Abstract
Background.
While ex vivo lung perfusion (EVLP) has become established in lung transplantation, the cellular processes occurring during this period are not yet fully understood. Prior studies demonstrated that donor leukocytes (DLs) migrate from the graft into the perfusate during EVLP, but the distribution of DLs in graft and perfusate compartments has not been characterized. Moreover, cell death of DLs has been implicated in mediating graft injury during EVLP, but the underlying mechanisms have not been elucidated. We hypothesized the following: (1) there is a nonspecific migration of DLs from the graft into perfusate and (2) cell death of DLs releases damage-associated molecular patterns (DAMPs) that contribute to the inflammatory milieu during EVLP.
Methods.
EVLP was performed on rat lungs for 3 hours (N = 6). At the end of EVLP, flow cytometry was used to quantify the distribution of different DL cell types in both the graft and perfusate compartments. During EVLP, the perfusate was also sampled hourly to measure levels of DAMPs and downstream inflammatory cytokines generated during EVLP.
Results.
At the conclusion of EVLP, there was a significantly higher proportion of T and B cells present in the perfusate compartment compared with the graft compartment. There was a time-dependent increase in extracellular DNA and tumor necrosis factor α in the perfusate during EVLP.
Conclusions.
T cells and B cells are enriched in the perfusate compartment during EVLP. Cell death of DLs contributes to an accumulation of DAMPs during EVLP.
Lung transplantation is the gold standard therapy for patients with end-stage lung disease. Despite efforts to increase donor utilization, there remains a significant shortage of donor lungs suitable for transplantation. Additionally, the survival of lung transplant recipients continues to lag behind other solid organ transplant recipients, reflecting the severity of illness and also limitations related to primary graft dysfunction (PGD) and chronic lung allograft dysfunction.1-3
Although ex vivo lung perfusion (EVLP) was initially utilized to assess donor lungs with marginal function,4,5 some forms of EVLP have become established alternatives to standard cold storage in lung preservation.6,7 A recent randomized trial comparing EVLP to standard cold storage demonstrated a reduction in the incidence of grade 3 PGD with EVLP.8 Moreover, EVLP provides a unique platform for performing therapeutic interventions to improve graft outcomes,9-12 which continues to be the subject of significant ongoing research.
An emerging body of literature in lung transplantation has highlighted the deleterious effects of donor leukocytes (DLs) as mediators of PGD and graft rejection.13,14 In the setting of EVLP, DLs have been shown migrate from the graft into the perfusate.15 Moreover, pyroptotic cell death of DLs during EVLP has recently been implicated in provoking graft injury.16 What remains unknown is the distribution of DL cell types in the graft and perfusate at the conclusion of EVLP, as well as the mechanisms by which cell death of DLs produces graft injury.
A possible mechanistic link between DL cell death and graft injury has emerged from recent studies focused on damage-associated molecular patterns (DAMPs) released during machine perfusion of lung and liver grafts.17,18 DAMPs are a heterogeneous group of molecules released by dying cells that activate proinflammatory cascades by binding to innate immune receptors.19-21 In lung transplantation, Hashimoto et al17 demonstrated an association between levels of M30 and high-mobility group box 1 (HMGB1) released during EVLP with the occurrence of PGD following human lung transplantation. In liver transplantation, we demonstrated that elevated levels of HMGB1 and extracellular DNA (exDNA) during machine perfusion of liver grafts were associated with the induction of inflammatory genes and increased histological injury following graft reperfusion.18
In the context of these recent observations, the primary objective of this study was to analyze the distribution of DL cell types in both graft and perfusate compartments following EVLP. We hypothesized that DLs migrate from the graft into the perfusate in a nonspecific manner, with no preferential migration of specific cell types. The secondary objective of the study was to determine the time course of exDNA and HMGB1 release, 2 DAMPs associated with cell death.22 We hypothesized that DAMP levels would reflect the magnitude of DL cell death during EVLP.
MATERIALS AND METHODS
Experimental Design
EVLP was performed on rat lungs for 3 hours as depicted in Figure 1 (N = 6). At the end of EVLP, flow cytometry was used to quantify the distribution of DLs in both the graft and perfusate compartments. During EVLP, the perfusate was sampled hourly to measure levels of DAMPs and downstream inflammatory cytokines generated during EVLP.
FIGURE 1.
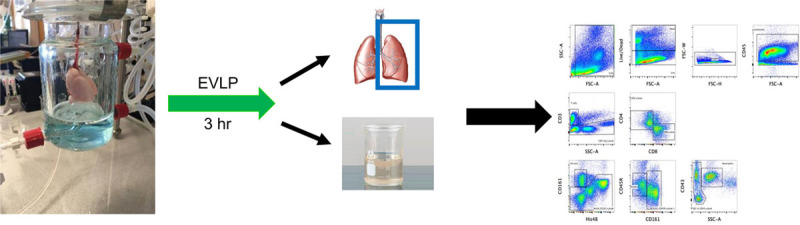
Experimental design and representative flow cytometry plot of major leukocyte populations in the rat. Antibody staining of leukocytes was performed as outlined in Table 1. EVLP, ex vivo lung perfusion; FSC-A, forward scatter area; FSC-H, forward scatter height; SSC-A, side scatter area.
Animals
The Duke University Institutional Animal Care and Use Committee approved all protocols. Animals were housed in standard pathogen-free conditions according to all institutional and governmental guidelines. Male Lewis (RT−1) rats (250–350 g; Charles River Laboratories, Wilmington, MA) were used in the following experiments.
Donor Lung Procurement
Rats were anesthetized with a mixture of 100% oxygen and isoflurane (2%), intubated and ventilated using positive pressure ventilation (tidal volume 10 mL/kg and respiratory rate of 60 breaths/min). The thoracic cavity was entered through a midline sternotomy. Heparin (1 U/g) was delivered through the inferior vena cava. The thymus was excised. The pulmonary artery (PA) was cannulated. The left atrium was incised to facilitate open drainage. The PA was then flushed with 20 mL of a cold (4°C) low potassium dextran electrolyte solution (Perfadex; XVIVO Perfusion, Gothenburg, Sweden). The heart and lungs were removed en bloc from the thoracic cavity and immersed in Perfadex at 4°C. The heart and lungs were then subjected to normothermic (37°C) EVLP as previously described.16,23,24
Ex Vivo Lung Perfusion
Normothermic EVLP was performed using a commercially available EVLP circuit (IL-2 Isolated Lung Perfusion System; Harvard Apparatus, Holliston, MA). The circuit was instilled with 100 mL of STEEN solution (XVIVO Perfusion, Gothenburg, Sweden), 40 mg of methylprednisolone (Solu-Medrol, Pfizer, Inc), 40 mg of cephalosporin, and 500 units of heparin. Circuit temperature (37°C) was regulated with a thermostatic water bath. The oxygenator in the circuit was used to deoxygenate the perfusate using a mixture of 8% CO2, 6% O2, and 86% N2. The perfusate was continuously recirculated for at least 30 minutes before organ perfusion.
Following donor lung procurement, the rat heart-lung blocks were mounted in the lung chamber and antegrade perfusion initiated. The incised left atrium facilitated open unobstructed drainage. Following a 20-minute rewarming period, ventilation was initiated. During EVLP, lung-protective ventilation strategies were utilized (positive end-expiratory pressure: 2.5–3 cm H2O, tidal volume: 5 mL/kg, respiratory rate: 60). The target perfusate flow rate was 20% of the cardiac output.24 Technical or procedural errors were defined by PA pressure >15 mm Hg, peak pressure > 20 cm H2O, or physical damage to the graft during initiation of EVLP and were excluded from the analysis. Of note, a leukocyte filter was not utilized in these experiments to allow a complete characterization of the distribution of DLs in the perfusate.
Physiological Measurements During EVLP
Physiological parameters of the perfused lung were measured during EVLP. Arterial pressure was measured using an arterial pressure transducer (P75, Harvard Apparatus). Inspiratory pressure was measured using a low-pressure transducer for respiratory flow (DLP2.5 Type 381, Harvard Apparatus) and a differential pressure transducer for airway pressure (MPX, Harvard Apparatus). Input signals from these transducers were recorded and analyzed using a Power Lab data acquisition system (ADI Instruments). The PA pressure, peak inspiratory pressure, and changes in compliance were continuously recorded.
Perfusate Collection
Perfusate (1.5 mL) was collected hourly to determine the partial pressure of oxygen/fraction of inspired oxygen (P/F) ratio using an iSTAT point-of-care blood gas analyzer (Abbott Labs, Chicago, IL) with the remainder spun and stored for cytokine and DAMP assays. At the end of the perfusion period, the heart and lung block was removed from the circuit and the remaining perfusate was collected. The circuit was washed with an equal volume of sterile PBS, and these were combined for perfusate flow cytometric analysis.
Tissue Collection
The heart and lungs were removed from the perfusion circuit following 3 hours of normothermic perfusion. The left lung was then isolated and immersed in Perfadex (4°C) before tissue processing and flow cytometric analysis.
Tissue Processing
The left lung was mechanically disrupted and enzymatically digested at 37°C for 30 minutes using the following digestion media: 10 mL of Roswell Park Memorial Institute media with 10% fetal bovine serum (Thermo Fischer), DNAse I (5 mg/mL), Collagenase D (30 mg/mL), and Dispase II (5 mg/mL) (Roche, Switzerland).
Flow Cytometry
Flow cytometry was used to quantify the distribution of different DL cell types in both the graft and perfusate compartments as previously described25 (Table 1). Isolated cells were washed and stained with Live/Dead dye (eBioscience) in PBS and then stained with the following antibodies: anti-CD45 (Biolegend), anti-CD3 (Miltenyi Biotec), anti-CD4 (BD Bioscience), anti-CD8 (eBioscience), anti-CD43 (Biolegend), anti-His48 (eBioscience), anti-CD161 (Biolegend), anti-CD45R (eBioscience). Cell counts (cells/mL) were determined using BD Trucount tubes. Flow cytometric compensation was performed using fluorescent compensation beads, and cells were analyzed using a multiparameter flow cytometer (LSRFortessa BD Biosciences). Analysis of flow results was performed with FlowJo Software (Version 10; FlowJo LLC, Ashland, OR).
TABLE 1.
Monoclonal antibodies for leukocyte subset characterization

Extracellular DNA
Perfusate exDNA levels were determined using a Quant-iT PicoGreen dsDNA kit (Molecular Probes, Eugene, OR) according to the manufacturer’s instructions.
High-Mobility Group Box 1
Extracellular HMGB1 levels were determined using the HMGB1 ELISA kit (Tecan, Morrisville, NC) according to the manufacturer’s instructions.
Perfusate Tumor Necrosis Factor α Levels
Perfusate tumor necrosis factor α (TNF-α) levels were measured using BD Cytometric Bead Array Rat TNF Flex sets (BD Bioscience, San Jose, CA) on a BD LSRFortessa X-20 analyzer (BD Bioscience).
Statistical Analysis
An unpaired Student’s t-test was applied for determination of statistical significance between groups and a paired t-test was used within groups. GraphPad Prism Software (Version 8; GraphPad Software Inc., La Jolla, CA) was used for statistical analysis. All values are presented as mean ± SEM. A probability of <0.5 (P < 0.05) was used for statistical significance.
RESULTS
Leukocyte Viability
DLs were isolated from both the left lung graft and perfusate following 3 hours of EVLP. The percentage of dead cells was significantly higher in the graft compared with the perfusate (11.7 ± 1.2% versus 1.9 ± 0.6%, P = 0.001, Figure 2).
FIGURE 2.
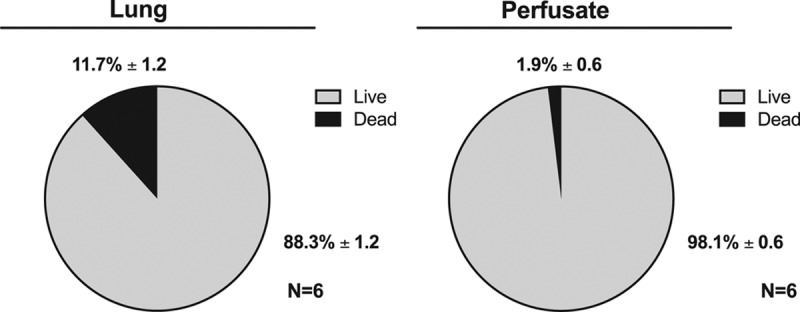
Quantification of the number of dead cells in graft and perfusate compartments following EVLP. Cells isolated from the lung graft and the perfusate at the conclusion of 3 h of EVLP were stained with live/dead dye and quantified using flow cytometry. EVLP, ex vivo lung perfusion.
Distribution of DLs by Compartment
Graft Compartment
In the graft, the percentage of T cells and B cells were analyzed in relation to the total DL population (Figure 3). The percentage of T cells was significantly higher than B cells (T cells 25.2 ± 1.9% versus B cells 11.2 ± 3.5%, P = 0.005).
FIGURE 3.
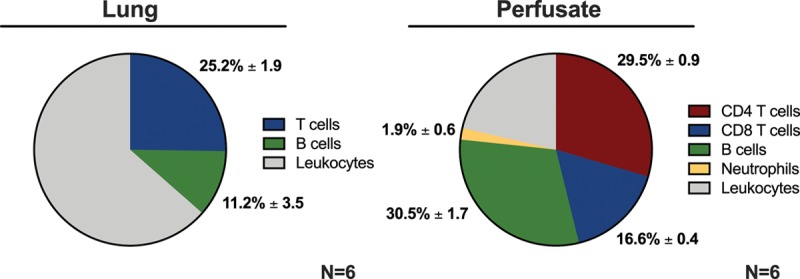
Distribution of donor leukocytes in graft and perfusate compartments following EVLP. Three hours of EVLP was performed, followed by flow cytometric analysis of leukocytes isolated from the lung graft and the perfusate compartments. EVLP, ex vivo lung perfusion.
Perfusate Compartment
In the perfusate, the percentage of T cells (CD4+, CD8+), B cells, and neutrophils were analyzed in relation to the total DL population (Figure 3). The percentage of T cells was significantly higher than B cells (T cells: 47.9 ± 0.8% versus B cells: 30.5 ± 1.7%, P = 0.001, Figure 3). Of the T-cell population, the percentage of CD4+ T cells was significantly higher than CD8+ T cells (CD4+ T cells: 29.5 ± 1.0% versus CD8+ T cells: 16.6 ± 0.4%, P = 0.001). The percentage of T cells and B cells in the perfusate was significantly higher than the percentage of neutrophils (P = 0.001).
Comparison of Graft Versus Perfusate Distribution of DL Cell Types
We hypothesized that there is a nonspecific migration of DLs from the graft into the perfusate during EVLP that would produce a similar distribution of cell types in the graft and perfusate compartments at the conclusion of EVLP. To test this, we compared the distribution of cell types in each compartment. Contrary to our hypothesis, this analysis demonstrated an enrichment of T and B cells in the perfusate compartment in comparison to the graft compartment.
The percentage of both T cells and B cells was significantly higher in the perfusate compared with the graft (T cells: perfusate 47.9 ± 0.8% versus graft 25.2 ± 1.9%, P = 0.0001; B cells: perfusate 30.5 ± 1.7% versus graft 11.2 ± 3.5%, P = 0.0006).
DAMPs and Inflammatory Cytokines
Extracellular DNA
There was a significant increase in the level of exDNA over the 3-h perfusion (1 h: 225.1 ± 7.4 ng/mL versus 2 h: 266.6 ± 6.7 ng/mL, P = 0.02; 2 h: 266.6 ± 6.7 ng/mL versus 3 h: 306.7 ±11.8 ng/mL, P = 0.01, Figure 4).
FIGURE 4.
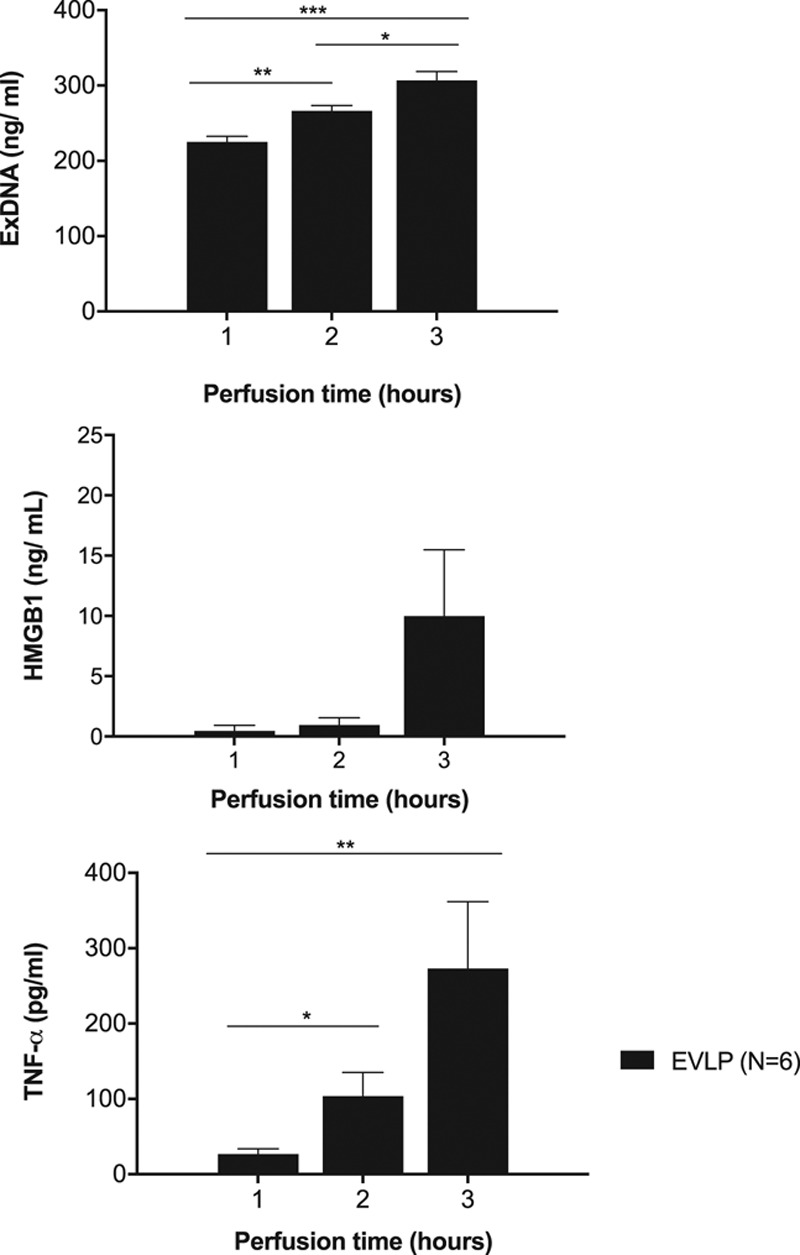
DAMP and cytokine release during EVLP. A, exDNA, (B) HMGB1, and (C) TNF-α levels were measured hourly in the perfusate during 3 h of EVLP. *P < 0.05, **P < 0.01, ***P < 0.001. DAMP, damage-associated molecular pattern; EVLP, ex vivo lung perfusion; exDNA, extracellular DNA; HMGB1, high-mobility group box 1; TNF-α, tumor necrosis factor α.
High-Mobility Group Box 1
HMGB1 levels in the perfusate increased over the 3-hour perfusion period, demonstrating a trend toward significance (1 h: 0.46 ± 0.5 ng/mL versus 3 h: 9.98 ± 5.5 ng/mL, P = 0.12; Figure 4).
Tumor Necrosis Factor α
There was a significant increase in the level of TNF-α over the 3-hour perfusion (1 h: 26.7 ± 7.1 pg/mL versus 2 h: 103.9 ± 31.3 pg/mL, P = 0.03; 1 h: 26.7 ± 7.1 pg/mL versus 3 h: 273.3 ± 88.6 pg/mL, P = 0.02, Figure 4).
Physiological Characteristics
Lung function remained stable over 3 hours of EVLP (Figure 5). There was an inverse correlation between TNF-α level in the perfusate and the P/F ratio (r = −0.82; P = 0.045; R2 = 0.67), demonstrating an association between elevated levels of TNF-α and diminished lung function during EVLP. Levels of exDNA and HMGB1 did not correlate with P/F ratio.
FIGURE 5.
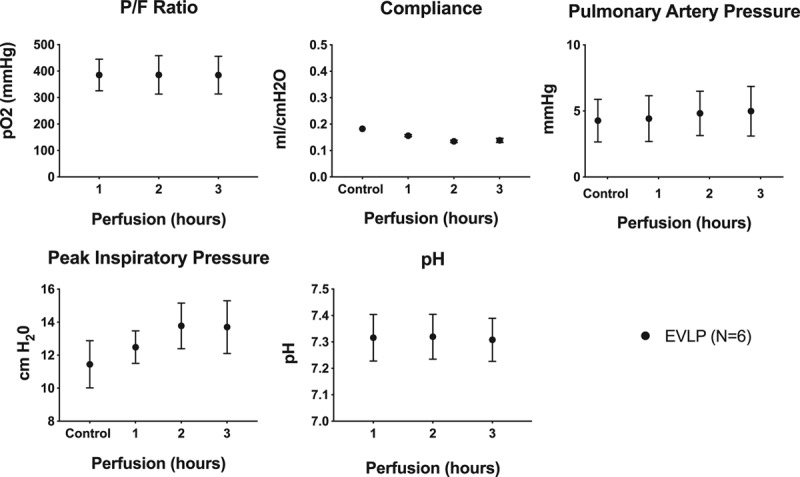
Lung function during EVLP. A, P/F ratio, (B) pulmonary arterial pressure, (C) lung compliance, and (D) peak airway pressure were measured hourly during EVLP. Data are represented as mean ± SEM. EVLP, ex vivo lung perfusion; P/F, partial pressure of oxygen/fraction of inspired oxygen; SEM, standard error of the mean.
The PA pressure increased during the 3 hours of EVLP (0 h: 4.3 ± 0.7 versus 3 h: 5.0 ± 0.8 mm Hg, P = 0.008). Similarly, the peak inspiratory pressure increased during the 3 hours of EVLP (0 h: 11.5 ± 0.6 versus 3 h: 13.7 ± 0.7 cm H2O, P = 0.035). Compliance decreased in the EVLP group over the 3 hours of EVLP (0 h: 0.20 ± 0.01 versus 3 h: 0.14 ± 0.01 mL/cm H2O, P = 0.005).
Perfusate pH was stable over time in the EVLP group (1 h: 7.32 ± 0.04 versus 3 h: 7.31 ± 0.03, P = 0.37).
DISCUSSION
In this study, we demonstrate an enrichment of T and B cells in the perfusate following 3 hours of EVLP. These data suggest a preferential migration of T and B cells out of the lung during EVLP, in contrast to our initial hypothesis that there is a nonspecific migration of all cell types. The significance of this finding is not yet fully understood, but recent literature suggests that donor-derived T and B cells mediate downstream effects on the alloimmune response, based on subtype. Harper et al26 demonstrated that donor-derived passenger CD4 T cells markedly enhance cellular and humoral alloimmunity leading to graft failure, while donor-derived regulatory CD4 T cells inhibit alloimmunity and prolong graft survival.27 Donor B cells present antigen in recipient lymphoid organs, activate naive T cells, and contribute to acute rejection.28 Thus, using EVLP to alter specific populations of graft leukocytes may have potential as a strategy to modulate the subsequent alloimmune response.
Defining the biological properties of DAMPs produced during machine perfusion is an active area of investigation and may provide a mechanism for recent observations linking DL cell death and graft inflammatory injury.16 Furthermore, Hashimoto et al17 demonstrated that DAMP levels during EVLP may serve as a biomarker for the development of PGD posttransplant. In this study, we demonstrated a time-dependent increase in levels of exDNA and the downstream inflammatory cytokine TNF-α. However, a limitation of this analysis is that we are unable to differentiate the specific cell types responsible for DAMP release (parenchymal versus DLs). Further study is also required to ascertain which DAMPs are most relevant as predictors of PGD and to define the actual inflammatory properties of DAMPs themselves.
In conclusion, there is a preferential migration of T cells and B cells from the graft into the perfusate during EVLP. This finding is contrary to our previous hypothesis that a nonspecific migration of DLs occurs during EVLP. This model may provide a foundation for future studies aiming to alter DL populations during EVLP to reduce donor lung immunogenicity.
Footnotes
Published online 10 February, 2020.
R.P.D. and J.Y. contributed equally.
The authors declare no funding or conflicts of interest.
R.P.D. involved in research design, performance of research, data analysis, and writing of paper. J.Y. involved in research design, performance of research, data analysis, and writing of paper. Q.G. involved in performance of research and data analysis. J.G. involved in performance of research and data analysis. U.S. involved in performance of research and data analysis. M.S. involved in performance of research and data analysis. M.Z. involved in performance of research and data analysis. W.P. involved in research design, data analysis, and writing of paper. J.L. involved in data analysis and writing of paper. M.G.H. involved in research design, data analysis, and writing of paper. A.S.B. involved in research design, data analysis, and writing of paper.
REFERENCES
- 1.van Suylen V, Luijk B, Hoek RAS, et al. A multicenter study on long-term outcomes after lung transplantation comparing donation after circulatory death and donation after brain death. Am J Transplant.. 2017; 17:2679–2686 [DOI] [PubMed] [Google Scholar]
- 2.Chambers DC, Yusen RD, Cherikh WS, et al. International Society for Heart and Lung Transplantation The registry of the International Society for Heart and Lung Transplantation: Thirty-Fourth Adult Lung and Heart-Lung Transplantation Report-2017; Focus Theme: allograft ischemic time. J Heart Lung Transplant.. 2017; 36:1047–1059 [DOI] [PubMed] [Google Scholar]
- 3.Maxwell BG, Levitt JE, Goldstein BA, et al. Impact of the lung allocation score on survival beyond 1 year. Am J Transplant.. 2014; 14:2288–2294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ingemansson R, Eyjolfsson A, Mared L, et al. Clinical transplantation of initially rejected donor lungs after reconditioning ex vivo. Ann Thorac Surg.. 2009; 87:255–260 [DOI] [PubMed] [Google Scholar]
- 5.Cypel M, Yeung JC, Liu M, et al. Normothermic ex vivo lung perfusion in clinical lung transplantation. N Engl J Med.. 2011; 364:1431–1440 [DOI] [PubMed] [Google Scholar]
- 6.Cypel M, Yeung JC, Machuca T, et al. Experience with the first 50 ex vivo lung perfusions in clinical transplantation. J Thorac Cardiovasc Surg.. 2012; 144:1200–1206 [DOI] [PubMed] [Google Scholar]
- 7.Slama A, Schillab L, Barta M, et al. Standard donor lung procurement with normothermic ex vivo lung perfusion: a prospective randomized clinical trial. J Heart Lung Transplant.. 2017; 36:744–753 [DOI] [PubMed] [Google Scholar]
- 8.Warnecke G, Van Raemdonck D, Smith MA, et al. Normothermic ex-vivo preservation with the portable Organ Care System Lung device for bilateral lung transplantation (INSPIRE): a randomised, open-label, non-inferiority, phase 3 study. Lancet Respir Med.. 2018; 6:357–367 [DOI] [PubMed] [Google Scholar]
- 9.Cypel M, Liu M, Rubacha M, et al. Functional repair of human donor lungs by IL-10 gene therapy. Sci Transl Med.. 2009; 1:4ra9. [DOI] [PubMed] [Google Scholar]
- 10.Nakajima D, Cypel M, Bonato R, et al. Ex vivo perfusion treatment of infection in human donor lungs. Am J Transplant.. 2016; 16:1229–1237 [DOI] [PubMed] [Google Scholar]
- 11.Motoyama H, Chen F, Hijiya K, et al. Plasmin administration during ex vivo lung perfusion ameliorates lung ischemia-reperfusion injury. J Heart Lung Transplant.. 2014; 33:1093–1099 [DOI] [PubMed] [Google Scholar]
- 12.Wang X, Wang Y, Parapanov R, et al. Pharmacological reconditioning of marginal donor rat lungs using inhibitors of peroxynitrite and poly (ADP-ribose) polymerase during ex vivo lung perfusion. Transplantation.. 2016; 100:1465–1473 [DOI] [PubMed] [Google Scholar]
- 13.Zheng Z, Chiu S, Akbarpour M, et al. Donor pulmonary intravascular nonclassical monocytes recruit recipient neutrophils and mediate primary lung allograft dysfunction Sci Transl Med. 2017; 9:eaal4508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stone JP, Critchley WR, Major T, et al. Altered immunogenicity of donor lungs via removal of passenger leukocytes using ex vivo lung perfusion. Am J Transplant.. 2016; 16:33–43 [DOI] [PubMed] [Google Scholar]
- 15.Stone JP, Sevenoaks H, Sjöberg T, et al. Mechanical removal of dendritic cell-generating non-classical monocytes via ex vivo lung perfusion. J Heart Lung Transplant.. 2014; 33:864–869 [DOI] [PubMed] [Google Scholar]
- 16.Noda K, Tane S, Haam SJ, et al. Targeting circulating leukocytes and pyroptosis during ex vivo lung perfusion improves lung preservation. Transplantation.. 2017; 101:2841–2849 [DOI] [PubMed] [Google Scholar]
- 17.Hashimoto K, Cypel M, Juvet S, et al. Higher M30 and high mobility group box 1 protein levels in ex vivo lung perfusate are associated with primary graft dysfunction after human lung transplantation J Heart Lung Transplant. 2017; 2498:31870–31873 [DOI] [PubMed] [Google Scholar]
- 18.Scheuermann U, Zhu M, Song M, et al. Damage-associated molecular patterns induce inflammatory injury during machine preservation of the liver: potential targets to enhance a promising technology. Liver Transpl.. 2019; 25:610–626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Land WG, Agostinis P, Gasser S, et al. Transplantation and damage-associated molecular patterns (DAMPs). Am J Transplant.. 2016; 16:3338–3361 [DOI] [PubMed] [Google Scholar]
- 20.Todd JL, Palmer SM. Danger signals in regulating the immune response to solid organ transplantation. J Clin Invest.. 2017; 127:2464–2472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Braza F, Brouard S, Chadban S, et al. Role of TLRs and DAMPs in allograft inflammation and transplant outcomes. Nat Rev Nephrol.. 2016; 12:281–290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Magna M, Pisetsky DS. The alarmin properties of DNA and DNA-associated nuclear proteins. Clin Ther.. 2016; 38:1029–1041 [DOI] [PubMed] [Google Scholar]
- 23.Nelson K, Bobba C, Eren E, et al. Method of isolated ex vivo lung perfusion in a rat model: lessons learned from developing a rat EVLP program J Vis Exp. 2015.. doi: 10.3791/52309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Noda K, Shigemura N, Tanaka Y, et al. Successful prolonged ex vivo lung perfusion for graft preservation in rats. Eur J Cardiothorac Surg.. 2014; 45:e54–e60 [DOI] [PubMed] [Google Scholar]
- 25.Barnett-Vanes A, Sharrock A, Birrell MA, et al. A single 9-colour flow cytometric method to characterise major leukocyte populations in the rat: validation in a model of LPS-induced pulmonary inflammation. PLoS One.. 2016; 11:e0142520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Harper IG, Ali JM, Harper SJ, et al. Augmentation of recipient adaptive alloimmunity by donor passenger lymphocytes within the transplant. Cell Rep.. 2016; 15:1214–1227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Harper IG, Gjorgjimajkoska O, Siu JHY, et al. Prolongation of allograft survival by passenger donor regulatory T cells. Am J Transplant.. 2019; 19:1371–1379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dijke EI, Platt JL, Blair P, et al. B cells in transplantation. J Heart Lung Transplant.. 2016; 35:704–710 [DOI] [PMC free article] [PubMed] [Google Scholar]


