Abstract
BACKGROUND AND PURPOSE: Systemic invasive aspergillosis involves the brain through hematogenous dissemination. A retrospective review of 18 patients with aspergillosis involving the brain was performed in order to present imaging findings and thereby broaden the understanding of the distribution and imaging characteristics of brain Aspergillus infection and to facilitate its early diagnosis.
METHODS: The neuroimaging studies of 17 biopsy- or autopsy-proved cases and one clinically diagnosed case were examined retrospectively by two neuroradiologists. The studies were evaluated for anatomic distribution of lesions, signal characteristics of lesions, enhancement, hemorrhage, and progression on serial studies (when performed). Medical records, biopsy reports, and autopsy findings were reviewed.
RESULTS: Thirteen of 18 patients had involvement of the basal nuclei and/or thalami. Nine of the 10 patients with lesions at the corticomedullary junction also had lesions in the basal nuclei or thalami. Callosal lesions were seen in seven patients. Progression of lesion number and size was seen in all 11 patients in whom serial studies had been performed. Enhancement was minimal or absent in most cases. There was gross hemorrhage in eight of the 18, and definite ring-enhancement in three.
CONCLUSION: Among our cases, lesions in perforating artery territories were more common than those at the corticomedullary junction. Ring enhancement and gross hemorrhage may be present, but are not necessary for the prospective diagnosis.
In the severely immunosuppressed host, pulmonary Aspergillus infection is invasive in nature, potentially gaining access to the systemic circulation and disseminating throughout the body. The brain is a common end organ of involvement. Conversely, in nearly all cases of cerebral aspergillosis, the portal of entry is the lung (1). While cerebral aspergillosis has historically had a dismal prognosis (2, 3), new antifungal therapies have made effective treatment possible (4, 5). Consequently, early and accurate diagnosis of this infection has become more important. This is particularly true as solid organ and bone marrow transplantation, with their concomitant immunosuppression, have become more prevalent.
Eighteen cases of hematogenously disseminated aspergillosis with brain involvement were reviewed retrospectively to assess the spectrum and frequency of involvement of specific anatomic sites and the imaging characteristics of the lesions to facilitate early, prospective diagnosis.
Methods
The 18 cases were identified over a period of 7 years by the authors during their clinical practice in a university medical center, which has a large immunosuppressed patient population, owing to busy transplantation and oncology services. Cases in which Aspergillus infection involved the brain via direct extension of sinus or mastoid infection, or via penetrating trauma, have been extensively described in the past and are therefore not included here. Aspergillus infection of the brain was proved by biopsy or autopsy in 17 cases; in one case, the patient had documented pulmonary aspergillosis, acute neurologic findings, typical CNS lesions on imaging, and response of pulmonary and neurologic disease to antifungal therapy, resulting in the clinical and radiologic diagnosis of CNS aspergillosis. Eight patients had liver transplants, two had bone marrow transplants, one a kidney transplant, one a simultaneous pancreas/kidney transplant, one a heart transplant, and one a lung transplant. In addition to the patients who were immunosuppressed for bone marrow transplantation, patients were immunosuppressed for the treatment of malignant disease, including one who had chronic lymphocytic leukemia and one who had breast carcinoma. One patient had steroid-dependent chronic obstructive pulmonary disease. The final patient had Duncan syndrome, a disease process consisting of a persistent deficit of cell-mediated immunity following a life-threatening Epstein-Barr viral infection (HIV had been rigorously excluded).
Twelve patients underwent MR imaging, including T1-, proton density–, and T2-weighted sequences (Resonex 0.38 T or GE Signa 1.5 T), all with gadolinium-containing contrast material at 0.1 mmol/kg; seven also had CT scans (Siemens DRH, GE 9800 Advantx, or GE CTi). Of those patients who had MR examinations, seven were imaged in the low-field scanner (reflecting limited scanner availability at the time of an institutional cluster of Aspergillus cases in the early 1990s) and five were imaged in the high-field scanner or in both scanners. Six patients had only CT examinations, four with intravenous iodinated contrast material. One patient's treatment with amphotericin B lipid complex resulted in long-term survival, so that long-term follow-up CT and MR studies were available. A second long-term survivor was lost to follow-up. Two neuroradiologists reexamined all imaging studies to form a consensus opinion. The studies were reviewed for anatomic distribution of lesions, signal characteristics of lesions, enhancement, hemorrhage, and progression on serial studies (when performed). Medical records, biopsy reports, and autopsy reports were reviewed, as well as tissue review when retained autopsy material was available.
Results
The basal nuclei or thalami were involved in 13 of the 18 patients (Figs 1 and 2). Three of these patients also had documented upper motor neuron signs corresponding to adjacent internal capsule involvement. The corpus callosum was involved in seven of the 18 patients (Fig 1). One patient had a focal midbrain lesion, centered in the left substantia nigra (Fig 3). Another patient had three mesencephalic lesions as well as a dorsal medullary lesion. Of the 10 patients with corticomedullary junction lesions, in only one was this the sole site of involvement (Fig 4); the nine other patients also had significant involvement of the basal nuclei, thalami, corpus callosum, or brain stem. One of the 18 patients had a large area of contiguous signal abnormality, corresponding to cerebritis at autopsy (Fig 5). Progression of the size and number of lesions over 2 to 10 days was seen in all 11 patients who underwent serial imaging studies.
fig 1.
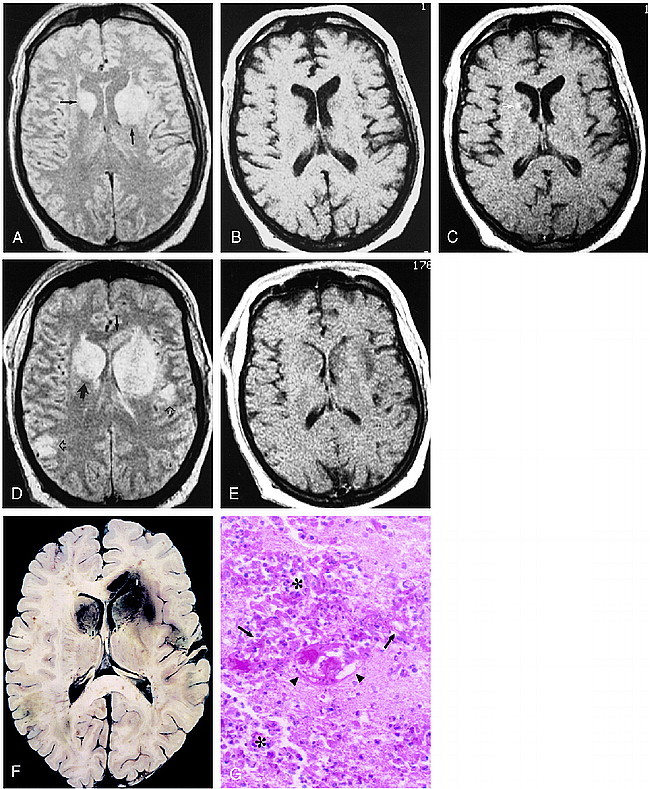
48-year-old man, 3 weeks after liver transplantation, with decline in mental status and flaccidity in bilateral lower extremities and left upper extremity.
A–C, Axial proton density–weighted (3000/25/2 [TR/TE/excitations]) (A) and unenhanced (500/20/2) (B) and contrast-enhanced (530/20/2) (C) T1-weighted spin-echo images. Areas of hyperintensity are present in the basal nuclei bilaterally, as well as in the left internal capsule on the proton density–weighted image (arrows, A), with corresponding modest hypointensity on the T1-weighted images. A small focus of enhancement is seen in the right caudate head (arrow, C).
D and E, Axial proton density–weighted (3000/25/2) and contrast-enhanced T1-weighted (530/20/2) spin-echo images obtained 6 days later show new involvement of the right internal capsule (large solid arrow) and callosal genu (small solid arrow). Cortical and subcortical lesions are also now seen (open arrows). Although the lateral ventricles are now effaced, there is rather little enhancement. The patient died several hours after this study was obtained.
F, The horizontally sectioned gross specimen shows red-brown areas of hemorrhagic necrosis corresponding to the abnormalities seen in D in the basal nuclei, and in the cortex and subcortical white matter. Hemorrhage is also seen in the genu of the corpus callosum.
G, Histologic section shows extensive necrosis with infiltration by polymorphonuclear leukocytes and macrophages (asterisks). Numerous Aspergillus hyphae are scattered throughout the section (arrows), and vascular fibrinoid necrosis is present (arrowheads) (PAS, hematoxylin; original magnification ×40).
fig 3.
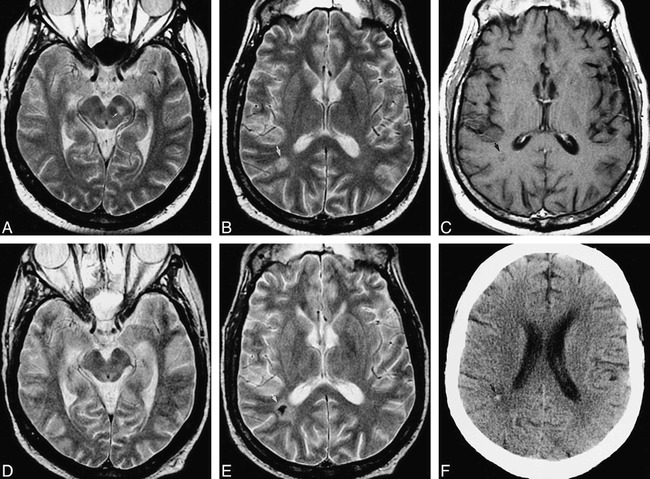
51-year-old man after bone marrow transplantation for IgA multiple myeloma with mental status decline. Pulmonary aspergillosis had been proved by bronchoalveolar lavage. A–C were obtained at neurologic presentation, D–F 5 months later.
A, Axial fast spin-echo T2-weighted (2200/84eff /1) image shows a mesencephalic lesion centered in the left substantia nigra (arrow).
B, Axial fast spin-echo T2-weighted (2200/84eff /1) image shows a hyperintense white matter lesion in the right parietal lobe (arrow). This patient also had severe (proved) bacterial sinusitis.
C, Axial fast spin-echo T1-weighted (600/12/2) image with contrast shows no definite enhancement of the lesion (arrow).
D, Axial fast spin-echo T2-weighted (2200/84eff /1) image of the midbrain shows return to normal after treatment with amphotericin B lipid complex.
E, Axial fast spin-echo T2-weighted (2200/84eff /1) image shows marked central hypointensity at the site of the parietal white matter lesion (arrow). There was again no enhancement of this lesion (image not shown).
F, Axial unenhanced CT scan shows high attenuation at the site of the parietal white matter lesion (arrow), confirming calcification of this lesion.
fig 4.
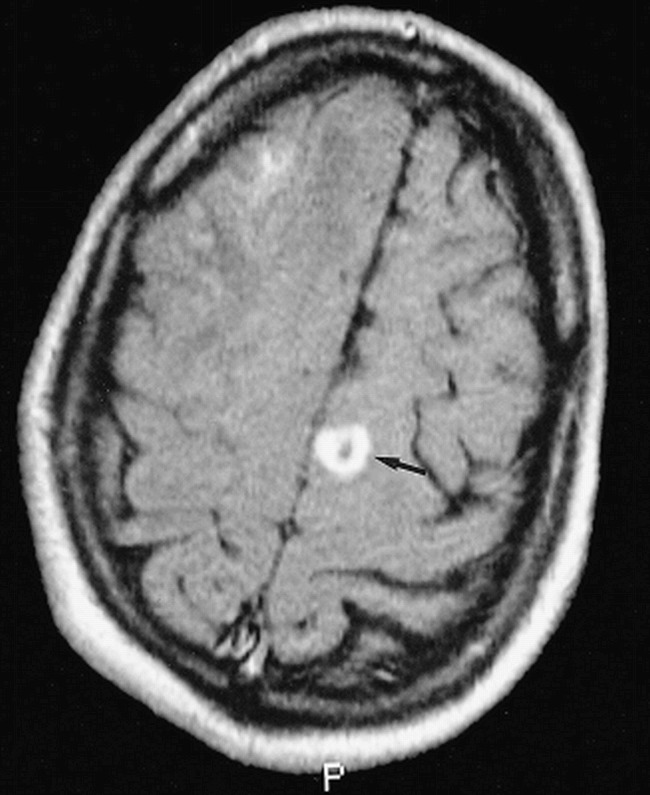
38-year-old man with seizures after a second liver transplantation. Axial contrast-enhanced spin-echo T1-weighted (630/20/2) image shows a ring-enhancing lesion at the corticomedullary junction of the left superior frontal gyrus (arrow). Biopsy of a corticomedullary junction lesion in the right frontal lobe (partially seen) confirmed cerebral aspergillosis
fig 5.
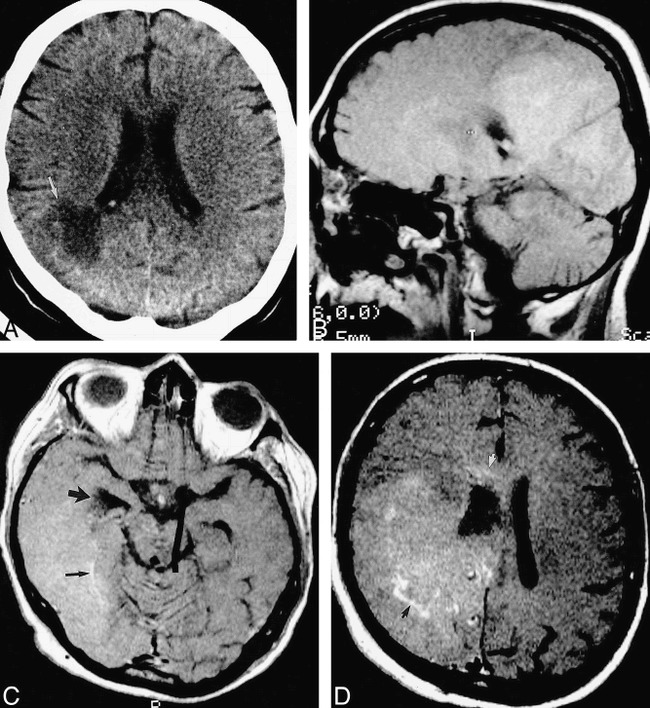
40-year-old woman after simultaneous pancreas/kidney transplantation had mental status decline and, later, a left homonymous hemianopia.
A, Axial unenhanced CT scan shows a large low-attenuation lesion (arrow) extending to the right lateral ventricle.
B, Parasagittal unenhanced spin-echo T1-weighted (630/20/2) MR image 10 days later shows a large, confluent area of diffuse, mild hyperintensity, suggesting petechial hemorrhage.
C, Axial contrast-enhanced T1-weighted (450/20/2) MR image shows involvement of the temporal and occipital lobes. The right temporal horn is obstructed (large arrow). There are serpentine foci of enhancement (small arrow).
D, Axial contrast-enhanced T1-weighted (450/20/2) MR image superior to C shows involvement of the frontal and parietal lobes, with focal areas of enhancement (black arrow). The abnormality extends into the corpus callosum (white arrow). The patient died 2 days later. At autopsy, this large abnormality was found to be Aspergillus cerebritis with scattered areas of petechial hemorrhage.
Gross intraparenchymal hemorrhage was identified by CT or MR imaging in eight of the 18 cases (Fig 6). In two of these cases, hemorrhage involved the corpus callosum. In two additional cases, a large area of subtle T1 shortening was thought to represent diffuse, mild hemorrhage (Fig 5). In three patients, white matter lesions were seen on proton density–and T2-weighted images, without significant associated enhancement (Figs 3 and 7). Definite ring enhancement was seen in three patients (Fig 4). Of the 12 patients who underwent MR imaging, seven had subtle, poorly defined peripheral enhancement (Fig 7). Another patient had only a minimal radiologic abnormality, an equivocal focus of enhancement on CT scans. No definite leptomeningeal or ependymal enhancement was evident in any of the 18 cases.
fig 6.
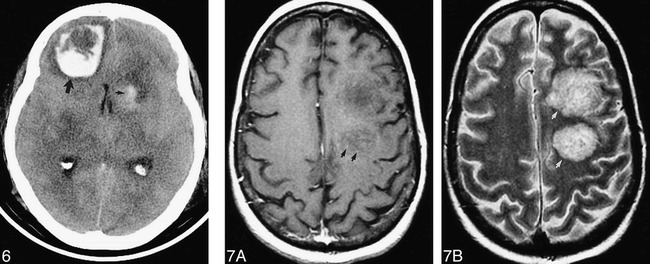
58-year-old woman after heart transplantation presented with obtundation and an unresponsive left pupil. Hemorrhagic lesions are seen on unenhanced CT scan in the left caudate head (small arrow) and right frontal lobe (large arrow). Aspergillosis was proved at autopsy.
fig 7. 43-year-old man after bone marrow transplantation for acute myelogenous leukemia, presented with mental status decline.
A, Axial fast spin-echo T1-weighted (600/12eff /2) MR image with contrast shows minimal punctate enhancement (arrows). There was no evidence of hemorrhage on an unenhanced T1-weighted MR image (not shown).
B, The hemispheric white matter lesions are conspicuously hyperintense (arrows) with fast spin-echo T2-weighting (2200/84eff /1). The patient died 2 days later. At autopsy, these lesions consisted of hemorrhagic, necrotizing encephalitis with numerous branching hyphae.
Sixteen of the 18 patients in our study died within several days of the diagnosis of CNS aspergillosis. At autopsy, all the lesions were necrotic (Fig 1). Hyphal elements were frequently identified within these necrotic lesions, as well as within vessel walls and lumina. Among the 13 cases in which autopsy was performed, gross or petechial hemorrhage was invariably seen. Varying degrees of polymorphonuclear and mononuclear inflammation were present in the vessel walls and brain parenchyma, with polymorphonuclear infiltrate typically predominating. Microabscesses were reported in three patients and granulomata in one. Ventriculitis was described in three patients, although ependymal enhancement had not been present. Leptomeningeal inflammation overlying cerebral and cerebellar lesions was generally present, although no leptomeningeal enhancement had been seen in any patient.
One patient was lost to follow-up 3 months after the biopsy diagnosis of cerebral aspergillosis and apparently successful antifungal therapy (Fig 3). Another patient, who had undergone bone marrow transplantation for IgA multiple myeloma, responded to early treatment and has survived to the time of writing, 10 months after the transplantation, 7 months after the confirmation of pulmonary aspergillosis, and 6½ months after the identification of brain lesions by MR imaging (no biopsy was performed). On follow-up studies 5 months after the initial scans, a lesion centered on the substantia nigra was no longer evident, and a parietal white matter lesion exhibited a focus of increased attenuation on CT scans and marked hypointensity on fast spin-echo T2-weighted MR images, consistent with calcification.
Discussion
Pyogenic abscesses and metastases to the brain have a known predilection for the corticomedullary junction (6, 7) owing to the hematogenous route of dissemination of bacteria and malignant cells and to the vascular anatomy of this interface. While Aspergillus also disseminates to the CNS hematogenously, most commonly but not exclusively from the lung (1), the pathophysiology is often different. Aspergillus may cause an infectious vasculopathy, leading initially to acute infarction or hemorrhage and later extending into surrounding tissue as an infectious cerebritis or occasionally evolving into an abscess (8). This vasculopathy-mediated septic infarction has an anatomic distribution and radiologic appearance significantly different from other infarcts and from primary cerebritis or abscess.
Three imaging patterns of cerebral aspergillosis in immunocompromised patients have been described by Ashdown and coworkers (9): 1) multiple cortical and subcortical areas of decreased CT attenuation or T2 lengthening (with or without hemorrhage), 2) multiple ring-enhancing lesions, and 3) dural enhancement with adjacent enhancing lesions of the paranasal sinuses or calvarial, or dural enhancement of the optic sheath with associated enhancement of the optic nerve and orbital fat. The last pattern represents direct extension of sinonasal disease, which is outside the focus of our study.
Characteristic involvement of the basal nuclei and thalami, indicating involvement of the lenticulostriate and thalamoperforator arteries, was frequent in our series, present in 13 of 18 cases. While corticomedullary involvement was similarly common (10 of 18 cases), neostriatal, thalamic, callosal, or brain stem disease was also present in nine of those 10 patients. Further, involvement of the lenticulostriate and thalamoperforating arteries, commonly without corresponding infarcts in the more distal middle or posterior cerebral artery territories, illustrates a pathophysiological difference between this fungal septic infarction and the common thromboembolic infarction. The apparent affinity of CNS aspergillosis for perforating artery distributions is most likely due to the invasive character of Aspergillus within the walls of the larger parent arteries to which it has spread hematogenously, subsequently compromising the origins of the perforating arteries. In addition, if diffuse vasculopathy is present, the perforating arteries may be the first to lose their patency simply because of their narrower diameter.
Pyogenic infection and thromboembolic infarction do not commonly involve the corpus callosum. Callosal lesions were seen in seven of these 18 cases (Fig 1). Therefore, callosal lesions may suggest the diagnosis of aspergillosis and again reflect the predisposition of perforating arteries to Aspergillus. Specifically, the corpus callosum is supplied by the medial lenticulostriates and perforating branches of the pericallosal arteries (10, 11).
The mesencephalic lesion depicted in Figure 3 was also within the distribution of small perforating arteries, in this case, from the basilar tip. The multiple mesencephalic lesions (not shown) seen in the steroid-dependent patient with chronic obstructive pulmonary disease were within the distribution of posterior cerebral artery (P1 and P2 segment) perforators. These lesions may have reflected the susceptibility of these perforator artery territories to lesions caused by Aspergillus vasculopathy, although they could have simply reflected the wide variety of lesions in this patient with a heavy disease burden.
The rapid growth of lesions may be explained by three possible mechanisms. First, the origins of the multiple perforator arteries are in proximity along the parent artery (eg, the M1 segment in the case of the lateral lenticulostriates), and are presumably vulnerable to an extending vasculopathy of the parent artery wall. There is experimental evidence suggesting that the necessary conditions for such a vasculopathy are present. First, endothelial cells in culture have been shown to engulf the organism (12), and Aspergillus has been seen to infiltrate and destroy the internal elastic lamina of major cerebral arteries (13). Second, any infarction, thromboembolic or septic, may have associated secondary edema, which increases during the first few days after the arterial occlusion. Third, Aspergillus hyphae were frequently observed within the parenchymal lesions at autopsy; infection of the infarcted tissue may be aggressive, and direct extension into the surrounding brain may progress quickly.
Subtle peripheral enhancement (Fig 7) was seen much more commonly than well-defined ring enhancement (Fig 4). This enhancement appears analogous to that seen during the evolution of a thromboembolic infarct. Well-defined ring enhancement was relatively uncommon in our series (three of 18 patients), in contrast to the findings of Ashdown and coworkers (9) (four of eight). This difference may be related to the patients' potentially different levels of immune system compromise, as edema and enhancement have been shown to be dependent on lymphocyte and total leukocyte counts (14), or it may be due to the temporal stage of the infection. The histologic appearance was similarly variable. The amount of inflammatory reaction depends on the stage of the infection and the immune status of the patient; lesions can sometimes contain hyphae without inflammatory reaction in an immunocompromised host (15). In our experience, these nonenhancing white matter lesions were most conspicuous on proton density–and T2-weighted images. We expect that fluid-attenuated inversion-recovery (FLAIR) images would also be of great utility, particularly as fast FLAIR has been shown to have better sensitivity than fast spin-echo T2 for the detection of radiologically subtle opportunistic infection, specifically HIV-related disease (16). The inclusion of FLAIR imaging in immunocompromised patients has since become routine at our institution. As these lesions represent septic infarction, diffusion-weighted images may also be of value in identifying early lesions.
One patient, an 80-year-old man with chronic lymphocytic leukemia (Fig 2), had a right-sided thalamic lesion on a CT scan performed 2 days antemortem. At autopsy, the anatomic extent of abnormality extended considerably beyond that seen on the CT examination, including a portion of the left thalamus, the fornices bilaterally, and the right basal nuclei. The anatomic distribution of these regions of hemorrhagic infarct, in conjunction with the histologic demonstration of branching septated hyphae in thrombosed veins and venules, including the internal cerebral veins and vein of Galen (Fig 2), indicate that the dominant pathophysiology of this patient's lesions was that of venous infarction.
fig 2.
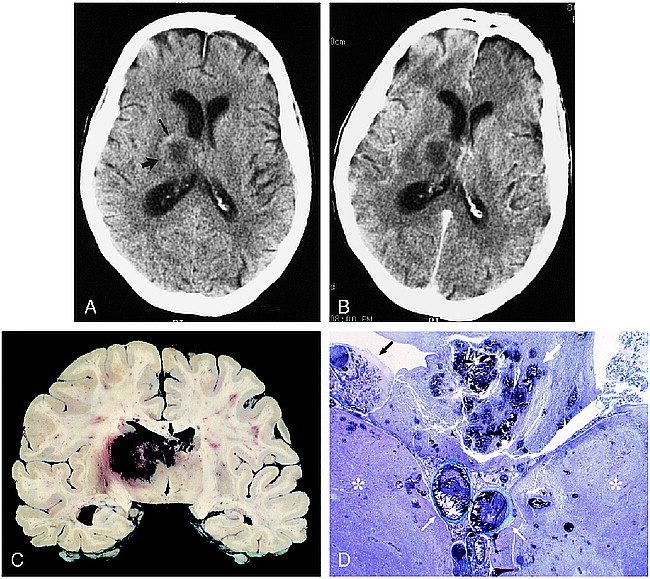
80-year-old man with chronic lymphocytic leukemia presented with disorientation and incontinence.
A, Axial unenhanced CT scan shows a solitary lesion in the right thalamus (large arrow) with surrounding vasogenic edema. Attenuation is slightly increased in the anterolateral aspect of the lesion, indicating petechial hemorrhage (small arrow). Note central hypoattenuation.
B, Corresponding axial contrast-enhanced CT scan shows no appreciable enhancement. The patient died 2 days later.
C, Coronally sectioned brain shows bilateral hemorrhagic necrosis of the thalami (right greater than left) and fornices (arrow). Smaller hemorrhagic foci are present in the corpus callosum.
D, Whole-mount coronal section shows thalamic necrosis bilaterally (asterisks). Hemorrhagic necrosis is present in the fornices (large white arrow). Thrombosis of the internal cerebral veins is present (small white arrows). A Gomori methenamine silver–stained section (not shown) revealed hyphae throughout the internal cerebral vein thrombus and walls and in the necrotic brain parenchyma. This anatomic distribution of lesions and the histologic findings indicate the primary pathophysiology is venous infarction with associated fungal encephalitis. Choroid plexitis is also seen (black arrow) (trichrome, original magnification ×1).
The subtle T1 shortening (Fig 5) seen in two patients may represent the petechial hemorrhage often seen in the evolution of an infarct. The occasional case with minimal imaging findings may also be analogous to the minimal findings seen on CT scans in early thromboembolic infarcts. As radiologically evident intraparenchymal hemorrhage was seen in a minority of our cases, and often in the late stages of disease, its absence should not exclude aspergillosis from the differential diagnosis.
The follow-up imaging studies of the patient with long-term survival indicate that with successful treatment small lesions can regress, leaving virtually no residual imaging abnormality. Larger lesions may calcify, as with other granulomatous processes (Fig 3). Miaux et al (17) described a lesion in a patient several weeks after surgical aspiration and antifungal treatment that had a hypointense rim on T2-weighted MR images.
Although leptomeningeal involvement was documented at autopsy in 12 of the 13 cases in which autopsy was performed, no specific pial imaging abnormalities were identified, even on focused retrospective review. Furthermore, of the three cases in which ventriculitis was present at autopsy, no ependymal enhancement was seen, possibly because of the immunocompromised state of the patients.
In immunocompromised patients, there must be a high index of suspicion for the diagnosis of aspergillosis in the workup of mental status changes, seizures, or focal neurologic findings. Candidiasis is also commonly seen in this group, but imaging studies typically show numerous ring-enhancing microabscesses, measuring less than 3 mm, at the corticomedullary junction, basal nuclei, and cerebellum. However, vasculitis, hemorrhage, and thrombotic infarction can be seen (18), and could potentially be confused with aspergillosis. A ring-enhancing Aspergillus lesion could resemble a Toxoplasma abscess. As no polymorphonuclear impairment (compromising fungicidal ability) is typically present in HIV/AIDS, invasive aspergillosis is relatively rare in this patient group. More at risk are patients with neutropenia and those on corticosteroids (8).
Aspergillosis should be a consideration when lesions of the basal nuclei or thalami are seen, as illustrated in Figure 1. Acute thromboembolic infarction must be a diagnosis of exclusion in this patient group; indeed, Miaux et al (19) have recommended antifungal treatment in patients at risk for invasive aspergillosis in whom acute infarct has been diagnosed, even without documented pulmonary disease. The corpus callosum is involved in relatively few other processes (eg, high-grade astrocytoma, cerebral lymphoma, multiple sclerosis, and Marchiafava-Bignami disease), which can be readily differentiated from aspergillosis on the basis of other imaging findings and the clinical context. Intraparenchymal hemorrhage in an immunocompromised patient should also raise serious suspicion of aspergillosis. Alternative causes include hemorrhagic metastases in a patient with neutropenia from chemotherapy, candidiasis, hemorrhage due to thrombocytopenia or hepatic insufficiency, or hypertensive hemorrhage in a patient receiving cyclosporin or other hypertension-inducing drugs.
Conclusion
The spectrum of CNS infection caused by hematogenous dissemination of Aspergillus includes cerebritis and abscess, as commonly seen with other organisms. However, the present series also includes many cases with a pathophysiology unique to hematogenous Aspergillus: infarction or hemorrhage as the early radiologic presentation, due to the angioinvasive nature of the infection. Infarction and hemorrhage in an uncommon distribution, and infectious lesions with unusual enhancement characteristics, may result from this vasculopathy. Involvement of the basal nuclei, thalami, corpus callosum, and other perforator artery territories, pronounced signal abnormalities on proton density–and T2-weighted MR images, often minimal peripheral enhancement, and rapid progression in size and number of lesions were typical findings in our series of patients with CNS aspergillosis. Any of these findings in the immunocompromised patient, whether or not a diagnosis of pulmonary aspergillosis has been established, should suggest a prospective diagnosis of aspergillosis, so that antifungal therapy in the appropriate dosing regimen may be instituted early in the disease course.
Footnotes
Presented in part at the annual meeting of the American Society of Neuroradiology, Vancouver, May 1993.
Address reprint requests to W. Douglas Brown, MD, Department of Radiology, E3/311, University of Wisconsin Hospital and Clinics, 600 Highland Ave, Madison, WI 53792.
References
- 1.Torre-Cisneros J, Lopez OL, Kusne S, et al. CNS aspergillosis in organ transplantation: a clinicopathological study. J Neurol Neurosurg Psychiatry 1993;56:188-193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pagano L, Ricci P, Montillo M, et al. Localization of aspergillosis to the central nervous system among patients with acute leukemia: report of 14 cases. Gruppo Italiano Malattie Ematologiche dell'Adulto Infection Program. Clin Infect Dis 1996;23:628-630 [DOI] [PubMed] [Google Scholar]
- 3.Cox J, Murtagh FR, Wilfong A, Brenner J. Cerebral aspergillosis: MR imaging and histopathologic correlation. AJNR Am J Neuroradiol 1992;13:1489-1492 [PMC free article] [PubMed] [Google Scholar]
- 4.Khoury H, Adkins D, Miller G, Goodnaugh L, Brown R, DiPerso J. Resolution of invasive central nervous system aspergillosis in a transplant recipient. Bone Marrow Transplant 1997;20:179-180 [DOI] [PubMed] [Google Scholar]
- 5.Schwartz S, Milatovic D, Thiel E. Successful treatment of cerebral aspergillosis with a novel triazole (voriconazole) in a patient with acute leukemia. Br J Haematol 1997;97:663-665 [DOI] [PubMed] [Google Scholar]
- 6.Hansman Whiteman ML, Bowen BC, Donovan Post MJ, Bell MD. Intracranial infection. In: Atlas SW, ed. Magnetic Resonance Imaging of the Brain and Spine 2nd ed. Philadelphia: Lippincott-Raven; 1996;707-772
- 7.Atlas SW, Lavi E. Intra-axial brain tumors. In: Atlas SW, ed. Magnetic Resonance Imaging of the Brain and Spine 2nd ed. Philadelphia: Lippincott-Raven; 1996;315-422
- 8.Harris DE, Enterline DS. Fungal infections of the central nervous system. Neuroimaging Clin N Am 1997;7:187-198 [PubMed] [Google Scholar]
- 9.Ashdown BC, Tien RD, Felsberg GJ. Aspergillosis of the brain and paranasal sinuses in immunocompromised patients: CT and MR imaging findings. AJR Am J Roentgenol 1994;162:155-159 [DOI] [PubMed] [Google Scholar]
- 10.Osborn AG. Diagnostic Neuroradiology. St Louis: Mosby; 1994;128-133
- 11.Gentry LR, Thompson B, Godersky JC. Trauma to the corpus callosum: MR features. AJNR Am J Neuroradiol 1988;9:1129-1138 [PMC free article] [PubMed] [Google Scholar]
- 12.Paris S, Boisvieux-Ulrich E, Crestani B, et al. Internalization of Aspergillus fumigatus conidia by epithelial and endothelial cells. Infect Immun 1997;65:1510-1514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chou SM, Chong YY, Kinkel R. A proposed pathogenetic process in the formation of Aspergillus mycotic aneurysm in the central nervous system. Ann Acad Med Singapore 1993;22: (3 Suppl): 518-525 [PubMed] [Google Scholar]
- 14.Yuh WT, Nguyen HD, Gao F, et al. Brain parenchymal infection in bone marrow transplantation patients: CT and MR findings. AJR Am J Roentgenol 1994;162:425-430 [DOI] [PubMed] [Google Scholar]
- 15.Scaravilli F, Cook GC. Parasitic and fungal infections. In: Graham DI, Lantos PL, eds Greenfield's Pathology 6th ed. London: Arnold; 1997;2:65-112 [Google Scholar]
- 16.Thurnher MM, Thurnher SA, Fleischmann D. Comparison of T2-weighted and fluid-attenuated inversion-recovery fast spin-echo MR sequences in intracerebral AIDS-associated disease. AJNR Am J Neuroradiol 1997;18:1611-1616 [PMC free article] [PubMed] [Google Scholar]
- 17.Miaux Y, Guermazi A, Bourrier P, Singer B, Leder S. MR of cerebral aspergillosis: different patterns in the same patient. AJNR Am J Neuroradiol 1994;15:1193-1195 [PMC free article] [PubMed] [Google Scholar]
- 18.Lai PH, Lin SM, Pan HB, Yang CF. Disseminated miliary cerebral candidiasis. AJNR Am J Neuroradiol 1997;18:1303-1306 [PMC free article] [PubMed] [Google Scholar]
- 19.Miaux Y, Ribaud P, Williams M, Guermazi A, et al. MR of cerebral aspergillosis in patients who have had bone marrow transplantation. AJNR Am J Neuroradiol 1995;16:555-562 [PMC free article] [PubMed] [Google Scholar]


