Abstract
BACKGROUND AND PURPOSE: Tortuous, engorged veins can be identified on the venous phase of the brain circulation in patients with venous congestion related to an intracranial dural arteriovenous fistula (DAVF). The term pseudophlebitic pattern (PPP) has been used to describe this finding. The purpose of this study was to determine the prevalence of PPP in patients with intracranial DAVF and to analyze the relationship of this sign to presentation, location of the fistula, presence of retrograde leptomeningeal venous drainage, and MR findings.
METHODS: We retrospectively reviewed the charts and imaging findings of 130 patients with intracranial DAVF. In 122 patients the venous phase of the brain circulation was adequately assessed. The PPP was graded as mild, moderate, or severe.
RESULTS: PPP was found in 51 patients (42%). Thirty-two (73%) of the 44 patients who had a hemorrhage, neurologic deficit, or seizure had PPP as compared with 16 (21%) of the 75 who had a bruit or orbital signs. The three patients with either congestive heart failure or increasing head circumference had PPP. Fourteen (88%) of the 16 who had fistula of the superior sagittal sinus, straight sinus, or superior petrosal sinus had PPP. PPP was seen in 46 (81%) of 57 patients who had retrograde leptomeningeal venous drainage and in five (8%) of the 65 who had only sinosal drainage. Fourteen (88%) of the 16 who had white matter T2 hyperintensity on MR images had severe PPP.
CONCLUSION: The PPP reflects venous congestion and is associated with an aggressive presentation with or without retrograde leptomeningeal venous drainage. PPP may be a useful prognostic indicator and should be considered in treatment decisions.
Intracranial dural arteriovenous fistulas (DAVFs) have been grouped into benign or aggressive categories on the basis of the presence or absence of retrograde leptomeningeal venous drainage (RLVD) (1, 2). The sine qua non in management of intracranial DAVFs is that patients with RLVD must be cured and those without may be followed up clinically or partially treated for symptom palliation (1, 2). This raises the question of whether the presence or absence of RLVD should be the only factor to consider when planning management. In this study we looked at the frequency of engorged, tortuous pial veins in patients with intracranial DAVF and correlated its presence or absence to presentation, location of the fistula, RLVD, and MR findings. We use the term pseudophlebitic pattern (PPP) to characterize the tortuous, engorged veins identified on the venous phase of the brain circulation. Lasjaunias and Berenstein (3, 4) have used the term pseudophlebitic to describe the dilated, serpiginous veins in patients with venous congestion related to vein of Galen aneurysmal malformations and brain arteriovenous malformations. This finding has been noted in the literature but has not been systematically analyzed (3–6).
Methods
The clinical records and imaging studies of 130 patients with intracranial DAVFs explored and/or treated at our institution between 1984 and July 1998 were reviewed. The following data were recorded: age, sex, presentation, location, angioarchitecture, venous sinus patency, Borden classification, MR findings, treatment, and follow-up. Presentation was considered the dominant event that led to the diagnosis. For the purpose of the analysis, presentation was grouped into the following categories: hemorrhage, neurologic deficit, seizure, bruit, orbital signs, and congestive heart failure/increasing head circumference. Hemorrhage was either subdural, intracerebral, or subarachnoid; some resulted in a neurologic deficit. Deficit included a transient or progressive nonhemorrhagic neurologic deficit, excluding cranial nerve palsies related to the cavernous sinus. Bruit included tinnitus and chronic headaches; patients who were asymptomatic at presentation were also grouped into this category. Orbital signs included proptosis, chemosis, conjunctival injection, and a cavernous sinus syndrome. Congestive heart failure/increasing head circumference was found in the pediatric age group. When multiple separate intracranial DAVFs were present, the symptomatic location was documented for the analysis.
Analysis of the angioarchitecture was done by two neuroradiologists and included location of the shunt, presence of retrograde flow in the involved dural sinus, patency of the sinuses, presence of RLVD, and presence of PPP. PPP was based on an analysis of the venous drainage of the cerebral or cerebellar hemispheres and was classified as mild, moderate, or severe (Fig 1). In this retrospective study we had no way to determine accurately subtle changes in the circulation time. Mild PPP had small serpiginous collateral veins bridging the superior and inferior hemispheric veins. Moderate PPP had numerous serpiginous bridging veins, and the cortical veins showed slight tortuosity. Severe PPP was characterized by a marked delay in the circulation time, an extensive network of collateral vessels, and marked tortuosity of the veins draining either the cerebellum or cerebral hemispheres (Figs 2 and 3). MR findings included prominence of the pial vessels (Fig 2), hydrocephalus (Fig 2), hindbrain herniation, and parenchymal hyperintensity on T2-weighted images.
fig 1.
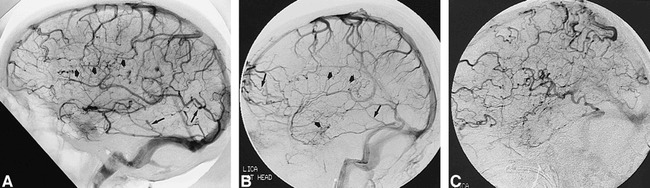
Representative cases showing severity of PPP.
A–C, Examples of mild (A), moderate (B), and severe (C) PPP on lateral views of the venous phase of cerebral angiogram. Mild PPP has small tortuous collateral veins (short wide arrows, A and B) with slight irregularity of the cortical veins (long thin arrows, A and B). These changes are more evident in moderate PPP and more dramatic in severe PPP.
fig 2.
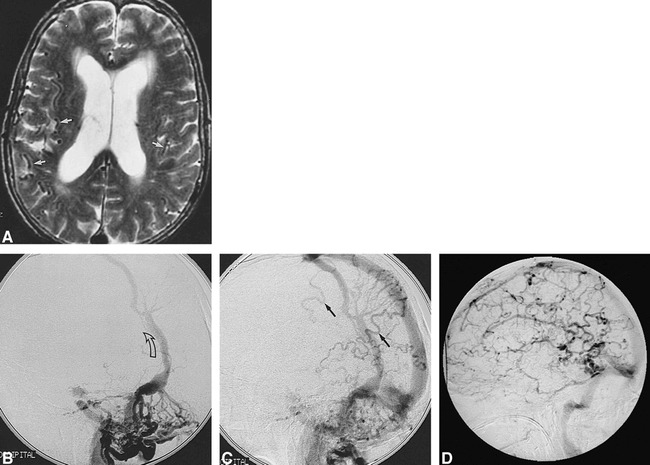
60-year-old woman with dementia.
A, Axial T2-weighted MR image shows hydrocephalus and a plethora of prominent subarachnoid vessels (arrows).
B and C, Lateral views of the arterial (B) and venous (C) phases of left occipital angiogram show a complex DAVF involving the distal transverse sinus with retrograde flow in an anomalous parietal dural venous sinus (arrow, B) and RLVD (arrows, C).
D, Lateral view of the venous phase of right internal carotid angiogram shows severe PPP.
fig 3.
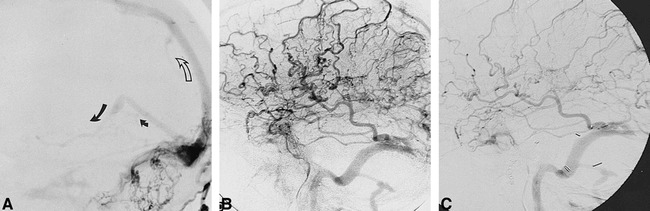
51-year-old man with dementia after removal of an acoustic neuroma.
A, Lateral view of a right occipital angiogram shows a DAVF involving an occluded right transverse sinus with retrograde flow in the superior sagittal sinus (open arrow) and RLVD (solid arrows) into the straight sinus and basal vein of Rosenthal.
B and C, Lateral views of venous phase of left internal carotid angiograms done before (B) and 4 years after (C) treatment show less marked PPP at follow-up, consistent with clinical improvement.
Results
The study population included 68 male and 62 female patients ranging in age from 4 months to 80 years (average age, 56 years) (Fig 4). There were five children, ages 4 months, 2 years, 4 years, 6 years, and 12 years, respectively. Location of the shunts was as follows: superior sagittal sinus (n = 7), transverse sinus/torcular (n = 49), cavernous sinus (n = 45), tentorium (n = 4), anterior cranial fossa (n = 5), superior petrosal sinus (n = 4), straight sinus (n = 4), and foramen magnum (n = 12). Four patients had multiple intracranial DAVFs. According to the Borden classification, 65 were type 1, 36 type 2, and 29 type 3. Thirteen patients had venous sinus occlusions; eight in the transverse sinus, three in the superior sagittal sinus, and two in the straight sinus. Fifteen patients had hemorrhage as the dominant presenting event, 28 had deficit, 45 had orbital signs, 35 had bruit (including two of the children), and four had seizure; two of the children had congestive heart failure and one had increasing head circumference. Dementia (n = 7) and ataxia (n = 6) were the most common signs in the group with deficit. Papilledema and raised intracranial pressure (n = 4) were included in the deficit group.
fig 4.
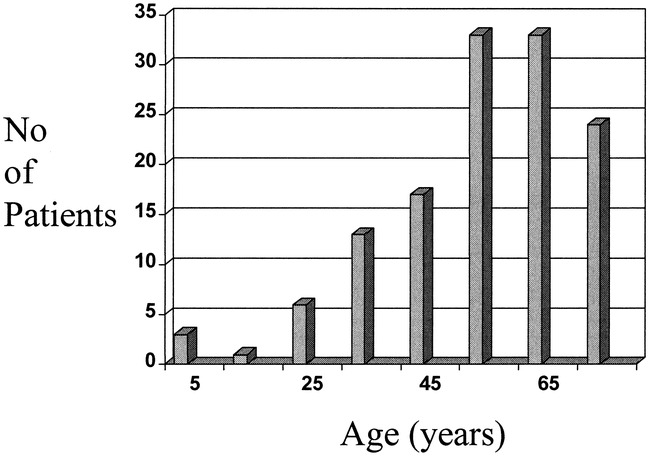
Age distribution of 130 patients with intracranial DAVF
Adequate angiographic assessment of the venous phase of the brain circulation was available in 122 of the 130 patients. In these, PPP was evident in 51 (42%). RLVD was found in 57 (47%) of the 122 patients, and 46 (81%) of these had PPP. PPP was categorized as mild in 15, moderate in 20, and severe in 16.
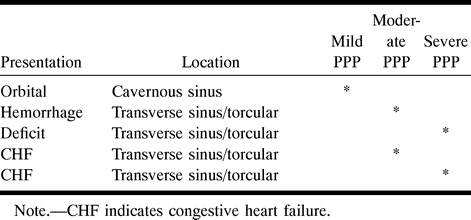
Tables 1 to 3 illustrate the correlation between PPP and presentation, RLVD, and location of the intracranial DAVF, respectively. Table 1 shows that 32 (73%) of the 44 patients who presented with a hemorrhage, deficit, or seizure had PPP. In contrast, 16 (21%) of the 75 patients who presented with orbital symptoms or bruit had PPP. All three patients with congestive heart failure or an enlarging head had PPP. Table 1 shows that presentation with a deficit or hemorrhage accounted for 13 (93%) of the 14 adult patients with severe PPP. Table 2 shows that nine (22%) of 41 patients with cavernous sinus DAVFs had PPP, whereas 14 (88%) of 16 patients with superior sagittal, superior petrosal, or straight sinus locations had PPP. RLVD was found in 27% of the patients with cavernous sinus DAVFs, compared with 100% of those with tentorial, anterior cranial fossa, superior petrosal, and straight sinus DAVFs. Table 3 highlights the relationship between presentation and location of PPP in five patients who did not have RLVD.
Table 1:
Relationship of presentation and severity of pseudophlebitic pattern (PPP) (n = 122 patients)
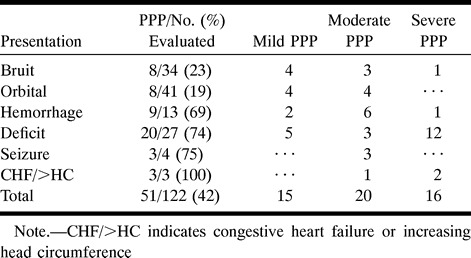
Table 2:
Relationship of location, retrograde leptomeningeal venous drainage (RLVD) and pseudophlebitic pattern (PPP) (n = 122 patients)
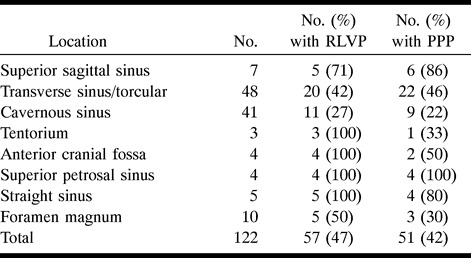
Table 3:
Relationship between presentation, location, and severity of pseudophlebitic pattern (PPP) in five patients without retrograde leptomeningeal venous drainage
MR imaging was done in 52 patients, 42 of whom had prominent pial vessels (Fig 2). Thirty-eight of the 42 patients had RLVD and 31 had PPP. PPP was present in two of the four patients who had prominent pial vessels but no RLVD. MR findings were normal in two patients with RLVD. Hydrocephalus and hindbrain herniation were evident on MR images in four adults and one child, four of whom had severe PPP. The four adults presented with a deficit, the child with increasing head circumference. MR images showed hyperintensity on T2-weighted sequences in 11 patients, 10 of whom presented with a deficit. Ten of these 11 had PPP, and in six it was severe.
Seventy-four patients were treated either by embolization alone (n = 32), by embolization and surgery (n = 23), or by surgery alone (n = 19). In 51 patients, follow-up angiography within 1 month of treatment showed no change in the PPP. Angiography showed less marked PPP in 11 of the 17 patients who had follow-up studies more than 6 months after treatment (Fig 3).
Discussion
In patients with intracranial DAVFs, RLVD and venous ectasia were first associated with intracranial hemorrhage by Houser et al (7) in 1972 and was later supported by Castaigne et al (8). In 1984, a metaanalysis by Malik et al (9) found RLVD, venous ectasia, and lesion location “outside a major sinus” to be associated with intracranial hemorrhage on presentation. In 1986, Lasjaunias et al (10) showed that focal neurologic deficits were related to the territory of the venous reflux. In 1987, Ishii et al (5) identified a subgroup of patients who were at high risk for hemorrhage and dementia because of venous overload exacerbated by occlusive changes in the transverse sinus. In their report, Ishii et al noted that engorged pial veins on angiography were related to the venous overload. Awad et al (11), in a review of 360 cases from the literature prior to 1990, found that RLVD, venous ectasia, and vein of Galen drainage correlated with intracranial hemorrhage and nonhemorrhagic neurologic deficit at presentation. In 1993, Lalwani et al (12) developed a grading system for transverse/sigmoid sinus DAVFs based on the severity of the venous restrictive disease.
A comprehensive classification of intracranial DAVFs based on angioarchitecture was first proposed by Djindjian et al (13) in 1977. In 1995, this scheme was modified by Cognard et al (14), who, in a review of their own series of 205 patients, were able to show a relationship between type of intracranial DAVF and presentation. A similar but simplified version of this classification was proposed by Borden et al (15). In 1996, Davies et al (16) confirmed the validity of the Borden and Cognard classification systems by showing a highly significant correlation with presentation.
The presentation of benign intracranial DAVFs often relates to the location of the fistula, and includes tinnitus, cranial nerve palsies, and/or signs related to venous congestion in the orbit (1, 11). Intracranial DAVFs with RLVD may have a similar presentation to the benign type or may present with an intracranial hemorrhage, neurologic deficit, or seizure (2, 5, 10–12, 17–21). Direct RLVD has a greater prevalence of hemorrhage as compared with sinosal drainage with RLVD (14, 22). Aneurysmal pial venous drainage has a greater prevalence of hemorrhage on presentation than does such drainage without aneurysmal enlargement (11, 14, 17). Sinosal drainage with retrograde flow in the venous sinuses but without RLVD can result in raised intracranial pressure (14, 23–25). The study by Davies et al (1, 2) of the natural history of intracranial DAVFs confirmed the benign course of those with sinosal drainage only and the aggressive course of those with RLVD.
The pathophysiology of nonhemorrhagic neurologic deficits and seizures in intracranial DAVFs relates to venous congestion (5, 6, 10, 19, 23). Venous congestion may be evident on MR images as bilateral or unilateral diffuse T2 hyperintensity in the white matter of either the cerebral or cerebellar hemispheres (6). These MR findings may be partially reversible after treatment (6). Thalamic and brain stem venous congestion can result from those fistulas refluxing into the straight sinus and deep veins (16). This clinicopathologic picture has been referred to as venous congestive encephalopathy (6, 19). PPP represents a response to venous congestion with the development of enlarged, tortuous collateral veins and venous rerouting. Rerouting may be into dilated transosseous venous channels or may be retrograde into the orbital veins.
Many reports have noted an association between presentation and location of intracranial DAVFs (1, 2, 10, 11, 17). An aggressive neurologic course is seen least often with transverse/sigmoid and cavernous sinus locations and most often with tentorial DAVFs. This corresponds to the frequency with which we found RLVD in each location (Table 2). RLVD was present in 100% of our tentorial, anterior cranial fossa, superior petrosal, and straight sinus locations. Cavernous and transverse sinus/torcular DAVFs were associated with RLVD in 27% and 42% of patients, respectively. A similar proportion of our patients had RLVD and PPP at each location (Table 2). Fourteen (88%) of our 16 patients with DAVFs located within the superior sagittal, straight, and superior petrosal sinuses had PPP, as compared with nine (22%) of 41 patients with cavernous sinus DAVFs. No location of an intracranial DAVF was immune to either RLVD or PPP.
Five patients with PPP had no RLVD (Table 3). In the literature there are a number of reports of intracranial DAVFs with only sinosal drainage producing raised intracranial pressure (14, 23–25). From these reports and our observation that five patients had PPP with sinosal drainage only, it could be suggested that these patients may have had venous hypertension within the involved sinuses, resulting in venous congestion. Four of these five patients had moderate or severe PPP, and their fistulas were all located in the transverse sinus/torcular. Two of the five were children who presented with congestive heart failure. Since PPP indicates venous congestion, its presence may be a factor to consider in management, especially in those patients without RLVD and in whom conservative management may be an option. Presentations with a hemorrhage or deficit in two of these five patients support the hypothesis that PPP without RLVD may be a sign that aggressive symptoms can occur despite the absence of RLVD.
Hydrocephalus and hindbrain herniation are not well-known findings in intracranial DAVFs; they are thought to be related to venous hypertension in the superior sagittal sinus, interfering with CSF absorption (3, 10). Previous subarachnoid hemorrhage could also interfere with CSF absorption (10). Lasjaunias and Berenstein (3, 4) referred to this pathophysiology as a hydrovenous disorder. Five of our 130 patients had hydrocephalus and hindbrain herniation. Two of the five had white matter hyperintensity on T2-weighted MR images. Their presentations included dementia in three, ataxia in one, and increasing head circumference in a 4-month-old child. All five patients with hydrocephalus had PPP, and in four it was severe. Hydrocephalus and hindbrain herniation are more commonly seen in children with a high-flow fistula, such as a vein of Galen aneurysmal malformation (10). Treatment of this hydrovenous disorder should be directed toward eliminating the fistula instead of ventricular shunting.
Cognard et al (26) reported worsening of venous drainage in seven partially treated patients with intracranial DAVFs and advised close follow-up of these patients. Davies et al (1) did not encounter any worsening of symptoms in 55 untreated intracranial DAVFs with sinosal venous drainage only (Borden type 1), which were followed up for a mean of 33 months. Long-term follow-up is required for conservatively managed or partially treated patients and for those treated by surgical disconnection alone (1, 2, 27). The presence of moderate or severe PPP may be useful in highlighting a subgroup of patients in whom aggressive symptoms or signs may develop. Interval improvement in PPP may be a reassuring angiographic sign in the follow-up of partially treated patients (Fig 3).
Catheter angiography in the investigation of intracranial DAVFs must include good visualization of the venous phase of the brain circulation. The ideal study would be done under general anesthesia to obtain perfect subtractions; however, in most patients, this is not practical. Excellent visualization of the venous phase is needed to detect subtle findings, which may include pial or medullary collateral veins, focal regions of delayed circulation, and venous rerouting to the orbit or to transosseous veins. Objective assessment of the circulation time would be a useful adjunct in the assessment of venous congestion.
Conclusion
The PPP is a common finding in intracranial DAVFs that can be identified if the venous circulation of the brain is carefully scrutinized. Analysis of PPP in relation to presentation, RLVD, location of the DAVF, and MR findings shows that the severity of PPP reflects the venous congestion. PPP tends to be associated with aggressive presentation and RLVD. PPP may be a useful angiographic sign to consider in treatment decisions.
Acknowledgments
We gratefully acknowledge M. C. Wallace and M. Tymianski for their important contribution to the neurosurgical care of many of these patients and for their collaboration in the University of Toronto Brain Vascular Malformation Study Group.
Footnotes
Address reprint requests to Robert A. Willinsky, MD, FRCPC.
References
- 1.Davies MA, Saleh J, terBrugge K, Willinsky RA, Wallace MC. The natural history and management of intracranial dural arteriovenous fistulas, 1: benign lesions. Intervent Neuroradiol 1997;3:295-302 [DOI] [PubMed] [Google Scholar]
- 2.Davies MA, terBrugge K, Willinsky RA, Wallace MC. The natural history and management of intracranial dural arteriovenous fistulas, 2: aggressive lesions. Intervent Neuroradiol 1997;3:303-311 [DOI] [PubMed] [Google Scholar]
- 3.Lasjaunias P. Vascular Diseases in Neonates, Infants and Children: Interventional Neuroradiology Management.. Berlin: Springer; 1997:105–142
- 4.Berenstein P, Lasjaunias P. Surgical Neuroangiography, 4: Endovascular Treatment of Cerebral Lesions.. Berlin: Springer; 1992:62–79, 280–288
- 5.Ishii K, Goto K, Ihara K, et al. High-risk dural arteriovenous fistulae of the transverse and sigmoid sinuses. AJNR Am J Neuroradiol 1987;8:1113-1120 [PMC free article] [PubMed] [Google Scholar]
- 6.Willinsky RA, terBrugge K, Montanera W, Mikulis D, Wallace MC. Venous congestion: an MR finding in dural arteriovenous malformations with cortical venous drainage. AJNR Am J Neuroradiol 1994;15:1501-1507 [PMC free article] [PubMed] [Google Scholar]
- 7.Houser OW, Baker HL, Rhoton Al, Okazaki H. Intracranial dural arteriovenous malformations. Radiology 1972;105:55-64 [DOI] [PubMed] [Google Scholar]
- 8.Castaigne P, Bories J, Brunet P, Merland JJ, Meininger V. Les fistules arterio-veneuses meningees pures a drainage veineux cortical. Rev Neurol 1976;132:169-181 [PubMed] [Google Scholar]
- 9.Malik GM, Pearce JE, Ausman JI, Mehta B. Dural arteriovenous malformations and intracranial haemorrhage. Neurosurgery 1984;15:332-339 [DOI] [PubMed] [Google Scholar]
- 10.Lasjaunias P, Chiu M, terBrugge K, Tolia A, Hurth M, Bernstein M. Neurological manifestations of intracranial dural arteriovenous malformations. J Neurosurg 1986;64:724-730 [DOI] [PubMed] [Google Scholar]
- 11.Awad IA, Little JR, Akrawi WP, Ahl J. Intracranial dural arteriovenous malformations: factors predisposing to an aggressive neurological course. J Neurosurg 1990;72:839-850 [DOI] [PubMed] [Google Scholar]
- 12.Lalwani A, Dowd C, Halbach VV. Grading venous restrictive disease in patients with dural arteriovenous fistulas of the transverse/sigmoid sinus. J Neurosurg 1993;79:11-15 [DOI] [PubMed] [Google Scholar]
- 13.Djindjian R, Merland JJ, Theron J. Super-Selective Arteriography of the External Carotid Artery.. New York: Springer; 1977:606–628
- 14.Cognard C, Gobin YP, Pierot L, et al. Cerebral dural arteriovenous fistulas: clinical and angiographic correlation with a revised classification of venous drainage. Radiology 1995;194:671-680 [DOI] [PubMed] [Google Scholar]
- 15.Borden JA, Wu KW, Shucart WA. A proposed classification for spinal and cranial dural arteriovenous fistulous malformations and implications for treatment. J Neurosurg 1995;82:166-179 [DOI] [PubMed] [Google Scholar]
- 16.Davies M, terBrugge K, Willinsky RA, Coyne T, Saleh J, Wallace MC. The validity of classifications for the clinical presentations of intracranial dural arteriovenous fistulas. J Neurosurg 1996;85:830-837 [DOI] [PubMed] [Google Scholar]
- 17.Brown R, Wiebers DO, Nichols D. Intracranial dural arteriovenous fistulae: angiographic predictors of intracranial hemorrhage and clinical outcome in nonsurgical patients. J Neurosurg 1994;81:531-538 [DOI] [PubMed] [Google Scholar]
- 18.Brunereau L, Gobin YP, Meder J, Cognard C, Tubiana J, Merland JJ. Intracranial dural arteriovenous fistulas with spinal venous drainage: relationship between clinical presentation and angiographic findings. AJNR Am J Neuroradiol 1996;17:1549-1554 [PMC free article] [PubMed] [Google Scholar]
- 19.Hurst R, Bagley L, Galetta S, et al. Dementia resulting from dural arteriovenous fistulas: the pathologic findings of venous hypertensive encephalopathy. AJNR Am J Neuroradiol 1998;19:1267-1273 [PMC free article] [PubMed] [Google Scholar]
- 20.Pierot L, Chiras J, Meder J, Rose M, Rivierez M, Marsault C. Dural arteriovenous fistulas of the posterior fossa draining into subarachnoid veins. AJNR Am J Neuroradiol 1992;13:315-323 [PMC free article] [PubMed] [Google Scholar]
- 21.Vinuela FV, Fox AJ, Pelz DM, Drake CG. Unusual clinical manifestations of dural arteriovenous malformations. J Neurosurg 1986;64:554-558 [DOI] [PubMed] [Google Scholar]
- 22.Barnwell SL, Halbach VV, Dowd CF, Higashida RT, Hieshima GB, Wilson CB. A variant of arteriovenous fistulas within the wall of dural sinuses: results of combined surgical and endovascular therapy. J Neurosurg 1991;74:199-204 [DOI] [PubMed] [Google Scholar]
- 23.Cognard C, Casasco A, Toevi M, Houdart E, Chiras J, Merland JJ. Dural arteriovenous fistulas as a cause of intracranial hypertension due to impairment of cranial venous outflow. J Neurol Neurosurg Psychiatry 1998;65:308-316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gelwan M, Choi IS, Berenstein A, Pile-Spellman J, Kupersmith M. Dural arteriovenous malformations and papilledema. Neurosurgery 1988;22:1079-1084 [DOI] [PubMed] [Google Scholar]
- 25.Lamas E, Lobato R, Esparza J, Escudero L. Dural posterior fossa AVM producing raised sagittal sinus pressure: case report. J Neurosurg 1977;46:804-810 [DOI] [PubMed] [Google Scholar]
- 26.Cognard C, Houdart E, Casasco A, Gabrillargues J, Chiras J, Merland JJ. Long-term changes in intracranial arteriovenous fistulae leading to worsening in the type of venous drainage. Neuroradiology 1997;39:59-66 [DOI] [PubMed] [Google Scholar]
- 27.Thompson BG, Doppman JL, Oldfield EH. Treatment of cranial dural arteriovenous fistulae by interruption of leptomeningeal venous drainage. J Neurosurg 1994;80:617-623 [DOI] [PubMed] [Google Scholar]


