Abstract
BACKGROUND AND PURPOSE: Diffuse axonal injury (DAI) accounts for a significant portion of primary intra-axial lesions in cases of traumatic brain injury. The goal of this study was to use diffusion-weighted MR imaging to characterize DAI in the setting of acute and subacute traumatic brain injury.
METHODS: Nine patients ranging in age from 26 to 78 years were examined with conventional MR imaging (including fast spin-echo T2-weighted, fluid-attenuated inversion-recovery, and gradient-echo sequences) as well as echo-planar diffusion-weighted MR imaging 1 to 18 days after traumatic injury. Lesions were characterized as DAI on the basis of their location and their appearance on conventional MR images. Trace apparent diffusion coefficient (ADC) maps were computed off-line with the diffusion-weighted and base-line images. Areas of increased signal were identified on the diffusion-weighted images, and regions of interests were used to obtain trace ADC values.
RESULTS: In the nine patients studied, isotropic diffusion-weighted images showed areas of increased signal with correspondingly decreased ADC. In one case, decreased ADC was seen 18 days after the initial event.
CONCLUSION: Decreased ADC can be demonstrated in patients with DAI in the acute setting and may persist into the subacute period, beyond that described for cytotoxic edema in ischemia.
Diffuse axonal injury (DAI) and cortical contusions constitute the vast majority of primary intra-axial lesions in cases of traumatic brain injury and are associated with significant morbidity. DAI results from rotational acceleration and deceleration forces producing diffuse shear-strain deformations of brain tissue, usually at the gray-white junction, in the corpus callosum, and at the dorsolateral aspect of the upper brain stem (1, 2). Cortical contusions are caused by direct contact between the skull and brain parenchyma, most often in the temporal and frontal lobes (1, 2).
Conventional T2*-weighted MR imaging is more sensitive than CT for detecting DAI, although both techniques most likely underestimate the extent of DAI (3). Diffusion-weighted MR imaging is based on the detection of a change in the random motion of protons in water. This imaging method has been described extensively in the evaluation of acute ischemia, and numerous studies have shown that diffusion-weighted imaging is more sensitive than conventional MR imaging in the detection of acute ischemic changes, in that it can reveal a decrease in the apparent diffusion coefficient (ADC) (4–8). Diffusion-weighted imaging has also been studied in experimental animal models of traumatic brain injury, although with different conclusions regarding changes in ADC in the areas of injury (9–12). We hypothesized that ADC values would be decreased acutely in DAI lesions. We present our findings of brain parenchymal injury related to DAI as seen on diffusion-weighted images obtained in the acute to subacute setting.
Methods
The study group consisted of nine patients (eight men and one woman; 26 to 78 years old; mean age, 39 years) who were selected over a period of 6 months from patients presenting to our medical center with recent trauma in whom diffusion-weighted imaging was performed and revealed areas of abnormally increased signal. The patients were imaged between 1 and 18 days after injury, which included motor vehicle accidents (n = 6), falls (n = 2), and being hit by a tram (n = 1). Imaging was performed on a 1.5-T clinical unit equipped with an Echospeed gradient system for echo-planar imaging. Sequences consisted of a sagittal T1-weighted localizer image followed by fast spin-echo T2-weighted (TR/TE 4000/98), fluid-attenuated inversion-recovery (FLAIR) (11000/125, TI = 2200), and gradient-echo (750/40, flip angle = 10°) acquisitions of the entire brain. Diffusion-weighted imaging was performed using a gradient-echo echo-planar sequence (10,000/125, field of view = 24, matrix = 128 × 128, b-value = 1000 s/mm2). The diffusion gradients were applied sequentially in three orthogonal directions to generate three sets of axial diffusion-weighted images (Sx, Sy, and Sz), in addition to a base-line image (S0) with no diffusion gradients. The diffusion images were transferred to an Ultrasparc 1 (Sun Microsystems, Mountain View, CA) station for off-line processing using software developed in IDL (Research Systems Inc, Boulder, CO). Isotropic diffusion-weighted images were calculated using the relationship
Diffusion trace maps were computed from the isotropic diffusion image and the base-line image on a pixel-by-pixel basis using the relationship
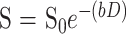 |
where S is the isotropic diffusion-weighted signal, S0 is the base-line signal intensity without diffusion gradients, D is the average trace, and b is the gradient attenuation factor. Regions of interest (ROI) were drawn on the diffusion-weighted images based on areas of abnormally increased signal intensity, and were applied to the trace maps to obtain the corresponding trace ADC values. In one case, an ROI could not be drawn owing to the small size of the lesion. In this case, the ADC measurements were obtained directly from a pixel map of the trace images. In addition, ADC measurements were obtained from normal-appearing white matter in all subjects. These values were compared with white matter ADC values, which we have compiled in our institution from measurements taken in healthy volunteers.
Lesions were characterized as DAI by two neuroradiologists on the basis of their location and their characteristics on conventional imaging sequences. In general, lesions at the gray-white junction, in the corpus callosum, and at the dorsolateral aspect of the upper brain stem were characterized as DAI.
Results
In each of the nine patients studied, isotropic diffusion-weighted images showed lesions with increased signal intensity and corresponding decreased ADC. Five DAI lesions were located in the splenium of the corpus callosum, one was in the body of the corpus callosum, one was in the caudate, and two were at the gray-white junction (see Table). Representative images appear in Figures 1 and 2.
Summary of lesion location and apparent diffusion coefficient (ADC) values in nine patients with diffuse axonal injury
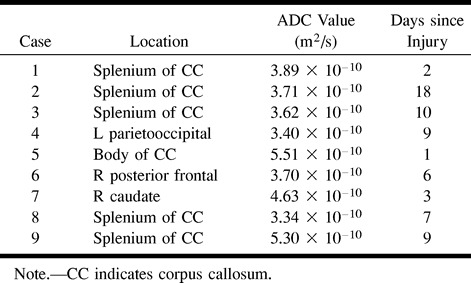
fig 1.
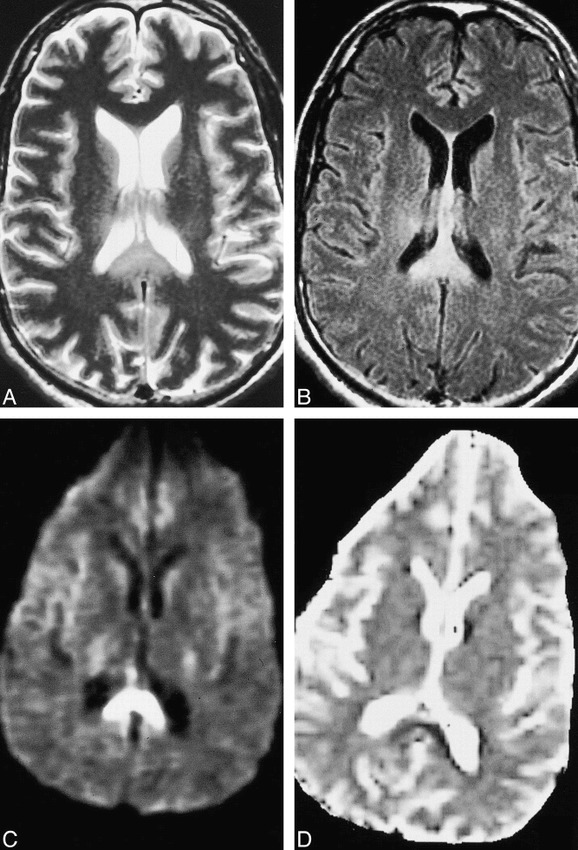
Case 2: 52 year-old man with intracranial injuries sustained in a motor vehicle accident. Brain MR imaging was performed 18 days after the accident.
A and B, Axial fast spin-echo T2-weighted (4000/98/1) (A) and FLAIR (11000/125/1) (B) images both show evidence of diffuse axonal injury, as evidenced by hyperintense signal in the splenium.
C and D, Isotropic diffusion-weighted (10000/125/1) (C) and diffusion trace (D) images show hyperintense signal and decreased ADC values, respectively, consistent with cellular edema. Of particular interest in this case is that ADC values were decreased well into the subacute period after the injury.
The ADC measurements obtained in all cases were greater than 2 SD below the ADC (mean, 6.79 × 10−10 m2/s; SD, 5.25 × 10−11) for normal-appearing white matter in these same patients. Several reports in the literature suggest that white matter that appears normal on conventional MR imaging sequences, such as T2-weighted or FLAIR images, may in fact be abnormal on magnetization transfer images or when using MR spectroscopy in a number of disease processes (13–16). Therefore, the ADC measurements in the areas of brain injury were compared with normal white matter ADC values in healthy volunteers. The measurements were also 2 SD below the ADC values for white matter in a group of five patients with no brain injury (6.73 × 10−10 m2/s; SD, 9.2 × 10−11). The lesions in the splenium measured more than 2 SD below ADC values for the splenium in the healthy volunteers (6.84 × 10−10 m2/s; SD, 6.00 × 10−11). In all cases, increased signal was seen in the affected areas on T2-weighted and FLAIR images. Of particular note, one patient (case 2) had decreased ADC 18 days after the initial injury (Fig 1).
Discussion
In the setting of traumatic brain injury, DAI and cortical contusions are the major causes of primary intra-axial lesions. Microscopically, shearing injury initially produces characteristic axonal bulbs, or retraction balls, with subsequent progression to microglial clusters and then long-tract degeneration. DAI is more commonly associated with impairment of consciousness than are other types of primary brain injury.
Previous studies have presented a confusing picture of the nature of ADC changes in the setting of traumatic brain injury (9–12, 17). Using an animal model, Hanstock et al (10) concluded that traumatic brain injury results in increased water diffusion rates with the directionality indicative of bulk flow of extracellular fluid toward the lateral ventricles (ie, vasogenic edema). In their experimental model of trauma, these authors showed that focal areas of brain injury displayed increased ADC values, as measured up to 4 hours after the injury, and suggested that diffusion-weighted imaging has the potential to differentiate ischemic from nonischemic traumatic brain injury. In contrast, using a similar experimental animal model, Alsop et al (9) concluded that diffusion-weighted imaging was able to depict significant decreases in ADC values at the site of the primary cortical contusion injury when imaging was performed up to 150 minutes after the insult. Using an impact-acceleration injury model in rats, Barzo et al (11) demonstrated an initial increase in ADC values at the site of the primary injury up to 1 hour after the insult. This was followed by a progressive decline in ADC values, reaching a minimum 1 to 2 weeks after the injury. These investigators concluded that after the first hour, cellular edema is the major contributor to post-traumatic brain swelling in diffuse closed head injury. Some of the conflicting results in these experimental studies may be due to the use of different head injury models (ie, fluid percussion versus closed head injury versus impact acceleration). Also, in reporting the ADC changes, distinctions were not drawn between areas of cortical contusion and shearing injuries. These lesions are caused by distinct mechanisms, and corresponding diffusion changes may have altered temporal characteristics.
Our study differs from prior reports in several respects: our study group consisted of human subjects whereas earlier studies were performed in experimental animal models, and the time course of imaging was later than in most of the aforementioned studies. Whereas imaging was performed in the hyperacute period for the experimental animal model studies, imaging in our study was performed in the acute to subacute period (1 to 18 days after the initial injury). In addition, in our study, diffusion-weighted imaging was performed with the echo-planar technique, whereas the other studies used modified spin-echo pulse sequences. Echo-planar imaging allows for much more rapid acquisition of the images, permitting diffusion imaging to be performed in three orthogonal directions (with computation of trace ADC maps). In most of the spin-echo studies, the diffusion gradients were only applied in one direction (acquisition of multisection, multidirectional images can take several hours with spin-echo methods). ADC values are known to vary with fiber orientation and diffusion gradient direction (18). This represents a potential source of error for these single-direction measurements. Trace ADC values, however, are rotationally invariant, and provide a more accurate measure of the diffusion rate within a voxel. Also of note is that, in all our cases, signal abnormalities were detected on FLAIR and/or fast spin-echo T2-weighted images. The presence of these signal alterations on conventional sequences does not affect ADC measurements; however, it does indicate that imaging was not performed in the hyperacute period, with sufficient time having elapsed for edema to be detectable on the conventional sequences.
We have shown that, in humans, significant decreases in ADC can be demonstrated in regions of DAI up to 18 days after initial injury to the brain. This finding may represent a reflection of cellular swelling, or cytotoxic edema, in the setting of acute brain injury. Our results are supported by the findings of Barzo et al (11), who used an experimental animal model that included imaging findings obtained up to 42 days after trauma. These authors demonstrated a progressive decline in ADC values after the hyperacute period (first hour) and well into the subacute period (several weeks). The mechanism for decreased ADC values remains uncertain, although other studies have also pointed toward cellular edema as a significant component of brain swelling in traumatic brain injury (11, 12). Cytotoxic edema is believed to be the cause of decreases in ADC values in the setting of acute ischemia (4–8). Water diffusion is relatively restricted within the intracellular compartment, with lower ADC values for the intracellular space as compared with those for the extracellular space. A flux of water protons into the intracellular compartment will result in a net decrease in ADC values.
DAI is often associated with small areas of hemorrhage. These lesions are frequently identified on the basis of signal blooming from magnetic susceptibility on gradient-echo images. The effect of blood products on ADC, however, is uncertain. Ebisu et al (19) reported decreased ADC values in hemorrhagic versus nonhemorrhagic infarcts, but could not explain the reason for this finding. They postulated that 1) acute hemorrhage may significantly impede water mobility, because of high viscosity, and that concentrated intact blood cells and the formation of a fibrin clot may cause a decrease in ADC; or 2) cytotoxic edema may be present, similar to a nonhemorrhagic stroke. In their study, several hemorrhagic lesions (without infarction) were studied, which also displayed persistently decreased ADC. Although the ADCs were decreased in both hemorrhagic and nonhemorrhagic stroke, the values were relatively lower in the hemorrhagic lesions.
In case 2 in our study, a low ADC was found 18 days after the initial traumatic event. Interestingly, this lesion did not show evidence of overt hemorrhage on the conventional imaging sequences. It is uncertain whether persistently decreased ADC represents ongoing ischemic change, cytotoxic edema, or demyelination, or whether it is more indicative of the underlying intrinsic process of traumatic brain injury. This is in contrast to the decline in ADC values seen with acute ischemia, which tends to persist for the first week and then normalize and ultimately rise. Although cytotoxic edema is the favored mechanism for decreases in ADC related to acute ischemia, the findings in this case are problematic given the time span over which the decreased ADC can be observed. In the setting of acute ischemia, the ADC values tend to reverse within 9 to 10 days (20–22). Continued cytotoxic edema in the brain for several weeks (as would need to be postulated for this case) is not supported by well-established cellular and physiological energy considerations for brain tissue in primate models (23–25). Furthermore, in experimental models of traumatic brain injury, the transient reductions seen in cerebral blood flow are not of sufficient magnitude to produce metabolic failure and associated influx of water (11, 12, 26). In the study by Barzo et al (11), the reductions in ADC extending out to several weeks, as seen in their experimental animal model, are postulated to result from neurotoxic edema. There is experimental evidence that parenchymal damage in the setting of traumatic brain injury may be related to the loss of calcium and potassium homeostasis (27–31), the release of excitotoxic amino acids (29, 30, 32, 33), free radicals (29, 33–35), and tissue acidosis (11, 36, 37). This slower form of cellular swelling, resulting from neurotoxic edema, may be responsible for the extended period of time over which decreased ADC values can be observed in traumatic brain injury (11). An alternative explanation may be related to the presence of microscopic hemorrhage and ruptured axons with membrane fragmentation in DAI, increasing the barriers to the free movement of water molecules, and thereby producing a decrease in ADC. A similar model has been reported in wallerian degeneration and the observed change in the magnetization transfer ratio (MTR) of white matter axons. Lexa et al (38) demonstrated that within the first 2 weeks after a controlled injury, the MTR increases, corresponding histologically to a period of axonal collapse, with an increase in Schmidt-Lantermann incisures, and physical destruction of myelin.
Potential limitations of our study include the small sample size and the lack of histologic confirmation of the findings. The lesions, however, were in characteristic locations for DAI. Further investigation is necessary to determine the frequency with which ADC values are decreased in the setting of traumatic brain injury, and whether these values are affected by injury type, severity, and duration.
Conclusion
Significant decreases in ADC values can be found in the presence of DAI well into the subacute period in humans. While cytotoxic edema may be a contributor, the decreased ADC extends beyond that described for cytotoxic edema with infarcts, suggesting alternative mechanisms at work in DAI.
fig 2.
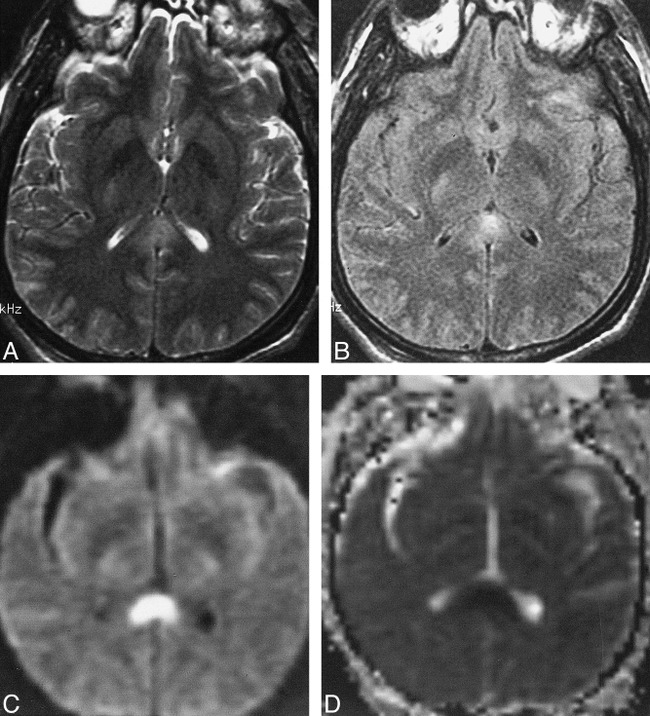
Case 8: 40 year-old man examined with brain MR imaging 7 days after injury.
A and B, Axial fast spin-echo (4000/98/1) (A) and FLAIR (11000/125/1) (B) images at the level of the splenium confirm diffuse axonal injury, with hyperintense signal seen on both pulse sequences. Also note the small bilateral frontal subdural hematomas and a small contusion in the left anterior temporal lobe.
C and D, Isotropic diffusion-weighted (10000/125/1) (C) and diffusion trace (D) images show hyperintense signal and decreased ADC values, respectively.
Footnotes
Supported in part by NIH grant NS-08803 (R.I.G.).
Address reprint requests to Joseph A. Maldjian, MD, Ground Floor Founders, Department of Radiology, Hospital of the University of Pennsylvania, 3400 Spruce St, Philadelphia, PA 19104.
References
- 1.Adams J, Graham D, Scott G, Parker L, Doyle D. Brain damage in fatal non-missile head injury. J Clin Pathol 1980;33:1132-1145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gentry L. Imaging of closed head injury. Radiology 1994;191:1-17 [DOI] [PubMed] [Google Scholar]
- 3.Mittl R, Grossman R, Hiehle J, et al. Prevalence of MR evidence of diffuse axonal injury in patients with mild head injury and normal head CT findings. AJNR Am J Neuroradiol 1994;15:1583-1589 [PMC free article] [PubMed] [Google Scholar]
- 4.Chien D, Kwong K, Gress D, Buonanno F, Buxton R, Rosen B. MR diffusion imaging of cerebral infarction in humans. AJNR Am J Neuroradiol 1996;13:1097-1102 [PMC free article] [PubMed] [Google Scholar]
- 5.Benveniste H, Hedlund L, Johnson G. Mechanism of detection of acute cerebral ischemia in rats by diffusion-weighted magnetic resonance microscopy. Stroke 1992;23:746-754 [DOI] [PubMed] [Google Scholar]
- 6.Sevick R, Kanda F, Mintorovitch J, et al. Cytotoxic brain edema: assessment with diffusion-weighted MR imaging. Radiology 1992;185:687-690 [DOI] [PubMed] [Google Scholar]
- 7.Moseley M, Cohen Y, Mintorovoitch J, et al. Early detection of regional cerebral ischemia in cats: comparison of diffusion and T2-weighted MRI and spectroscopy. Magn Reson Med 1990;14:330-346 [DOI] [PubMed] [Google Scholar]
- 8.Lutsep H, Albers G, DeCrespigny A, Kamat G, Marks M, Moseley M. Clinical utility of diffusion-weighted magnetic resonance imaging in the assessment of ischemic stroke. Ann Neurol 1997;41:574-580 [DOI] [PubMed] [Google Scholar]
- 9.Alsop D, Murai H, Detre J, McIntosh T, Smith D. Detection of acute pathologic changes following experimental traumatic brain injury using diffusion-weighted magnetic resonance imaging. J Neurotrauma 1996;13:515-521 [DOI] [PubMed] [Google Scholar]
- 10.Hanstock C, Faden A, Bendall M, Vink R. Diffusion-weighted imaging differentiates ischemic tissue from traumatized tissue. Stroke 1994;25:843-848 [DOI] [PubMed] [Google Scholar]
- 11.Barzo P, Marmarou A, Fatouros P, Hayasaki K, Corwin F. Contribution of vasogenic and cellular edema to traumatic brain swelling measured by diffusion-weighted imaging. J Neurosurg 1997;87:900-907 [DOI] [PubMed] [Google Scholar]
- 12.Ito J, Marmarou A, Barzo P, Fatouros P, Corwin F. Characterization of edema by diffusion-weighted imaging in experimental traumatic brain injury. J Neurosurg 1996;84:97-103 [DOI] [PubMed] [Google Scholar]
- 13.Cecil K, Hills E, Sandel M, et al. Proton magnetic resonance spectroscopy for detection of axonal injury in the splenium of the corpus callosum of brain-injured patients. J Neurosurg 1998;88:795-801 [DOI] [PubMed] [Google Scholar]
- 14.Filippi M, Rocca M, Martino G, Horsfield M, Comi G. Magnetization transfer changes in the normal appearing white matter precede the appearance of enhancing lesions in patients with multiple sclerosis. Ann Neurol 1998;43:809-814 [DOI] [PubMed] [Google Scholar]
- 15.Kimura H, Meaney D, McGowan J, et al. Magnetization transfer imaging of diffuse axonal injury following experimental brain injury in the pig: characterization by magnetization transfer ratio with histopathologic correlation. J Comput Assist Tomogr 1996;20:540-546 [DOI] [PubMed] [Google Scholar]
- 16.Loevner L, Grossman R, Cohen J, Lexa F, Kessler D. Microscopic disease in normal-appearing white matter on conventional MR images in patients with multiple sclerosis: assessment with magnetization-transfer measurements. Radiology 1995;196:511-515 [DOI] [PubMed] [Google Scholar]
- 17.Assaf Y, Beit-Yannai E, Shohami E, Berman E, Cohen Y. Diffusion and T2-weighted MRI of closed-head injury in rats: a time course study and correlation with histology. Magn Reson Imaging 1997;15:77-85 [DOI] [PubMed] [Google Scholar]
- 18.Pierpaoli C, Jezzard P, Basser P, Barnett A, DiChiro G. Diffusion tensor MR imaging of the human brain. Radiology 1996;201:637-648 [DOI] [PubMed] [Google Scholar]
- 19.Ebisu T, Tanaka C, Umeda M, et al. Hemorrhagic and nonhemorrhagic stroke: diagnosis with diffusion-weighted and T2-weighted echo-planar MR imaging. Radiology 1997;203:823-828 [DOI] [PubMed] [Google Scholar]
- 20.Warach S, Moseley M, Sorensen A, Koroshetz W. Time course of diffusion imaging abnormalities in human stroke (letter). Stroke 1996;27:1254-1256 [PubMed] [Google Scholar]
- 21.Warach S, Benfield A, Edelman R. Time course of abnormal apparent diffusion coefficient in human stroke. Proc Soc Magn Reson 1995;1:82 [Google Scholar]
- 22.Schlaug G, Siewert B, Benfield A, Edelman R, Warach S. Time course of the apparent diffusion coefficient (ADC) abnormality in human stroke. Neurology 1997;49:113-119 [DOI] [PubMed] [Google Scholar]
- 23.Ginsberg M. The new language of cerebral ischemia. AJNR Am J Neuroradiol 1997;18:1435-1445 [PMC free article] [PubMed] [Google Scholar]
- 24.Astrup J, Symon L, Branston N, Lassen N. Cortical evoked potential and extracellular K+ and H+ at critical levels of brain ischemia. Stroke 1977;8:51-57 [DOI] [PubMed] [Google Scholar]
- 25.Branston N, Symon L, Crockhard H. Recovery of the cortical evoked response following temporary middle cerebral artery occlusion in baboons: relation to local blood flow and PO2. . Stroke 1976;7:151-157 [DOI] [PubMed] [Google Scholar]
- 26.Barzo P, Marmarou A, Fatouros P, Corwin F, Dunbar J. Magnetic resonance imaging-monitored acute blood-brain barrier changes in experimental traumatic brain injury. J Neurosurg 1996;85:1113-1121 [DOI] [PubMed] [Google Scholar]
- 27.Choi D. Calcium-mediated neurotoxicity: relationship to specific channel types and role in ischemic damage. Trends Neurosci 1988;11:465-469 [DOI] [PubMed] [Google Scholar]
- 28.Hovda D, Becker D, Katayama Y. Secondary injury and acidosis. J Neurotrauma 1992;9: (Suppl 1): S47-S60 [PubMed] [Google Scholar]
- 29.Katayama Y, Becker D, Tamura T, Ikezaki K. Early cellular swelling in experimental traumatic brain injury: a phenomenon mediated by excitatory amino acids. Acta Neurochir Suppl 1990;51:271-273 [DOI] [PubMed] [Google Scholar]
- 30.Katayama Y, Becker D, Tamura T, Hovda D. Massive increases in extracellular potassium and the indiscriminate release of glutamate following concussive brain injury. J Neurosurg 1990;73:889-900 [DOI] [PubMed] [Google Scholar]
- 31.Kimmelberg H, Rutledge E, Goderie S, Charniga C. Astrocytic swelling due to hypotonic or high K+ medium causes inhibition of glutamate and aspartate uptake and increases their release. J Cereb Blood Flow Metab 1995;15:409-416 [DOI] [PubMed] [Google Scholar]
- 32.Kettenmann H, Schachner M. Pharmacological properties of gamma-aminobutyric acid-glutamate, and aspartate-induced depolarization in cultured astrocytes. J Neurosci 1985;5:3295-3301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kimmelberg H, Pang S, Treble D. Excitatory amino acid-stimulated uptake of 22NA+ in primary astrocyte cultures. J Neurosci 1989;9:1141-1149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Faden A, Demediuk P, Panter S, Vink R. The role of excitatory amino acids and NMDA receptors in traumatic brain injury. Science 1989;244. [DOI] [PubMed]
- 35.Kontos H. Oxygen radicals in CNS damage. Chem Biol Interact 1989;72:229-255 [DOI] [PubMed] [Google Scholar]
- 36.Sjesko B, Katsura K, Mellegard P. Acidosis-related brain damage. Prog Brain Res 1993;96:23-48 [PubMed] [Google Scholar]
- 37.Suzuki S, Ishii M, Ottomo M, Iwabuchi T. Changes in the subarachnoid space after experimental subarachnoid haemorrhage in the dog: scanning electron microscopic observation. Acta Neurochir 1977;39:1-14 [DOI] [PubMed] [Google Scholar]
- 38.Lexa F, Grossman R, Rosenquist A. MR of wallerian degeneration in the feline visual system: characterization by magnetization transfer rate with histopathologic correlation. AJNR Am J Neuroradiol 1994;15:201-212 [PMC free article] [PubMed] [Google Scholar]


