5 × 20-mm Big NC Ranger balloon angioplasty catheter (SciMed Life Systems) was then prepared and used to postdilate the stent. This was performed distally to conform the stent to the vessel distal to the stenosis and proximally at the waist. The result was satisfactory, with preservation of the right PICA and no thromboembolic complications (Fig 2C). The groin site was closed uneventfully using an 8F Pro Star XL suture device (Perclose), and the patient awoke neurologically intact. Heparinization was continued for 24 hours, and the patient was discharged on clopidogrel 75 mg and aspirin 325 mg every day.
The patient has been asymptomatic since the procedure. A 3-month follow-up angiogram showed mild intimal growth through the struts of the stent with a satisfactory appearance of the site (Fig 2D).
Case 3
A 47-year-old right-handed man presented on an emergency basis to the hospital with a month-long history of episodic progressive dizziness, diplopia, perioral tingling, and visual disturbances. He had a history of heavy smoking and significant coronary artery disease that had been treated by a coronary artery bypass procedure. On admission on this occasion, diffusion MR imaging showed multiple small acute infarctions of the posterior circulation, including the cerebellum and thalamus. MR angiography revealed absence of flow signal in the distal intradural right vertebral artery and a short-segment severe stenosis of the distal intradural left vertebral artery. A diagnostic angiogram confirmed these findings, showing the left vertebral artery lesion to be a threadlike stenosis proximal to the origin of the left PICA (Fig 3A). The right vertebral artery was occluded. Collateral flow from the posterior communicating arteries was minimal. The patient was maintained on heparin with PTT in the range of 60 to 80 seconds for a week, during which time recurrent symptoms were reported. Consideration was given as to whether medical management along with Coumadin and/or aspirin would be sufficient to prevent further strokes. However, the severity of his stenotic lesion and his relatively young age argued in favor of attempting to revascularize the vertebrobasilar system by means of endovascular techniques.
fig 3.
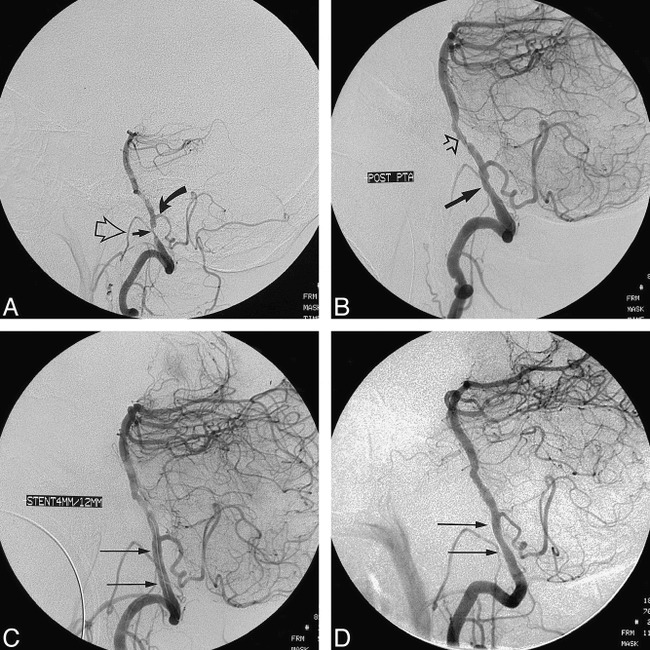
Case 3.
A, Lateral projection of the left vertebral artery shows a critically tight stenosis (straight arrow) of the vessel proximal to the origin of the PICA (curved arrow). An ipsilateral stenosis of the external carotid artery causes opacification of the collateral vessels, resulting in superimposition of the hypoglossal branch (open arrow) of the neuromeningeal trunk on the stenosis of the left vertebral artery.
B, After slow PTA with 2-mm, 3-mm, and 3.5-mm balloons, a satisfactory appearance of the stenosis site is seen (closed arrow). Distal disease in the proximal basilar artery (open arrow) is more apparent now. It was decided during the procedure not to treat the upper site.
C, An AVE 4 × 12-mm GFX stent was deployed in the stenosis site (arrows indicate placement) proximal to the left PICA. The Mailman wire is still across the lesion site within the stent. Conventional planar and 3D views (not shown) were used with and without subtraction to determine that the stent expansion was satisfactory, precluding the need to risk further postdilatation.
D, 3-month poststent angiogram shows mild intimal growth through the struts of the stent (arrows) but no evidence of hemodynamically significant restenosis.
The patient was premedicated for 3 days in advance with clopidogrel 75 mg by mouth, and the procedure was performed under general anesthesia following application of a strip of topical nitropaste to the skin. Because of the relatively small caliber and tortuosity of the cervical segment of the left vertebral artery, a 6F Cordis 90-cm Brite-Tip sheath was selected and introduced uneventfully at the right common femoral artery. However, even this size sheath caused relatively significant obstruction of flow in the left vertebral artery, and the sheath had to be retracted to approximately the C4 level. This subsequently proved to be a technical disadvantage during stent placement. The patient was heparinized to an ACT level of 250 to 300 seconds.
The vertebrobasilar system was infused with papaverine hydrochloride 90 mg, which was prophylactically delivered via the introducer sheath to reduce the possibility of vasospasm. A bolus of abciximab (ReoPro) followed by the start of a 12-hour infusion was given. The stenotic lesion was crossed with some difficulty using a 300-cm ACS Hi-Torque Balance wire (Advanced Cardiovascular Systems), which was then advanced distally in the right posterior cerebral artery. A 2 × 20-mm Ranger balloon catheter (SciMed Life Systems) was prepared and tracked across the lesion easily. It was gently inflated in a slow punctuated fashion to a final maximum of 4 atm. Occlusion times were approximately 1 minute. As in the previous cases, hand injections were made around the deflated balloon prior to withdrawal. This was to facilitate quick reinflation of the balloon should the vessel wall have ruptured. Subsequently, tediously slow repeated inflations across the lesion were made with a 3 × 20-mm and a 3.5 × 20-mm Ranger balloon (SciMed Life Systems), not exceeding 3 atm. Post-PTA images were satisfactory, with no evidence of complications (Fig 3B).
An AVE 4 × 12-mm GFX stent (Arterial Vascular Engineering) was prepared and advanced easily through the sheath to the level of the skull base. However, the degree of tortuosity of the left vertebral artery and the lesser support provided by the proximally placed sheath made the final turn of the stent course difficult to negotiate. To give greater wire support, a 300-cm Mailman wire (SciMed Life Systems) was placed across the lesion to the proximal basilar artery. In this position, the stent could then be advanced smoothly over the Mailman wire. The stent was inflated slowly to the desired diameter, as defined by roadmapping and DSA runs, using 3.5 atm of pressure. Poststent imaging showed no evidence of complications or need for further dilatation (Fig 3C). The wire was maintained across the lesion for a further 15 minutes before removal. The patient awoke uneventfully from general anesthesia, neurologically at preprocedure baseline. He was continued on the remainder of the infusion of abciximab and daily clopidogrel with aspirin. A CT scan obtained the next day to evaluate for possible petechial or hemorrhagic complications related to the abciximab showed no complications or new lesions. Three months after the procedure, he remains asymptomatic, and a follow-up angiogram showed no evidence of significant restenosis (Fig 3D).
Discussion
The use of stents in the treatment of stenotic disease of the coronary (4, 5), renal (12, 13), and iliac (14) arteries has become accepted as preferable to PTA alone. Published rates of complications, difficulties, and long-term patency all indicate that stent technology represents a significant step forward in the treatment of vascular disease. Interest has turned, therefore, to the use of this technology in the extracranial carotid and vertebral arteries, with satisfactory results (9–11, 15, 16). With reference to the intracranial circulation, application of this technology has been stalled by the apparent high-risk nature of the procedure. Nevertheless, some isolated cases of successful stent deployment in the petrous carotid artery or intradural vertebral artery, as an adjunct to coil occlusion of aneurysms or for atherosclerotic disease, have been reported (16–24). The adventitia and elastic layers of the intracranial arteries are more sparse than those of the extracranial vessels (25), implying that the intracranial vessels are more prone to rupture during angioplasty. However, these concerns notwithstanding, the risks associated with angioplasty elsewhere in the body, which are diminished with the use of stents, should be similarly diminished in the intracranial circulation, in theory, if the technology is suitable.
Based on our experience with coronary angioplasty and stenting, it is our opinion that potent platelet inhibitors enhance the safe deployment of intracranial stents. The thienopyridine derivatives ticlodipine (Ticlid, Roche) and clopidogrel (Plavix, Sanofi) have been shown to be beneficial in the prevention of ischemic stroke and myocardial ischemia (26, 27). These agents act by preventing the binding of adenosine diphosphate (ADP) to platelet receptors and thus diminish ADP-related platelet aggregation. In our department, patients are premedicated for at least three doses with these agents prior to stenting of the cephalic vessels and are continued on one of these agents for at least a month afterward. Furthermore, complications related to stent placement in the coronary arteries are reduced significantly by use of platelet glycoprotein IIb/IIIa receptor antagonists (28–31). The most potent of these, abciximab (ReoPro), has been available for a longer period of time in the United States. It has additional binding features related to the vitronectin receptor of platelets and endothelial and smooth muscle cells. Newer agents, eptifibatide (Integrelin, Key, Cor) and tirofiban (Aggrastat, Merck) have the theoretical advantage of being shorter acting than abciximab and potentially more easily reversible in the event of complications. These drugs are given in the form of a bolus calculated by the patient's weight, followed by a prolonged intravenous infusion. In the case of abciximab, the infusion is given for 12 hours. This agent was not used in the first case described because of the presence of multiple acute infarcts and the concern for hemorrhagic complications. Use of this agent in the third patient, who had multiple small posterior circulation infarcts, did not cause any complications.
Most of the coronary angioplasty balloons can be delivered through a 6F catheter, although for subsequent delivery of a stent or contrast injection around the shaft of the balloon catheter, a larger delivery system is preferable.
There were many circumstantial and technical factors that undoubtedly played a part in the successful placement of stents in these patients. Probably the most important was the use of strong antiplatelet agents. Without adequate premedication, the likelihood of thromboembolic complications due to platelet adhesion within a small stent lumen is probably high. The patients were relatively young, which made the access vessels a little easier to navigate than would be the case in older patients. The use of general anesthesia contributed enormously to the safety of the procedures in allowing accurate deployment of the stents and facilitated immediate manipulation of blood pressure parameters during balloon inflation and vessel occlusion. The loss of the ability to evaluate patients neurologically during general anesthesia might be considered a potential hazard, but this was more than compensated for by the manner in which the devices could be advanced and deployed intracranially without having to cope with patient motion, agitation, or autonomic instability.
The rate of balloon inflation during these procedures was probably quite important. All inflations were made very slowly, with balloon pressures never exceeding 7 atm, well below the tolerated maximal pressure of all the balloons used. During angioplasty, the balloon was deflated repeatedly to allow resumption of flow and to minimize the periods of total occlusion. Slow inflation and stretching of the vessel may allow the viscoelastic qualities of the vessel wall to accommodate the balloon rather than force the wall to crack abruptly. The shear stress applied to a viscoelastic solid, as one might characterize an atherosclerotic vessel wall, is proportional to the rate of deformation (32). Hence, with reference to the risk of rupturing the vessel wall, the maximal pressure in the balloon may not be as important as the rate of inflation. Similarly, during deployment of the stents, no effort was made to force the stent to open quickly at the site of the waist. A slow, sustained, low-pressure inflation repeated a number of times eventually distended the stents to full diameter.
The question of what constitutes a safe inflation pressure in the intracranial vessels is important. High-pressure inflations up to 20 atm are favored in the coronary vessels so as to achieve full stent expansion and to minimize the risk of stasis and acute thrombosis around the stent. However, unless the vessel dimensions are known precisely, such pressures intracranially would be extremely hazardous. The ideal solution would involve full stent expansion at an extremely low pressure. For this reason, self-expanding stents might be considered for intracranial use. With balloon-expandable stents, as used in this series of patients, adequate predilatation of the vessel is crucial. Predilatation prepares a route of atraumatic passage for the balloon-mounted stent and reduces the resistance to expansion of the stent during inflation. Adequacy of stent expansion was judged in these patients from the angiographic runs. However, more accurate appraisal of the need for further stent expansion may be possible using intravascular sonography.
Could or should the use of stents have been avoided in these patients? The first case demonstrates how potentially catastrophic difficulties were encountered through an innate reluctance to use a stent unless it became absolutely necessary. Fortunately, provision for its use had been made and the unopened stent package was ready in the room, but a reluctance to use the stent at first almost led to a catastrophic complication. Although acute vasospasm after intracranial PTA is common, especially in the absence of premedication (33), this does not seem to have been the case in the first patient, in whom direct local papaverine and nitroglycerin failed to reopen the vessel. The culprit dissection was clearly evident on the pre- and poststent images, indicating the absolute need for the stent in this patient.
Does this near catastrophic complication in the first patient justify stent placement in the second and third patients? The answer is not known at this time. Clearly, the weight of emerging evidence suggests that so far the evolution of knowledge in the field of transluminal revascularization of the extracranial and intracranial circulation is an emulation of the experience gained in the coronary, renal, and iliac arteries. With use of inhibitors of the GP IIb/IIIa receptors during coronary intervention, acute and subacute complications related to stent placement are lower as compared with PTA alone, and long-term restenosis rates are also significantly better. A recent report indicates a 27% restenosis rate 6 months after coronary PTA as compared with a 10% rate after stent placement (4). A follow-up study of intracranial PTA for atherosclerotic disease indicated a similar rate of 28% restenosis in the intracranial vessels (34). All evidence available to date suggests that the experiences and lessons of extracranial and coronary transluminal revascularization bear some relevance to the intracranial circulation.
Intracranial atherosclerotic lesions have been recently classified by Mori et al (34, 35) into type A (<5 mm in length, annular or slightly eccentric); type B (5–10 mm, tubular, eccentric or totally occluded, <3 months old); and type C (diffuse, >10 mm, angulated segments, occlusions >3 months old) lesions. On the basis of this classification, Mori et al (35) have correlated more favorable rates of response to PTA, lower rates of restenosis after PTA, and lower rates of stroke with type A lesions than with type C lesions, type B lesions holding an intermediate position. The patients described in this article would be classified as type A (case 1) and type B (cases 2 and 3), demonstrating that the tubular type of lesion described by Mori et al can respond favorably to PTA and stent placement.
Conclusion
The technological and pharmacological advances made in coronary and peripheral percutaneous revascularization in recent years are considerable. It is likely that adaptation of these successes to the extracranial vessels and to some areas of the intracranial vessels is technically feasible. The role, safety, and efficacy of such procedures can now be defined.
Footnotes
Address reprint requests to P. Pearse Morris, MB, BCh, Interventional Neuroradiology, Department of Radiology, Wake Forest University School of Medicine, Medical Center Blvd, Winston-Salem, NC 27157.
References
- 1.McKenzie JD, Wallace RC, Dean BL, Flom RA, Khayata MH. Preliminary results of intracranial angioplasty for vascular stenosis caused by atherosclerosis and vasculitis. AJNR Am J Neuroradiol 1996;17:263-268 [PMC free article] [PubMed] [Google Scholar]
- 2.Purdy PO, Devous MD Sr, Unwin DH, Giller CA, Batjer HH. Angioplasty of an atherosclerotic middle cerebral artery associated with improvement in regional cerebral blood flow. AJNR Am J Neuroradiol 1990;11:878-880 [PMC free article] [PubMed] [Google Scholar]
- 3.Takis C, Kwan ES, Pessin MS, Jacobs DH, Caplan LR. Intracranial angioplasty: experience and complications. AJNR Am J Neuroradiol 1997;18:1661-1668 [PMC free article] [PubMed] [Google Scholar]
- 4.Erbel R, Haude M, Höpp HW, et al. Coronary-artery stenting compared with balloon angioplasty for restenosis after initial balloon angioplasty. N Engl J Med 1998;339:1672-1678 [DOI] [PubMed] [Google Scholar]
- 5.Serruys PW, de Jaegere P, Kiemeneij F, et al. A comparison of balloon-expandable-stent implantation with balloon angioplasty in patients with coronary artery disease. N Engl J Med 1994;331:489-495 [DOI] [PubMed] [Google Scholar]
- 6. EPISTENT Investigators. Randomised placebo-controlled and balloon-angioplasty controlled trial to assess safety of coronary stenting with use of platelet glycoprotein IIb/IIIa blockade. Lancet 1998;352:87-92 [DOI] [PubMed] [Google Scholar]
- 7.Hennequin LM, Joffre FG, Rousseau HP, et al. Renal artery stent placement: long term results with the Wallstent endoprosthesis. Radiology 1994;191:713-719 [DOI] [PubMed] [Google Scholar]
- 8.Palmaz JC, Laborde JC, Rivera FJ, Encarnacion CE, Lutz JD, Moss JG. Stenting of the iliac arteries with the Palmaz stent: experience from a multi-center trial. Cardiovasc Intervent Radiol 1992;15:291-297 [DOI] [PubMed] [Google Scholar]
- 9.Robbin ML, Lockhart ME, Weber TM, et al. Carotid artery stents: early and intermediate follow-up with Doppler US. Radiology 1997;205:749-756 [DOI] [PubMed] [Google Scholar]
- 10.Yadav JS, Roubin GS, Iyer S, et al. Elective stenting of the extracranial carotid arteries. Circulation 1997;95:376-381 [DOI] [PubMed] [Google Scholar]
- 11.Diethrich EB, Jdiaye M, Reid DB. Stenting in the carotid artery: initial experience in 110 patients. J Endovasc Surg 1996;3:42-46 [DOI] [PubMed] [Google Scholar]
- 12.Blum U, Krumme B, Flugel P, et al. Treatment of ostial renal-artery stenoses with vascular endoprostheses after unsuccessful balloon angioplasty. N Engl J Med 1997;336:459-465 [DOI] [PubMed] [Google Scholar]
- 13.Henry M, Armor M, Henry J, et al. Stent placement in the renal artery: three year experience with the Palmaz stent. J Vasc Interv Radiol 1996;7:343-350 [DOI] [PubMed] [Google Scholar]
- 14.Murphy KD, Encarnacion CE, Le VA, Palmaz JC. Iliac artery stent placement with the Palmaz stent: follow-up study. J Vasc Interv Radiol 1995;6:321-329 [DOI] [PubMed] [Google Scholar]
- 15.Vozzi CR, Rodriguez AO, Paulantonio D, Smith JA, Wholey MH. Extracranial carotid angioplasty and stenting: initial results and short-term follow up. Tex Heart Inst J 1997;24:167-172 [PMC free article] [PubMed] [Google Scholar]
- 16.Storey GS, Marks MP, Dake M, Norbash AM, Steinberg GK. Vertebral artery stenting following percutaneous transluminal angioplasty: technical note. J Neurosurg 1996;84:883-887 [DOI] [PubMed] [Google Scholar]
- 17.Lylyk P, Ceratto R, Hurvitz D, Basso A. Treatment of a vertebral dissecting aneurysm with stents and coils: technical case report. Neurosurgery 1998;43:385-388 [DOI] [PubMed] [Google Scholar]
- 18.Sekhon LHS, Morgan MK, Sorby W, Grinnell V. Combined endovascular stent implantation and endovascular coil placement for the treatment of a wide-necked vertebral artery aneurysm: technical case report. Neurosurgery 1998;43:380-384 [DOI] [PubMed] [Google Scholar]
- 19.Dorros G, Cohn JM, Palmer LE. Stent deployment resolves a petrous carotid artery angioplasty dissection. AJNR Am J Neuroradiol 1998;19:392-394 [PMC free article] [PubMed] [Google Scholar]
- 20.Higashida RT, Smith W, Gress D, et al. Intravascular stent and endovascular coil placement for a ruptured fusiform aneurysm of the basilar artery: case report and review of the literature. J Neurosurg 1997;87:944-949 [DOI] [PubMed] [Google Scholar]
- 21.Feldman RL, Trigg L, Gaudier J, Galat J. Use of coronary Palmaz-Schatz stent in the percutaneous treatment of an intracranial carotid artery stenosis. Cathet Cardiovasc Diagn 1996;84:883-887 [DOI] [PubMed] [Google Scholar]
- 22.Guterman LR, Budney JL, Gibbons KJ, Hopkins LN. Thrombolysis of the cervical internal carotid artery before balloon angioplasty and stent placement: report of two cases. Neurosurgery 1996;38:620-624 [PubMed] [Google Scholar]
- 23.Levy DI. Endovascular treatment of carotid artery occlusion in progressive stroke syndromes: technical note. Neurosurgery 1998;42:186-193 [DOI] [PubMed] [Google Scholar]
- 24.Al-Mubarak N, Gomez CR, Vitek JJ, Roubin GS. Stenting of symptomatic stenosis of the intracranial internal carotid artery. AJNR Am J Neuroradiol 1998;19:1949-1951 [PMC free article] [PubMed] [Google Scholar]
- 25.Scott GE, Neubuerger KT, Denst J. Dissecting aneurysm of intracranial arteries. Neurology 1960;10:22-27 [DOI] [PubMed] [Google Scholar]
- 26.Leon MB, Baim DS, Popma JJ, et al. A clinical trial comparing three antithrombotic-drug regimens after coronary artery stenting. N Engl J Med 1998;339:1665-1671 [DOI] [PubMed] [Google Scholar]
- 27. CAPRIE Steering Committee. A randomized, blinded trial of clopidrogel versus aspirin in patients at risk of ischaemic events (CAPRIE). Lancet 1996;348:1329-1339 [DOI] [PubMed] [Google Scholar]
- 28. EPILOG Investigators. Platelet glycoprotein IIb/IIIa receptor blockade and low dose heparin during percutaneous coronary revascularization. N Engl J Med 1887;336:1689-1696 [DOI] [PubMed] [Google Scholar]
- 29. Capture Investigators. Randomized placebo-controlled trial of abciximab before, during and after coronary intervention in refractory unstable angina: the CAPTURE study. Lancet 1997;349:1429-1435 [PubMed] [Google Scholar]
- 30.Braunwald E, Maseri A, Armstrong PW, et al. Rationale and clinical evidence for the use of GP IIb/IIIa inhibitors in acute coronary syndromes. Am Heart J 1998;135:S56-S66 [DOI] [PubMed] [Google Scholar]
- 31. EPISTENT Investigators. Randomised placebo-controlled and balloon-angioplasty-controlled trial to assess safety of coronary stenting with use of platelet glycoprotein IIb/IIIa blockade. Lancet 1998;352:87-92 [DOI] [PubMed] [Google Scholar]
- 32.Vogel S. Life in Moving Fluids. 2nd ed. Princeton, NJ: Princeton University Press; 1994;
- 33.Jimenez C, Duong H, Olarte M, Pile-Spellman J. Recurrent abrupt occlusion after transluminal angioplasty for basilar artery stenosis: case report. Neurosurgery 1999;44:210-215 [DOI] [PubMed] [Google Scholar]
- 34.Mori T, Fukuoka M, Kazita K, Mori K. Follow-up study after intracranial percutaneous transluminal cerebral balloon angioplasty. AJNR Am J Neuroradiol 1998;19:1525-1533 [PMC free article] [PubMed] [Google Scholar]
- 35.Mori T, Mori K, Fukuoka M, Arisawa M, Honda S. Percutaneous transluminal cerebral angioplasty: serial angiographic follow-up after successful dilatation. Neuroradiology 1997;39:111-116 [DOI] [PubMed] [Google Scholar]


