Abstract
BACKGROUND AND PURPOSE: To our knowledge, recurrent carotid blowout syndrome (rCBS) has not been well described. Our purpose was to review our institution's recent experience with patients who presented with multiple episodes of carotid blowout syndrome (CBS), and who were referred for emergent diagnostic angiography and endovascular therapy.
METHODS: We retrospectively reviewed the last 46 consecutive patients who had a clinical diagnosis of CBS. All patients were examined and treated prospectively according to a standardized protocol. Most patients (43 of 46) had undergone extensive primary and salvage radical surgery with intraoperative brachytherapy or external beam radiation or both. The remaining three patients had either traumatic or iatrogenic CBS.
RESULTS: Twelve patients (26%) in our series had more than one episode of CBS in which a total of 32 (20 recurrent) events were observed (average 2.7, range 2–4). Intervals of rCBS ranged from 1 day to 6 years. Thirteen (65%) of 20 recurrent events were attributed to progressive disease (PD), and seven (35%) of 20 to treatment failures (TFs). In the PD group, seven (54%) of 13 had recurrent ipsilateral disease, and six (46%) of 13 had recurrent contralateral disease. Etiologies of rCBS were as follows: seven exposed carotids; seven carotid pseudoaneurysms; eight small-branch pseudoaneurysms; five tumor hemorrhages; three hyperemic/ulcerated wounds; and one aortic arch rupture. Twenty-seven of 32 events were treated with endovascular therapy, which included the following: nine carotid occlusions; 11 small-branch embolizations; three transarterial tumor embolizations; one carotid stent; and two direct-puncture embolizations. Four of six TFs were retreated successfully with endovascular therapy; the remaining two TFs were managed successfully by surgery. In the PD group, hemorrhagic complications of rCBS were managed successfully in all but one patient, who died. No permanent neurologic or ophthalmologic complications occurred.
CONCLUSION: Recurrent CBS is a frequently encountered problem in which most cases are caused by PD resulting from both multifocal iatrogenic arteriopathy and occasional wound complications that are characteristic of aggressively managed head and neck surgical patients. Initial TFs are encountered often as well. Despite the diagnostic and therapeutic challenges of rCBS, most cases can be retreated effectively.
Rupture of the carotid artery, or “carotid blowout,” may occur in a variety of clinical scenarios, although most commonly it is an increasingly recognized complication of aggressive primary and salvage surgery for squamous cell carcinoma of the head and neck (1–3). Since rupture of the carotid artery first was described in the 1960s (4, 5), most reports have shown relatively high mortality (40%) and neurologic morbidity (60%) associated with this complication (4–10).
In reviewing the literature and our recent cumulative experience, we have shown that the various scenarios of carotid rupture manifest in a well-recognizable pattern of clinical presentations, termed the carotid blowout syndrome (CBS) (1, 2). Furthermore, our work and that of others have shown that CBS can be managed effectively by a well-coordinated, multidisciplinary protocol centered on endovascular therapy (1–3, 11, 12).
With our increasing experience in the management of CBS, we have observed that several previously treated patients develop recurrent episodes of CBS (rCBS). These patients typically are considered for additional endovascular or surgical management or both, presenting further challenges in diagnosis and delivery of optimal care. To our knowledge, rCBS has not been well described; therefore, we review our institution's cumulative experience over the last 4 years for the purpose of characterizing this subgroup of patients and evaluating therapeutic efficacy.
Methods
In 1993, our institution developed a standardized protocol for evaluating and treating CBS (1, 2). Carotid blowout syndrome is operationally defined as either an episode of acute hemorrhage (usually transoral or transcervical) or an exposure of a portion of the carotid arterial system (usually from wound dehiscence or devitalized musculocutaneous flap). These complications occur in the setting of either aggressive surgical management of cervical carcinoma (usually squamous cell carcinoma) or penetrating trauma to the neck.
In our experience, the clinical spectrum of CBS can be subclassified into three groups of affected patients, which are equivalent to many previously described variations of carotid rupture (1, 2, 7, 13, 14). These subtypes of CBS previously have been defined in detail (1, 2) as Group 1 (threatened carotid blowout), Group 2 (sentinel hemorrhage/impending carotid blowout), and Group 3 (acute carotid blowout). Briefly, the important features of each group are as follows. Group 1 patients have a visibly exposed segment of the carotid artery that invariably will rupture if not promptly covered with healthy, well-vascularized tissue. Group 2 patients present with a short-lived acute hemorrhage that resolves either spontaneously or with simple surgical packing. Group 3 patients present with an acute, profuse hemorrhage that is not self-limiting and is not well-controlled with surgical packing, invariably owing to complete rupture of the affected artery.
Between 1993–1997, 46 consecutive patients with a clinical diagnosis of CBS were referred for a total of 62 events to our interventional neuroradiology service for diagnosis and treatment. Forty-three of these patients had undergone extensive primary or salvage radical resections or both. Recurrent CBS was defined as either a repeat episode of self-limited or uncontrollable bleeding (Groups 2 and 3) that occurred within 12 hours of completed therapy for a previous episode of CBS, or a newly exposed portion of the carotid system (Group 1) that occurred any time after completed therapy for a previous episode of CBS.
According to protocol, all patients of Groups 2 and 3 were studied emergently by carotid angiography after being resuscitated aggressively in the surgical intensive care unit with crystalloid, blood products, and vasopressors, as needed. All neuroangiographic and neurointerventional procedures were completed under intensive monitoring with the support of an anesthesiology team.
Standard neuroangiographic techniques were used in which high-resolution (1024 × 1024 matrix) digital subtraction imaging of the cervical and intracranial carotid circulation was obtained bilaterally. Meticulous attention to possible sites of bleeding, such as pseudoaneurysm formation, active extravasation, and endoluminal irregularity, was directed at the common carotid artery (CCA), external carotid artery (ECA) and its branches, and the cervical internal carotid artery (ICA). If bleeding occurred within the lower neck, bilateral selective arteriography of the subclavian, costocervical, and thyrocervical arteries also was performed. Angiographic evaluation of the circle of Willis also was performed routinely.
Endovascular therapeutic intervention was performed only on the basis of angiographic findings that would be consistent with hemorrhagic complications resulting in either Group 2 or 3 CBS (2). Most commonly, these findings were either pseudoaneurysms of the carotid arterial system or tumor neovascularity (2). Patients with Group 1 CBS were scheduled electively for diagnostic angiography and balloon test occlusion (BTO) of the affected carotid system. In both emergent and elective settings, if PBO of the ICA was anticipated, a BTO was attempted, if the patient was clinically stable, by using a previously described standardized protocol (1, 15).
All patients managed by endovascular techniques were monitored closely in the neurointensive care unit. If therapeutic occlusion of the ICA was performed, the patient was anticoagulated with intravenous heparin (target partial thromboplastin time, 55–60 seconds) for 48 hours, and hydrated under close hemodynamic and neurologic monitoring. After discontinuing heparin, the patient subsequently was placed on daily aspirin therapy (325 mg q.i.d.).
Results
Of the 46 consecutive patients with CBS referred for evaluation and treatment to our interventional neuroradiology service, 12 (26%) fulfilled the definition of rCBS. A total of 32 events prompting diagnostic or therapeutic neuroangiographic procedures or both were encountered in the rCBS group, of which 20 were recurrent. Therefore, the average number of episodes of CBS in this group was 2.7. Considerable variability in intervals between episodes of rCBS was observed, ranging from 1 day to 6 years. A summary of the pertinent clinical data is provided in Table 1. Patients who presented with rCBS (including the index event) were subclassified as follows: Group 1, eight events in five patients; Group 2, 15 events in nine patients; and Group 3, nine events in seven patients.
TABLE: Summary of clinical data
In reviewing the patterns of recurrence in our patient population, two readily discernible categories could be identified based upon the actual or presumptive pathogenetic mechanisms of rCBS. The first category could be classified broadly as progressive disease (PD). All patients within this group developed independent episodes of either potential or actual hemorrhagic recurrences. These could be attributed to one or more of the following putative etiologies: 1) surgical wound dehiscence; 2) free pedicle or mobilized musculocutaneous flap necrosis; 3) iatrogenic mechanical vascular injury; 4) radiation-induced arteriopathy; 5) tumor invasion of a major arterial segment; and 6) recurrent tumor growth and invasion into adjacent mucosal surfaces. Recurrent CBS attributable to PD could be classified further into bleeding from vasculature that was either ipsilateral or contralateral to the index event (ie, first episode of CBS). This subclassification was useful in assessing the potential longitudinal complications and risks that may be encountered with subsequent events of rCBS.
The second subtype of cases could be designated as treatment failures (TFs). This group of patients was defined by the development of rCBS attributable to repetitive bleeding occurring in the same arterial segment or territory that previously had been treated by either endovascular or open surgery. This included recurrent bleeding from either a previously treated arterial pseudoaneurysm or tumor neovasculature. The period between recurrent events attributable to TFs was relatively short, varying between 1–10 days.
Based on the previously outlined definitions, we found that 13 (65%) of 20 recurrent events were attributable to PD. In the PD group, seven (54%) of 13 had recurrent ipsilateral disease and six (46%) of 13 had recurrent contralateral disease. The remaining seven (32%) of 20 recurrent events could be attributable to TFs.
A variety of angiographic-anatomic etiologies of rCBS were observed. This included seven exposed carotids (ie, Group 1 patients with threatened CBS), seven large-vessel pseudoaneurysms of the carotid system (ie, ICA, CCA, or innominate artery), five small arterial branch pseudoaneurysms of the ECA, three small arterial branch pseudoaneurysms of the brachiocervical arterial branches (eg, thyrocervical trunk, costocervical trunk, internal mammary), six tumor hemorrhages, and two unknown causes.
Twenty-seven of the 32 events occurring in the rCBS group were treated with endovascular therapy. The following categories of endovascular techniques were used: nine “composite” or internal carotid parent artery occlusions, 11 small-branch therapeutic occlusions, four tumor embolizations, one carotid stent, and two direct-puncture embolizations.
A variety of embolic devices were used to treat patients with rCBS. The selection of a device depended upon the specific pathoetiologic lesion identified by angiography and the prevailing cerebrovascular hemodynamics as determined by cerebral angiography and BTO. Detachable latex balloons (Goldvalve, Ingenor, France) mounted on uncoated microcatheters (Tracker-18 HiFlo Unibody, Target Therapeutics/Boston Scientific, Freemont, CA) frequently were used for therapeutic PBO of the larger segments of the carotid system harboring pseudoaneurysms, as previously reported (2). Furthermore, the previously described “composite” therapeutic occlusion of the carotid system (2), which typically uses detachable balloons and nonretrievable platinum-fibered microcoils (Tornado, Cook, Indianapolis, IN), usually was performed when lesions were found near the origin of the ICA, the CCA, or in multiple sites, and in all Group 1 patients. In cases where therapeutic occlusion of the ICA was planned, a distal occluding balloon was positioned within the petrous portion of the ICA, whenever possible, to minimize risk of postocclusive thromboembolism (2).
For cases requiring smaller vessel occlusion, such as pseudoaneurysms or injuries to the ECA and brachiocervical branches, both nonretrievable (Tornado; Vortex, Target Therapeutics/Boston Scientific, Freemont, CA) and retrievable (GDC, Target Therapeutics/Boston Scientific, Freemont, CA) platinum microcoils, polyvinyl alcohol (PVA) particles (Contour, ITC/Target Therapeutics/Boston Scientific), or cyanoacrylate embolic mixtures (Histoacryl, Braun, Germany) were used for embolic materials.
For patients with CBS from tumor hemorrhages, tumor devascularization was achieved with either conventional transarterial embolization using PVA, or direct-puncture tumor embolization with absolute alcohol or cyanoacrylate embolic mixtures. In one patient (CH), a novel method of large-artery pseudoaneurysm obliteration was performed using temporary balloon occlusion and direct-puncture acrylic embolization, which previously has been described (12).
The following shows the overall clinical outcomes. Within the group of patients with rCBS, 11 (92%) of 12 patients survived their multiple episodes of CBS through successful therapeutic intervention by either endovascular or open surgical approaches, or both. Ten (91%) of 11 patients had their rCBS ultimately successfully managed by endovascular therapy, and one patient required an additional surgical intervention after endovascular therapy (ie, craniotomy for distal trapping of the cavernous segment of the ICA). The one “attributable” fatality (ie, death directly the result of rCBS or a complication of its management) within this series was in a patient (DR) who developed multiple episodes of rCBS during a single hospitalization that ultimately led to septic mediastinitis and rupture of the aortic arch. Attempted surgical repair of this rupture was unsuccessful.
No fatalities were attributable to complications from therapeutic intervention. Also, no major neurologic morbidity was associated with endovascular therapy (0%), although two (17%) of 12 patients suffered minor neurologic morbidity attributable to therapy. This consisted of one patient (CH) who developed a completely reversible left hemiparesis within 24 hours after undergoing multiple periods of intermittent temporary balloon occlusion to control an acute carotid blowout (Group 3). This patient's large CCA pseudoaneurysm eventually was treated definitively by a direct-puncture cyanoacrylate injection (12). The other patient (EV) developed a completely asymptomatic, small watershed infarct (documented on MR imaging) of the left frontal lobe after serially undergoing combined endovascular and surgical trapping of an iatrogenic ICA pseudoaneurysm. There also were no ophthalmic ischemic complications.
One technical complication occurred within the current series (case # PD2), consisting of an inadvertent migration of a detached latex balloon that originally was deployed within the innominate artery. This inflated balloon migrated into the right common iliac artery, but successfully was retrieved with an endovascular forceps device without sequelae.
Despite undergoing successful endovascular therapeutic management of rCBS, one patient (FH) died prior to discharge owing to septic complications and respiratory failure related to surgical repair of a pharyngotracheal fistula. Another patient (WF) developed a neurologic deficit (mild left upper extremity weakness) because of a thromboembolic stroke from spontaneous occlusion of the right CCA. This spontaneous occlusion was believed to be the result of radiation-induced arteriopathy. Therefore, the rates of fatality and neurologic morbidity for patients with rCBS that was “nonattributable” to endovascular therapy were 8% each.
Discussion
The continuing advances and refinements in endovascular therapy of the cerebrovascular system are resulting in an ever-expanding role for this therapeutic technique to manage patients with various intracranial and extracranial vascular diseases. Arterial injuries resulting from aggressive treatment of head and neck cancer, and occasionally from penetrating injuries, which manifest as CBS, appear well-suited to endovascular therapeutic intervention. The specific role of endovascular therapy, relating to indications and appropriate selection of techniques and technology, is beginning to be better defined, though it continues to evolve (2, 12).
In the past, emergent operative ligation of the CCA or ICA typically was used to treat CBS (4–10, 16–18). This approach, however, usually was associated with high rates of major morbidity and death (average mortality of 40%, and average major neurologic morbidity of 60%) (1, 2, 6–9, 16–18). A few anecdotal reports of the use of exclusively endovascular detachable balloon techniques for the treatment of hemorrhagic complications associated with iatrogenic injury to the carotid system have shown generally good results related to feasibility and short-term efficacy within a very small number of patients (13, 19, 20).
Recently, our group's results of endovascularly managed CBS in a larger case series (1, 2) and those of Morrissey et al (3) have demonstrated excellent short-term efficacy in arresting or preventing hemorrhagic complications. These reports shared substantially reduced neurologic morbidity and mortality when compared with previously published surgical series. In these endovascular series, targeted therapeutic embolization techniques were used that were directed by specific pathologic-anatomic data obtained from meticulous angiography, and frequently combined with provocative testing in cases of planned large parent artery occlusion of the CCA or ICA.
Although endovascular therapeutic management of CBS has been shown to be largely successful, potential risks and limitations remain. A variety of technical and disease-related complications may occur, including intraoperative thromboembolic or foreign-body embolic stroke, reflex bradycardia and hypotension (from stimulation of the carotid body), catastrophic rehemorrhage (from rupture of a pseudoaneurysm or arterial perforation), and delayed cerebral ischemia after therapeutic ICA occlusion (which can occur from either hemodynamic insufficiency or thromboembotic phenomena) (2). Many strategies have been adopted to minimize these risks, which previously have been discussed in detail (2). Nonetheless, a formally unrecognized, additional limitation of endovascular therapeutic management of CBS would appear to include the risk of recurrent episodes of acute or threatened hemorrhages.
From our evolving cumulative experience with the management of CBS, we have encountered a surprisingly high proportion of patients (26%) who develop recurrent events that can be attributed to a variety of causes. To our knowledge, a detailed examination of the relevant clinical-pathologic parameters of this subpopulation of patients, which we have designated “rCBS,” has not been reported. Both our group (1, 2) and Morrissey et al (3) previously have reported very limited numbers of documented recurrent acute or threatened hemorrhages in patients presenting with CBS (2, 3, 12). Specific identification and analysis of these cases as a distinct clinical entity were not provided.
In reviewing the current clinical series, it appears that two distinct types of rCBS may be encountered that are related to presumptive etiologic mechanisms of this syndrome. The first category, which we have defined as recurrences that develop as a consequence of PD, was most frequently encountered (65% of cases). All patients within this group developed independent episodes of either potential or actual hemorrhagic recurrences that could be attributed to numerous putative etiologic mechanisms of CBS (2, 12). These mechanisms include the following: radiation injury resulting in medial necrosis (from both external beam and brachytherapy); inadvertent arterial wall injury and weakening (eg, adventitial and outer media) from multiple surgical dissections; carotid exposure owing to musculocutaneous flap necrosis; wound infections; chronic inflammatory infiltrate; pharyngocutaneous fistulae; lack of surrounding supporting tissues; and recurrent/persistent carcinoma (5–7, 10, 12, 16–18, 21–23). Despite our short-term ability to arrest or prevent hemorrhagic complications in patients presenting with rCBS, the various etiologic substrates that originally may have been responsible for the index event often persist chronically in this patient population. This persistence of etiologic factors, which frequently occur bilaterally, would explain the frequent occurrence of rCBS attributable to both ipsilateral and contralateral PD.
Within the PD group of patients, nearly half had an episode of rCBS attributable to disease occurring contralaterally to the index event. This surprisingly high rate of contralateral events potentially can create significant longitudinal complications when rCBS develops from a pseudoaneurysm of either the CCA or ICA in a patient who previously was treated with endovascular therapeutic occlusion of the contralateral ICA. In such cases, therapeutic occlusion of the remaining ICA is likely to result in catastrophic cerebral ischemia.
The second category of rCBS encountered in our series comprised cases of recurrent hemorrhagic complication that could be directly attributable to the failure of prior therapy (ie, TF). This group of patients developed recurrent events that were directly attributable to repetitive bleeding occurring in the same previously treated arterial segment or territory. Unlike the PD group, the intervals between events for rCBS attributable to TF were usually fairly short (range 1–10 days). Several specific limitations of management of patients with CBS may explain the associated high incidence of rCBS attributable to TF observed in our patient population. As observed in our series, occasional technical failures (eg, inability to cross the ostium of a pseudoaneurysm for endovascular trapping) or device failures (eg, premature balloon deflation) may occur. More commonly, endovascular TF may be the result of recurrent tumor hemorrhages that previously were treated by partial transarterial embolization with particulates. This technical approach to tumor devascularization has a higher probability of short-term failure owing to the frequent inability to achieve ideal superselective microcatheterization of all arterial pedicles supplying the tumor (24). As illustrated in one of our cases, this limitation can be overcome by the use of direct-puncture embolization techniques, using either cyanoacrylate or ethanol (24). Finally, the surgical TF in our series were most commonly the result of wound dehiscence and flap failures, resulting in Group 1 CBS (threatened). These types of surgical complications were not unexpected considering the extensive, often multiple, prior surgeries and frequent use of some form of radiation therapy that these patients receive. Consequently, the ability of the resection margins to accept an interposed graft often is impaired severely because of poor perfusion, oxygenation, and other cellular and molecular derangements (25, 26).
The apparent high incidence of rCBS observed in our patient population raises some important issues regarding assumptions of durability and optimalization of endovascular therapeutic management. Based upon our original experience, we had recommended that pseudoaneurysms involving the ICA or CCA were approached best by parent artery occlusion techniques (typically using detachable balloons and embolic coils). Although “composite” therapeutic occlusion in such situations theoretically will protect against ipsilateral rCBS involving the major segments of the carotid system (ie, CCA, ICA, and proximal ECA), no protection from contralateral PD is provided by such an intervention. Furthermore, prior therapeutic occlusion of the ICA may create significant difficulties in the future management of rCBS because of a pseudoaneurysm of the contralateral CCA or ICA. In such cases, therapeutic occlusion of the remaining ICA generally would not be possible without significant risk of devastating neurologic morbidity.
Because it is not infrequent that parent artery sacrifice of either the ICA or CCA is a primary consideration for the management of CBS (1, 2), we continue to make every attempt to perform a conventional BTO of the ICA (ie, without blood flow measurements) in patients presenting with rCBS who may require therapeutic occlusion of the carotid system for effective short- and long-term management. Although not perfect, this provocative test has proved useful in detecting most patients who will not tolerate permanent occlusion of the ICA (24, 27, 28). It is fully acknowledged that this provocative test will not enable the interventional surgeon to recognize a small subset of patients who may develop delayed hemodynamic ischemia after therapeutic occlusion of the ICA because of collateral reserve failure.
Nonetheless, it is highly questionable that additional provocative adjuncts (ie, hypotensive challenge and various methods of measuring regional CBF) are of any significant benefit in this patient population (29), because many of our patients present emergently for control of life-threatening hemorrhage, often are relatively unstable for additional provocative testing, and usually are not candidates for extracranial to intracranial bypass (1, 2).
In contrast to our previous reports (1, 2), we are encountering more frequently situations in which patients may not be candidates for conventional therapeutic ICA occlusion because of the following reasons: 1) failure to tolerate BTO; 2) spontaneous occlusion of the contralateral ICA/CCA secondary to concurrent occlusive arteriopathy; and 3) prior contralateral therapeutic ICA occlusion in rCBS. As previously noted, the usual option of extracranial to intracranial bypass in such individuals usually is not possible because of the combination of technical limitations in performing this surgery in patients with prior radical neck surgery and irradiation, and the frequent urgency of presentation (2). We encountered one such patient in our series of rCBS (CH), in whom we initially attempted to treat a large pseudoaneurysm by endoluminal exclusion by using overlapping self-expanding stents. Although this initially was successful in arresting an episode of acute CBS (Group 3), the patient rebled 24 hours later. This prompted us to attempt an unconventional heroic intervention (ie, direct-puncture acrylic embolization), which was successful. Such a technical approach, however, has potential limitations and risks that make it unsuitable for a routine primary therapeutic approach (12).
In lieu of the potential limitations of therapeutic ICA occlusion in this patient population, there is an impetus to consider alternative parent-artery preservation techniques. As illustrated in one of our patients, a promising possibility is the use of either “uncovered” or “covered” intravascular stents, which increasingly are being used for a variety of diseases that affect the extracranial carotid system (12, 30–34). The rationale for endovascular stenting techniques specifically applied to the treatment of aneurysms originally derived from animal studies of experimentally constructed side-wall aneurysms (35–39). These studies showed that the alterations in flow dynamics within aneurysms after stent placement could result in eventual thrombosis of an aneurysm with neointimalization of the aneurysm ostium. Nevertheless, the time required for aneurysm obliteration can be substantial when using uncovered stents. This prompted some investigators to advocate the conjoint use of thrombogenic coils placed within the aneurysm by navigating a microcatheter through the stent mesh to promote rapid thrombotic occlusion (38, 39). This strategy has been used successfully in a limited number of clinical cases (40–42), although its application in the treatment of large pseudoaneurysms is questionable (12).
Covered stents, using either native venous grafts or synthetic materials (eg, PTFE) sewn into the inner portion of the metallic frame, have the advantage of being more likely to produce immediate exclusion of an aneurysm from the parent arterial blood flow. This effectively results in an endoluminal bypass of the aneurysm. The major disadvantages of these devices, however, currently are their relatively larger size (and therefore, requirements for larger delivery systems), requirements for surgical harvesting of a vein graft, and overall restricted availability. Despite these limitations, Parodi et al (34) successfully treated a large traumatic pseudoaneurysm of the CCA with placement of an endoluminal stent wrapped with a basilic vein graft.
Although it is now technically possible to approach many pseudoaneurysms of the ICA and CCA by endoluminal bypass by using a covered stent, our approach to CBS patients with this disease is only to consider those who are not good candidates for conventional ICA occlusion (ie, those patients who either fail BTO or have an existing contralateral ICA/CCA occlusion). We believe that a more liberal application of this parent-artery preservation technique may be premature, because results from extensive case series and from long-term follow-up of the use of covered stents placed within the carotid circulation are not available. With increasing clinical experience and improved technologic development of endoluminal bypass techniques, it is possible this approach will become more widely used and advocated (12).
Despite the diagnostic and therapeutic challenges encountered in our series of rCBS managed primarily by endovascular therapy, the observed clinical outcomes compare very favorably with our original series (2) and that of Morrissey et al (3). The multidisciplinary management of rCBS centered on endovascular therapy achieved a 92% success rate in ultimately controlling these recurrent hemorrhagic complications. This primary outcome endpoint was accomplished with an attributable mortality rate of 8% (due to a TF), and major neurologic morbidity rate of 0%, which continues to be better than most previously reported surgical series. An additional 17% incidence of minor or transient neurologic morbidity was encountered; in both cases, there was an excellent functional neurologic outcome (Glascow Outcome Score = 5).
We conclude that rCBS is a frequently encountered problem for patients who have been previously managed by endovascular or surgical intervention or both. Most cases appear to be due to disease progression related to multifocal iatrogenic arteriopathy and occasional wound complications that are characteristic of aggressively managed head and neck surgical patients. Nevertheless, short-term TFs frequently are encountered as well. Despite the diagnostic and therapeutic challenges of rCBS, most cases can be retreated effectively by endovascular techniques. As was the case in our original case series, a well-organized multidisciplinary protocol is an excellent management approach to this problem, with a high rate of both technical and clinical success and an acceptable rate of complication.
fig 1.
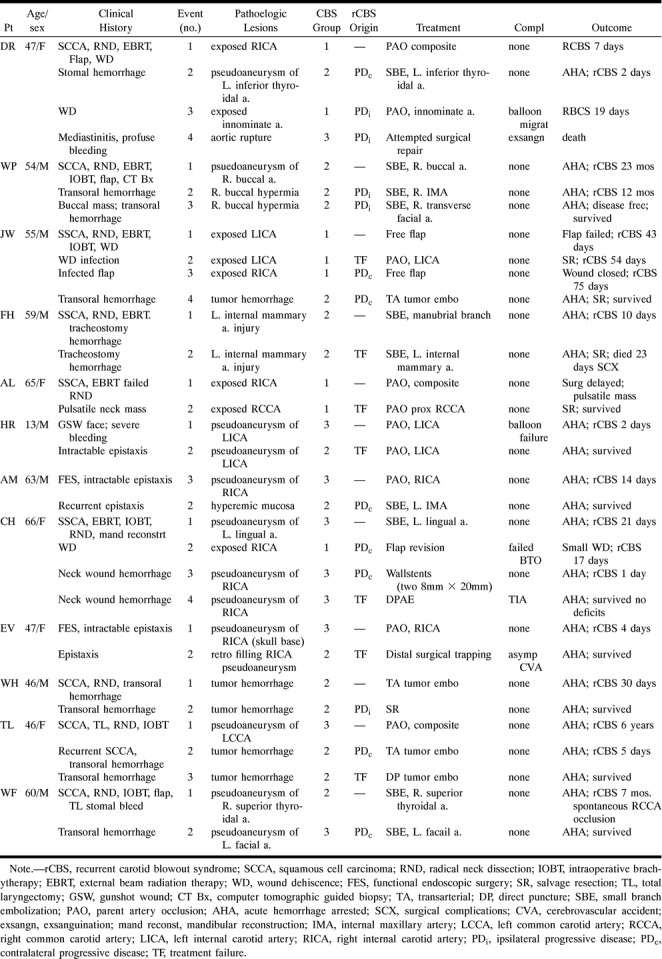
Illustrative case 1 of the spectrum of rCBS and its management.
A and B, Forty-six-year-old woman (patient TL) initially presents with CBS (Group 3). Lateral view from left common carotid injection (A) shows a pseudoaneurysm (arrow), which successfully was treated with therapeutic balloon occlusion (B).
C and D, Six years later, the patient develops a second episode of CBS. Lateral view from right external carotid injection (C) shows a hypervascular tumor of the oropharynx and hypopharynx (arrows) that is responsible for bleeding. Lateral view from superselective injection of the ascending palatine artery (D) shows significant supply to the tumor (arrows), which successfully was embolized with PVA.
E, Five days later, the patient develops a third episode of CBS, related to recurrent tumor hemorrhage. She was taken to the operating room in which, after induction of general anesthesia and oral retraction, the tumor was punctured under direct visualization and fluoroscopic guidance with a 23-gauge Chiba needle. Lateral fluoroscopic spot film shows the needle coursing through the tumor (long arrow) with extensive filling of neovasculature upon injection of absolute ethanol mixed with metrizamide (small arrows).
fig 2.
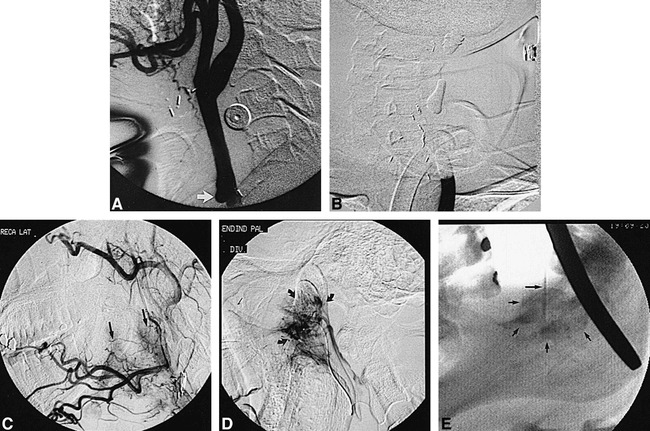
Illustrative case 2 of the spectrum of rCBS and its management.
A and B, Sixty-six-year-old woman (patient CH) initially presents with CBS (Group 3). Lateral views from superselective injection of a facial arterial branch (short arrow) in early (A) and late arterial phases (B) show a ruptured pseudoaneurysm with extensive extravasation (long arrows). This successfully was treated with coil embolization.
C, Twenty-one days later, the patient develops a second episode of CBS (Group 1) due to a flap dehiscence. Oblique view from right CCA injection shows no evidence of pseudoaneurysm and prior ligation of the ECA. The patient failed BTO at this time, prompting a flap revision.
D, Seventeen days later, the patient develops a third episode of CBS (Group 3). Oblique view from right CCA injection shows a large pseudoaneurysm of that vessel. Acute hemorrhage initially was arrested by placement of two overlapping 8 × 20-mm Wallstents across the rent of the artery (not shown).
E and F, One day later, the patient develops a fourth episode of CBS (Group 3) due to a TF of the previously deployed stents. After inflation of a balloon occlusion catheter across the carotid rent, the pseudoaneurysm was directly punctured and embolized with cyanoacrylate. Fluoroscopic spot film (E) and subtracted-control angiography (F) from right CCA injection (oblique view) shows complete obliteration of the pseudoaneurysm with cyanoacrylate (arrow) and patency of the parent artery.
Footnotes
This study was presented at the 36th Annual Meeting of the American Society of Neuroradiology, May 1998, Philadelphia, PA.
Address reprint requests to John C. Chaloupka, Interventional Neuroradiology Service, Department of Radiology; University of Iowa Hospitals and Clinics, 200 Hawkins Drive, JPP 3891, Iowa City, IA 52242.
References
- 1.Citardi MJ, Chaloupka JC, Son YH, Sasaki CT. Management of carotid artery rupture by monitored endovascular therapeutic occlusion (1988–1994). Laryngoscope 1995;1086-1092 [DOI] [PubMed]
- 2.Chaloupka JC, Putman CM, Citardi MJ, Ross DA, Sasaki CT. Endovascular therapy of carotid blowout syndrome in head and neck surgical patients: evolving diagnostic and management considerations. AJNR Am J Neuroradiol 1996;17:843-852 [PMC free article] [PubMed] [Google Scholar]
- 3.Morrissey DD, Andersen PE, Nesbit GM, Barnwell SL, Everts EC, Cohen JI. Endovascular management of hemorrhage in patients with head and neck cancer. Arch Otolaryngol Head Neck Surg 1997;123:15-19 [DOI] [PubMed] [Google Scholar]
- 4.Borsany SJ. Rupture of the carotids following radical neck surgery in irradiated patients. Ear Nose & Throat Monthly 1962;41:531-533 [PubMed] [Google Scholar]
- 5.Ketcham AS, Hoye RC. Spontaneous carotid artery hemorrhage after head and neck surgery. Am J Surg 1965;110:649-655 [DOI] [PubMed] [Google Scholar]
- 6.Shumrick DA. Carotid artery rupture. Laryngoscope 1973;83:1051-1061 [DOI] [PubMed] [Google Scholar]
- 7.Heller KS, Strong EW. Carotid artery hemorrhage after radical head and neck surgery. Am J Surg 1979;138:607-610 [DOI] [PubMed] [Google Scholar]
- 8.Razack MS, Sako K. Carotid artery hemorrhage and ligation in head and neck cancer. J Surg Oncology 1982;19:189-192 [DOI] [PubMed] [Google Scholar]
- 9.Porto DP, Adams GL, Foster C. Emergency management of carotid artery rupture. Am J Otolaryng 1986;7:213-217 [DOI] [PubMed] [Google Scholar]
- 10.Maran AGD, Amin M, Wilson JA. Radical neck dissection: a 19 year experience. J Laryngol Otol 1989;103:760-764 [DOI] [PubMed] [Google Scholar]
- 11.Walker AT, Chaloupka JC, Putman CM, Abrahams JJ, Ross DA. Sentinel transoral hemorrhage from a pseudoaneurysm of an internal maxillary artery branch; a complication of CT guided biopsy of the masticator space. AJNR Am J Neuroradiol 1996;17:377-381 [PMC free article] [PubMed] [Google Scholar]
- 12.Roth TC, Chaloupka JC, Putman CM, et al. Percutaneous direct puncture acrylic embolization of a pseudoaneurysm after failed carotid stenting for the treatment of acute carotid blowout. AJNR Am J Neuroradiol 1998;19:912-916 [PMC free article] [PubMed] [Google Scholar]
- 13.Sanders EM, Davis KR, Whelan CS, Deckers KR. Threatened carotid rupture: a complication of radical neck surgery. J Surg One 1986;33:190-193 [DOI] [PubMed] [Google Scholar]
- 14.Zimmerman MC, Mickel RA, Kessler DJ, et al. Treatment of impending carotid rupture with detachable balloon embolization. Arch Otolaryngol Head Neck Surg 1987;113:1169-1175 [DOI] [PubMed] [Google Scholar]
- 15.Chaloupka JC, Awad IA. Strategies and armamentarium of treatment options. In: Awad IA, Barrow DL. Giant Intracranial Aneurysms. Park Ridge, Ill: AANS Publications; 1995;91–116
- 16.Marchetta FC, Sako K, Maxwell W. Complications after radical head and neck surgery performed through previously radiated tissues. Am J Surg 1967;114:835-838 [DOI] [PubMed] [Google Scholar]
- 17.Stell PM. Catastrophic haemorrhage after major neck surgery. Br J Surg 1969;56:525-527 [DOI] [PubMed] [Google Scholar]
- 18.Coleman JJ. Treatment of the ruptured or exposed carotid artery: a rational approach. South Med J 1985;78:262-267 [DOI] [PubMed] [Google Scholar]
- 19.Osguthorpe JD, Hungerford GD. Transarterial carotid occlusion: case report and review of the literature. Arch Otolaryngol Head Neck Surg 1984;110:694-696 [DOI] [PubMed] [Google Scholar]
- 20.Khoo CTK, Molyneux AJ, Rayment R, Saad MN. The control of carotid arterial haemorrhage in head and neck surgery by balloon catheter tamponade and detachable balloon embolisation. Br J Plast Surg 1986;39:72-75 [DOI] [PubMed] [Google Scholar]
- 21.Swain RE, Biller HF, Ogura JH. An experimental analysis of causative factors and protective methods in carotid artery rupture. Arch Otolaryngol Head Neck Surg 1974;99:235-241 [DOI] [PubMed] [Google Scholar]
- 22.Huvos AG, Learning RH, Moore OS. Clinicopathologic study of the resected carotid artery: analysis of 64 cases. Am J Surg 1973;126:570-574 [DOI] [PubMed] [Google Scholar]
- 23.Ariyan S, Marfaggi RA, Harden G, et al. An experimental model to determine the effects of adjuvant therapy on the incidence of postoperative wound infection. I. Evaluating preoperative radiation therapy. Plast Reconstr Surg 1980;328-337 [DOI] [PubMed]
- 24.Chaloupka JC, Putman CM. Endovascular therapy of surgical diseases of the cranial base. Clin Plastic Surg 1995;22:417-450 [PubMed] [Google Scholar]
- 25.Goldberg J, Sepka RS, Perona BP, Pederson WC, Klitzman B. Laser doppler blood flow measurements of common cutaneous donor sites for reconstructive surgery. Plast Reconstr Surg 1990;85:581-586 [DOI] [PubMed] [Google Scholar]
- 26.Jones NF. Intraoperative and postoperative monitoring of microsurgical free tissue transfers. Clin Plast Surg 1992;19:783-797 [PubMed] [Google Scholar]
- 27.Berenstein A, Ransohoff J, Kupersmith M, Flamm E, Graeb D. Transvascular treatment of giant aneurysms of the cavernous carotid and vertebral arteries. Functional investigation and embolization. Surg Neurol 1984;21:3-12 [DOI] [PubMed] [Google Scholar]
- 28.Higashida R, Halbach V, Dowd C, et al. Endovascular detachable balloon embolization therapy of cavernous carotid artery aneurysms: results in 87 cases. J Neurosurg 1990;72:857-863 [DOI] [PubMed] [Google Scholar]
- 29.Dare AO, Chaloupka JC, Putman CM, Fayad PB, Awad IA. Failure of the hypotensive provocative test during temporary balloon test occlusion of the internal carotid artery to predict delayed hemodynamic ischemia after therapeutic carotid occlusion. Surg Neurol 1998;50:147-156 [DOI] [PubMed] [Google Scholar]
- 30.Diethrich EB, Ndiaye M, Reid DB. Stenting in the carotid artery: initial experience in 110 patients. J Endovasc Surg 1996;3:42-62 [DOI] [PubMed] [Google Scholar]
- 31.Yadav JS, Roubin GS, Iyer S, et al. Elective stenting of the extracranial carotid arteries. Circulation 1996;5:1-6 [DOI] [PubMed] [Google Scholar]
- 32.Horowitz MB, Miller G, Meyer Y, Carstens G, Purdy PD. Use of intravascular stents in the treatment of internal carotid and extracranial vertebral artery pseudoaneurysms. AJNR Am J Neuroradiol 1996;16:693-696 [PMC free article] [PubMed] [Google Scholar]
- 33.Marks MP, Dake MD, Steinberg GK, Norbash AM, Lane B. Stent placement for arterial and venous cerebrovascular disease: preliminary experience. Radiology 1994;191:441-446 [DOI] [PubMed] [Google Scholar]
- 34.Parodi JC, Ferreira M, Estol CJ. Treatment of carotid artery disease with an endoluminal stent-venous graft. J Neurovasc Dis 1996;1:27-31 [Google Scholar]
- 35.Graves VB, Strother CM, Partington CR, Rappe A. Flow dynamics of lateral carotid artery aneurysms and their effects on coils and balloons: an experimental study in dogs. AJNR Am J Neuroradiol 1992;13:189-196 [PMC free article] [PubMed] [Google Scholar]
- 36.Wakhloo AK, Schellhammer F, de Vries J, Haberstroh J, Schumacher M. Self-expanding and balloon-expandable stents in the treatment of carotid aneurysms: an experimental study in a canine model. AJNR Am J Neuroradiol 1994;15:493-502 [PMC free article] [PubMed] [Google Scholar]
- 37.Geremia G, Haklin M, Brennecke L. Embolization of experimentally created aneurysms with intravascular stent devices. AJNR Am J Neuroradiol 1994;15:1223-1231 [PMC free article] [PubMed] [Google Scholar]
- 38.Turjman F, Massoud TF, Ji C, Guglielmi G, Vinuela F, Robert J. Combined stent implantation and endosaccular coil placement for treatment of experimental wide-neck aneurysms: a feasibility study in swine. AJNR Am J Neuroradiol 1994;15:1087-1090 [PMC free article] [PubMed] [Google Scholar]
- 39.Szikora I, Guterman LR, Wells KM, Hopkins LN. Combined use of stents and coils to treat experimental wide-neck carotid aneurysms: preliminary results. AJNR Am J Neuroradiol 1994;15:1091-1102 [PMC free article] [PubMed] [Google Scholar]
- 40.Higashida RT, Smith W, Gress D, et al. Intravascular stent and endovascular coil placement for a ruptured fusiform aneurysm of the basilar artery. Case report and review of the literature. J Neurosurg 1997;87:944-949 [DOI] [PubMed] [Google Scholar]
- 41.Matsuura JH, Rosenthal D, Jerius H, Clark MD, Owens DS. Traumatic carotid artery dissection and pseudoaneurysm treated with endovascular coils and stent. J Endovasc Surg 1997;4:339-343 [DOI] [PubMed] [Google Scholar]
- 42.Manninen HI, Koivisto T, Saari T, et al. Dissecting aneurysms of all four cervicocranial arteries in fibromuscular dysplasia: treatment with self-expanding endovascular stents, coil embolization, and surgical ligation. AJNR Am J Neuroradiol 1997;18:1216-1220 [PMC free article] [PubMed] [Google Scholar]


