Abstract
Summary: We review the clinical history and imaging (CT and/or MR) studies in three patients with histologically proved extraorbital inflammatory pseudotumor of the head and neck. The imaging findings in all three cases were nonspecific, mimicking a malignant tumor or granulomatous disease.
Inflammatory pseudotumor (IPT) is a rarely occurring lesion with no identifiable local or systemic cause (1). The condition was first described in 1905 by Birch-Hirschfield, but remains somewhat of an enigma despite multiple ophthalmologic, radiologic, and pathologic reports (2). The term pseudotumor was coined because these lesions mimic expansive, invasive, malignant tumors, both clinically and radiologically (3–5). IPT most commonly involves the lung and orbit, but has been reported to occur in nearly every site in the body (1, 6, 7), although it is rarely found in the head and neck outside the orbits. The condition may mimic a malignant lesion, and may occur at sites that hinder or preclude biopsy, possibly exposing the patient to the risk of unnecessary and potentially mutilating surgery. Our three cases did not disclose a specific imaging feature of IPT. The distribution of the mass lesions suggested a perineural spread pattern along the maxillary, mandibular, and hypoglossal nerve. One of the cases was complicated by internal carotid artery (ICA) occlusion.
The combined clinical history, biochemical findings, and imaging features of an infiltrating soft-tissue mass should lead to the possibility of IPT; radical surgery should be avoided before there is histologic proof of a malignant tumor. In confirmed cases of IPT, conservative steroid therapy may relieve symptoms.
Case Reports
Case 1
A 16-year-old girl had a 10-day history of pain in the left temporal area, radiating to the throat. She also reported fullness in the left ear and left-sided tinnitus. A CT study of the paranasal sinuses obtained at a regional hospital showed inflammatory changes in the ethmoidal and right maxillary sinus. A mass within the left nasopharyngeal soft tissues was also noted, which was thought to be due to reactive lymphoid hyperplasia (Fig 1A). A diagnosis of sinusitis with atypical pain distribution and reactive inflammation in the fossa of Rosenmüller was made, and oral antibiotics were administered. No relief of the symptoms was obtained. One month later, the patient experienced numbness and tingling paresthesias of the left side of the face. A CT study of the maxillofacial region showed resolving sinusitis in the paranasal sinuses. Because of continuing neurologic symptoms, an MR study of the brain and skull base was performed, revealing a soft-tissue mass in the posterolateral left side of the nasopharynx that abutted the base of the skull, extended into the parapharyngeal space, and involved the pterygoid muscles, consistent with a nasopharyngeal tumor (Fig 1B and C). A biopsy specimen, however, revealed only lymphoid hyperplasia, without signs of malignancy or specific inflammation. Repeat biopsies returned no evidence of malignancy, and the antibiotic treatment was continued. Two months later, the pain and numbness had became worse and a repeat MR study was performed, which showed further growth of the nasopharyngeal lesion. Treatment with antibiotics was continued. A follow-up MR study performed 2 months later revealed further progression of the lesion with extension into the floor of the middle cranial fossa (Fig 1D).
fig 1.
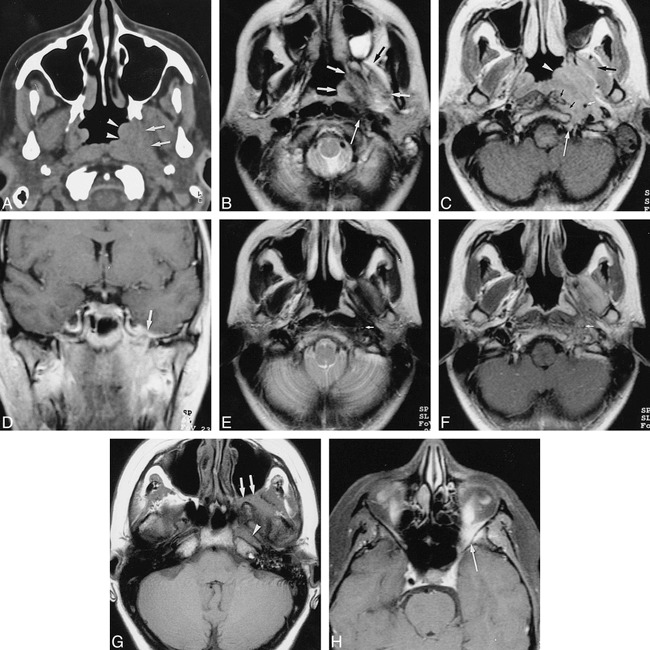
Case 1.
A, Unenhanced axial CT scan through the nasopharyngeal region shows thickening of the nasopharyngeal tissues at the level of the left torus tubarius and fossa of Rosenmüller (arrowheads) associated with infiltration of the left parapharyngeal space (arrows).
B, Axial T2-weighted MR image through the nasopharyngeal region, obtained 1 month after A. A poorly defined mass lesion (short arrows) is visible in the left nasopharynx and parapharyngeal space. The lateral pterygoid muscle and the origin of the medial pterygoid muscle show high signal intensity, more or less isointense with fat (compare with opposite side). A region of lower signal intensity is also visible within the lesion, extending between the prevertebral muscles and the ICA (long arrow). A left-sided mastoidal effusion is also present.
C, Axial contrast-enhanced T1-weighted MR image through the nasopharyngeal region, obtained at same time as B, shows an enhancing soft-tissue mass (arrowhead) in the left nasopharyngeal and retrostyloid compartment of the parapharyngeal space, extending around the left ICA (small white arrow) into the skull base (region of hypoglossal canal, long white arrow), and into the prevertebral muscles (small black arrows). The anterocranial part of the pterygoid muscles also shows some enhancement (large black arrow).
D, Coronal contrast-enhanced T1-weighted image, obtained 4 months after B and C, shows further progression of the lesion, now extending up to the foramen ovale with associated enhancement on the floor of the middle cranial fossa (arrow).
E and F, Axial T2-weighted (E) and contrast-enhanced T1-weighted (F) images, obtained 4 months after D, after a course of corticosteroids, show the mass lesion is clearly reduced in size. In E, low signal intensity is seen in the left parapharyngeal space, in the fat plane between the lateral pterygoid and temporalis muscle, and around the ICA (arrow). In F, enhancement is visible in and around the left lateral pterygoid muscle and around the left ICA; the normal signal void is not seen in this artery (arrow).
G and H, Axial unenhanced (G) and contrast-enhanced fat-suppressed (H) T1-weighted images, obtained 5 months after E and F, when symptoms recurred. The lesion now extends into the left retromaxillary fat and pterygopalatine fossa (arrows, G) and into the orbital apex. Soft-tissue enhancement can be seen posterolaterally in the left orbit (long arrow, H). Absence of normal signal void is evident in the left ICA in the horizontal part of the left carotid canal (arrowhead, G) and in the left cavernous sinus.
The patient had continuing severe facial pain after unsuccessful treatment with antibiotics. She consulted her family general practitioner, who initiated a course of high-dose corticosteroids. The symptoms rapidly improved. A follow-up MR study, a few months later, revealed reduction in the size of the lesion (Fig 1E and F). The signal intensity on T2-weighted spin-echo (SE) images was decreased as compared with the previous MR studies; the normal signal void in the left ICA was now absent, suggesting slowing of blood flow. At that time, 1½ years after the onset of symptoms, the patient was referred to our hospital for further evaluation. The corticosteroid therapy was tapered and discontinued to see if the lesion would reappear. Three months later, a new MR study was performed; the lesion remained largely unchanged relative to the last MR examination. Given the dramatic response to corticosteroid therapy, the hypothesis of an IPT was formulated. A cytoplasmic antineutrophil cytoplasm antibody (c-ANCA) test, to rule out Wegener disease, was negative. A new biopsy was performed via a Caldwell-Luc approach to the pterygopalatine fossa. Biopsy revealed fibrous tissue with multiple vessels. Around and between the vessels was a dense inflammatory cellular infiltrate, composed mainly of mature lymphocytes and plasma cells. There was no evidence of vasculitis. Immunohistochemical investigations showed an admixture of T- and B-lymphocytes. The histologic appearance was compatible with IPT. All the cultures were negative.
Because the patient had almost no signs or symptoms at that time, the steroid treatment was discontinued. Two months later, however, she reported a return of a severe left-sided headache, retroorbital pressure with diplopia, and vomiting. An MR study (Fig 1G and H) now revealed extension in the left pterygomaxillary fissure and the left orbital apex, compatible with recrudescence of IPT. The ICA flow void was absent, and there was increased contrast enhancement in its place, suggesting occlusion. Treatment with high-dose corticosteroids was started, and the symptoms diminished within 24 hours, almost completely resolving within 3 days. A month later, the patient was admitted to the emergency department because of paresthesias in the right forearm and hand. An MR examination of the brain, including an angiographic study, showed occlusion of the left ICA and an ischemic zone in the left parietooccipital area, presumably a complication of the ICA occlusion. The symptoms disappeared over time. At present, the patient is not on any medication, and is without any symptoms 2½ years after the onset of symptoms.
Case 2
A 39-year-old man was admitted with a 2-month history of headache in the left temporal region. He had also been experiencing occasional short episodes (about 30 seconds in duration) of paresthesias in the left temporal and supraorbital region. Two dental extractions and left antral lavage was performed at another institution without any effect on the symptoms. One week before admission to the hospital, the patient experienced diplopia. Clinical examination revealed a complete left external ophthalmoplegia, without ptosis. Biochemically, there was no elevation of the erythrocyte sedimentation rate. Thyroid function was normal, and the c-ANCA test was negative. Examination of lumbar CSF revealed no abnormalities; a CT study of the brain was also normal.
At this time, a diagnosis of cavernous sinus thrombosis and Tolosa-Hunt syndrome was postulated, and an MR study was performed, revealing a mass lesion in the left pterygopalatine fossa that extended through the inferior orbital fissure via the apex of the left orbit to the superior orbital fissure (Fig 2). Some enhancement of the maxillary nerve in the foramen rotundum was seen. The differential diagnosis included a neoplastic lesion and IPT.
fig 2.
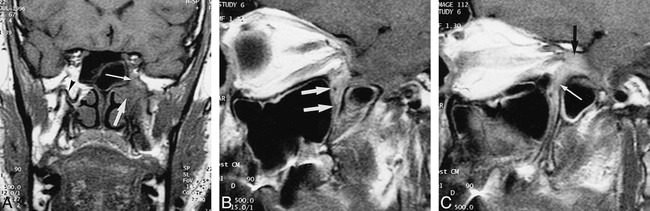
Case 2.
A, Coronal unenhanced T1-weighted image through the base of the skull. The left pterygopalatine fossa appears infiltrated by soft tissue (thick arrow), isointense with muscle, extending into the inferior orbital fissure (thin arrow). On the opposite side, the pterygopalatine fossa displays its normal high T1 signal intensity, with the internal maxillary artery (arrowhead) visible as a signal void.
B and C, Sagittal contrast-enhanced T1-weighted images through the left pterygopalatine fossa: C is 4 mm medial to B. The enhancing mass lesion (arrows, B) is seen to grow through the inferior orbital fissure (white arrow, C) in the orbital apex and into the superior orbital fissure (black arrow, C).
Results of an endoscopic biopsy of the retromaxillary region showed dense fibrous tissue with some aggregates of mature lymphoid tissue and some foci of calcification. In addition to the lymphoid aggregates, a few lymphoplasmocytic cells were present throughout the fibrous tissue. There was no evidence of malignancy. These histologic findings were compatible with IPT. The cultures were negative.
A treatment of high-dose corticosteroids was started. The left temporal headache disappeared within 5 days, and the eye motility disturbances and associated diplopia improved substantially. The dose of corticosteroids was gradually tapered. Currently, 1½ years after the onset of symptoms, the patient is asymptomatic.
Case 3
An 18-year-old girl was admitted with a 2-month history of trismus and swelling of the right submandibular region. A CT study of the neck showed a poorly defined, enhancing soft-tissue mass around an enlarged right submandibular salivary gland, extending into the masticator space (Fig 3A–C). An MR study (Fig 3D–G) confirmed the mass lesion in the right masticator space, which appeared slightly hyperintense relative to muscle on T2-weighted SE images, and clearly enhancing on postcontrast T1-weighted SE images. The differential diagnosis included inflammation of the submandibular salivary gland and surrounding tissues or a neoplastic lesion.
fig 3.
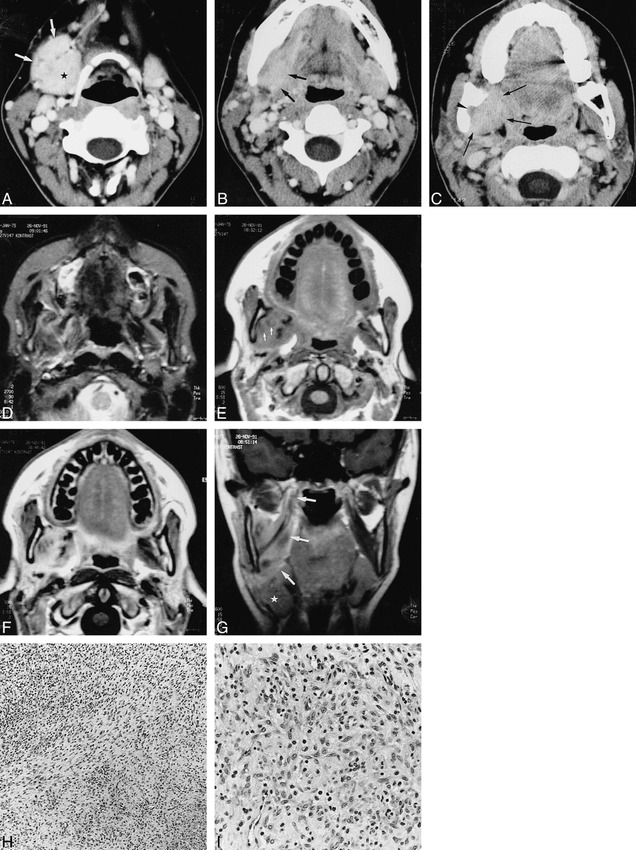
Case 3.
A–C, Axial contrast-enhanced CT scans show an enhancing soft-tissue mass (arrows, A) lying against the enlarged and enhancing right submandibular salivary gland (star). At a higher level (B), the mass (arrows) cannot be distinguished from this gland. In C, the soft-tissue mass extends into the medial pterygoid muscle (arrows), abutting the right oropharyngeal wall; there is infiltration of the fat in the mandibular foramen (arrowhead) and associated sclerosis of the mandibular ramus, which appears slightly deformed.
D, Axial T2-weighted MR image shows a poorly defined soft-tissue structure, more or less isointense with fat, in the right masticator space.
E, Unenhanced T1-weighted image shows that the mass is largely isointense with muscle, with some small low-intensity areas (arrows); some signal loss is evident in the right mandibular ramus.
F, After injection of contrast material, there is clear enhancement within the mass, except for the previously indicated low-signal areas, which possibly correspond to areas of fibrosis.
G, Coronal T1-weighted image shows the mass infiltrating the medial pterygoid muscle up to its attachment at the skull base (arrows); the submandibular salivary gland is displaced inferiorly (star).
H, Low-power histologic section shows spindle cells embedded in a collagenous background and intermingled with mononuclear inflammatory cells (H and E, original magnification ×125).
I, At high power, the admixture of the inflammatory cells and the plump (myo)fibroblastlike cells is seen to a better extent (H and E, original magnification ×325).
A biopsy specimen revealed reactive inflammatory tissue with fibrous tissue and inflammatory cells. A lymphoid infiltrate and multiple vascular structures were recognized. There was no evidence of a lymphoma or vasculitis, and treatment with antibiotics was started. A follow-up CT study 5 months later showed no changes. Because a neoplastic lesion could not be excluded with certainty, partial excision of the mass was performed. Histologic examination showed a fibrous background with spindle (myo)fibroblastlike cells admixed with a lymphoplasmocytic infiltrate, compatible with IPT (Fig 3H and I). The patient's clinical condition gradually improved without treatment, and at present, 5 years after surgery, the patient is asymptomatic on no medication.
Discussion
IPT is an inflammatory lesion of unknown origin (1, 3–5, 8–10), although various stimuli may act as triggers for its development, such as unrecognized microorganisms (1, 3, 6, 7), minor trauma (1, 5), smoking (1, 11), and chronic irritation caused by cocaine abuse (7). The characteristic feature of IPT might not lie in the inciting agent, but in the way the response to such a triggering agent develops. According to Williams et al (12), whatever the cause, a localized derangement in the immune response after the initial insult may be an underlying mechanism. Other authors contend that most of the features of IPT can be related to the production of mediators of inflammation, such as cytokines and, particularly, interleukin-1 (7). This substance, which is produced mainly by monocytes, macrophages, and, to a lesser extent, by many other cell types, has a wide range of local and systemic effects. Locally, it stimulates the proliferation of fibroblasts, the extravasation of neutrophils, and the activation and increase of procoagulant activity of the vascular endothelium. Systemically, it induces production of acute-phase reactants by hepatocytes, proteolysis, and also neurologic disturbances.
IPT has been reported to occur in nearly every site in the body (6). In the head and neck region, it is commonly found in the orbit, but has been reported in such locations as the nasal cavity, the nasopharynx, the maxillary sinuses (3), the larynx, and the trachea (6). The local symptoms in the head and neck depend on the site of involvement (6, 11, 13, 14). The most frequent symptom in all locations is local swelling and pain. Different anatomic sites can be involved within the orbit, with a corresponding clinical picture; the most frequent orbital symptoms are pain, proptosis, diplopia, and disturbance of the extraocular muscle motility (2, 7). In extraorbital head and neck locations, the most frequent local symptoms are pain and obstruction of the involved tract (such as the pharynx [7], the larynx [11], and the sinuses [3]). Involvement of the skull base and intracranial extension, resulting in cranial nerve neuropathy and/or ischemic insults, has been reported (4, 15, 16).
Constitutional symptoms, such as fever, anorexia, weight loss, malaise, and somnolence, have been reported in 15% to 30% of patients with IPT (6). The unifying histologic feature of this lesion is the highly variable admixture of bland-looking spindle cells and inflammatory cells (16). The spindle cells, which can predominate, usually represent a mixture of fibroblasts and myofibroblasts. Histiocytes are variably present. The inflammatory component is polymorphous, but lymphocytes, eosinophils, or plasma cells (hence the previously used term plasma cell granuloma) may constitute the major inflammatory component (6, 17). Because of the wide histologic spectrum, the terminology used for this group of lesions is confusing (inflammatory myofibroblastic lesion, inflammatory myofibrohistiocytic proliferation, inflammatory fibromyxoid tumor, xanthomatous pseudotumor, postinflammatory pseudotumor, plasma cell–histiocytoma complex, inflammatory fibrosarcoma) (17–19).
CT has proved valuable in defining the extension of IPT and its response to treatment and, perhaps, in suggesting its pathogenesis (2, 19). Usually, a soft-tissue mass is seen, which may appear circumscribed; however, especially in orbital locations, infiltration of the surrounding fat may be evident (2, 6). This mass lesion shows a strong enhancement in most cases (8). In orbital locations, retrobulbar fatty infiltration, proptosis, extraocular muscle enlargement, and apical fat edema are the most frequent radiologic characteristics. Uveal and scleral thickening is seen in 33% of orbital cases, and is thought to be a specific sign of orbital IPT (8, 13). Bone remodeling is only seen in 5% of orbital locations (3, 13). In extraorbital head and neck locations, a mass lesion with strong enhancement is consistently reported. On imaging studies, IPT in the maxillary sinus has a more aggressive appearance than does that in orbital locations (3, 15). Bone involvement, such as erosion, remodeling, sclerosis, and thickening, is much more common than in its orbital counterpart. Some bony alterations (of the mandibular ramus) were also seen in our third case. Skull base involvement and intracranial extension can be detected with CT or MR imaging (3).
The mass lesions in our cases were isointense with muscle on T1-weighted SE images and isointense with fat on T2-weighted SE images at first presentation. These signal patterns are analogous to those reported for orbital pseudotumor (20) and Tolosa-Hunt syndrome (21), an inflammatory condition of the cavernous sinus with a similar histopathologic appearance as orbital pseudotumor. In all our cases, enhancement after injection of contrast material was seen on T1-weighted images.
All three of our cases showed findings that can be interpreted as perineural involvement: in the first case, extension of the tissue abnormalities in the hypoglossal canal and through the foramen ovale (mandibular nerve) was seen; in the second case, extension in the superior orbital fissure and along the foramen rotundum (maxillary nerve) was observed; and in the third case, infiltration of the mandibular foramen (inferior alveolar nerve) was present. To our knowledge, such perineural involvement has not been explicitly described in IPT.
Angiographic demonstration of slight narrowing of the common carotid artery by a surrounding IPT has been reported (22). We are unaware of any previous description of ischemic infarction caused by narrowing and occlusion of the extracranial ICA, such as seen in our first case. We speculate that this occlusion occurred as a result of a scarlike retraction of the mass induced by the steroid treatment; this increase in connective tissue may also be the reason the mass eventually appeared less intense on T2-weighted images. An alternative hypothesis is that the ICA was narrowed and encased by the inflammatory process, potentially deterring thrombosis.
Radiologically, IPT can mimic meningioma, malignant neoplasms (such as lymphoma and perineural spread of carcinoma), Wegener granulomatosis, and sarcoidosis. All these entities may show signal characteristics similar to those of IPT on MR images.
Whenever an infiltrating mass is seen in the extracranial head and neck, lymphoma is a possibility, even in the absence of regional adenopathy or another lymphatic or extralymphatic site of disease (23, 24). Furthermore, head and neck lymphoma may be responsive to the oral steroids that are used to treat IPT (8). Tissue sampling should anticipate the possibility of lymphoma (24). Immunohistochemical studies of T- and B-cell subpopulations may be helpful in distinguishing lymphoma from IPT (13). In IPT, T-cells as well as B-cells are found. In lymphoma, a (clonal) B- or T-cell population predominates. Furthermore, the heterogeneity of the inflammatory cell population in IPT excludes a lymphoma (15). Several cases of IPT have been reported that, over a period of time, were found to contain a lymphoma without any evidence of systemic disease (25). The mechanism by which this chronic inflammatory disease can occasionally lead to later neoplastic proliferation is not known.
Wegener granulomatosis primarily affects the upper respiratory tract, lungs, and kidneys. In 18% to 22% of the cases, it also involves the orbit. Wegener granulomatosis limited to the orbits is rare (14). The orbital symptoms are the same as those seen with orbital IPT, and patients usually also manifest constitutional symptoms. The vasculitic lesions and necrotizing granulomas, found in Wegener granulomatosis, are not seen in pseudotumor (4, 15). Furthermore, a positive titer for c-ANCA is a diagnostic criterion for Wegener granulomatosis. This cytoplasmic antibody directed at neutrophil cytoplasmic antigen is elevated during periods of active disease. In the absence of respiratory tract or renal lesions, the diagnosis is not confirmed until the elevated c-ANCA titers are identified (14). Therefore, the systemic evaluation of patients with a presumed diagnosis of IPT should include an assay of c-ANCA titers.
Patients with sarcoidosis will often have systemic symptoms, and meningiomas will not resolve with steroid therapy (21). Carcinoma of the head and neck may show perineural spread on imaging studies without correlating clinical findings or symptoms. In some cases, the symptoms caused by perineural tumor spread, such as facial pain or paresthesias, cranial nerve paresis, or decreased visual acuity, may be the presenting signs of a clinically occult, entirely submucosal carcinoma. A careful clinical and radiologic search should be performed for a primary lesion of the head and neck that may be the origin of perineural spread.
There are several possible treatments for IPT. Therapy with high-dose oral corticosteroids is the preferred treatment for suspected IPT (3, 8, 16). The acute IPTs will show a dramatic response to a high dose of corticosteroids. When the amount of collagenous connective tissue increases (as seen in more chronic forms of IPT), the sensitivity to corticosteroids decreases (11, 13, 15). Reports in which chemotherapy has been used to treat IPT are scarce; it has been applied successfully in a few patients, with or without associated corticosteroid administration (26).
Patients who respond poorly to steroid therapy, who experience a recurrence after tapering of steroids, or who have medical contraindications to prolonged use of high-dose corticosteroids (4, 9) can be treated by radiation therapy. A favorable response to this form of treatment has been reported in approximately 75% of patients (9).
Radical and mutilating surgery has been performed in patients in whom there was a strong clinical suggestion of malignancy or who had a lesion in an area that was difficult to access for biopsy, and in whom IPT was eventually diagnosed (5, 13). In patients who do not respond satisfactorily to steroid therapy, there may be a role for more limited surgery; for example, orbital decompression when vision is threatened (8, 13). IPT may show a tendency toward spontaneous regression, and in these cases, surgery should be as conservative as possible (13).
Conclusion
The likelihood of extraorbital IPT of the head and neck may be suggested by the combination of clinical signs and symptoms and radiologic findings of an infiltrative mass lesion; however, a definitive diagnosis cannot be obtained without considering a number of differential diagnoses, requiring further biochemical analysis and tissue sampling for histologic study.
Footnotes
Address reprint requests to Robert Hermans, MD, PhD, Department of Radiology, University Hospitals, Herestraat 49, B-3000 Leuven, Belgium.
References
- 1.Wenig BM, Devaney K, Bisceglia M. Inflammatory myofibroblastic tumor of the larynx: a clinicopathologic study of eight cases simulating a malignant spindle cell neoplasm. Cancer 1995;76:2217-2229 [DOI] [PubMed] [Google Scholar]
- 2.Flanders AE, Mafee MF, Rao VM, Choi KH. CT characteristics of orbital pseudotumors and other orbital inflammatory processes. J Comput Assist Tomogr 1989;13:40-47 [DOI] [PubMed] [Google Scholar]
- 3.Som PM, Brandwein MS, Maldjian C, Reino AJ, Lawson W. Inflammatory pseudotumor of the maxillary sinus: CT and MR findings in six cases. AJR Am J Roentgenol 1994;163:689-692 [DOI] [PubMed] [Google Scholar]
- 4.Keen M, Cho HT, Savetsky L. Pseudotumor of the larynx: an unusual cause of airway obstruction. Otolaryngol Head Neck Surg 1986;94:243-246 [DOI] [PubMed] [Google Scholar]
- 5.Kaye AH, Hahn JF, Craciun A, Hanson M, Berlin AJ, Tubbs RR. Intracranial extension of inflammatory pseudotumor of the orbit. J Neurosurg 1984;60:625-629 [DOI] [PubMed] [Google Scholar]
- 6.Coffin CM, Watterson J, Priest JR, Dehner LP. Extrapulmonary inflammatory myofibroblastic tumor (inflammatory pseudotumor): a clinicopathologic and immunohistochemical study of 84 cases. Am J Surg Pathol 1995;19:859-872 [DOI] [PubMed] [Google Scholar]
- 7.Hytiroglou P, Brandwein MS, Strauchen JA, Mirante JP, Urken ML, Biller HF. Inflammatory pseudotumor of the parapharyngeal space: case report and review of the literature. Head Neck 1992;14:230-234 [DOI] [PubMed] [Google Scholar]
- 8.Harr DL, Quencer RM, Abrams GW. Computed tomography and ultrasound in the evaluation of orbital infection and pseudotumor. Radiology 1982;142:395-401 [DOI] [PubMed] [Google Scholar]
- 9.Orcutt JC, Garner A, Henk JM, Wright JE. Treatment of idiopathic inflammatory orbital pseudotumors by radiotherapy. Br J Ophthalmol 1983;67:570-574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Inui M, Tagawa T, Mori A, Yoneda J, Nomura J, Fukumori T. Inflammatory pseudotumor in the submandibular region: clinicopathologic study and review of the literature. Oral Surg Oral Med Oral Pathol 1993;76:333-337 [DOI] [PubMed] [Google Scholar]
- 11.Sclafani AP, Kimmelman CP, McCormick SA. Inflammatory pseudotumor of the larynx: comparison with orbital inflammatory pseudotumor with clinical implications. Otolaryngol Head Neck Surg 1993;109:548-551 [DOI] [PubMed] [Google Scholar]
- 12.Williams SB, Foss RD, Ellis GL. Inflammatory pseudotumors of the major salivary glands: clinicopathologic and immunohistochemical analysis of six cases. Am J Surg Pathol 1992;16:896-902 [DOI] [PubMed] [Google Scholar]
- 13.Weisman RA, Osguthorpe JD. Pseudotumor of the head and neck masquerading as neoplasia. Laryngoscope 1988;98:610-614 [DOI] [PubMed] [Google Scholar]
- 14.Diamond JP, Bloom PA, Ragge N, Easty DL, Laszlo G. Localised Wegener's granulomatosis presenting as an orbital pseudotumour with extension into the posterior cranial fossa. Eur J Ophthalmol 1993;3:143-146 [DOI] [PubMed] [Google Scholar]
- 15.Pillai P, Saini JS. Bilateral sino-orbital pseudotumour. Can J Ophthalmol 1988;23:177-180 [PubMed] [Google Scholar]
- 16.Borruat FX, Vuilleumier P, Ducrey N, Fankhauser H, Janzer RC, Regli F. Idiopathic orbital inflammation (orbital inflammatory pseudotumour): an unusual cause of transient ischaemic attack. J Neurol Neurosurg Psychiatry 1995;58:88-90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chan JKC. Inflammatory pseudotumor: a family of lesions of diverse natures and etiologies. Adv Anat Pathol 1996;3:156-171 [Google Scholar]
- 18.Ryan GB, Cliff WJ, Gabbiani G, et al. Myofibroblasts in human granulation tissue. Hum Pathol 1974;5:55-67 [DOI] [PubMed] [Google Scholar]
- 19.Sciot R, Dal Cin P, Fletcher CDM, et al. Inflammatory myofibroblastic tumour of bone: report of two cases with evidence of clonal chromosomal changes. Am J Surg Pathol 1997;21:1166-1172 [DOI] [PubMed] [Google Scholar]
- 20.Atlas SW, Grossman RI, Savino PJ, et al. Surface-coil MR of orbital pseudotumor. AJNR Am J Neuroradiol 1987;8:141-146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yousem DM, Atlas SW, Grossman RI, Sergott RC, Savino PJ, Bosley TM. MR imaging of Tolosa-Hunt syndrome. AJNR Am J Neuroradiol 1989;10:1181-1184 [PMC free article] [PubMed] [Google Scholar]
- 22.Okamoto M, Takahashi H, Yamanaka J, Nemoto S, Kuno K, Ishii T. Sclerosing inflammatory pseudotumor arising from the carotid artery region. Auris Nasus Larynx 1997;24:315-320 [DOI] [PubMed] [Google Scholar]
- 23.Hermans R, Horvath M, De Schrijver T, Lemahieu SF, Baert AL. Extranodal non-Hodgkin lymphoma of the head and neck. J Belge Radiol 1994;77:72-77 [PubMed] [Google Scholar]
- 24.Mancuso A. Lymphoma, master of chicanery. AJNR Am J Neuroradiol 1998;19:1808-1809 [PMC free article] [PubMed] [Google Scholar]
- 25.Heersink B, Rodrigues MR, Flanagan JC. Inflammatory pseudotumor of the orbit. Ann Ophthalmol 1977;9:17-29 [PubMed] [Google Scholar]
- 26.Paris GL, Waltuch GF, Egbert PR. Treatment of refractory orbital pseudotumors with pulsed chemotherapy. Ophthal Plast Reconstr Surg 1990;6:96-101 [DOI] [PubMed] [Google Scholar]


