Supplemental Digital Content is available in the text.
Abstract
Background.
The approach to reducing nonspecific inflammation after islet allotransplantation has been designed to improve engraftment, typically using 1 agent. We report results with the use of combination inflammatory blockade consisting of anti-interleukin (IL)-1β and tumor necrosis factor (TNF)-α.
Methods.
Nine patients underwent islet allotransplantation under a prospective research protocol using double cytokine blockade with anti–TNF-α (etanercept, d 0, 3, 7, 10) and IL-1β (anakinra, d 0–7) at the time of each islet infusion. The primary endpoint, assessed 2 years after the last islet transplant, was the elimination of severe hypoglycemic events and hypoglycemia unawareness, with proper glycemic control, and detectable serum C-peptide.
Results.
No thrombotic events or infectious complications were associated with combined IL-1β and TNF-α blockade. Six patients became insulin independent, 2 had partial function, and 1 had primary nonfunction. After 24-month follow-up, 6 of 9 patients had excellent glycemic control, hemoglobin A1c ≤6.5%, and no episodes of hypoglycemia unawareness. Eight patients developed HLA alloantibodies at various time points (class 1, 5; class 2, 6), with enhanced T-cell alloreactivity. One patient retained good graft function despite having anti-glutamic acid decarboxylase 65 antibodies.
Conclusions.
The use of double cytokine blockade is safe, with reduction of inflammation at transplantation and presumably with better engraftment. However, it does not influence later islet loss from T-cell–mediated autoimmunity and alloimmunity, which require other strategies to maintain long-term islet function.
Islet allotransplantation is a treatment modality currently reserved for type 1 diabetic patients with poor glycemic control and hypoglycemia unawareness, refractory to maximal care with medication, insulin, diet, and follow-up.1 The success of the procedure depends on the infusion of good-quality islet preparations, good engraftment, and avoidance of islet loss due to immunologic events in which allogeneic and autologous immunity are involved, as well as nonimmunologic reactions.2 Islet loss has less of an impact if a good initial islet mass is engrafted. Studies in both animal models and in the clinical setting show that half of the islet mass is lost in the first days after islet infusion. A part of the loss is attributable to initial islet viability, but a more important factor in islet loss is instant blood-mediated inflammatory reaction.3,4 Tumor necrosis factor (TNF)-α and interleukin (IL)-1β are 2 key proinflammatory cytokines known to cause islet β cell death.5 The approach to reducing nonspecific inflammation has been designed to improve engraftment, typically using etanercept, a TNF-α blocker.6 The objective of this study is to use a combination inflammatory blockade consisting of anti-IL-1β and TNF-α in the early course after islet allotransplantation. Outcomes from our phase I/II clinical trial are reported.
MATERIALS AND METHODS
This study was a fully completed prospective phase I trial of 9 patients who underwent islet transplantation and completed 2 years of follow-up. Enrolled patients, >18 years of age, had diabetes mellitus and >5 years of hypoglycemia unawareness or frequent hypoglycemic episodes, despite maximal diabetes care. Patients had a body mass index ≤28 kg/m2, required ≤0.7 units of insulin per kilogram body weight, had a renal glomerular filtration rate of ≥60 mL/min (or serum creatinine <1.6 mg/dL), were not on chronic steroid therapy of prednisone >5 mg/d or equivalent, and had no liver disease by liver sonography, coagulation disorder, or portal hypertension, clinically and by Doppler sonography. Patients were evaluated according to a set protocol, approved by the Baylor Scott and White Research Institute institutional review board (IRB; approval number: 008-095). Individual patient data were assessed for risks due to immunosuppression therapy after transplant. Patients found eligible for islet allotransplantation were presented for approval by the common Kidney and Pancreas Selection Committee of Baylor Annette C. and Harold C. Simmons Transplant Institute (Dallas, TX). Eligible patients were placed on the deceased donor waiting list.
Deceased donors were evaluated and managed by the local organ procurement organization. Donor data were evaluated by the principal investigator before organ acceptance. Islet preparations were obtained from deceased organ donor pancreata according to the national organ allocation system. Following multiorgan procurement, the pancreas was brought to our Food and Drug Administration (FDA)–approved current good manufacturing practice facility for islet isolation. Following isolation, the islets were not placed in culture. Final review of the preparation included ABO blood type compatibility between donor and recipient, islet mass ≥4000 islet equivalents (IEq)/kg body weight, negative Gram stain and negative up-to-date donor cultures, endotoxin <5 units/kg recipient body weight, islet viability >70%, and islet purity >30%. Upon release from the laboratory, the islet preparations were taken to the interventional radiology suite at Baylor Scott and White All Saints Medical Center (Fort Worth, TX) and infused intraportally via the percutaneous, transhepatic route. Portal venous pressure was monitored throughout the infusion procedure. The transplant was performed after a negative donor–recipient flow crossmatch with no donor-specific antibodies (both for first and second infusions).
Anti-inflammatory blockade comprised etanercept (Enbrel) 50 mg intravenously on the day of islet infusion, followed by 25 mg subcutaneously on days 3, 7, and 10 postinfusion, and anakinra (Kineret) 100 mg subcutaneously on the day of infusion and daily for 7 days after infusion. The dosage was similar to the one used in inflammatory diseases.7 Of note, all patients received information about the FDA warning of increased risk of infections with administration of etanercept and anakinra as part of the informed consent before enrollment in the study. We limited the exposure to the anti-inflammatory medications to the early posttransplant period, as we surmise that IBMIR and the process of engraftment are self-limiting, and should not continue beyond that time.
Patients received immunosuppression induction therapy with anti-thymocyte globulin (Thymoglobulin) (first 7 patients) or alemtuzumab (Campath) (next 2 patients). Steroid therapy was used only for premedication before anti-thymocyte globulin infusion. Maintenance immunosuppression was achieved with a combination of tacrolimus, aiming for serum trough levels of 3–7 mg/dL, and mycophenolate.
Anticoagulation therapy was administered as a means of preventing portal vein thrombosis, with heparin 70 units/kg body weight in the islet preparation, followed by enoxaparin (Lovenox) subcutaneously 30 mg every 12 hours for 14 days.
Patients remained on insulin therapy as needed, with gradual weaning of insulin based on glycemic control. Patients who did not achieve insulin independence 6 weeks after the islet infusion or who became insulin free and subsequently insulin dependent in follow-up were considered for a second islet transplant. The same process used in the initial islet infusion was observed for a second infusion. While the protocol allowed a third islet infusion, this was not performed in the study.
Patients were monitored prospectively for 2 years after the last islet infusion for the study procedures and for safety thereafter. The islet infusion and follow-up protocol were approved and periodically reviewed by the IRB.
Primary and secondary endpoints were assessed at 2 years following the last islet transplant. The primary endpoint was the elimination of severe hypoglycemic events and hypoglycemia unawareness, with proper glycemic control (hemoglobin [Hb]A1c, ≤7%), with or without insulin independence, with detectable serum C-peptide. Secondary endpoints included proportion of insulin independence, HbA1c values, number of hypoglycemic events, stimulated blood glucose level, the area under the curve of blood glucose levels and serum C-peptide response during a mixed meal tolerance test, and the average amount of daily exogenous insulin injection per kilogram of patient body weight. Insulin independence was defined as fasting blood glucose levels of ≤126 mg/dL and 2-hour postprandial levels ≤180 mg/dL, without exogenous insulin.
RESULTS
Nine patients underwent islet allotransplantation in an IRB-approved protocol. All patients completed at least 2 years of follow-up (range, 2–6.5 y). All patients were Caucasian, with a median age at first infusion of 50 years (range, 27–63); body mass index of 23.9 kg/m2 (range, 19.1–28.1); duration of type 1 diabetes of 34 years (range, 19–44); insulin requirement of 0.4 units/kg (range, 0.3–0.7); and HbA1c of 7.8% (range, 6.0%–9.4%).
The immediate follow-up after islet infusion was quite unremarkable, except for 1 patient who underwent laparoscopic exploration the day after islet infusion for bleeding from the liver puncture site. There was no active bleeding at the time of surgery; a large hematoma was evacuated. The patient received a transfusion of 5 units of packed red blood cells. Liver enzymes and bilirubin were normal at the time, peaked at day 14, and normalized. The initial insulin dose was same as pretransplant baseline (30 units/d), and the patient became insulin independent at 3 months.
Routine Doppler sonogram of the liver performed the day after islet infusion showed no evidence of thrombus in the portal vein and its main branches in all patients. One patient had slow blood flow in the portal vein after the first islet infusion, which normalized during follow-up with sonogram, and a normal venous portogram at the second islet infusion. Routine Doppler sonogram in follow-up to 2 years showed no abnormalities in all patients.
Liver enzymes and bilirubin were obtained according to our protocol days 0, 1, 2, 3, 7, 14, and thereafter. The maximum total bilirubin was 0.6 ± 0.4 mg/dL, the maximum alkaline phosphatase was 80 ± 42 U/L, with aspartate aminotransferase 70 ± 53 U/L, and maximum alanine aminotransferase was 59 ± 48 U/L, observed at day 1 after islet infusion (Figure 1). Higher peak levels of alkaline phosphatase, aspartate aminotransferase, and alanine aminotransferase on day 1 post second infusion were observed in 3 out of 4 recipients with second transplant when compared with those post first infusion; however, the elevations were within grade 1 of Common Terminology Criteria for Adverse Events version 4.0 scale. Beyond day 1 posttransplant, the maximum bilirubin was 0.7 ± 0.3 mg/dL, the maximum alkaline phosphatase was 113 ± 49 U/L, with aspartate aminotransferase 101 ± 61 U/L, and the maximum alanine aminotransferase was 136 ± 81 U/L, observed at days 6–14 after islet infusion. Second islet infusions resulted in higher alkaline phosphatase and aspartate aminotransferase levels in only half of the cases, and only 25% had higher alanine aminotransferase levels with a second infusion. The enzymes normalized in all cases after each infusion.
FIGURE 1.
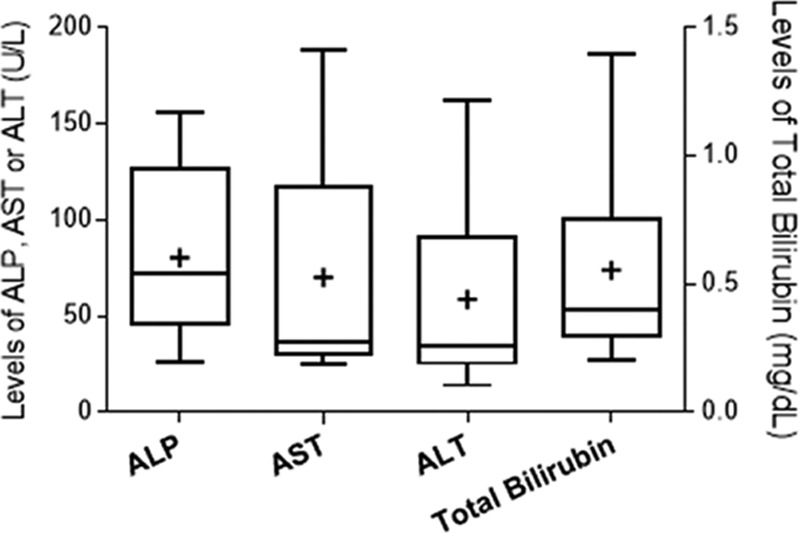
Peak liver enzymes and bilirubin after islet transplantation procedures. Plots are shown as boxes for the 25–75th percentiles, central lines for the median, bars for range. and “+” for average. ALP, alkaline phosphatase; ALT, maximum alanine aminotransferase; AST, aspartate aminotransferase.
The mean islet infusion dose was 8967 IEq/kg body weight (range, 3582–6395), for a total dose per patient of 12 999 IEq/kg (range, 6842–24 829) in 1 or 2 preparations (5 and 4 patients, respectively) (Table 1). Mean portal vein pressure, obtained by direct manometry, was 8.7 ± 4.2 mm Hg at the start of the procedure, and 15.3±4.8 mm Hg at the end, with a rise of 7.9 ± 5.4 mm Hg, which was not clinically significant. Pressures were similar for the first and the second infusion.
TABLE 1.
Transplanted islet mass
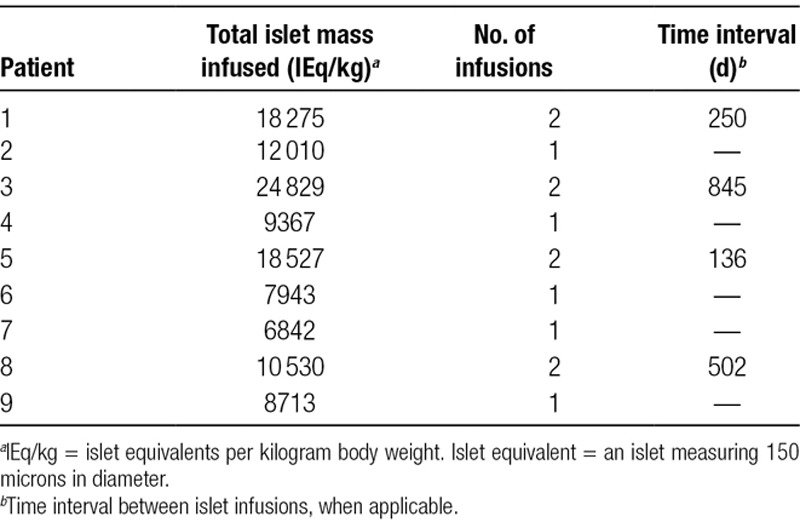
Figure 2 shows the function of the transplants for each of the 9 patients, and Figure 3 shows their insulin requirements. Four patients received 2 islet infusions. Patient 1 became insulin independent on day 49 after the first islet infusion; she returned to insulin on day 154, to become insulin independent again on day 63 after the second transplant, after receiving a total of 18 275 IEq/kg. Patient 3, who received a total islet dose of 24 829 IEq/kg from 2 infusions, achieved insulin independence by day 93, returned to insulin at the 2-year mark, and became insulin independent again 128 days after the second transplant. Patients 5 and 8 achieved insulin independence only after the second islet infusion. Patient 5 maintained insulin independence 2.5 years after the last islet infusion (total islet mass, 18 527 IEq/kg). Unfortunately, patient 8, who became insulin independent 54 days after the second transplant (total 10 530 IEq/kg), died suddenly at home while insulin independent for 108 days, with no known hypoglycemic episodes, presumably of heart disease, based on limited history from family (no autopsy was performed).
FIGURE 2.
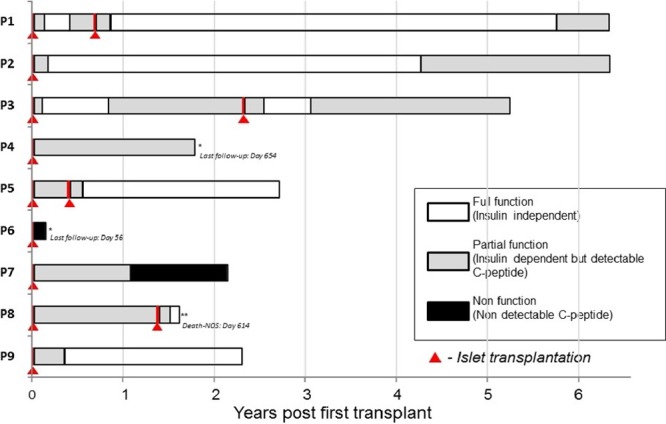
Summary of islet transplant procedures and outcome: number of infusions, interval, graft function. *Patients 4 and 6 have dropped out of this study due to personal reasons. **Subject with previous total pancreatectomy followed by autologous islet transplantation for chronic pancreatitis.
FIGURE 3.
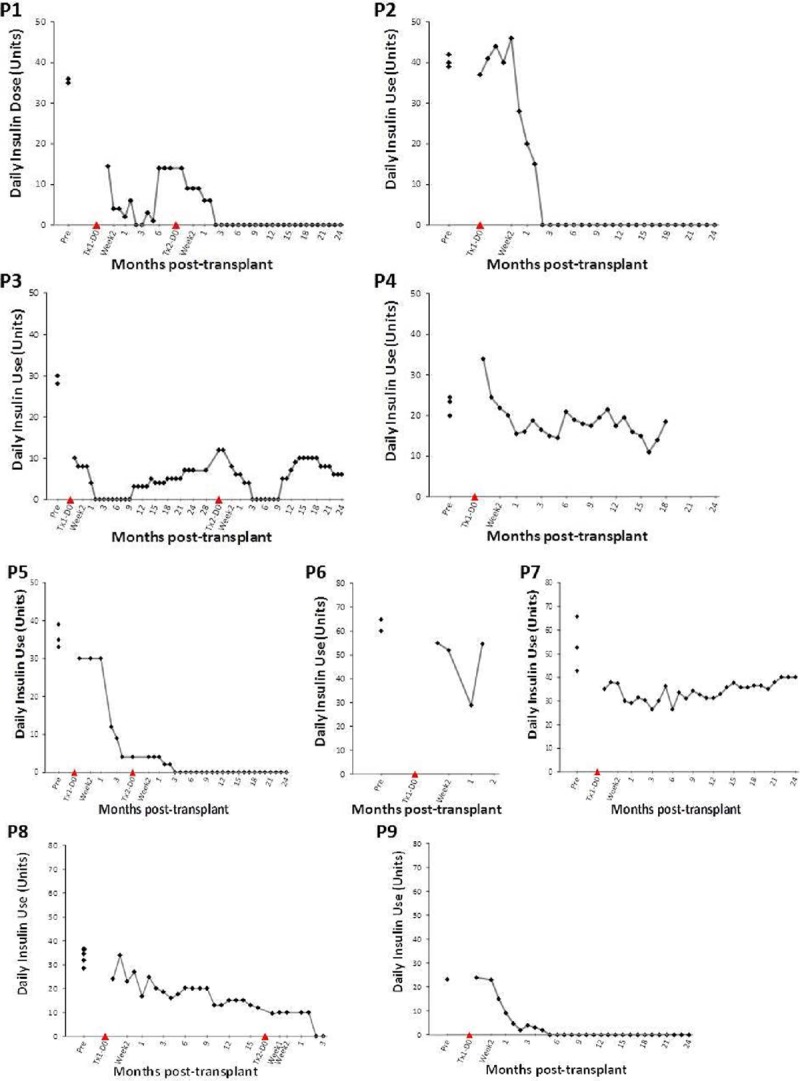
Daily insulin requirements after islet transplantation. Six out of 9 patients attained transient insulin independence.
Another 5 patients received 1 islet infusion. Patients 2 and 9 achieved insulin independence for 2–4 years (islet dose 12 010 and 8713 IEq/kg, respectively), with patient 2 returning to insulin >4 years after transplant. Patient 4, despite receiving 9367 IEq/kg, did not achieve insulin independence and was listed for a second infusion; however, the patient decided to drop out of the study due to side effects of immunosuppression 18 months after the initial islet infusion. Patient 6 had primary nonfunction and patient 7 had acute loss of islet function at 13 months after the islet infusion—both with elevated levels of anti-glutamic acid decarboxylase (GAD) 65 antibody. Both were not considered for a second islet infusion.
Six out of 9 patients had excellent glycemic control at 2 years, defined by fasting blood glucose <126 mg/dL, a negative intravenous glucose tolerance test, HbA1c of <6% (Figure 4), and no hypoglycemia unawareness. Kaplan-Meier estimate did not show a difference in glycemic control (P = 0.85) (Figure 5A) or insulin independence with 1 or 2 islet infusions (P = 0.32) (Figure 5B).
FIGURE 4.
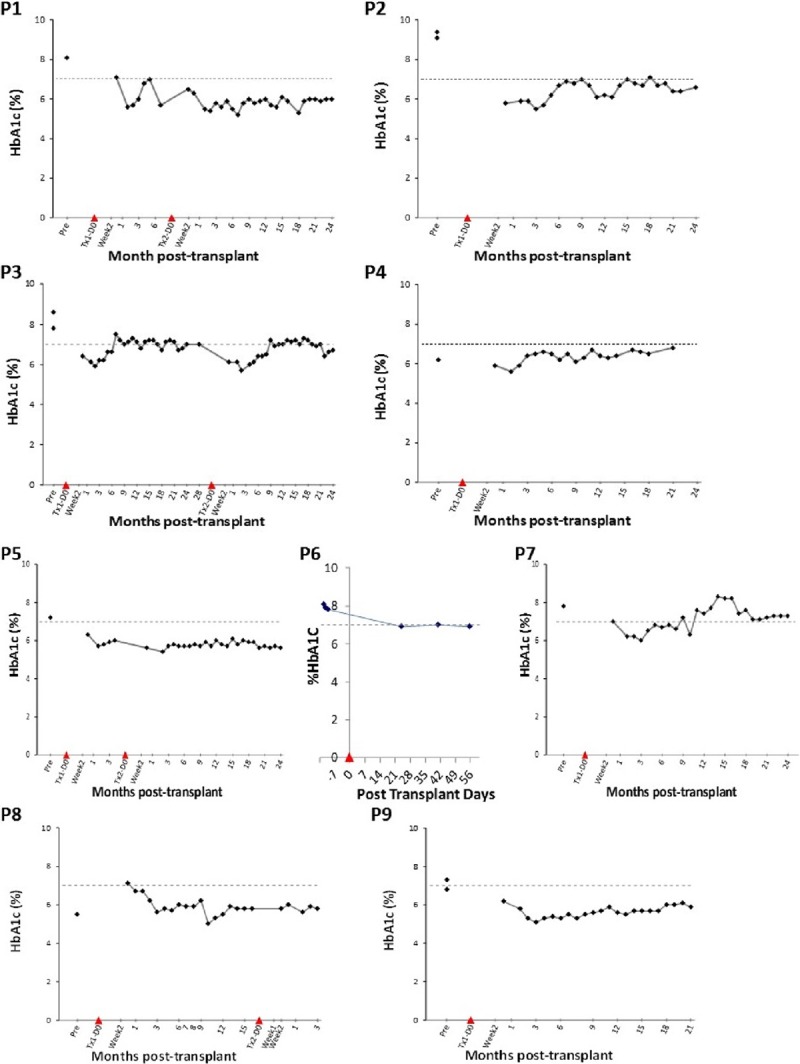
Hemoglobin A1c (HbA1c) (%) in follow-up after islet transplantation. Threshold marked at 7%. All patients achieved good glycemic control.
FIGURE 5.
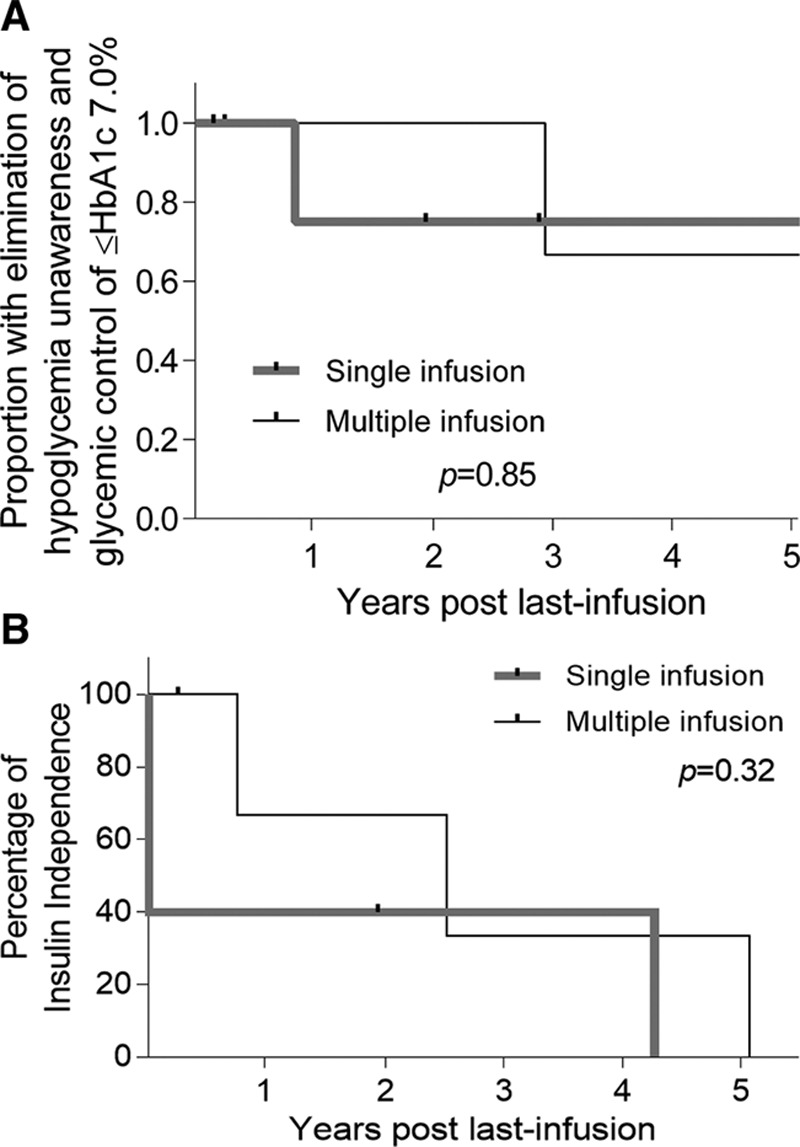
A, Kaplan-Meier for glycemic control as primary endpoint. B, Kaplan-Meier for insulin independence with single islet infusions (5 patients) and multiple islet infusions (4 patients). Good glycemic control includes no hypoglycemia unawareness, hemoglobin A1c (HbA1c) ≤7%, and detectable serum C-peptide.
Despite double cytokine blockade, none of our patients had infectious episodes during the entire study.
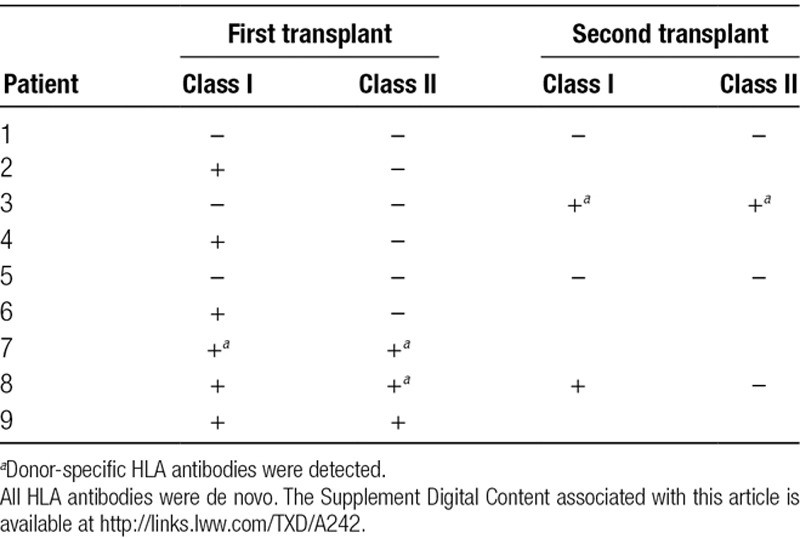
HLA antibodies were monitored with Single Antigen Bead Luminex assay (One Lambda, Canoga Park, CA). None of the patients had preformed donor-specific antibodies before the transplant. Either class I or II HLA antibodies were detected in all patients except patients 1 and 5 during the study period. Donor-specific antibodies, all of which were de novo, were observed in patients 3, 7, and 8. Patient 3 had multiple antibodies, all after the second islet infusion, some with mean fluorescence intensity up to 9000 developing 9 months after, and decreasing thereafter. Five patients developed persistent antibodies (54.5%): 2 against class I, and 3 against both class I and II. Except for patient 3, all antibodies observed had 1000–4000 mean fluorescence intensity. The development of alloantibodies did not correlate with islet function (Table 2).
TABLE 2.
Detection of HLA antibodies in the 9 study patients who underwent islet allotransplantation
Antibodies to GAD65, islet antigen-2, microinsulin autoantibody, and zinc transporter 8 were obtained at the time of transplant and through follow-up (Figure 6). Patient 4 had anti-GAD65 antibodies at the time of transplant. She did not gain insulin independence and ultimately dropped out of the study. Patient 6, with pretransplant anti-GAD65, developed primary nonfunction; patient 7, who developed anti-GAD65 early (d 28), lost the graft as well. However, patient 3, who developed anti-GAD65 9 months after her second infusion, lost insulin independence but has graft function.
FIGURE 6.
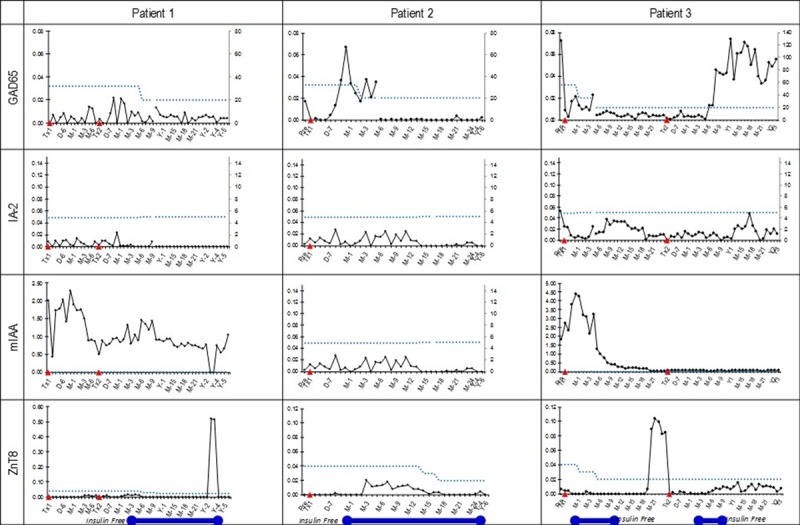
Trends of islet autoantibodies in follow-up after transplantation. The blue bar represents periods of freedom from insulin. Autoantibody levels showed no correlation with graft function.
Immunosuppression was maintained with tacrolimus and mycophenolate. Minimum tacrolimus levels were 3.7 ± 1.1 ng/mL, and maximum levels were 7.95 ± 1.7 ng/mL. The trough levels were not different than the average (higher or lower) before return to insulin use.
DISCUSSION
Our results suggest that the use of early double cytokine blockade (against IL-1β and TNF-α) is associated with diminished intrahepatic inflammation, as indicated by only slight elevation of liver enzymes and no hyperbilirubinemia. There were no early infectious complications after islet allotransplantation and no serious adverse events related to infections throughout the 2-year follow-up, despite immunosuppression. Six out of 9 patients achieved excellent glycemic control, with no hypoglycemia unawareness.
Islet allotransplantation is a treatment option in type 1 diabetics with poor glucose control, especially those with frequent hypoglycemic events and hypoglycemia unawareness, despite maximal care by a dedicated diabetes team and despite proper patient compliance.1,8 Hypoglycemic events can be incapacitating, significantly altering life, with patients refraining from driving or operating machinery. Unfortunately, hypoglycemia unawareness is associated with mortality in follow-up or on the waiting list for transplantation.9 While initially the goal was to obtain insulin independence,10 currently the primary accepted objective for islet transplantation is resolution of hypoglycemia unawareness, with or without insulin independence.
Compared with solid organ transplantation, islet transplantation has lower morbidity and mortality.11 However, compared with insulin treatment, islet transplantation requires immunosuppressive therapy with its associated risks of infection, malignancy,12 hypertension, nephrotoxicity,13 and sensitization with islet loss.14,15 There is gradual islet loss over time, which can be multifactorial (immediate loss, autoimmunity, alloimmunity, toxicity). The Edmonton group recognized the need for a good islet preparation to attain insulin independence and described a threshold of islet mass infused, aiming for a total islet dose of ≥10 000 IEq/kg body weight, from 2 separate islet infusions from 2 separate donors.16 Subsequently, insulin independence was achieved with single islet infusions of ≥7000 IEq/kg.17,18 Once instant blood-mediated inflammatory reaction was recognized as an important factor in islet loss immediately after transplantation,3,19 emerging islet transplant protocols included anti-inflammatory agents such as etanercept, which were associated with better engraftment.6,20
As the nonspecific inflammatory response is multifactorial, we sought the added blockade of IL-1 (as IL-2 is affected by tacrolimus used for immunosuppression maintenance). Anakinra, an IL-1β inhibitor, is FDA approved for refractory rheumatoid arthritis. The use of etanercept and anakinra in combination has been reported in an animal model21 and in 2 patients of human islet transplantation in Japan.22 However, the FDA issued a warning not to use it in conjunction with etanercept, citing the risk of serious infections in patients with rheumatoid arthritis.7 Our patients were specifically informed about this risk. We surmised that islet transplantation is a procedure during which there is minimal infectious contamination, if any, and requires a relatively short hospital stay, which lowers the infectious risk. Also, the administration of etanercept and anakinra is restricted to the first 7–0 days after islet transplantation compared with longer treatment regimens in autoimmune diseases, spanning months (24 wk), and use in patients on steroids and methotrexate.22 Based on this difference, we assumed that the infectious risk in our patients would not be as high as with other indications of treatment. Indeed, none of our patients experienced serious infections in the follow-up, even while they received induction immunosuppression therapy, which is a known, independent risk factor for serious infections. We did not notice any side effects related to etanercept, anakinra, or the combination thereof. There were no serious adverse events related to infections during the 2-year study period. Of note, our maintenance immunosuppression aimed for the lowest doses/levels possible. We have published preliminary results comparing 3 patients using double cytokine blockade, compared with 3 patients who received etanercept only. Double blockade resulted in insulin independence using a single islet infusion, compared with 2 infusions with etanercept blockade only.23 Our group has been using the combination of etanercept and anakinra in islet autotransplantation with the good safety profile.24
The initial administration of a high quality and quantity of islets and reduction of inflammation are important factors in the achievement and maintenance of good glycemic control. However, these factors are not a guarantee of long-term function, where other factors come into play. We have shown that almost all patients develop alloantibodies to their graft at some point in the follow-up. However, only 1 of our patient developed transiently very significant sensitization. Some also develop autoantibodies, such as anti-GAD65, with various consequences, from the abrupt loss of the islet graft to 1 case with maintenance of islet function (with loss of insulin independence). We have not found a consistent correlation between antibody development and graft outcome. Not only are there no effective agents in use to mitigate these factors, but a complete list of factors that contribute to later islet use is yet to be determined. Our maintenance immunosuppression was tacrolimus-based, with trough levels of 3–7 ng/dL, in an attempt to avoid over immunosuppression (and possible islet toxicity from tacrolimus). Return to insulin did not correlate with the trough levels of tacrolimus. However, low maintenance tacrolimus could trigger the emergence of alloantibody or even autoantibody.
Our study has limitations—as the number of subjects is small and we had no control group. While we transplanted patients using etanercept without anakinra in the past, that group could not have been used as historical controls because they also received a different immunosuppression regimen. Ultimately, graft survival is multifactorial, and it is very hard to assess the benefit of anti-inflammatory agents in isolation. It is conceivable that offering the best engraftment potential improves the long-term results.
The use of double cytokine blockade at the time of islet allotransplantation is safe, with reduction of inflammation at transplantation and presumably with better engraftment. However, it does not influence later islet loss from T-cell–mediated autoimmunity and alloimmunity, which require different strategies to maintain long-term islet function.
Footnotes
Published online 10 February, 2020.
This study has been supported in part by the Baylor Scott & White Dallas Foundation.
The authors declare no conflicts of interest.
Clinical Trial Notation: This study was registered as NCT00530686 in ClinicalTrials.gov.
N.O. participated in research design, performance of the research, data analysis, and writing of the article. M.T. participated in performance of the research, data analysis, and writing of the article. M.F.L. and B.N. participated in research design, performance of the research, data analysis, and writing of the article. All authors reviewed and accepted the final article for the submission.
Supplemental digital content (SDC) is available for this article. Direct URL citations appear in the printed text, and links to the digital files are provided in the HTML text of this article on the journal’s Web site (www.transplantationdirect.com).
REFERENCES
- 1.Shapiro AM, Pokrywczynska M, Ricordi C. Clinical pancreatic islet transplantation. Nat Rev Endocrinol.. 2017; 13:268–277 [DOI] [PubMed] [Google Scholar]
- 2.Balamurugan AN, Naziruddin B, Lockridge A, et al. Islet product characteristics and factors related to successful human islet transplantation from the Collaborative Islet Transplant Registry (CITR) 1999-2010. Am J Transplant.. 2014; 14:2595–2606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Naziruddin B, Iwahashi S, Kanak MA, et al. Evidence for instant blood-mediated inflammatory reaction in clinical autologous islet transplantation. Am J Transplant.. 2014; 14:428–437 [DOI] [PubMed] [Google Scholar]
- 4.Vivot K, Langlois A, Jeandidier N, et al. Instant blood-mediated inflammatory reaction during islet transplantation: the role of toll-like receptors signaling pathways. Transplant Proc.. 2011; 43:3192–3194 [DOI] [PubMed] [Google Scholar]
- 5.Barshes NR, Wyllie S, Goss JA. Inflammation-mediated dysfunction and apoptosis in pancreatic islet transplantation: implications for intrahepatic grafts. J Leukoc Biol.. 2005; 77:587–597 [DOI] [PubMed] [Google Scholar]
- 6.Bellin MD, Kandaswamy R, Parkey J, et al. Prolonged insulin independence after islet allotransplants in recipients with type 1 diabetes. Am J Transplant.. 2008; 8:2463–2470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Genovese MC, Cohen S, Moreland L, et al. 20000223 Study Group Combination therapy with etanercept and anakinra in the treatment of patients with rheumatoid arthritis who have been treated unsuccessfully with methotrexate. Arthritis Rheum.. 2004; 50:1412–1419 [DOI] [PubMed] [Google Scholar]
- 8.Hering BJ, Clarke WR, Bridges ND, et al. Clinical Islet Transplantation Consortium Phase 3 trial of transplantation of human islets in type 1 diabetes complicated by severe hypoglycemia. Diabetes Care.. 2016; 39:1230–1240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lee MH, Ward GM, MacIsaac RJ, et al. Mortality in people with type 1 diabetes, severe hypoglycemia, and impaired awareness of hypoglycemia referred for islet transplantation. Transplant Direct.. 2018; 4:e401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shapiro AM, Ricordi C, Hering BJ, et al. International trial of the Edmonton protocol for islet transplantation. N Engl J Med.. 2006; 355:1318–1330 [DOI] [PubMed] [Google Scholar]
- 11.Shapiro AM. State of the art of clinical islet transplantation and novel protocols of immunosuppression. Curr Diab Rep.. 2011; 11:345–354 [DOI] [PubMed] [Google Scholar]
- 12.Peters A, Olateju T, Deschenes J, et al. Posttransplant lymphoproliferative disorder after clinical islet transplantation: report of the first two cases. Am J Transplant.. 2017; 17:2474–2480 [DOI] [PubMed] [Google Scholar]
- 13.Ojo AO, Held PJ, Port FK, et al. Chronic renal failure after transplantation of a nonrenal organ. N Engl J Med.. 2003; 349:931–940 [DOI] [PubMed] [Google Scholar]
- 14.Campbell PM, Senior PA, Salam A, et al. High risk of sensitization after failed islet transplantation. Am J Transplant.. 2007; 7:2311–2317 [DOI] [PubMed] [Google Scholar]
- 15.Rickels MR, Kearns J, Markmann E, et al. HLA sensitization in islet transplantation Clin Transpl. 2006413–420 [PMC free article] [PubMed] [Google Scholar]
- 16.Shapiro AM, Lakey JR, Ryan EA, et al. Islet transplantation in seven patients with type 1 diabetes mellitus using a glucocorticoid-free immunosuppressive regimen. N Engl J Med.. 2000; 343:230–238 [DOI] [PubMed] [Google Scholar]
- 17.Al-Adra DP, Gill RS, Imes S, et al. Single-donor islet transplantation and long-term insulin independence in select patients with type 1 diabetes mellitus. Transplantation.. 2014; 98:1007–1012 [DOI] [PubMed] [Google Scholar]
- 18.Hering BJ, Kandaswamy R, Ansite JD, et al. Single-donor, marginal-dose islet transplantation in patients with type 1 diabetes. JAMA.. 2005; 293:830–835 [DOI] [PubMed] [Google Scholar]
- 19.Nilsson B, Ekdahl KN, Korsgren O. Control of instant blood-mediated inflammatory reaction to improve islets of Langerhans engraftment. Curr Opin Organ Transplant.. 2011; 16:620–626 [DOI] [PubMed] [Google Scholar]
- 20.Gangemi A, Salehi P, Hatipoglu B, et al. Islet transplantation for brittle type 1 diabetes: the UIC protocol. Am J Transplant.. 2008; 8:1250–1261 [DOI] [PubMed] [Google Scholar]
- 21.McCall M, Pawlick R, Kin T, et al. Anakinra potentiates the protective effects of etanercept in transplantation of marginal mass human islets in immunodeficient mice. Am J Transplant.. 2012; 12:322–329 [DOI] [PubMed] [Google Scholar]
- 22.Matsumoto S, Noguchi H, Takita M, et al. ET-Kyoto ductal injection and density-adjusted purification combined with potent anti-inflammatory strategy facilitated single-donor islet transplantation: case reports. Transplant Proc.. 2010; 42:2159–2161 [DOI] [PubMed] [Google Scholar]
- 23.Matsumoto S, Takita M, Chaussabel D, et al. Improving efficacy of clinical islet transplantation with iodixanol-based islet purification, thymoglobulin induction, and blockage of IL-1β and TNF-α. Cell Transplant.. 2011; 20:1641–1647 [DOI] [PubMed] [Google Scholar]
- 24.Naziruddin B, Kanak MA, Chang CA, et al. Improved outcomes of islet autotransplant after total pancreatectomy by combined blockade of IL-1β and TNFα. Am J Transplant.. 2018; 18:2322–2329 [DOI] [PubMed] [Google Scholar]


