Abstract
BACKGROUND AND PURPOSE: The prevalence of hippocampal sclerosis in the general nonepileptic patient population is not well described. While reports of its association with partial complex seizures are abundant, its absence in nonafflicted patients is generally presumed but not well documented. To test the hypothesis that hippocampal sclerosis is specific for epilepsy, we reviewed the MR imaging studies of 207 patients referred for hearing loss to determine whether high-resolution MR imaging could detect unsuspected hippocampal sclerosis in nonepileptic patients.
METHODS: Our institution screens patients with hearing loss by using high-resolution coronal and axial temporal bone MR imaging that includes the hippocampus within the imaging volume. We retrospectively reviewed 207 studies randomly selected from this database.
RESULTS: The hippocampus was normal in 205 patients; in the remaining two patients we identified one or more primary determinants for hippocampal sclerosis. Subsequent retrospective chart review revealed that both patients had had previously diagnosed seizure disorders.
CONCLUSION: The imaging determinants of hippocampal sclerosis are not prevalent in nonepileptic patients. Incidental identification of hippocampal sclerosis on MR images is uncommon and significant, and should prompt further clinical investigation to exclude a seizure disorder.
Epilepsy is a common affliction. While the association of hippocampal sclerosis with epilepsy is well described (1–3), little has been reported on the prevalence and thus significance of hippocampal sclerosis in the general population. If hippocampal sclerosis is specific for epilepsy, then it should not be identified in nonepileptic patients.
Diagnostic algorithms for many nonepileptic disorders, such as hearing loss, skull base masses, pituitary dysfunction, and cranial neuropathies, include MR imaging as the initial diagnostic tool. These studies frequently include the temporal lobes and hippocampi within the imaging volume, and may reveal an incidental unexpected abnormality. Recognition of these unsuspected findings by the radiologist is essential so that appropriate clinical management decisions can be made.
We retrospectively reviewed the MR imaging studies of 207 patients to test the hypothesis that incidental identification of hippocampal sclerosis on MR images in nonepileptic subjects is uncommon and significant.
Methods
Patient Demographics
Two hundred seven MR imaging studies were examined retrospectively for hippocampal abnormalities. Subjects were selected randomly from the collective database of patients with hearing loss who were undergoing a screening MR examination to exclude acoustic schwannoma. One hundred nine patients were female and 98 were male; the mean age was 52 years (range, 3 to 90 years). Patient selection was blinded to history except for hearing loss. Retrospective chart review, consultation with the referring physician, and/or personal communication with the patient were performed for positive cases.
Imaging Protocol
All 207 patients underwent high-resolution 2D axial and coronal T2-weighted fast spin-echo (FSE) MR imaging of the temporal bone. The imaging protocol was as follows: 4000/80/3 (TR/TE/excitations), an echo train length of 32, a 512 × 384 matrix, a 20 × 15-cm field of view (FOV), 2.0-mm-thick sections with a 1.0-mm overlap, and a 6-minute acquisition time on a 1.5-T MR scanner using a 3-inch bilateral temporomandibular joint phase-array coil.
One patient (case 2) underwent additional high-resolution imaging of the hippocampus with a T2-weighted FSE sequence with parameters of 4860/100/2, an echo train length of 8, a 512 × 512 matrix, a 16-cm FOV, 3.1-mm-thick sections with no overlap, and a 10-minute acquisition time, as well as a T1-weighted spoiled gradient-echo (SPGR) sequence with parameters of 23/4, a 45° flip angle, a 256 × 192 matrix, a 16-cm FOV, and 3.1-mm-thick sections with no overlap. These images were acquired using a dedicated 6-inch phase-array temporal lobe coil (4).
Criteria for Determining Hippocampal Sclerosis
Three primary qualitative diagnostic criteria were used: hippocampal volume loss, T2 signal hyperintensity, and/or loss of internal hippocampal architecture. Three secondary findings were considered when included in the imaging volume: asymmetric fornices, choroidal fissure widening, or temporal horn dilatation.
Image Interpretation
Three neuroradiologists participated in the retrospective review of all study images. Readers were blinded to patients' clinical history except regarding hearing loss. All imaging studies were independently reviewed by two of the three readers, with complete agreement achieved in 198 patients. Minor disagreements regarding the remaining nine studies were arbitrated by the third neuroradiologist, and a group consensus was achieved in all cases.
Literature Database Search
A dedicated Medline search using the Pubmed (U.S. National Library) search engine was performed using the World Wide Web. No citations describing radiologically evident hippocampal sclerosis in nonepileptic patients were identified.
Exclusion Criteria
All 207 patients reviewed had technically adequate imaging studies; no patient with a technically adequate examination was excluded from the investigation. Excluded studies were incomplete, compromised by motion or artifact, or did not include the hippocampus.
Results
Hippocampal sclerosis was identified in two patients; the hippocampus was normal (Fig 1) in the remaining 205 patients.
fig 1.
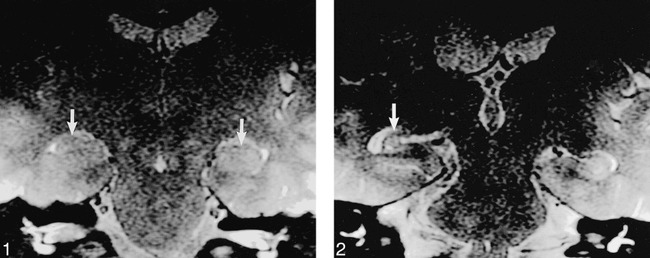
19-year-old nonepileptic patient with hearing loss. T2-weighted FSE (4000/80/3) image shows normal hippocampi bilaterally (arrows). Incidentally noted is a right acoustic schwannoma.
fig 2. Case 1: 29-year-old woman with new seizure onset 17 months after AVM hemorrhage. T2-weighted image (4000/80/3) obtained using the screening ear protocol 17 months after initial AVM hemorrhage shows unilateral right hippocampal sclerosis (arrow) with ipsilateral temporal lobe volume loss. No confirmatory high-resolution temporal lobe imaging was performed because the patient was not considered to be a surgical candidate.
The first positive finding was in a 29-year-old woman (case 1) who presented acutely with intracranial hemorrhage caused by a right parietal arteriovenous malformation (AVM). She reported hearing loss following resection. MR imaging performed 17 months after initial presentation was negative for acoustic schwannoma but revealed incidental right hippocampus and fornix volume loss with concordant enlargement of the ipsilateral temporal horn and choroidal fissure (Fig 2). Subsequent retrospective chart review revealed that the patient had new onset of seizures since the hemorrhage.
The other positive finding (in case 2) revealed unilateral hippocampal volume loss, loss of internal architecture, and T2 hyperintensity (Fig 3A). Subsequent dedicated high-resolution temporal lobe MR images (Fig 3B–C) confirmed unilateral hippocampal sclerosis. A retrospective review with the referring physician and a telephone conversation with the patient revealed a long-standing seizure disorder, currently being treated with phenytoin. The presence of hippocampal sclerosis was not known prior to the patient's screening MR examination; consequently, seizure management is now being reconsidered.
fig 3.
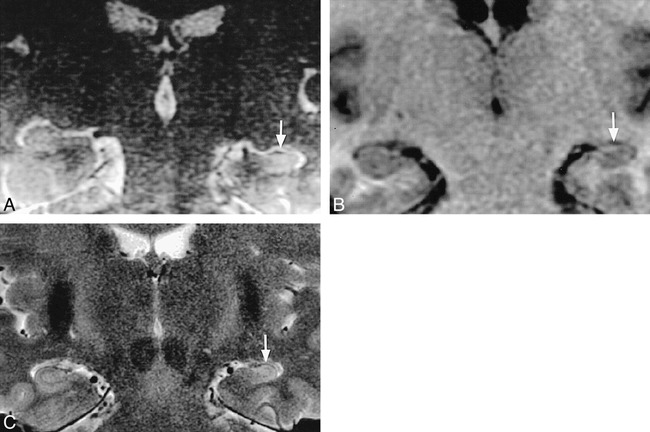
Case 2: 46-year-old woman with seizures.
A, T2-weighted image (4000/80/3) obtained using the screening ear protocol shows unilateral left hippocampal sclerosis (arrow) with volume loss, poor delineation of internal architecture, and T2 hyperintensity.
B and C, Subsequent dedicated high-resolution T1-weighted SPGR image (23/4, 45° flip angle) (B) and T2-weighted FSE image (4860/100/2) (C) confirm hippocampal sclerosis (arrows). There is no forniceal volume loss.
Discussion
The radiologic appearance of the hippocampus in healthy subjects (5–7) and in epileptic patients (2, 3) has been described extensively. The radiologic-pathologic correlation between hippocampal sclerosis and partial complex seizures is also well accepted (3). What is not well described, however, is whether hippocampal sclerosis can be identified radiologically in nonepileptic patients. If hippocampal sclerosis is specific to epilepsy, particularly to partial complex seizures, then it should not be seen in nonepileptic patients. Therefore, the incidental identification of hippocampal sclerosis in a patient not known to have epilepsy would prompt further clinical evaluation to exclude an undetected seizure disorder.
Additionally, the hippocampus is commonly imaged in asymptomatic patients because it may be included within the imaging volume on brain MR studies obtained for nonepileptic complaints. If hippocampal sclerosis can be found in asymptomatic patients, then it should be intermittently identified within this large patient group. Yet, our anecdotal experience in examining several hundred studies prior to this report revealed no positive cases, and we know of no large MR imaging series describing hippocampal sclerosis in nonepileptic patients.
To investigate the prevalence of hippocampal sclerosis in the general population, we retrospectively examined 207 temporal bone MR studies performed for an unrelated clinical problem (hearing loss) to exclude hippocampal sclerosis. To avoid bias, the reviewers were blinded to all history besides hearing loss. Only two abnormal studies were found. The normal hippocampal structures identified in 205 of these 207 patients provided reassurance that hippocampal sclerosis is not commonly recognized when imaging for nonepileptic complaints. Conversely, the two patients whose images revealed unilateral hippocampal sclerosis were both retrospectively determined to have partial complex seizures following chart review or physician/patient consultation. The independent detection of hippocampal sclerosis in these two patients supports the conclusion that unexpected imaging detection of hippocampal sclerosis is a significant finding that merits further clinical investigation.
In contrast to the radiologic literature, there are several nonradiologic series spanning the period from 1954 to 1991 in which hippocampal sclerosis was reported in nonepileptic patients. Unfortunately, these reports are confusing and contradictory (8–13). In contrast to our imaging experience, these small historical postmortem pathologic studies suggest that 10% (12, 13) or more (8, 9) of nonepileptic patients may have hippocampal sclerosis at autopsy. There are several possible explanations for this radiologic-pathologic disparity. First, the resolution of imaging studies is inherently less than that of histologic examination, and subtle hippocampal sclerosis apparent on microscopic specimens may not be appreciated on MR images. Second, the determination of epilepsy in these patients was made by EEG and clinical examination of the patients. Given that many of these patients were mentally impaired and institutionalized, it was very difficult to confirm the absence of partial complex seizure activity clinically.
There are also significant differences between our study population and the pathologic series that seriously hinder their comparison. Patient selection in these historical studies was based in large part on postmortem availability for study. Their underlying mental or psychiatric disorders also make them a poor control group from which to form conclusions pertinent to the general population. Also, since most of these patients were autopsied 30 or 40 years ago, no CT or MR imaging of the brain was available for comparison with the pathologic examination. Conversely, the patients in our series were all ambulatory outpatients selected for a common unrelated chief complaint. For these reasons, it is difficult to make cogent imaging recommendations regarding hippocampal sclerosis on the basis of these historical studies.
Our study has two possible limitations. First, only qualitative T2-weighted FSE imaging determinants were used to identify hippocampal sclerosis, and no quantitative volume measurements were attempted. Therefore, it is possible that some patients with either bilateral or subtle unilateral hippocampal volume loss could be erroneously unrecognized because their subtle hippocampal abnormalities could not be resolved by our imaging technique. However, two previously published MR series have described quantitative techniques for determining hippocampal volume in a total of 42 control subjects (healthy nonepileptic volunteers) (14, 15). Careful review of these studies revealed no reported cases of incidental hippocampal sclerosis. In the future, MR spectroscopy may play an adjunctive role in identifying physiological hippocampal dysfunction in the radiologically normal hippocampus.
The second possible limitation is that only one patient (case 2) underwent dedicated high-resolution imaging specific to the hippocampus. Nevertheless, we do not think this invalidates our conclusions. Even though the initial study was directed to the vestibulocochlear auditory system, the hippocampus was satisfactorily imaged with high-resolution imaging techniques in all 207 patients, including a small FOV, multiple acquisitions, and a local phase-array surface receiver coil. Although the dedicated temporal lobe study done with the 6-inch phase-array coil will produce superior resolution and signal-to-noise ratio, it is not feasible to image a large screening population with a dedicated hippocampal imaging protocol. All images of patients in the screening group obtained with the 3-inch temporomandibular joint coil had good signal-to-noise ratio and resolution, with excellent coverage of the hippocampus.
Conclusion
The imaging determinants of hippocampal sclerosis are not prevalent in nonepileptic patients. Although future advances in MR technology may reveal subtle hippocampal abnormalities in clinically nonepileptic patients, incidental identification of hippocampal sclerosis using current MR imaging techniques should be considered uncommon and significant, and should prompt further clinical investigation to exclude an undiagnosed seizure disorder.
Footnotes
Presented at the annual meeting of the American Society of Neuroradiology, Philadelphia, May 1998.
Address reprint requests to Kevin R. Moore MD, Department of Radiology, Section of Neuroradiology, University of Utah School of Medicine, 50 N Medical Dr, Salt Lake City, UT 84132.
References
- 1.Gloor P. The Temporal Lobe and Limbic System. . New York: Oxford University Press; 1997
- 2.Kuzniecky R, Burgard S, Bilir E,, et al. Qualitative MRI segmentation in mesial temporal sclerosis: clinical correlations. . Epilepsia 1996;37:433-439 [DOI] [PubMed] [Google Scholar]
- 3.Meiners L, Gils A, Jansen G,, et al. Temporal lobe epilepsy: the various MR appearances of histologically proven mesial temporal sclerosis. . AJNR Am J Neuroradiol 1994;15:1547-1555 [PMC free article] [PubMed] [Google Scholar]
- 4.Hayes C, Tsuruda J, Mathis C. Temporal lobes: surface MR coil phased-array imaging. . Radiology 1993;189:918-920 [DOI] [PubMed] [Google Scholar]
- 5.Naidich T, Daniels D, Haughton V, Williams A, Pojunas K, Palacios E. Hippocampal formation and related structures of the limbic lobe, II: anatomic-MR correlation. . Radiology 1987;162:755-761 [DOI] [PubMed] [Google Scholar]
- 6.Naidich T, Daniels D, Haughton V, Williams A, Pojunas K, Palacios E. Hippocampal formation and related structures of the limbic lobe, I: anatomic-MR correlation. . Radiology 1987;162:747-754 [DOI] [PubMed] [Google Scholar]
- 7.Miller M, Mark L, Ho K, Haughton V. MR appearance of the internal architecture of Ammon's horn. . AJNR Am J Neuroradiol 1996;17:23-26 [PMC free article] [PubMed] [Google Scholar]
- 8.Morel F, Wildi E. Colleque de Marsedies, les Lesions de L'Epilepsie. . Bruxelles: Acta Medical Belgica; 1954
- 9.Crome L. A morphological critique of temporal lobectomy. . Lancet 1955;1:882-884 [DOI] [PubMed] [Google Scholar]
- 10.Margerison J, Corsellis J. Epilepsy and the temporal lobes: a clinical, electroencephalographic, and neuropathologic study of the brain in epilepsy, with particular reference to the temporal lobes. . Brain 1966;89:499-530 [DOI] [PubMed] [Google Scholar]
- 11.Meencke HJ, Veith G. Hippocampal sclerosis in epilepsy. . In: Luders H, ed Epilepsy Surgery New York: Raven; 1991;705-715
- 12.Peiffer J. Morphologische Aspekte der Epilepsien. . Berlin: Springer; 1963
- 13.Peiffer J. Probleme der Krampfschadigung Beim Menschen. . Epilepsie 1988;88:257-267 [Google Scholar]
- 14.Free S, Bergin P, Cook M, Shorvon S, Stevens J. Methods for normalization of hippocampal volumes measured with MR. . AJNR Am J Neuroradiol 1995;16:637-643 [PMC free article] [PubMed] [Google Scholar]
- 15.Hasboun D, Chantome M, Zouaoui A,, et al. MR determination of hippocampal volume: comparison of three methods. . AJNR Am J Neuroradiol 1996;17:1091-1098 [PMC free article] [PubMed] [Google Scholar]


