Abstract
BACKGROUND AND PURPOSE: The suboccipital cavernous sinus, a vertebral venous plexus surrounding the horizontal portion of the vertebral artery at the skull base, provides an alternative pathway of cranial venous drainage by virtue of its connections to the cranial dural sinuses, the vertebral venous plexus, and the jugular venous system. Knowledge of the anatomy of this system facilitates interpretation of images and might reduce the number of false-positive diagnoses of lesions, such as adenopathy or schwannoma. We hypothesized that this circulation could be visualized on contrast-enhanced, fat-suppressed T1-weighted MR images.
METHODS: The craniocervical junctions of 14 patients were scanned using fat-suppressed, contrast-enhanced, T1-weighted MR sequences and evaluated for visibility of the following venous structures: suboccipital cavernous sinus, vertebral artery venous plexus, anterior and posterior condylar veins, vertebral venous plexus, internal jugular vein, and the marginal sinus. Both the right and left sides were assessed in at least two planes. The venous diameters were also measured.
RESULTS: All the evaluated venous structures were seen routinely in all three planes, with the exception of the posterior condylar vein, known to be variably present, which was seen only one third of the time in the sagittal plane and two thirds of the time in the other planes. The posterior condylar vein also showed the greatest variability in size and symmetry.
CONCLUSION: The suboccipital cavernous sinus and most of its associated venous circulation at the skull base are easily identified on contrast-enhanced, fat-suppressed T1-weighted MR images. The posterior condylar vein, known to be variably present, was not well seen in the sagittal plane and displayed the greatest variability in size and symmetry.
The venous circulation at the craniocervical junction has been described primarily in the neurosurgical, anatomic, and angiographic literature, with most cross-sectional imaging reports limited to descriptions of isolated emissary vessels or lesions, mainly vascular malformations, that cause venous enlargement (1–21). Arnautovic et al (1) recently evaluated and reviewed the venous circulation at the craniocervical junction. These authors diagrammed the numerous interconnections of an entity they called the suboccipital cavernous sinus, which they defined as the venous compartment surrounding the horizontal segment of the vertebral artery at the skull base (Fig 1). Knowledge of this skull base venous anatomy is important in the understanding of pathologic processes and in differentiating normal structures from lesions. We hypothesized that this venous circulation could be well seen on contrast-enhanced, fat-suppressed T1-weighted MR images.
fig 1.
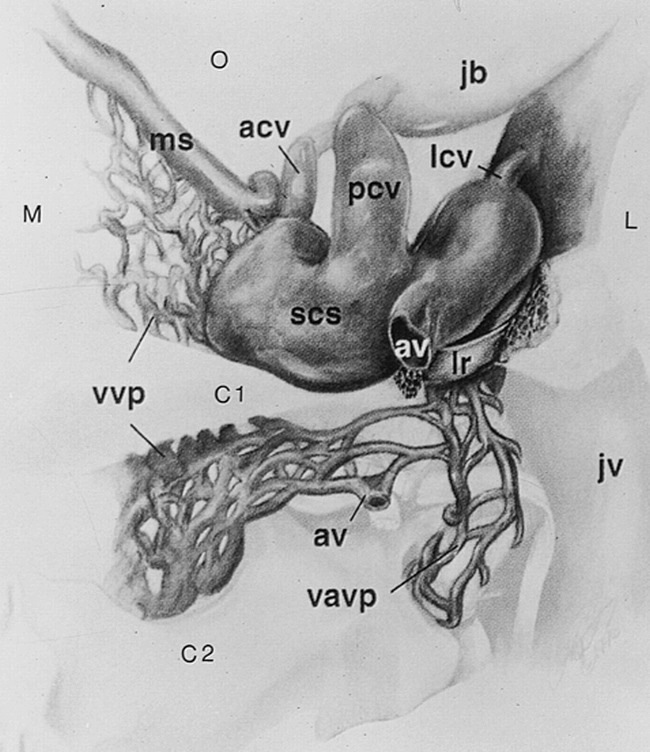
Posterior view of the right suboccipital cavernous sinus and its venous communications at the skull base. The vertebral artery has been removed. The left suboccipital cavernous sinus would form a mirror image, with direct connection of the marginal sinus and vertebral venous plexus across the midline. M indicates medial; L, lateral; C1, atlas; C2, axis; O, occipital region; acv, anterior condylar vein; av, anastomotic vein; jb, jugular bulb; jv, jugular vein; lcv, lateral condylar vein; lr, lateral ring; ms, marginal sinus; pcv, posterior condylar vein; scs, suboccipital cavernous sinus; vavp, vertebral artery venous plexus; vvp, vertebral venous plexus. Adapted from (1) with permission
Methods
The venous circulation at the craniocervical junction in 14 patients was evaluated on all MR images (obtained at 1.5 T) over a 16-month period that met the following criteria: 1) craniocervical junction coverage to lower C2 in at least two planes, 2) T1-weighted spin-echo technique with fat suppression, 3) contrast enhancement (0.1 mM/kg of gadolinium chelate for 12 patients and half that dose for two patients), 4) section thickness of 3 to 4 mm, and 5) no evidence of venous occlusive disease or arteriovenous shunting. Imaging was performed for a variety of clinical problems, the most common being neurosensory hearing loss. Four images were evaluated retrospectively and 10 prospectively.
Images were evaluated independently by four faculty neuroradiologists for conspicuity of the suboccipital cavernous sinus and the following structures that communicated with it: 1) vertebral artery venous plexus (except in the sagittal plane in two patients), 2) anterior condylar vein, 3) posterior condylar vein, 4) internal vertebral venous plexus, and 5) marginal sinus. The internal jugular vein, including the bulb, was also evaluated. Of the 14 patients, seven were women and seven men; average ages were 48 and 36 years, respectively, with most patients in their fourth and fifth decades. One patient had imaging studies in all three planes; the remaining 13 in two planes. Both sides were evaluated in each plane for a total of 28 coronal, 18 axial, and 12 sagittal assessments in the 14 patients. Disagreements among the four physicians were settled by a majority vote; in all instances, a consensus was reached.
In addition to the above determinations, the venous structures were measured on a computer monitor using electronic calipers; their relative degree of bilateral symmetry was assessed.
Results
Tables 1 and 2 summarize our results. The venous structures studied could be detected reliably in all three planes (Figs 2–5) with the exception of the posterior condylar vein in the sagittal plane, which was detectable only a third of the time; conversely, it was seen two thirds of the time in the other planes. Generally, the axial and coronal planes proved to depict the venous anatomy to greatest advantage.
Table 1:
Normal suboccipital venous structures seen in 14 patients using contrast-enhanced T1-weighted fat-suppressed sequences*
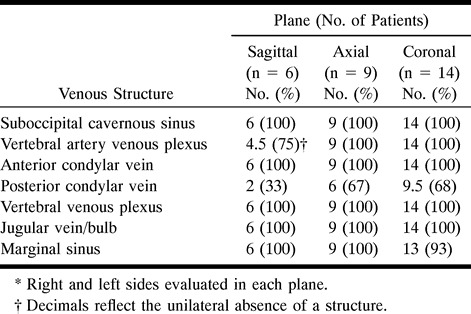
Table 2:
Summary of measured diameters of normal suboccipital venous structures in 14 patients using contrast-enhanced T1-weighted fat-suppressed MR sequences*
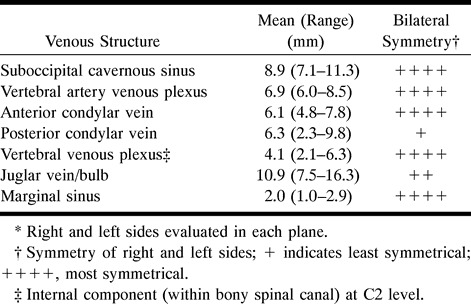
fig 2.
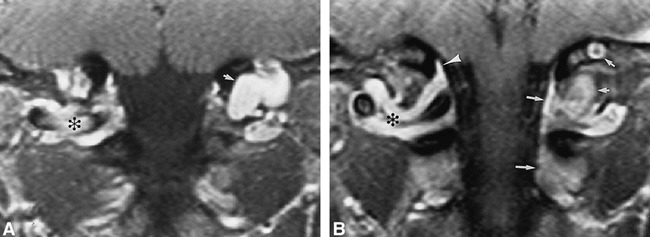
45-year-old man referred for follow-up of a presumed schwannoma posterior to the left occipital condyle, shown to be the left posterior condylar vein.
A and B, Contrast-enhanced fat-suppressed T1-weighted (640/14/3) coronal images (A posterior to B) show the suboccipital cavernous sinus (asterisk), marginal sinus (arrowhead), posterior condylar vein (short arrows), and vertebral venous plexus (long arrows). The right posterior condylar vein is diminutive.
fig 3.
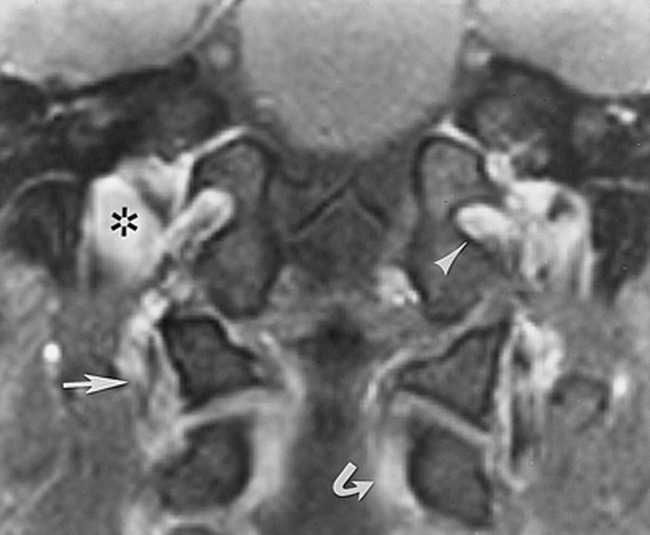
43-year-old man. Contrast-enhanced fat-suppressed T1-weighted (950/12/2) coronal image in a plane anterior to figure 2 shows the anterior condylar vein (arrowhead) and the jugular vein (asterisk) at the jugular tubercle, the vertebral venous plexus (curved arrow), and the vertebral artery venous plexus (straight arrow)
fig 4.
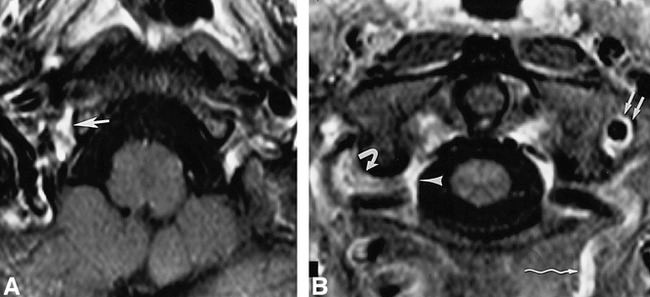
68-year-old woman.
A and B, Contrast-enhanced fat-suppressed T1-weighted (630/20/2) axial images (superior to inferior) show the anterior condylar vein (large straight arrow), suboccipital cavernous sinus (curved arrow), vertebral venous plexus (arrowhead), vertebral artery venous plexus (small straight arrows), and anastomotic vein (wavy arrow).
fig 5.
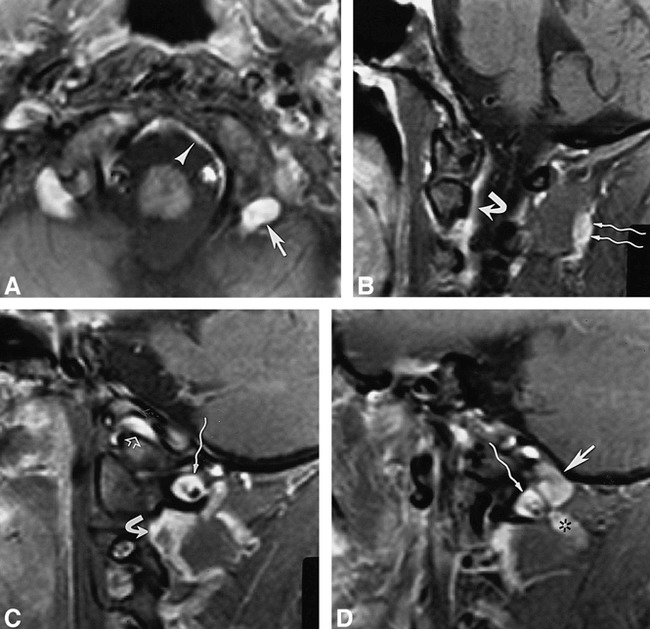
34-year-old woman.
A–D, Contrast-enhanced fat-suppressed T1-weighted (820/12/2) axial image (in a plane between fig 4A and B) (A) and T1-weighted (700/12/2) sagittal images (medial to lateral) (B–D) show the posterior condylar vein (straight arrow, A and D) at the posterior aspect of the occipital condyle, marginal sinus (arrowhead, A), suboccipital cavernous sinus (wavy arrow, C and D), vertebral venous plexus (curved arrow, B and C), suboccipital venous plexus (wavy arrows, B), anterior condylar vein (open arrow, C), and anastomotic vein (asterisk, D).
The vertebral artery venous plexus, suboccipital cavernous sinus, and anterior condylar vein showed the least variation from patient to patient; the posterior condylar vein and jugular vein and bulb showed the most. The posterior condylar vein and, to a lesser extent, the jugular vein and bulb displayed the greatest asymmetry. The remaining vessels were remarkably symmetrical. In some patients, the vertebral artery venous plexus and suboccipital cavernous sinuses were mildly asymmetrical, which was related to the size of their corresponding vertebral arteries and their foramina. Usually, the variation in size of the venous structure was less than that of the artery within it.
Discussion
Arnautovic et al (1) recently reviewed and analyzed the venous anatomy at the craniocervical junction. These investigators emphasized the importance of the suboccipital cavernous sinus, a term they proposed to describe the venous compartment surrounding the horizontal portion of the vertebral artery at the skull base (Fig 1). They described its many communications, including the following: 1) anterior condylar vein, connecting to the jugular bulb via the anterior condylar (hypoglossal) canal in the region of the jugular tubercle; 2) posterior condylar vein, connecting to the jugular bulb via the posterior condylar canal at the posterior aspect of the occipital condyle; 3) lateral condylar vein, which connects to the internal jugular vein at the lateral aspect of the occipital condyle; 4) vertebral artery venous plexus, the venous plexus surrounding the vertical segment of the vertebral artery at the level of the axis, which connects to the vertebral venous plexus and continues inferiorly as vertebral veins; 5) marginal sinus, the sinus in the dura leaves at the margin of the atlantooccipital ligament, which connects to the occipital and basilar venous sinuses superiorly and to the vertebral venous plexus inferiorly; 6) vertebral venous plexus, consisting of internal (epidural veins within the bony spinal canal) and external (other paravertebral and intravertebral veins) components, which interconnect at each level and also communicate with the vertebral artery venous plexus, the marginal sinus, and with the opposite suboccipital cavernous sinus; and 7) anastomotic vein, which connects to the suboccipital venous plexus, a variable plexus posterior to the suboccipital cavernous sinus, located between the deep and intermediate muscle layers and having interconnections of its own.
Previous investigators have shown that the vertebral venous plexus performs in concert with the jugular venous system in the drainage of the cranial cavity in healthy patients, providing an important additional pathway (2–5). It has been noted on angiograms in both monkeys and humans to provide the majority of cranial drainage in the upright position (2–5); this probably relates to a relative increase in intrathoracic pressure (3). The plexus is important as a reservoir and as an auxiliary drainage pathway in certain situations, such as during the Valsalva maneuver and when the jugular circulation is compromised (2–5). Venous enlargement may occur in a variety of lesions, including vascular malformations and venous obstructive lesions (4–11). An instance of greatly enlarged craniocervical epidural veins after craniectomy has recently been reported (11). Epidural venous congestion and enlargement may accompany intracranial hypotension (12, 13).
This study represents a work in progress, with several attendant limitations. Our study population was relatively small. We did not study the lateral condylar vein, owing to its very small size, or the anastomotic vein and suboccipital venous plexus, because we did not include the posterior soft tissues in the coronal plane; the intradural venous circulation was not studied. The posterior condylar vein, previously reported by Ginsberg (14) and Weissman (15) to be commonly asymmetrical or absent, was the most difficult vein to evaluate, owing to its frequent variation in size and symmetry and its potential, when small, for partial volume averaging with the suboccipital cavernous sinus. It was seen in only one third of the sagittal evaluations and two thirds of the coronal and axial evaluations. The remaining vessels were easy to identify in all planes because of their specific relationships to bony landmarks.
Our observations only relate to the resting patient in the supine position. A small portion of the “venous” enhancement of the suboccipital cavernous sinus and vertebral artery venous plexus may represent flow-related enhancement of the vertebral artery or enhancement of its vasa vasorum. Dural enhancement may contribute a small component. Pressure from the surface coil on the neck may affect venous filling. Our measurements may have been affected by volume averaging.
Conclusion
The venous circulation of the craniocervical junction, anchored by the suboccipital cavernous sinus, is well seen on contrast-enhanced, fat-suppressed T1-weighted MR images. The axial and coronal planes provide the best view. All the venous structures studied were easily identifiable in all patients, except for the posterior condylar vein, which was highly variable in terms of size, symmetry, and presence. The jugular vein was frequently asymmetrical. The remaining vessels were remarkably symmetrical. The least variation from patient to patient was displayed by the vertebral artery venous plexus, suboccipital cavernous sinus, and anterior condylar vein; the most variation by the posterior condylar vein and jugular vein.
Acknowledgments
We are grateful to John P. Griffin for his technical assistance and helpful suggestions.
Footnotes
Presented at the annual meeting of the American Society of Head and Neck Radiology, Phoenix, April 1998.
Address reprint requests to Ronald D. Caruso, MD.
References
- 1.Arnautovic KI, Al-Mefty O, Pait TG, Krisht AF, Husain MM. The suboccipital cavernous sinus. J Neurosurg 1997;86:252-262 [DOI] [PubMed] [Google Scholar]
- 2.Epstein HM, Linde HW, Crampton AR, Ciric IS, Eckenhoff JE. The vertebral venous plexus as a major cerebral venous outflow tract. Anesthesiology 1970;32:332-337 [DOI] [PubMed] [Google Scholar]
- 3.Eckenhoff JE. The vertebral venous plexus. Can Anaesth Soc J 1971;18:487-4955094099 [Google Scholar]
- 4.Dilenge D. The physiologic role of the meningorachidian plexus. In: Theron J, Moret J, eds. Spinal Phlebography. Berlin: Springer; 1978:3–23
- 5.Theron J, Moret J. Cervical phlebography. In: Theron J, Moret J, eds. Spinal Phlebography. Berlin: Springer; 1978:119–127
- 6.Friedman DP, Flanders AE, Tartaglino LM. Vascular neoplasms and malformations, ischemia, and hemorrhage affecting the spinal cord: MR imaging findings. AJR Am J Roentgenol 1994;162:685-692 [DOI] [PubMed] [Google Scholar]
- 7.Mascalchi M, Scazzeri F, Prosetti D, et al. Dural arteriovenous fistula at the craniocervical junction with perimedullary venous drainage. AJNR Am J Neuroradiol 1996;17:1137-1141 [PMC free article] [PubMed] [Google Scholar]
- 8.Willinsky R, terBrugge K, Lasjaunias P, Montanera W. The variable presentations of craniocervical and cervical dural arteriovenous malformations. Surg Neurol 1990;34:118-123 [DOI] [PubMed] [Google Scholar]
- 9.Cahan LD, Higashida RT, Halbach VV, Hieshima GB. Variants of radiculomeningeal vascular malformations of the spine. J Neurosurg 1987;66:333-337 [DOI] [PubMed] [Google Scholar]
- 10.Blomquist MH, Barr JD, Hurst RW. Isolated unilateral hypoglossal neuropathy caused by dural arteriovenous fistula. AJNR Am J Neuroradiol 1998;19:951-953 [PMC free article] [PubMed] [Google Scholar]
- 11.Caruso RD, Smith MV, Chang JK, Wasenko JJ, Rosenbaum AE. Giant cervical epidural veins after craniectomy for head trauma. AJNR Am J Neuroradiol 1998;19:903-906 [PMC free article] [PubMed] [Google Scholar]
- 12.Rabin BM, Roychowdhury S, Meyer JR, Cohen BA, LaPat KD, Russell EJ. Spontaneous intracranial hypotension: spinal MR findings. AJNR Am J Neuroradiol 1998;19:1034-1039 [PMC free article] [PubMed] [Google Scholar]
- 13.Moayeri NN, Henson JW, Schaeffer PW, Zervas NT. Spinal dural enhancement on magnetic resonance imaging associated with spontaneous intracranial hypotension. J Neurosurg 1998;88:912-918 [DOI] [PubMed] [Google Scholar]
- 14.Ginsberg L. The posterior condylar canal. AJNR Am J Neuroradiol 1994;15:969-972 [PMC free article] [PubMed] [Google Scholar]
- 15.Weissman JL. Condylar canal vein: unfamiliar normal structure as seen at CT and MR imaging. Radiology 1994;190:81-84 [DOI] [PubMed] [Google Scholar]
- 16.Braun JP, Tournade A. Venous drainage in the craniocervical region. Neuroradiology 1977;13:155-158 [DOI] [PubMed] [Google Scholar]
- 17.Wen HT, Rhoton AL, Katsuta T, Oliveira ED. Microsurgical anatomy of the transcondylar, supracondylar, and paracondylar extensions of the far lateral approach. J Neurosurg 1997;87:555-585 [DOI] [PubMed] [Google Scholar]
- 18.Johnson MH. Head and neck vascular anatomy. In: Gentry LR, ed. Normal Anatomy of the Head and Neck: Neuroimaging Clin N Am 1998;8:119–142 [PubMed]
- 19.McKinnon SG. Anatomy of the cerebral veins, dural sinuses, sella, meninges, and cerebrospinal fluid spaces. In: Gentry LR, ed. Normal Anatomy of the Head and Neck: Neuroimaging Clin N Am 1998;8:101–118 [PubMed]
- 20.Smoker WRK. Normal anatomy of the neck. In: Som PM, Curtin HD, eds. Head and Neck Imaging 3rd ed. St Louis: Mosby; 1996;2:711–737
- 21.Cure JK, Van Tassel P, Smith MT. Normal and variant anatomy of the dural venous sinuses. Semin Ultrasound CT MR 1994;15:499-519 [DOI] [PubMed] [Google Scholar]


