Abstract
Summary: Capillary malformations or telangiectasias of the brain usually exhibit a benign clinical course, although occassionally they may be associated with mild to moderate symptomatology of uncertain origin. We report a case of an exceptionally aggressive capillary telangiectasia in a child, which was associated with progressive neurologic deterioration resulting in death.
Capillary malformations, or telangiectasias of the brain, are a distinct category of cerebral vascular malformations, consisting of localized collections of multiple thin-walled vascular channels interposed between normal brain parenchyma (1, 2). Most capillary telangiectasias are discovered incidentally either at autopsy or on MR imaging, which suggests that they are usually clinically benign lesions (3, 4).
With the increased use of MR imaging, numerous reports have appeared describing lesions that have been mostly presumed to be capillary malformations, because surgical or pathologic confirmation or both is usually lacking. Although “typical” and “atypical” features have been described, there is considerable variation in these features (2–5).
We report a case of a pathologically documented capillary malformation of the brain stem presenting in childhood with an exceptionally aggressive clinical course that was eventually fatal. An autopsy was performed, affording a rare opportunity for radiologic-pathologic correlation.
Case Report
A young female infant, progressing through normal milestones, experienced a febrile seizure (38° C) and later strabismus at the age of 15 months. She experienced a second prolonged febrile seizure (40° C) at the age of 22 months, followed shortly thereafter by deterioration in speech noted by a decline in her vocabulary, though comprehension of speech was unaffected. She ambulated with an ataxic gait. Evaluation at that time showed a nonspecified pontine lesion (reportedly suspicious for hemorrhage or glioma). An EEG was obtained showing no abnormality. At 28 months of age, seizure activity recurred. Evoked potentials revealed a mild conductive hearing loss. Imaging studies showed no apparent change in the pontine lesion.
At approximately 4 and a half years of age, the patient again experienced a prolonged seizure prompting evaluation by a pediatric neurologist. Physical examination was markedly abnormal, revealing truncal and gait ataxia, multiple cranial nerve abnormalities (including diminished extraocular movements, ptosis, bilateral facial weakness, absent gag reflex, and poor palatal elevation). An obvious dysarthria was noted. Muscle tone and strength were normal.
All pertinent laboratory examinations were normal. MR imaging of the brain was obtained using standard spin-echo T1-weighted (700/17/1 [TR/TE/excitations]), proton density–weighted (2200/35/1), and T2-weighted (2200/90/1) sequences. Gadolinium was administered for axial T1-weighted imaging. On the short TR/TE sequences, there was mild asymmetric atrophy of the lower basis pontis and left inferior cerebellar peduncle, with a subtle area of decreased intensity involving the midline and left pons (Fig 1A). The remaining portions of the pons, and the adjacent mesencephalon and medulla showed no obvious signal abnormality. On the long TR/TE sequences, there were more extensive areas of signal abnormality within different portions of the brain stem. At the level of the pontomesencephalic junction, an oval-shaped region of abnormally low signal intensity was seen ventrally, involving primarily the midline and left lateral structures. At the level of the pontomedullary junction, a focal area of abnormally high signal intensity was seen involving much of the left ventral portion of the brain stem, in the region of the inferior olivary nucleus (Fig 1B). The full magnitude of brain stem involvement was best illustrated on gadolinium-enhanced short TR/TE sequences. Large geographic areas of intense, homogeneous enhancement were seen extending from the level of the inferior colliculi caudally through the pons and into the upper medulla (Fig 1C). At the pontomedullary junction, the margins of enhancement extended into the middle cerebellar peduncles, becoming more ill-defined and linear.
fig 1.
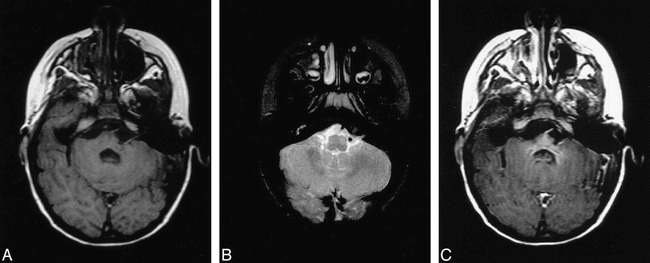
A, Axial T1-weighted (700/17/1) MR image shows mild asymmetry of the lower basis pontis and middle cerebellar peduncle on the left. Note the geographic region of subtle hypointensity centrally and to the left of midline and questionable punctate hyperintense foci near the midline.
B, Axial T2-weighted (2200/90/1) image showing abnormal hyperintensity within the left ventral portion of the brain stem at the level of the inferior cerebellar peduncle/medulla. This focus is slightly caudal to the region of hypointensity seen in figure 1B.
C, Post-gadolinium T1-weighted (700/19/1) axial image reveals homogeneous enhancement within the rostral pons and adjacent cerebellar peduncles. The magnitude of contrast enhancement is disproportionately greater than the focal signal changes seen on the T2-weighted images (see figure 1B).
The child was lost to any further clinical follow-up. She reportedly died at home while asleep, at the age of 5 years. An autopsy revealed a diffuse capillary malformation of the midbrain, pons, and medulla. Involvement was most extensive at the level of the mid-pontine level where the malformation involved nearly the entire cross-sectional area of the pons, with further lateral extension into the middle cerebellar peduncles (Fig 2A). Microscopic histopathologic analysis showed innumerable thin-walled, endothelium-lined vascular channels (50–150 microns in diameter) replacing much of the basis pontis and pontine tegmentum (Fig 2B). These abnormal vascular channels extended caudally into the medullary pyramids, olives, and medial lemniscus, with marked neuronal loss and chronic astrocytosis. Myelin staining revealed extensive axonal loss within the corticospinal and corticobulbar tracts. There was also extensive neuronal drop-out within multiple brain stem nuclei. Other findings were multiple abnormal small-draining venous channels and occasional focal calcium deposits within the tegmentum. There was no evidence of recent or remote hemorrhage. The final diagnosis was diffuse capillary malformation with anomalous venous drainage involving the pons, middle cerebellar penduncles, and medulla. The constellation of histopathologic changes affecting the brain stem nuclei and long tracts were compatible with chronic ischemic injury, presumably secondary to a “steal” phenomenon from the malformation.
fig 2.
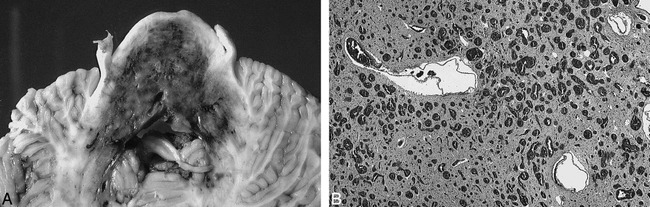
A, Gross anatomic specimen showing axial section through mid-pontine level. The capillary malformation is clearly demarcated by the extensive bluish discoloration involving nearly the entire cross-sectional area of the basis pons and tegmentum with extension into the middle cerebellar peduncles. There are patchy areas of relatively more dense discoloration representing increased density of telangiectasia.
B, Microscopic specimen showing the basis pontis. Vast numbers of blood-filled endothelium-lined vascular channels measuring 50–150 microns in diameter have replaced much of the brain parenchyma. There is also gliosis and neuronal drop-out, but no evidence of hemorrhage.
Discussion
The true incidence of capillary malformations or telangiectasias of the brain is difficult to discern because most are likely to be clinically asymptomatic. Estimates from autopsy series suggest they are not uncommon, representing approximately 16% to 20% of all CNS vascular malformations (6). Capillary malformations represent histologically benign collections of dilated capillaries interposed within normal brain parenchyma (1). The area of involvement of the brain is typically small, ranging from several millimeters to 2 centimeters in size (2, 4). Common sites of involvement include the pons, cerebral hemispheres, and spinal cord (1, 4).
The clinical manifestations related to capillary malformations are variable, although typically they are regarded as quiescent lesions occasionally presenting with headaches, confusion, weakness, dizziness, visual changes, vertigo, tinnitus, or seizures (2, 4). The precise cause of symptoms is often not known because only rarely have there been reports of apoplectic hemorrhage developing from a capillary telangiectasia (7). Rarely, hemorrhage may be the initial presentation of a capillary malformation (6, 7). Detection of hemorrhage on imaging studies should prompt consideration of a coexisting lesion (eg, cavernous malformation), or may indicate a “mixed” form of lesion (3, 8). Reports of progressive symptoms from telangiectasias are lacking in the literature.
Cases of so-called “progressive telangiectasias” occasionally have been described, although these all have been specific to dermal lesions (9). To our knowledge, there are no cases of pure telangiectasias of the brain associated with an aggressive or progressive clinical course. The reason for the aggressive clinical behavior of a capillary malformation is uncertain, although a few mechanisms are possible based upon prior investigations. As with other cerebral vascular malformations, apoplectic or recurrent microscopic hemorrhage into the adjacent brain parenchyma may occur, producing both primary and secondary neuronal injury. Blood-pool shifting or possibly true arteriovenous shunting through a capillary telangiectasia may also occur (3), producing local or regional disturbances in cerebral perfusion (8). Such disturbances may lead to ischemic injury. Because no gross or microscopic evidence of hemorrhage was observed in this case, we speculate that the extensive nature of this malformation may have produced regional hemodynamic alterations within the brain stem. These alterations may have resulted in the “steal” phenomenon that ultimately led to chronic ischemic injury to the brain stem and possibly seizure activity.
Diagnosis of cerebral capillary malformations is now possible with MR imaging. Review of the literature reveals considerable inconsistency of the MR features. Lee et al (4) reported that most lesions in their series were not detectable on both the T1- and T2-weighted images, but were consistently identified as regions of pronounced loss of signal intensity on the gradient-echo images, which they considered essential for making the diagnosis. Unfortunately, gradient-echo images were not obtained in this case. All capillary malformations in the Lee series showed mild contrast enhancement. Barr et al (2) reported slight T2 hyperintensity and contrast enhancement as the most common finding in their series; T1 hypointensity or T1 and T2 isointensity were uncommon presentations. Gradient-echo images were useful for depicting the lesion in those cases where signal abnormalities were absent on conventional T1- and T2-weighted images. In earlier work by Rigamonti et al (5), lesions suspected of being cerebral telangiectasias appeared on MR imaging as “predominantly decreased signal intensity.” The imaging parameters used, however, were not described. In light of the considerable variability in reported appearances of capillary malformations on MR imaging, it is our opinion that these lesions in fact have no “classic” or typical features that could be considered pathognomonic.
The unusually extensive involvement of the midbrain, pons, and medulla produced a dramatic bluish discoloration on gross inspection, which superficially resembled chronic hemorrhage (Fig 2A). These regions of discoloration were shown on microscopic analysis to be dilated vessels containing blood, without any evidence of extravasation or hemosiderin deposition. These findings at least in part explain why there was relatively little abnormal low signal intensity observed on the long TR image sequences for most of the affected brain stem.
The malformation was demarcated most accurately on the gadolinium-enhanced images, revealing a diffuse pattern of enhancement throughout the brain stem. This correlated with gross and microscopic histopathologic examinations showing extensive involvement of the brain stem from the level of the inferior colliculi to the upper medulla. The intensity of enhancement was variable at some levels, which may be a reflection of the relative density of abnormal vasculature within the affected parenchyma.
The extensive enhancement of the malformation observed in this case is not surprising, considering the magnitude of brain stem involvement. We speculate that this malformation had relatively low flow, allowing for visualization of contrast agent within the intravascular compartment. This was supported further by the lack of observable flow-voids on nonenhanced sequences. It is likely that the regions of well-defined enhancement reflect the “vascular blood pool” of the malformation. As for the areas of more ill-defined enhancement (ie, the “brush border” appearance), it is uncertain what is responsible for this finding.
Interestingly, large areas of the affected brain stem appeared to have no appreciable signal abnormality on either the short or long TR imaging sequences. The exception to this was oval-shaped regions of low signal intensity within the middle and left anterolateral portions of the pontomesencephalic junction (Fig 1B), and within the left pontomedullary junction. There was also abnormal focal high signal intensity seen involving the region of the inferior olivary nucleus and pyramids (Fig 1C). Unfortunately no clear histopathologic findings, either gross or microscopic, specifically correlated with these imaging findings.
We postulate that the focus of T2 hyperintensity within the pontomedullary junction involving the left ventral brain stem and olivary nuclei may have resulted from a relatively high concentration of intracellular oxyhemoglobin within the vascular spaces, either alone or in combination with surrounding gliosis. The focus of T2 hypointensity within the pontomesencephalic junction and rostral pons could be explained by yet another mechanism. Histopathologic specimens showed focal deposits of calcium in this location, which may appear as a focus of hypointensity on the T2-weighted MR images. Alternatively, it is possible that the T2 hypointensity was due to increased levels of deoxyhemoglobin. This form of hemoglobin is found in higher concentrations relative to oxyhemoglobin in low-oxygen environments, such as those seen in areas of regional oligemia (10–12). Although gradient-echo images were not obtained in this case, it is our opinion that the significant quantities of deoxyhemoglobin that would have been within a malformation of this size would have been sufficient to produce notable T2 shortening.
It is clear that the focal regions of signal alteration were grossly disproportionate to the size of the overall lesion demarcated by contrast enhancement that could be explained by flow characteristics and resulting microcirculatory physiology. It is likely that this was a consequence of visualization of intravascular contrast agent within relatively slow-flowing vessels of the capillary malformation (10).
Two focal regions within the malformation may have had flow conditions different from the others, which consequently produced alterations in signal intensity on nonenhanced imaging. Regarding the low signal intensity detected in the left pontomesencephalic junction on long TR sequences, it is possible that there was diminished blood flow resulting in stagnation and hemoglobin desaturation. The presence of sufficient quantities of deoxyhemoglobin may have produced the observed T2 shortening. As for the abnormally focal high intensity in the pontomedullary junction, it is possible that there was relatively higher density of telangiectasias with similar blood flow to adjacent regions of the malformation, producing a relatively higher concentration of oxyhemoglobin and high signal intensity changes on the T2-weighted images.
In conclusion, we believe this case represents a highly unusual form of capillary malformation (telangiectasia) from both a clinical and imaging standpoint. It is conceivable that the extensive brain stem involvement produced significant alterations in local cerebral hemodynamics, resulting in a perfusion “steal” phenomenon that ultimately led to secondary ischemic brain stem injury and, less likely, to seizures. These hemodynamic alterations are at least partially supported by MR imaging and histopathologic findings. A capillary malformation should be considered in the differential diagnosis when areas of geographic enhancement of the brain are observed to occur in association with varied signal alterations on standard spin-echo MR imaging sequences.
Footnotes
Address reprint requests to John C. Chaloupka, MD, Director, Interventional Neuroradiology, Department of Radiology JPP 3891, the University of Iowa College of Medicine, 200 Hawkins Drive, Iowa City, IA 52242-1077.
References
- 1.Okazaki H. Cerebrovascular Disease. In: Fundamentals of Neuropathology-Morphologic Basis of Neurologic Disorders. 2nd ed. New York: IGAKU-SHOIN Medical Publishers; 1989;27-94
- 2.Barr RM, Dillon WP, Wilson CB. Slow-Flow Vascular Malformations of the Pons: Capillary Telangiectasias? AJNR Am J Neuroradiol 1996;17:71-78 [PMC free article] [PubMed] [Google Scholar]
- 3.Chang SD, Steinberg GK, Rosario M, Crowley RS, Hevner RF. Mixed arteriovenous malformation and capillary telangectasia: a rare subset of mixed vascular malformations (case report). J Neurosurg 1997;86:699-703 [DOI] [PubMed] [Google Scholar]
- 4.Lee RR, Becher MW, Benson ML, Rigamonti D. Brain Capillary Telangiectasia: MR Imaging Apearance and Clinicohistopathologic Findings. Radiology 1997;205:797-805 [DOI] [PubMed] [Google Scholar]
- 5.Rigamonti D, Johnson PC, Spetzler RF, Hadley MN, Drayer BP. Cavernous Malformations and Capillary Telangiectasia: a spectrum within a single pathological entity. Neurosurgery 1991;28:60-64 [PubMed] [Google Scholar]
- 6.Chaloupka JC, Huddle DC. Classification of Vascular Malformations of the Central Nervous System. Neuroimaging Clin North Am 1998;8:295-321 [PubMed] [Google Scholar]
- 7.Bland LI, Lapham LW, Ketonen L, Okawara S. Acute Cerebellar Hemorrhage Secondary to Capillary Telangiectasia in an Infant. A Case Report. Arch Neurol 1994;51:1151-1154 [DOI] [PubMed] [Google Scholar]
- 8.Awad IA, Robinson JR Jr, Mohanty S, Estes ML. Mixed Vascular Malformations of the Brain: Clinical and Pathogenic Considerations. Neurosurgery 1993;33:179-188 [DOI] [PubMed] [Google Scholar]
- 9.Torok L, Borka I, Szabo G. Waldernstrom's macroglobulinemia presenting wth cold urticaria and cold purpura. Clin Exp Dermatol 1993;18:277-279 [DOI] [PubMed] [Google Scholar]
- 10.Wolf GL. Paramagnetic Contrast Agents for MR Imaging of the Brain. In: Latchaw RE, ed. MR and CT Imaging of the Head, Neck, and Spine. 2nd ed. St. Louis: Mosby Yearbook; 1991;95-108
- 11.Bradley WG Jr. MR Appearance of Hemorrhage in the Brain. Radiology 1993;189:15-26 [DOI] [PubMed] [Google Scholar]
- 12.Bradley WG Jr. Hemorrhage and hemorrhagic infections in the brain. Neuroimaging Clin North Am 1994;4:707-32 [PubMed] [Google Scholar]


