Abstract
BACKGROUND AND PURPOSE: The management of the clinically negative neck (N0) remains controversial because the incidence of occult metastases is high and the prognostic difference between elective treatment and a “wait and see” approach remains unclear. This study was undertaken to assess the role of sonographically guided aspiration cytology for the selection of the initial-management strategy for the neck and for the early detection of neck metastases during follow-up of patients with N0.
METHODS: Seventy-seven clinically and cytologically confirmed N0 patients, who underwent a transoral tumor excision and no neck treatment, were followed up for 1 to 4 years by both palpation and sonographically guided aspiration cytology.
RESULTS: Fourteen patients (18%) had recurrent neck tumor; 10 (71%) of these necks were salvaged. Of the 14 neck failures, six were detected before being palpable and nine were detected within 7 months. Eleven of the 19 aspirated tumor-positive nodes had a minimal diameter smaller than 1 cm, and all four patients who eventually died had lymph node metastases larger than 14 mm.
CONCLUSION: With sonographically guided aspiration cytology, the risk of missing occult metastases was 18%, which is less than expected after palpation only. Sonographically guided aspiration cytology is an effective technique for following up on the status of the neck after transoral tumor excision, and should be used at frequent intervals if no elective neck treatment is performed.
The assessment of the clinically negative neck (N0) by different radiologic techniques has been studied widely. The risk of occult metastases can be diminished by an accurate staging method that enables detection of small nonpalpable lymph node metastasis in the neck. In spite of improvements of radiologic techniques and staging criteria for metastases (1), no author has used imaging findings as a guideline for the selection of neck management nor for follow-up of the neck. In most institutions, the clinically negative neck is dissected electively or irradiated if the estimated risk of occult metastasis, based on characteristics of the primary tumor, exceeds 20% (2). Imaging could influence the strategy if positive imaging findings ensure timely treatment, and if negative imaging findings provide a greater degree of certainty that no occult metastases are present. If this is the case, and the risk of occult metastases can be brought below 20%, a “wait and see” strategy could be used (3). Nevertheless, only very few clinicians have altered their treatment strategy toward the N0 neck based on negative imaging findings.
CT and MR imaging have been reported to show some 40–60% of all nonpalpable metastases; however, these techniques also have a 25–35% false-positive rate in this population (4–11). Although necrosis and irregular enhancement on CT and MR images are very important criteria, and are present in almost 75% of all neck sides with metastases, they unfortunately are seldom visible in nodes smaller than 1 cm, which comprise the majority of metastatic nodes in palpably N0 necks (1). Recently, Curtin et al showed that irregular enhancement is rare in nonpalpable lymph node metastases smaller than 1 cm, and hardly contributes to the sensitivity of CT and MR imaging (11). It is probably because irregular enhancement becomes rarer in small lymph node metastases that the sensitivity and specificity of CT and MR images are somewhat disappointing in the N0 neck.
The accuracy of positron emission tomography (PET) for staging the N0 neck has been studied by only a few investigators (12–14). PET, however, might become a valuable tool for detecting small occult metastases, although its limited availability and costs will hamper widespread use for this indication. In all likelihood, its main indication will be for the detection of occult primary tumors and recurrences after radiotherapy. An initial report on PET in 14 patients showed a sensitivity of 78% in the N0 neck (13). In another comparative study, tyrosine-PET proved to be superior to CT and MR for the detection of lymph node metastases (14).
In a previous study, we found that sonographically guided fine-needle aspiration cytology was more accurate for the assessment of the clinically N0 neck than was CT or MR imaging (6, 15). Sonographically guided aspiration cytology has gained popularity for staging the clinically N0 neck of patients with head and neck carcinomas. The reported sensitivity of sonographically guided aspiration cytology in the N0 neck ranges from 48% by Takes (16), 50% by Righi (9), to 73% by our group (6). The specificity approaches 100%, as false-positive cytologic results of lymph nodes are very rare. A sensitivity of 73% in the N0 necks means that 73% of all neck sides with nonpalpable metastases can be upstaged correctly. This implies that if the initial risk of occult metastases is 40%, as in T2 tongue carcinomas, this risk diminishes to approximately 15% after negative aspiration cytology results. Calculation for 100 patients is (40–73% × 40) ÷ [60 + (40–73% × 40)] = (11 ÷ 71 = 15.49%).
In our clinic, since 1992, we gradually changed our policy for patients treated with transoral excisions without metastases detected with palpation or sonographically guided aspiration cytology. In the past, these patients frequently would have had an elective neck dissection, as now they are followed at regular intervals with palpation and sonographically guided fine-needle aspiration cytology. This study describes the outcome of these patients initially staged N0 by sonographically guided fine-needle aspiration cytology and the cytologic findings during follow-up.
Methods
A retrospective study was performed in 77 patients treated between 1991 and 1997 with 79 head and neck squamous cell carcinomas without palpable neck nodes (N0). There were 44 men and 33 women of an average age of 61 years. Two patients had two primary carcinomas. Primary tumor location was as follows: 34 mobile-tongue, 24 floor-of-mouth, eight lip and buccal mucosa, three alveolar ridge, five palate, three oropharynx, and two supraglottic (transoral CO2 laser excision). The 79 primary tumors were staged T1 in 43, T2 in 33, T3 in two, and T4 in one case. All patients were treated surgically with a transoral excision of the primary tumor. None of the patients underwent elective neck dissection or were irradiated before or immediately after excision of the primary tumor.
At presentation, all 77 patients had a sonographic examination of their 154 neck sides. In 75 neck sides in 53 patients, an aspirate was obtained from one or more lymph nodes at risk of harboring metastasis. In five neck sides, the initial aspirate was nondiagnostic. In these cases, a transoral tumor excision was performed, and the first repeat sonographically guided fine-needle aspiration cytology was planned within 4 weeks. In 79 neck sides no aspirate was obtained, as no lymphadenopathy fulfilling size criteria was visible sonographically. Cytology results were considered tumor-positive if the cytologist saw atypical epithelial cells suspicious for or showing proof of squamous cell carcinoma. Cytology results were considered negative if no nodes larger than 3–4 mm were visible, and thus no aspirate was obtained, or if the aspirate showed enough material but no cells suspicious for squamous cell carcinoma. Because only T1 and T2 oral cavity tumors and superficial T1 and T2 oropharyngeal tumors that were eligible for transoral excision were entered in the study, no further CT or MR examinations were performed for primary tumor staging.
Real-time, B-scan sonographic examinations were performed by one of the authors (J.A.C.) using a 7-MHz linear-array transducer and an Acuson 128 system (Acuson Corp., Mountain View, CA). All nodes that were made visible by sonography were measured on screen. Because the minimal axial diameter is the most accurate diameter to use for predicting tumor-positive lymph nodes, this measurement was used to select the most suspicious lymph nodes (17). Levels 1 through 5 of both sides of the neck were examined fully for the presence of enlarged lymph nodes. Level 1 corresponds to the submandibular and submental triangle, levels 2 through 4 correspond to the high-, mid-, and low-jugular chain lymph nodes, whereas level 5 corresponds to the posterior triangle.
Lymph nodes as small as 5 mm in minimal axial diameter were aspirated in level 2, whereas 4 mm was used as a cutoff size criterion for all other levels. The most suspicious (largest) lymph node(s), in the first or second echelon (lymph node drainage level) was aspirated. At the maximum, three lymph nodes per side were aspirated. Most often, lymph nodes were aspirated twice to ensure that sufficient material was obtained. A 0.6-mm × 25-mm needle was introduced into the skin about 1 cm from the transducer. After visualization of the needle point inside the lymph node, aspiration was started and continued by moving the needle up and down inside the lymph node. The smears were immediately fixed using 70% ethanol and were stained with Papanicolaou and air-dried and stained with May-Grunwald-Giemsa solution. The needle and syringe were washed into carbowax to obtain additional smears.
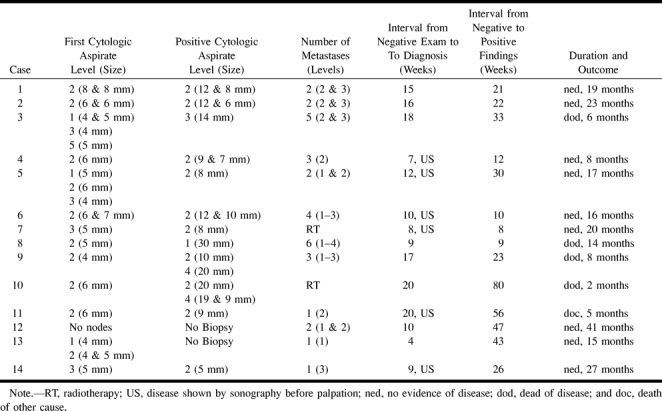
The follow-up period of the patients who survived varied from 13 to 58 months (mean, 31 months; median, 30 months). In the first 2 years, apart from palpation at regular intervals, songraphically guided fine-needle aspiration cytologies of the neck were performed as well. Follow-up visits were performed at 4–8-week intervals in the first year, gradually increasing the interval thereafter. Aspiration cytology was performed every two to five visits, depending on the clinician involved and the estimated risk of occult metastases (Table 1). The number of cytologic aspirates obtained during follow-up varied from one to six per patient.
TABLE 1:
Characteristics of the 14 patients with recurrent neck tumor
Results
All preoperative initial sonographically guided fine-needle aspiration cytology results were negative. Of the 77 patients, 14 (18%) patients had neck tumor; 10 of these were successfully treated (71%). The T stage of the primary tumor in these 14 cases was as follows: eight T1, five T2, and one T3. Of the 14 recurrent neck tumors, four were detected within 3 months after treatment of the primary tumor, five between 4 and 7 months, and five after more than 7 months. Of the four patients who died of their tumor, two neck recurrences were detected within 4 months, one after 8 months and one after 19 months (Table 1). Six regional failures of the neck were found with sonographically guided fine-needle aspiration cytology, whereas eight were detected by palpation. All these eight patients previously had negative cytology results. In six of these eight patients, sonographically guided cytology was used to confirm clinical suspicion, whereas for the other two cases no cytologic confirmation was obtained.
In 11 of the 14 patients, the preoperative sonographically guided cytology was obtained from the correct level in the neck, as the later metastasis occurred at this level. During the follow-up of these 14 patients, 54 tumor-negative lymph nodes were aspirated, varying in size from 3 to 18 mm in minimal axial diameter, whereas a total of 19 metastatic lymph nodes were aspirated, varying in size from 6 to 30 mm in minimal axial diameter (Fig 1). Most metastases were detected at level 2 (Table 1). There was only minor discordance between the levels indicated by the sonographer and the pathologist (Table 1). For the 10 patients who had undergone both sonographically guided aspiration cytology and histopathologic analysis (Table 1), the comparison shows that metastases at level 1 were missed by the sonographer three out of four times. In at least three cases, lymph nodes that the sonographer classified to be at level 2 were classified to be at level 3 by the pathologist.
fig 1.
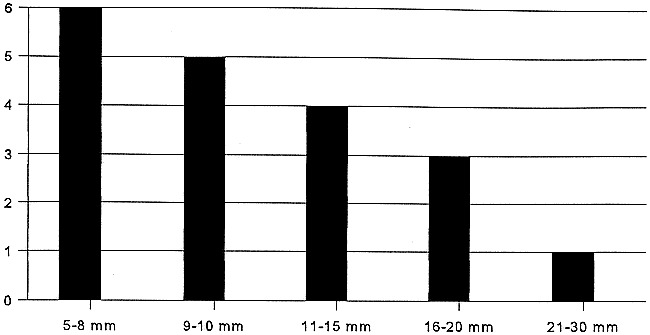
Size (minimal diameter) of the 19 metastases, as measured by sonography
Of these 14 patients with regional failure, 12 were treated with a neck dissection, and 10 of these also received postoperative radiotherapy. One patient with one 8-mm metastasis was treated only with curative-intent radiotherapy, and one patient with distant metastases was radiated for palliation only. In the 12 salvage-neck dissection specimens, there were 32 histologically proved positive lymph nodes found at levels 1 through 4 (Table 1). Of these 32 nodes, 13 showed extranodal tumor spread in nine patients.
Of the 14 patients who developed neck failure, five patients died (Table 1). Four patients died of their cancer, whereas one patient died after 5 months of another cause and was cancer-free. The disease-free survival in the 10 patients salvaged ranged from 5 to 48 months (mean and median, 26 months). The lymph node size in the patients who died of the regional failure was 14 mm or larger, whereas it was smaller in the group of 10 patients who were salvaged. In one patient (number 8), only 2 months after his transoral excision, a 30-mm submandibular node was palpated that was not seen on the previous sonographically guided needle cytology. In patient 3, two follow-up sonographically guided examinations were performed, and 18 weeks after the last negative cytology, a metastasis at level 3 was detected by palpation and measured at 14 mm by sonography. Although there was extranodal spread, this patient refused postoperative radiotherapy and died. In case 9, only one, and in case 10, only two follow-up sonographically guided aspiration cytologies were performed at long intervals, and metastases were found by palpation.
Discussion
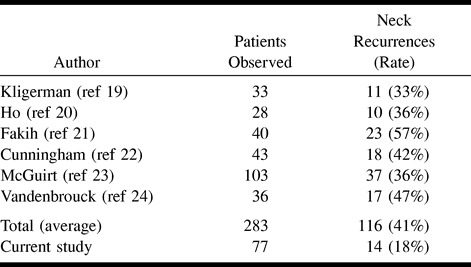
In a previous study, we found sonographically guided fine-needle aspiration cytology to be significantly more accurate than palpation, CT, or MR imaging for the assessment of the neck, and especially the N0 neck (6). Because of this, we now rely on this technique for the early detection and exclusion of lymph node metastases in patients treated with transoral excision. As occult metastases will inevitably develop into neck failures when not treated, the risk of occult metastases is studied best by looking at series that adhered to a “wait and see” strategy. Studies that looked at the incidence of occult metastases by examining the histopathologic neck-dissection specimen are hampered by the fact that routine histopathologic analysis overlooks between 5% and 10% of all metastases (18). The failure rate in the neck in this study was 18%, which compares favorably to the incidence of occult metastases quoted in the literature for patients treated with transoral tumor excision and a “wait and see” follow-up strategy for the neck. In most series on transoral tumor excision for T1–2 oral carcinomas that use palpation for staging and follow-up, the incidence of occult metastases varies and is between 24% and 57% (19–24). Although the differences are quite large, the mean percentage of neck node recurrences found in these series is 41% (Table 2). In a previous study, we found a histopathologic incidence of palpably occult metastases of 41% as well (6). If an estimate of occult metastases missed by palpation is 41% in this patient population, and those missed by sonographically guided fine-needle aspiration cytology is 18%, the sensitivity of the sonographically obtained cytology in this study can be calculated to be 68%. Calculation for 100 patients is: (False Negatives) ÷ (100 − 41+ False Negatives) = 18%; False negatives = 13; Sensitivity = (41−13) ÷ 41 = 68%. This figure of 68% for the palpably N0 neck is in the same range as that found in our earlier studies (6, 15) and is somewhat higher than the sensitivity of 48% found by Takes (16) or of 50% found by Righi (9).
TABLE 2:
Summary of results in some comparable series of patients treated with transoral excision of T1-2 tumors and clinically N0 necks in whom a ``wait and see'' management strategy for the neck was conducted
This study also shows that sonographically guided fine-needle aspiration cytology often ensures detection of neck recurrences at an early stage. Six out of the 14 neck recurrences were detected before being palpable, and most of these nodal metastases were smaller than 1 cm (Table 1, Fig 1). Furthermore, almost all regional failures were detected in the first year (Fig 2). Unfortunately, sonographically guided fine-needle aspiration cytology did not enable detection of all these early-stage recurrences and in some patients, even within a couple of months after a previous negative examination, a large nodal metastases was present. Although it will never be possible to detect all early-stage neck failures, in three out of the four patients who died of their disease, a too-long interval between the follow-up sonographic examinations might have contributed to the spread of the disease. This, once again, emphasizes the need for regular follow-up sonographically guided aspiration cytology in the first year. It also is clear from the literature that most neck failures occur within the first 12 months of initial treatment. In the series of Fakih (21), 22 of 23 neck recurrences were found within the first year and Vandenbrouck (24) also diagnosed 16 of the 17 neck recurrences in the first 18 months. Probably because of this early detection of neck failures, we were able to salvage 10 (71%) of these 14 patients. This figure compares favorably to reported salvage rates, which vary from 27% to 82% (average 50%) after a regional recurrence (19–24). Nevertheless, our follow-up is still limited. As expected, the most important differences in patients salvaged from those who died of their disease were the size and number of metastases (25, 26). The four patients who died had three or more metastases and had nodes of 14 mm or larger. In three of these four patients, distant metastases already were present. In some of these patients, the interval between the follow-up sonographically guided cytology was quite long, whereas a submandibular metastases was missed in one. It has been reported previously that sonography is more difficult to perform at level 1 nodes, probably because of the mandible and sometimes the distinction between lymph nodes and the submandibular gland (1, 16). For this area, palpation seems crucial during follow-up, although awareness and special attention of the sonographer might improve the results. It needs to be studied further to determine if CT or MR imaging can play a more prominent role at this level.
fig 2.
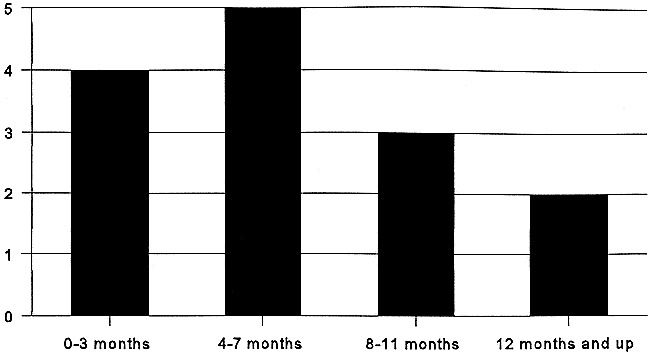
Delay between excision of the primary tumor and neck failure in 14 patients
In this study, 14 (18%) of 77 patients had false-negative cytology results. Reasons for these false negatives can be multiple. The metastasis can be too small and missed by the needle, or a single tumor cell can be overlooked by the cytopathologist (18). It is also possible that the wrong lymph node is aspirated. Although no definitive clues can be given, the fact that the correct level initially was aspirated in 11 of the 14 patients suggests that either the metastases inside the aspirated lymph nodes were too small initially, or a wrong node in the correct level was aspirated. We recently started to test whether or not a combination of the sentinel-node procedure (27), using both sonographically and scintigraphically guided aspiration, can improve the detection and aspiration of the lymph node at most risk to harbor metastasis.
In conclusion, sonographically guided fine-needle aspiration cytology reduced the incidence of occult metastases to 18%. Furthermore, in this study, sonographically guided cytology contributed to the early detection and possibly the salvage rate of neck failures. Although the optimal interval between the follow-up sonographically guided cytologies cannot be deducted from this study, the data are suggestive that this interval should be in the range of 2–3 months the first year after excision of primary neck tumor.
Footnotes
Address reprint requests to Michiel W.M. van den Brekel, MD, PhD, Free University Hospital, Boelelaan 1117, 1081 HV Amsterdam, The Netherlands.
References
- 1.van den Brekel MWM, Castelijns JA, Snow GB. The size of lymph nodes in the neck on sonograms as a radiologic criterion for metastasis: how reliable is it? AJNR Am J Neuroradiol 1998;19:695-700 [PMC free article] [PubMed] [Google Scholar]
- 2.Weiss MH, Harrison LB, Isaacs RS. Use of decision analysis in planning a management strategy for the stage N0 neck. Arch Otolaryngol Head Neck Surg 1994;120:699-702 [DOI] [PubMed] [Google Scholar]
- 3.Baatenburg de Jong RJ, Knegt P, Verwoerd CDA. Reduction of the number of neck treatments in patients with head and neck cancer. Cancer 1993;71:2312-2318 [DOI] [PubMed] [Google Scholar]
- 4.Stern WBR, Silver CE, Zeifer BA, Persky MS, Heller KS. Computed tomography of the clinically negative neck. Head Neck 1990;12:109-113 [DOI] [PubMed] [Google Scholar]
- 5.Friedman M, Mafee MF, Pacella BL Jr. Strorigl TL, Dew LL, Toriumi DM. Rationale for elective neck dissection in 1990. Laryngoscope 1990;100:54-59 [DOI] [PubMed] [Google Scholar]
- 6.van den Brekel MWM, Castelijns JA, Stel HV, Golding RP, Meyer CJLM, Snow GB. Modern imaging techniques and ultrasound-guided aspiration cytology for the assessment of neck node metastases: a prospective comparative study. Eur Arch Otorhinolaryngol 1993;250:11-17 [DOI] [PubMed] [Google Scholar]
- 7.Hillsamer PJ, Schuller DE, McGhee RB, Chakeres D, Young DC. Improving diagnostic accuracy of cervical metastases with computed tomography and magnetic resonance imaging. Arch Otolaryngol Head Neck Surg 1990;116:1297-1301 [DOI] [PubMed] [Google Scholar]
- 8.John DG, Anaes FC, Williams SR, et al. Palpation compared with ultrasound in the assessment of malignant cervical lymph nodes. J Laryngol Otol 1993;107:821-823 [DOI] [PubMed] [Google Scholar]
- 9.Righi PD, Kopecky KK, Caldemeyer KS, Ball VA, Weisberger EC, Radpour S. Comparison of ultrasound fine needle aspiration and computed tomography in patients undergoing elective neck dissection. Head Neck 1997;19:604-610 [DOI] [PubMed] [Google Scholar]
- 10.Yucel T, Saatci I, Sennaroglu L, Cekirge S, Aydingoz U, Kaya S. MR imaging in squamous cell carcinoma of the head and neck with no palpable lymph nodes. Acta Radiol 1997;38:810-814 [DOI] [PubMed] [Google Scholar]
- 11.Curtin HD, Ishwaran H, Mancuso AA, Dalley BW, Caudry DJ, McNeil BJ. Comparison of CT and MR imaging in staging of neck metastases. Radiology 1998;207:123-130 [DOI] [PubMed] [Google Scholar]
- 12.Mc Guirt WF, Greven K, Williams 3rd DW , et al. PET scanning in head and neck oncology: a review. Head Neck 1998;20:208-215 [DOI] [PubMed] [Google Scholar]
- 13.Myers LL, Wax MK, Nabi H, Simpson GT, Lamonica D. Positron emission tomography in the evaluation of the N0 neck. Laryngoscope 1998;108:232-236 [DOI] [PubMed] [Google Scholar]
- 14.Braams JW, Pruim J, Nikkels PG, Roodenburg JL, Vaalburg W, Vermey A. Nodal pread of squamous cell carcinoma of the oral cavity detected with PET-tyrosine, MRI and CT. J Nucl Med 1996;37:897-901 [PubMed] [Google Scholar]
- 15.van den Brekel MWM, Castelijns JA, Stel HV, et al. Occult metastatic neck disease: detection with US and US-guided fine-needle aspiration cytology. Radiology 1991;180:457-461 [DOI] [PubMed] [Google Scholar]
- 16.Takes RP, Righi P, Meeuwis CA, et al. The value of ultrasound guided fine needle aspiration biopsy compared to computed tomography in the detection of regional metastases in the clinically negative neck. Int J Radiat Oncol Biol Phys 1998;40:1027-1032 [DOI] [PubMed] [Google Scholar]
- 17.van den Brekel MWM, Stel HV, Castelijns JA, et al. Cervical lymph node metastasis: Assessment of radiologic criteria. Radiology 1990;177:379-384 [DOI] [PubMed] [Google Scholar]
- 18.van den Brekel MWM, van der Waal I, Meyer CJLM, Freeman JL, Castelijns JA, Snow GB. The incidence of micrometastases in neck dissection specimens obtained from elective neck dissections. Laryngoscope 1996;106:987-991 [DOI] [PubMed] [Google Scholar]
- 19.Kligerman J, Lima RA, Soares JR, et al. Supraomohyoid neck dissection in the treatment of T1/T2 squamous cell carcinoma of oral cavity. Am J Surg 1994;168:391-394 [DOI] [PubMed] [Google Scholar]
- 20.Ho CM, Lam KH, Wei WI, Lau SK, Lam LK. Occult lymph node metastasis in small oral tongue cancers. Head Neck 1992;14:359-363 [DOI] [PubMed] [Google Scholar]
- 21.Fakih AR, Rao RS, Patel AR. Prophylactic neck dissection in squamous cell carcinoma of oral tongue: a prospective randomized study. Semin Surg Oncol 1989;5:327-330 [DOI] [PubMed] [Google Scholar]
- 22.Cunningham MJ, Johnson JT, Myers EN, Schramm VL, Thearle PB. Cervical lymph node metastasis after local excision of early squamous cell carcinoma of the oral cavity. Am J Surg 1986;152:361-366 [DOI] [PubMed] [Google Scholar]
- 23.Mc Guirt WF, Jr. Johnson JT, Myers EN, Rothfield R, Wagner R. Floor of mouth carcinoma. The management of the clinically negative neck. Arch Otolaryngol Head Neck Surg 1995;121:278-282 [DOI] [PubMed] [Google Scholar]
- 24.Vanden Brouck C, Sancho-Garnier H, Chassagne D, Saravane D, Cachin Y, Micheau C. Elective versus therapeutic radical neck dissection in epidermoid carcinoma of the oral cavity: results of a randomized clinical trial. Cancer 1980;46:386-390 [DOI] [PubMed] [Google Scholar]
- 25.Spiro RH, Alfonso AE, Farr HW, Strong EW. Cervical node metastasis from epidermoid carcinoma of the oral cavity and oropharynx; A critical assessment of current staging. Am J Surg 1974;128:562-567 [DOI] [PubMed] [Google Scholar]
- 26.Leemans CR, Tiwari RM, Nauta JJP, Waal van der I, Snow GB. Regional lymph node involvement and its significance in the development of distant metastases in head and neck carcinoma. Cancer 1993;71:452-456 [DOI] [PubMed] [Google Scholar]
- 27.Koch WM, Choti MA, Civelek AC, Eisele DW, Saunders JR. Gamma probe-directed biopsy of the sentinel node in oral squamous cell carcinoma. Arch Otolaryngol Head Neck Surg 1998;124:455-459 [DOI] [PubMed] [Google Scholar]


