Abstract
Atrial fibrillation (AF) is an important risk factor for stroke. Although anticoagulation is effective in mitigating this risk, many high-risk patients are not anticoagulated in routine practice. Furthermore, as many as 50% of those who are prescribed an anticoagulant stop treatment within a year. This under treatment may be due, in part, to difficulty in navigating difficult decisions about initiating potentially lifelong therapy with significant costs, potential risks, and impact on daily life. To address these challenges, the most recent American guidelines issued a class I recommendation to use shared decision-making (SDM) to individualize patients’ antithrombotic care. The call by the major cardiovascular organizations for SDM is in an effort to improve quality of care by promoting decisions that reflect what is best for an individual patient based on their stroke and bleeding risks, as well as their comorbid conditions and socio-personal context. SDM is readily applicable to current cardiovascular practice, but ongoing work will be needed to determine whether brief, evidence-based, and patient-oriented tools are able to support thoughtful, patient-centered decision-making and, ultimately, improve the rates of appropriate treatment initiation and adherence.
Keywords: Shared decision-making, Atrial fibrillation, Stroke, Bleeding
1. Introduction
Nonvalvular chronic atrial fibrillation is the most common cardiac arrhythmia, [1] affecting ~3 million Americans [2] and accounting for ~US$26 billion/year in health care costs, largely due to thromboembolic strokes [3]. Patients have a strong desire to prevent strokes [4, 5], and anticoagulation can help reduce their risk [6]. Yet, less than half of high-risk patients with AF receive anticoagulant treatment, and of those who start anticoagulation, 30–50% stop treatment within a year [7]. Multiple factors contribute to this underuse. Both patients and clinicians are often highly concerned about a major bleeding due to anticoagulation use [8], and clinicians with low-risk tolerance may be less likely to prescribe appropriate anticoagulation [9]. Furthermore, patients have trouble implementing anticoagulation treatment in their lives, often due to factors such as the impact on daily routine [10], associated dietary or lifestyle restrictions, or direct out of pocket costs [11]. Even when anticoagulation seems feasible for an individual, patients often lack access to reliable and up-to-date information about risks and benefits of treatment around which the decision-making conversation is centered.
In response to these challenges of under use and decision-making, the 2014 guidelines from the American Heart Association, American College of Cardiology, and the Heart Rhythm Society for the management of patients with AF formulated a class I recommendation for patients and clinicians to use shared decision-making to individualize patients’ antithrombotic care [12]. In SDM, patients and clinicians work together to identify the best way forward to address the patient’s situation, i.e., an approach that maximally supports meeting patient’s goals, such as cure or better quality of life, while minimally disrupting their lives[13]. They work through the best available evidence and the patient’s views, expectations, and preferences along with the practical and emotional demands and constraints of treatment to identify an approach that makes the most intellectual, practical, and emotional sense [14]. This means respectively, that the care is consistent with evidence-based practice, can be implemented and sustained in a patient’s day-to-day life, and it addresses, supports, and advances the emotional experience of illness and treatment. Care that makes sense integrates, balances, and adapts the intellectual, emotional, and practical elements to the demands of the patient’s situation. Both patients and clinician come to know and understand that what they will do is, for now, the best way forward, it feels right, and it can be implemented in the life of the patient. SDM shifts the focus from care for patients like this, to care for this patient [14]. The call by the three major cardiovascular organizations for SDM opens an opportunity to improve quality of patient care by making anticoagulation decisions that reflect what is best for this patient, based on their individualized stroke and bleeding risks, as well as their situation, including comorbid conditions and socio-personal context.
2. SDM in atrial fibrillation
SDM is a conversation between a patient and a clinician with the purpose of identifying, tailoring and initiating treatment best suited to the patient and their situation. This conversation usually contains three key elements before making a final decision [13, 15, 16]. The first element is to foster choice awareness, that is, to acknowledge that there is more than one way forward, that a decision needs to be made and that the patient’s views matter. The second element is to introduce the options and their evidence-based potential harms and benefits along with their likelihood. The third element is to talk through how the available options would affect issues that emotionally and practically matter to the patient and their ability to fit treatment in their life.
In the last few decades, tools have been developed to help patients and clinicians with these often difficult conversations. Some of these SDM tools—called “patient decision aids”— take SDM outside the encounter and ask patients to review evidence and think about their preferences by themselves, in preparation for the encounter [17]. A recent systematic review found that most SDM tools tested are in fact patient decision aids, and that they are associated with increased patient knowledge, increased likelihood of making a decision, and lower decisional conflict [18]. However, none of the tools identified in the review are currently widely used. Also, as patient decision aids ask patients to prepare, to educate themselves, to read about their disease and possible treatments, to watch educational videos, hear about other patients’ experiences, and weigh the pros and cons of treatments by themselves, these aids may place more burden on patients. Those who already feel overwhelmed by their illness or demands of medical care may not have the capacity to review or absorb the information provided by these tools [19].
Other kinds of SDM tools are called ‘conversation aids,’ designed for use within the clinical encounter to directly support the patient-clinician conversation in choosing a best way forward. These tools help patients and clinicians work together, and because they are used in collaboration in the context of the encounter and in the presence of a clinician’s expertise, they do not necessarily need to be as informationally comprehensive and time-demanding as a tool used outside the clinical encounter. Moreover, they offer the flexibility to individualize care, enabling patients and clinicians to tailor the use of the tool to the needs of the situation.
3. Working through SDM using a conversation aid: AF example
Our group has developed and is currently testing an atrial fibrillation anticoagulation conversation aid in a randomized clinical trial: anticoagulation choice [20]. This tool was designed to promote a patient-clinician conversation during a clinical encounter, building on the clinician’s expertise on the medical issues, and the patient’s expertise on how treatment options would fit in their life. Although the tool is currently being validated in a trial, and is undergoing continual revision and improvement, we will use the current iteration of our design to illustrate the use of a SDM conversation tool in practice. This tool will be evaluated in 1000 patients with atrial fibrillation, about half of whom are considering initiation of anticoagulation and half of whom are currently being treated with anticoagulation. We will evaluate whether the tool improves patient-clinician communication, decisional conflict, patient involvement in decision-making, and whether the tool has an impact on the rates of adherence to therapy and long-term outcomes. The current version of the tool can be found at https://anticoagulationdecisionaid.mayoclinic.org/.
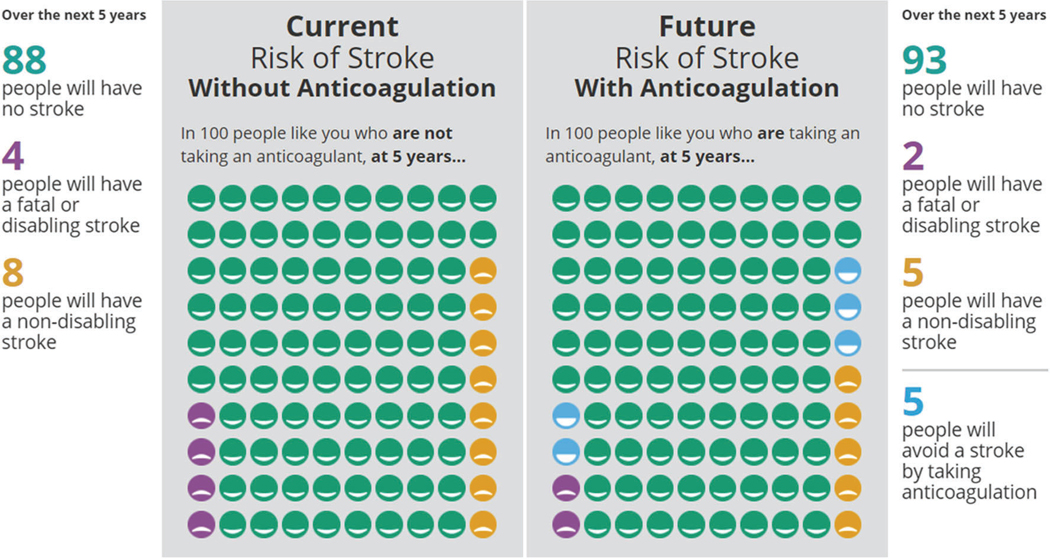
The tool consists of two parts, first to help patients and clinicians decide whether to start (or continue) anticoagulation treatment, and second to help them decide which treatment would be best suited to the patient and their life. In order to decide whether starting or continuing anticoagulation is appropriate for this patient and their situation, the tool facilitates calculation of an individualized risk of stroke, based on the medical situation of the patient (using the CHA2DS2-VASc scoring system, i.e., gender, age, history of hypertension, congestive heart failure, strokes, history of vascular disease, diabetes mellitus) [6, 12]. These risks for disabling or fatal strokes can be presented in a 1-year or a 5-year time horizon and are displayed through numbers, colors, and icon arrays, framed both positively (3 out of 100 people will have a stroke) and negatively (97 out of 100 people will not have a stroke) to help patients and clinicians in understanding this risk. Alongside these risks of stroke without treatment, the tool displays the risk of stroke with anticoagulation (Fig. 1).
Fig. 1.
The risk of stroke, with and without anticoagulation, are depicted using a visual representation of 100 patients, each with similar stroke risk based on CHA2DS2-VASc score. A representative proportion of this a population who would be expected to have either a fatal/disabling stroke or a non-disabling stroke are colored in yellow or purple. Individuals who would be spared an anticipated stroke with anticoagulation are colored in blue
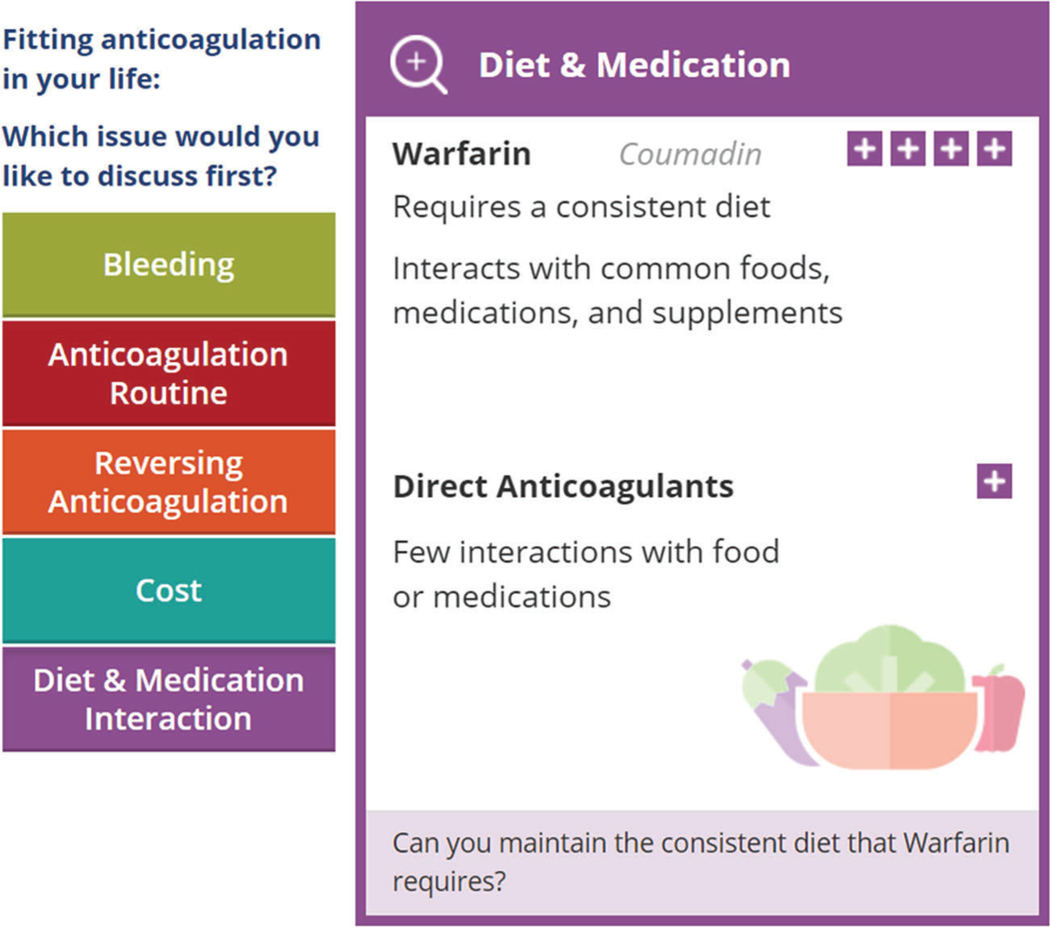
If patients and clinicians consider the reduction in risk of stroke significant, and they agree the patient’s situation requires action, the tool moves on to supporting a conversation on which anticoagulant would be best. It helps patients and clinicians proceed through the three key elements described previously, starting with fostering choice awareness: indicating that there is more than one option, and in order to choose between different anticoagulation approaches, patient and clinicians need to think about how the options would fit in the patient’s life. Next, the tool offers specific issues that may be relevant in working through what an option would mean for the patient and their life. Topics offered for conversation are the individualized risk of bleeding, the routine of taking anticoagulation, the availability of reversal agents, costs, restrictions in activities, and interactions with diet and medication (Fig. 2). Evidence is provided to help them with this part of the conversation.
Fig. 2.
After the “choice awareness” step, the discussion turns to issues of interest to the patient including exploration of bleeding risks, anticoagulation routine, reversing anticoagulation, cost, and diet and medication interactions (left tab for diet and medication interaction is expanded in this example)
Through the conversation, patients and clinicians may conclude that one treatment approach (if any) makes the most intellectual, emotional, and practical sense for this individual patient and their situation. The tool offers the option to select the final decision, and it provides a summary of the calculated risks of stroke with and without anticoagulation and the most important pros and cons of the available options presented on the issue cards. This final decision can be documented or a summary can be printed and given to the patient or scanned to an electronic medical record.
4. Challenges of SDM
SDM conversations may be challenging for patients and clinicians, in part because they may be novel, but foremost because AF and its treatment has significant implications on the patient’s life, and working through what to do in each situation should not be taken lightly. Time constraints, patients’ incapability, or the clinical situation are often cited as barriers for SDM [21]. Although on average, clinical encounters take < 10% longer when patient decision aids are employed outside the clinical encounter, most studies on the use of within-encounter conversation aids do not show an increase in length of the encounter [22]. However, when using such conversation aids, we need to be mindful that simply walking through a tool is not a surrogate for a meaningful conversation. Ideally, these conversation aids should be used to support, and not replace, discussions already happening in busy practice in order to conduct a deliberate and thoughtful conversation with the patient to figure out what is best for this patient.
Approaching SDM as a conversation limits the informational burden and the decisional weight placed on the patient, ensuring that all patients are capable of participating in SDM, not only those who are more empowered, higher educated, or less overwhelmed by their illness. Although the level of engagement may vary by individual, both parties, patient and clinicians, are encouraged to participate in an open and productive conversation that limits the bias of patient’s preconceived notions or the clinician’s own preferences. Following a meaningful conversation in which clinicians learn about this patient and their lives, clinicians can recommend treatment approach that fits best, even if patients are not willing or able to make the final decision themselves.
The goals of SDM are to help patients and clinicians make a shared and informed decision integrating the known risks and benefits of a treatment with the patient’s context and preferences. Hence, the goal is not to convince a patient to choose a specific treatment. Further, SDM is intended to enhance communication and not a checklist of completing a specific task recommended by a class 1 guideline. As SDM tools are developed and implemented in real-world clinical practice, a critical aspect will be to assess the degree that SDM transpired. Ongoing trials assess the quality of SDM with measures including the decisional conflict scale, measures of patient and clinician satisfaction, and by annotation of key components of the process by direct observation of recorded patient encounters.
Lastly, although clinical operational demands initially appear to be a barrier for SDM, working together in conversation to address the threat of AF and the thorny issues of bringing anticoagulation into a person’s life is necessary and guideline-supported. SDM and supporting tools such as anticoagulation choice offer support and structures for this everyday clinical work.
Although there is still some imprecision and controversy as to the various untreated risks, patients and clinician still need to make treatment decisions. Approximate risks are practically useful in conversation to help decide whether treatment should be initiated, as the issue at hand is not precision but what to do. It is important to stress that while SDM tools have a tendency to formalize the communication of risk and benefit of therapy, this should not be mistaken for increased certainty or universality of risk estimates. This is particularly true for direct comparisons of the various non-vitamin K oral anticoagulants (NOACs), since there are no prospective, randomized trials directly comparing these drugs. Estimates of relative safety and efficacy of the NOACs are largely derived from observational data sources or indirect comparisons of clinical trial data.
5. Conclusion
AF is a common condition, with a devastating complication of stroke in a small percentage of patients. Anticoagulation can lower the risk of stroke by 2/3, yet many high-risk patients do not start or continue anticoagulation treatment. Deciding about anticoagulation treatment is difficult, both for patients and clinicians. In a SDM process, they can work together to identify a treatment option that makes intellectual, emotional, and practical sense: an approach for anticoagulation treatment or no treatment that fits best in the life of the individual patient. SDM tools can help patients and clinicians with these difficult conversations. Future studies will evaluate whether brief, evidence-based, and patient-oriented tools are able to support thoughtful, patient-centered consideration of management options, and their impact on treatment initiation, and adherence.
References
- 1.Go AS, Mozaffarian D, Roger VL, Benjamin EJ, Berry JD, Blaha MJ, et al. Heart disease and stroke statistics–2014 update: a report from the American Heart Association. Circulation. 2014;129:e28–e292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Williams BA, Honushefsky AM, Berger PB. Temporal trends in the incidence, prevalence, and survival of patients with atrial fibrillation from 2004 to 2016. Am J Cardiol. 2017;120:1961–5. [DOI] [PubMed] [Google Scholar]
- 3.Kim MH, Johnston SS, Chu BC, Dalal MR, Schulman KL. Estimation of total incremental health care costs in patients with atrial fibrillation in the United States. Circ Cardiovasc Qual Outcomes. 2011;4:313–20. [DOI] [PubMed] [Google Scholar]
- 4.Haynes RB, Devereaux PJ, Guyatt GH. Clinical expertise in the era of evidence-based medicine and patient choice. Vox Sang. 2002;83(Suppl 1):383–6. [PubMed] [Google Scholar]
- 5.Lahaye S, Regpala S, Lacombe S, Sharma M, Gibbens S, Ball D, et al. Evaluation of patients’ attitudes towards stroke prevention and bleeding risk in atrial fibrillation. Thromb Haemost. 2014;111:465–73. [DOI] [PubMed] [Google Scholar]
- 6.Kirchhof P, Benussi S, Kotecha D, Ahlsson A, Atar D, Casadei B, et al. 2016 ESC Guidelines for the management of atrial fibrillation developed in collaboration with EACTS. Eur Heart J. 2016;37: 2893–962. [DOI] [PubMed] [Google Scholar]
- 7.Yao X, Abraham NS, Alexander GC, Crown W, Montori VM, Sangaralingham LR, et al. Effect of adherence to oral anticoagulants on risk of stroke and major bleeding among patients with atrial fibrillation. J Am Heart Assoc. 2016;5(2):pii: e003074 10.1161/JAHA.115.003074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Eckman MH. Decision-making about the use of non-vitamin K oral anticoagulant therapies for patients with atrial fibrillation. J Thromb Thrombolysis. 2016;41:234–40. [DOI] [PubMed] [Google Scholar]
- 9.Raptis S, Chen JN, Saposnik F, Pelyavskyy R, Liuni A, Saposnik G. Aversion to ambiguity and willingness to take risks affect therapeutic decisions in managing atrial fibrillation for stroke prevention: results of a pilot study in family physicians. Patient Prefer Adherence. 2017;11:1533–1539. 10.2147/PPA.S143958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jacobs MS, Schouten JF, de Boer PT, Hoffmann M, Levin LA, Postma MJ. Secondary adherence to non-vitamin-K antagonist oral anticoagulants in patients with atrial fibrillation in Sweden and the Netherlands. Curr Med Res Opin. 2018;34(10):1839–1847. 10.1080/03007995.2018.1459528. [DOI] [PubMed] [Google Scholar]
- 11.Obamiro KO, Chalmers L, Lee K, Bereznicki BJ, Bereznicki LR. Adherence to oral anticoagulants in atrial fibrillation: an Australian survey. J Cardiovasc Pharmacol Ther. 2018;23:337–43. [DOI] [PubMed] [Google Scholar]
- 12.January CT, Wann LS, Alpert JS, Calkins H, Cigarroa JE, Cleveland JC Jr, et al. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the Heart Rhythm Society. J Am Coll Cardiol. 2014;64:e1–76. [DOI] [PubMed] [Google Scholar]
- 13.Kunneman M, Montori VM, Castaneda-Guarderas A, Hess EP. What is shared decision making? (and what it is not). Acad Emerg Med. 2016;23:1320–4. [DOI] [PubMed] [Google Scholar]
- 14.Kunneman M, Henselmans I, van Laarhoven HWM et al. Shared decision-making for good clinical care: better, but not easier. NEJM Catalyst. 2017. https://catalyst.nejm.org/shared-decision-makinggood-clinical-care/. Accessed 27 Aug 2018. [Google Scholar]
- 15.Stiggelbout AM, Pieterse AH, De Haes JC. Shared decision making: concepts, evidence, and practice. Patient Educ Couns. 2015;98: 1172–9. [DOI] [PubMed] [Google Scholar]
- 16.Elwyn G, Durand MA, Song J, et al. A three-talk model for shared decision making: multistage consultation process. BMJ. 2017;359: j4891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Montori VM, Kunneman M, Brito JP. Shared decision making and improving health care: the answer is not in. JAMA. 2017;318:617–8. [DOI] [PubMed] [Google Scholar]
- 18.O’Neill ES, Grande SW, Sherman A, Elwyn G, Coylewright M. Availability of patient decision aids for stroke prevention in atrial fibrillation: a systematic review. Am Heart J. 2017;191:1–11. [DOI] [PubMed] [Google Scholar]
- 19.Hargraves I, LeBlanc A, Shah ND, Montori VM. Shared decision making: the need for patient-clinician conversation, not just information. Health Aff (Millwood). 2016;35:627–9. [DOI] [PubMed] [Google Scholar]
- 20.Kunneman M, Branda ME, Noseworthy PA, Linzer M, Burnett B, Dick S, et al. Shared decision making for stroke prevention in atrial fibrillation: study protocol for a randomized controlled trial. Trials. 2017;18:443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Legare F, Stacey D, Briere N, et al. A conceptual framework for interprofessional shared decision making in home care: protocol for a feasibility study. BMC Health Serv Res. 2011;11:23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stacey D, Legare F, Lewis K, et al. Decision aids for people facing health treatment or screening decisions. Cochrane Database Syst Rev. 2017;4:CD001431. [DOI] [PMC free article] [PubMed] [Google Scholar]