Tobacco smoke off-gassing from prior exposure releases contaminants that are equivalent to several cigarettes of secondhand smoke.
Abstract
The contamination of indoor nonsmoking environments with thirdhand smoke (THS) is an important, poorly understood public health concern. Real-time THS off-gassing from smokers into a nonsmoking movie theater was observed with online and offline high-resolution mass spectrometry. Prominent emission events of THS tracers (e.g., 2,5-dimethylfuran, 2-methylfuran, and acetonitrile) and other tobacco-related volatile organic compounds (VOCs) coincided with the arrival of certain moviegoers and left residual contamination. These VOC emission events exposed occupants to the equivalent of 1 to 10 cigarettes of secondhand smoke, including multiple hazardous air pollutants (e.g., benzene and formaldehyde) at parts-per-billion concentrations. Nicotine and related intermediate-volatility nitrogen-containing compounds, which vaporized from clothes/bodies and recondensed onto aerosol, comprised 34% of observed functionalized organic aerosol abundance. Exposure to THS VOC emission events will be considerably enhanced in poorly ventilated or smaller spaces in contrast with a large, well-ventilated theater—amplifying concentrations and potential impacts on health and indoor chemistry.
INTRODUCTION
Decades of research have demonstrated the adverse effects of fine-mode particulate matter (i.e., PM2.5) and volatile organic compounds (VOCs) from tobacco smoke (i.e., environmental tobacco smoke) on human health, with no “safe” level of exposure (1, 2). Regulations, some of which extend smoking restrictions to within 25 feet of a building’s doors, windows, and air intakes, have decreased nonsmokers’ exposure to secondhand smoke (SHS) (3). Yet, with worldwide smoking rates at 22% (4), exposure to hazardous pollutants from tobacco smoke remains a major risk for nonsmokers, and thirdhand smoke (THS) has been identified as a major exposure pathway (1, 5, 6).
THS originates from the direct contamination of surfaces (e.g., smokers’ bodies and clothes, indoor furnishings and surfaces, and building materials) with hazardous organic compounds from tobacco combustion but does not include airborne primary particles. When VOCs and larger intermediate- or semi-volatile compounds (I/SVOCs; e.g., nicotine) sorb to these surfaces, they can accumulate in a persistent organic layer (1, 7, 8). From the organic layer, they can dynamically repartition to the gas phase and then condense onto aerosols (9, 10), dust (11), or other surfaces (12). Surface-deposited THS can also participate in chemistry with common oxidants (e.g., ozone, nitrous acid) to form oxidation by-products, such as highly carcinogenic tobacco smoke nitrosamines (TSNAs) (11, 13).
THS exposure can occur via inhalation of evaporated gases (6), resuspended dusts (14), or I/SVOCs condensed onto aerosols (9, 10), along with ingestion (1) or dermal exposure (1, 15) via surfaces or dust. THS presents health risks to nonsmokers (6, 14), especially infants and children, who represent particularly vulnerable populations (5). THS-related genotoxicity, carcinogenicity, and oxidative stress has resulted in cytotoxicity (i.e., cell death) in a variety of cultured cells and caused physiological and developmental effects in live mice (1, 16). Even when nonsmokers were exposed to SHS, the health impacts of THS were estimated to be 5 to 60% of the combined disease burden from SHS and THS exposure (6). The share of THS contribution to total disease burden will be greater for individuals who minimize SHS exposure.
THS contamination of surfaces and dust is prevalent and has been observed (often via nicotine measurements) in a wide variety of locations with past smoking and even some nonsmoking environments (1, 11, 14). Elevated gas-phase concentrations of 17 tobacco-related VOCs (relative to outdoor air) were also observed in a smoker’s home long after the dissipation of SHS, indicating the persistent release of these VOCs by surfaces (6). Previous studies have also demonstrated adsorption and desorption of tobacco smoke VOCs to/from clothing (17, 18), clothing’s ability to accumulate nicotine (15), the rate of decrease of select VOCs in smokers’ breath over minutes to days (17, 19, 20), and aerosol uptake of semivolatiles for redistribution within a building (9). However, studies on THS-related VOCs in nonsmoking environments do not exist, and while THS transport to nonsmoking sites has been proposed theoretically (1), no studies have yet observed or quantified the active transport and emission of THS VOCs and IVOCs from people into nonsmoking environments.
This paper demonstrates real-time emissions of THS from people into a nonsmoking indoor environment. Our objectives are to (i) evaluate the dynamics of real-world THS emission events that increase indoor concentrations of hazardous pollutants, (ii) chemically characterize gas- and aerosol-phase THS contributions using a combination of online and offline high-resolution mass spectrometry (MS) techniques, and (iii) estimate the magnitude of these emissions relative to SHS emissions to contextualize the findings. This case study took place in a well-ventilated, well-maintained, and modern movie theater environment where occupants cannot actively smoke. It is an ideal case study location, where contributors to THS events could only be exposed to SHS before entering the theater building (either as a smoker or from being in the presence of smokers), entered the theater at a fixed time, and then remained there for multiple hours throughout the movie. Yet, this observed effect translates to any indoor environment that people occupy after tobacco smoke exposure.
RESULTS
Dynamics of THS-related VOC emissions and concentrations and their differences with audience demographics
The movie theater where the study took place strictly enforces German laws banning indoor smoking in theaters and has not allowed smoking for 15 years. The theater was supplied 100% fresh air for the purposes of this study (i.e., no recirculation), and its air intakes are at or near the roofline (approximately 20+ m above the base of the building), keeping them further away from potential street-level sources. This configuration effectively reduced outdoor SHS exposure and minimized any contribution from other parts of the building during this study. In addition, intake air was passed through a filter (equivalent to MERV 12) and underwent humidity and temperature adjustments.
Through four consecutive days (27 to 30 January 2017) of real-time measurements with online high-resolution MS [i.e., proton transfer reaction–time-of-flight (PTR-TOF) MS], 35 different VOCs previously associated with THS or tobacco smoke were observed at considerable concentrations in the theater, including furanoids, aromatics, aldehydes, alkenes, and nitrogen-containing species, confirmed via offline gas chromatography with MS (GC-MS) as listed in Table 1 (6, 21, 22). Most changes in these VOC concentrations trended together over the course of each day, punctuated by synchronized, sharp increases of known THS tracers (e.g., 2,5-dimethylfuran, 2-methylfuran, and acetonitrile) and other THS-related VOCs (Fig. 1). These concentration spikes occurred repeatedly at the start of films during audience arrival, concurrent with sharp changes in markers of human occupancy, including decamethylcyclopentasiloxane (D5) and CO2.
Table 1. Emission rates of known THS compounds in online PTR-TOF MS data.
All compounds reported here have been previously observed in tobacco smoke (6, 21, 22). For the isomer distributions for C8 aromatics, C9 aromatics, and C10 aromatics, consult Fig. 2D.
| Compounds |
Average emission rate during THS events (mg/hour) |
Emission rate regression to 2,5-dimethylfuran |
Emission rate regression to benzene |
t test: R-rated versus G-rated emission rates |
||||
| Mean ± SD* | r | Slope | r | Slope | t | P value† | ||
| Aromatics | Benzene‡§ | 3.46 ± 1.79 | 0.87 | 4.73 ± 0.73 | 1.00 | 1.00 ± 0.00 | 4.14 | 0.001 |
| Toluene‡§ | 5.91 ± 2.78 | 0.74 | 6.14 ± 1.53 | 0.93 | 1.42 ± 0.15 | 2.79 | 0.009 | |
| C8 aromatics‡§ | 4.84 ± 2.11 | 0.74 | 5.00 ± 1.26 | 0.92 | 1.15 ± 0.14 | 2.81 | 0.010 | |
| C9 aromatics‡ | 3.12 ± 1.37 | 0.72 | 3.28 ± 0.88 | 0.91 | 0.77 ± 0.10 | 4.36 | <0.001 | |
| C10 aromatics‡ | 0.91 ± 0.42 | 0.75 | 1.03 ± 0.25 | 0.91 | 0.23 ± 0.03 | 4.62 | <0.001 | |
| Phenol‡§ | 0.57 ± 0.32 | 0.85 | 0.74 ± 0.13 | 0.77 | 0.13 ± 0.03 | 2.60 | 0.011 | |
| Styrene‡§ | 0.71 ± 0.35 | 0.72 | 0.84 ± 0.22 | 0.83 | 0.18 ± 0.03 | 5.07 | <0.001 | |
| Benzaldehyde‡ | 0.24 ± 0.10 | 0.59 | 0.18 ± 0.07 | 0.57 | 0.03 ± 0.01 | 2.98 | 0.006 | |
| Cresols‡§ | 0.26 ± 0.14 | 0.87 | 0.34 ± 0.05 | 0.81 | 0.06 ± 0.01 | 2.93 | 0.006 | |
| Naphthalene‡§ | 0.34 ± 0.16 | 0.88 | 0.40 ± 0.06 | 0.88 | 0.07 ± 0.01 | 2.78 | 0.008 | |
| Furanoids | Furan | 0.43 ± 0.40 | 0.90 | 0.90 ± 0.12 | 0.74 | 0.14 ± 0.03 | 1.83 | 0.045 |
| 2-Methylfuran | 0.83 ± 0.75 | 0.98 | 1.92 ± 0.11 | 0.85 | 0.31 ± 0.05 | 3.15 | 0.006 | |
| 2,5-Dimethylfuran§ | 0.48 ± 0.36 | 1.00 | 1.00 ± 0.00 | 0.87 | 0.16 ± 0.02 | 3.76 | 0.002 | |
| Furfural‡ | 0.60 ± 0.36 | 0.90 | 0.86 ± 0.11 | 0.80 | 0.14 ± 0.03 | 2.29 | 0.020 | |
| Furfuryl alcohol‡ | 0.31 ± 0.14 | 0.93 | 0.37 ± 0.04 | 0.83 | 0.06 ± 0.01 | 3.37 | 0.003 | |
| Carbonyls | Formaldehyde§ | 0.94 ± 0.50 | 0.87 | 1.25 ± 0.20 | 0.83 | 0.22 ± 0.04 | 3.84 | 0.001 |
| Acetaldehyde§ | 9.07 ± 6.51 | 0.90 | 15.44 ± 2.20 | 0.84 | 2.64 ± 0.50 | 2.86 | 0.009 | |
| Acrolein§ | 0.73 ± 0.57 | 0.94 | 1.43 ± 0.14 | 0.87 | 0.24 ± 0.04 | 3.31 | 0.004 | |
| Acetone∥ | 20.45 ± 10.45 | 0.69 | 19.54 ± 6.43 | 0.72 | 3.75 ± 1.14 | 3.06 | 0.010 | |
| Methacrolein | 0.61 ± 0.43 | 0.93 | 1.10 ± 0.12 | 0.85 | 0.18 ± 0.03 | 3.14 | 0.004 | |
| 2,3-Butanedione | 1.00 ± 0.68 | 0.90 | 1.68 ± 0.23 | 0.72 | 0.25 ± 0.07 | 3.59 | 0.003 | |
| Other | Acetonitrile§ | 1.20 ± 0.86 | 0.96 | 2.24 ± 0.19 | 0.80 | 0.35 ± 0.07 | 3.27 | 0.004 |
| Acetic acid∥ | 8.40 ± 2.21 | 0.68 | 5.84 ± 1.99 | 0.69 | 1.09 ± 0.36 | 3.10 | 0.010 | |
| Isoprene | 4.55 ± 2.89 | 0.84 | 6.81 ± 1.22 | 0.77 | 1.15 ± 0.26 | 2.93 | 0.006 | |
| Monoterpenes‡∥ | 2.14 ± 1.91 | 0.58 | 2.62 ± 1.05 | 0.46 | 0.41 ± 0.23 | 0.99 | 0.179 | |
*Emission rates were calculated from the THS emission events of 10 R-rated movie showings unless noted otherwise. High SDs indicate high movie-to-movie variability. Emission rates include isomers and ionization products not mentioned above (e.g., methyl vinyl ketone with methacrolein).
†P values in bold represent those that are statistically significant (P < 0.05) for the unpaired two-sample t test, evaluating whether the emission rates were higher in the 10 R-rated movie screenings than the five family movies. See section S1 for more details.
‡Compounds were identified with an offline GC-MS method for ≥C6 compounds using standards and the National Institute of Standards and Technology library.
§Hazardous air pollutants (HAPs) as assigned by the EPA or in the case of 2,5-dimethylfuran, showed cytotoxicity in previous studies (1).
∥Emission factors use data from days 1 to 3 for acetone and acetic acid because large non-THS related emissions from cleaning were observed before the start of day 4 for these compounds, which biased calculations. The monoterpenes values exclude the third film on day 1 because of a large non-THS spike after the start of the film.
Fig. 1. Real-time concentrations of known THS compounds from PTR-TOF MS over a weekend (Friday to Sunday) of films.
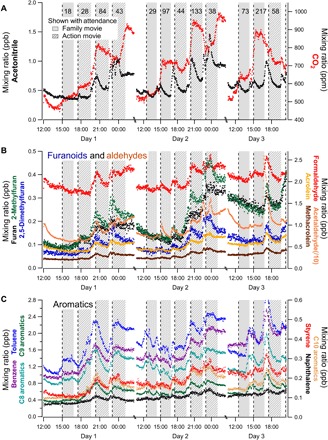
Major repeated emission events of THS tracers and known tobacco-related compounds, including (A) acetonitrile, (B) furanoids and aldehydes, and (C) aromatics, are observed near the start of R-rated action films, while only minor enhancements are present for family films. (A) includes CO2 as a marker of human occupancy and displays attendance data from ticket sales (along the top), movie start times (dotted lines), and movie duration (shading). The shading also denotes generic movie category—family movie (Wendy) or R-rated action movie (Resident Evil). Concentrations are shown as 2-min averages. A change in ventilation mode led to the sudden increases in CO2 at around midnight each night. Figure S5 includes Monday’s data, along with D5, which represents an additional marker for human occupancy changes complementary to CO2. ppm, parts per million; ppb, parts per billion.
The increase in THS tracer concentrations was a function of audience demographics for both movie type and movie showtime. While movie rating (G-rated versus R-rated) may not explicitly represent the audience demographic, it was a proxy for both audience age and the likelihood that individuals in the audience were exposed to smoke at some point before entry. A much more pronounced enhancement was observed for the showings of R-rated action movies (e.g., Resident Evil and Irre Helden), while similar abrupt increases were minor and only occasionally present in the family movie screenings, even with large audiences of 70 to 220 people (e.g., Wendy). Concentration spikes due to THS emissions are largest for later showtimes.
We determined the overall chemical composition of the emission events during R-rated films (Fig. 2D) and calculated each VOC’s effective gas-phase emission rates (hereafter, “emission rates”) for each film by using a box model similar to the one used by Stönner et al. (23) (see the Supplementary Materials). THS emission rates are statistically higher for R-rated action movies than for family movies for most THS compounds (P < 0.01 in one-tailed t tests) (Table 1).
Fig. 2. Composition and dynamics of THS emission events observed at the theater.
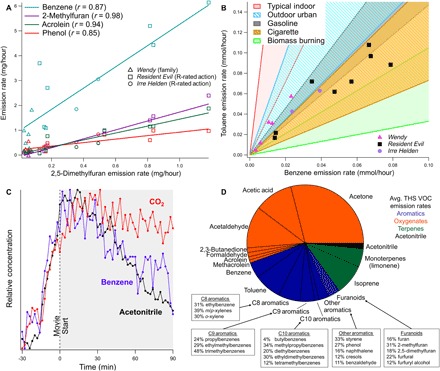
(A) Regressions between emission rates of VOCs commonly found in THS with 2,5-dimethylfuran, a commonly used tracer for THS and environmental tobacco smoke. (B) Observed toluene versus benzene emission rates compared to literature data (see the Supplementary Materials) for tobacco smoke and other sources and environments. (C) Close-up of a single THS emission event, with acetonitrile, benzene, and CO2 concentrations shown on relative scales for comparison (day 2, 20:20 showing of Resident Evil). Concentrations increase simultaneously; CO2 comes to steady state because of constant emissions, while acetonitrile and benzene decay as a result of decreased off-gassing from occupants. (D) The average composition of THS-related emissions during THS events, colored by compound type. Isomer speciation is derived from offline TD-GC-MS and may not add up to 100% because of rounding.
In sharp contrast with the respiration-dependent CO2 signal, THS VOC concentrations decay throughout the film because of both the known exponential decay in initial peak THS off-gassing (17, 19) and their ventilation from the room (Figs. 1 and 2C). The effective air exchange rate (AER) reported in previous works at the same theater is 1.5 hour−1 (23, 24), which would correspond to a 63% concentration decrease (i.e., ) in 40 min (for an instantaneous emissions spike). In this study, the observed 63% decay in acetonitrile concentrations from peak THS emissions at the start of the film was 42 to 50 min (fig. S1A). THS VOC concentrations generally increased over the course of multiple R-rated films and the weekend (Fig. 1) because of (i) continued off-gassing from the audience throughout the film (albeit at lower rates), (ii) persistent THS repartitioning within the theater room, and/or (iii) insufficient time between THS emission events for ventilation to dilute concentrations back to their initial baseline levels.
Emissions from late arrivals, observed as concentration spikes of THS tracers during films (well after their start), contributed noticeable concentration enhancements multiple times during 4 days of online MS measurements (fig. S1B). These spikes indicate that subsets of audience members can contribute appreciable emissions of THS-related compounds upon entry into the screening room, and the positive pressure of fresh outdoor air supplied to the screening room ensures that these spikes are not the result of air intrusion from other parts of the building. We also observed smaller concentration spikes occurring at the ends of some R-rated films as the audience leaves (fig. S5). These may be attributable to higher breathing rates during departure or agitation of clothes that may have been inaccessible to airflow while seated. In principle, entry/exit by audience members could lead to the resuspension of aerosol/dust containing persistent THS from the theater’s surfaces, or the warming of seats with sorbed persistent THS could cause thermal repartitioning, but the observed THS VOC emissions were minimally affected by these because the large audiences for family films did not produce similar emission spikes.
Emissions of key tobacco smoke tracers are well correlated, and relative VOC composition is consistent with THS off-gassing
Some of the most prominent tobacco smoke markers are furanoids (6, 22, 25). One furanoid in particular, 2,5-dimethylfuran (C6H8O), is an ideal gas-phase marker for tobacco smoke in indoor environments because (i) smoking emits a substantial amount of 2,5-dimethylfuran (average of 210 μg per cigarette) (22), (ii) emissions of 2,5-dimethylfuran are unique to tobacco smoke (22), (iii) its concentration in outdoor air is negligible (6), and (iv) its isomers are not present at sufficient concentrations to interfere with measurements (6, 22, 25). 2-Methylfuran and acetonitrile are also notable THS tracers for similar reasons (1, 6) and changed simultaneously with 2,5-dimethylfuran in the movie theater. The other furanoids identified in the PTR data—furan, furfural, and furfuryl alcohol—varied in parallel with 2,5-dimethylfuran (Fig. 1B and fig. S2A). Calculated emission rates of 2,5-dimethylfuran correlated very strongly with previously reported tobacco smoke VOCs acrolein, a cytotoxin (1), and 2-methylfuran (r = 0.94 and 0.98, respectively), suggesting THS as the movie theater’s primary source of these and other well-correlated VOCs that are known to originate from tobacco smoke (Fig. 2A).
The ratio of THS tracers 2-methylfuran to 2,5-dimethylfuran can indicate the approximate age of THS emissions and the contribution of fresh versus aged THS to observed concentrations (1, 6). The slope of the emission rate regression between 2-methylfuran and 2,5-dimethylfuran during R-rated films was 1.9 ± 0.1, which is indicative of less-aged THS (Fig. 2A, Table 1, and fig. S4A). Yet, the overall concentration mass ratio (i.e., not just fresh emissions) generally ranged between 1.0 and 2.2 throughout the 4 days, notably increasing over the course of R-rated movies (fig. S4A). In the work of Sleiman et al. (6), this mass ratio in a simulated room chamber decreased from 2.4 ± 0.3 to 1.8 ± 0.3 after 2 hours of aging and further decreased to 1.2 ± 0.2 after 18 hours. In the theater, the ratio decreased and approached the 18-hour ratio reported by Sleiman et al. during family film showings, which supports the case for persistent background THS present at the case study site from previous emission events (fig. S4A). On Friday (day 1), baseline 2,5-dimethylfuran concentrations before the THS emission events were 64 ± 6 parts per trillion (ppt; including during the family film), while average concentrations across the THS emission events in the last two R-rated films were 123 ± 24 ppt. Even during these fresh THS emission events, the persistent THS accounted for half of the THS exposure represented by 2,5-dimethylfuran.
Nonfuranoid aromatics were also present in large quantities (Fig. 1C). Both toluene and benzene are well documented in tobacco smoke emissions (22, 26), and together, they comprised 3% of total VOCs present in this study and 12.6% of THS VOCs (Fig. 2D). Similarly, C8 to C10 aromatics and functionalized aromatics (e.g., phenol and benzaldehyde) are well-reported VOCs from cigarettes (22) and observed at the theater. To demonstrate that benzene and toluene emissions (and other correlated aromatics) were predominantly from THS and not from the transport of other emissions, we compared their emission ratios to the 2,5-dimethylfuran THS tracer. Emission rates correlated strongly with 2,5-dimethylfuran (e.g., r = 0.87 and 0.85 for benzene and phenol, respectively) (Fig. 2A and Table 1), and ratios were consistent with previous THS measurements (fig. S4B). The ratio of benzene to 2,5-dimethylfuran in THS emission rates in the theater was 4.7 ± 0.7 (Table 1), which was similar to the 4.8 ± 0.7 ratio for THS and significantly different from the 2.8 ± 0.4 ratio for fresh tobacco smoke in the simulated room chamber of Sleiman et al.(6) (fig. S4B).
Literature toluene:benzene concentrations and molar emission ratios (mol mol−1) vary as follows: typical nonsmoking conditions indoors, 3.59 to 14.57; urban outdoors, 1.75 to 4.95; biomass burning emissions, 0.33 to 1.05; gasoline emissions, 1.47 to 2.30; and cigarette smoke emissions, 0.73 to 1.70 (table S2). Here, the toluene-to-benzene molar emission rate ratios were within the ratios expected for cigarette smoke and were markedly different from past indoor environments without fresh tobacco smoke or THS (Fig. 2B). The largest six emission events observed fell within the cigarette smoke range, and 72% of the total benzene emissions observed during THS events fell within this range. All but one showing of Resident Evil (i.e., seven of eight) and one of two showings of Irre Helden (R-rated) fell in the cigarette smoke range with the remaining two showings having slightly higher toluene emission rates. However, these two films had large correlated spikes in THS tracers, so shifts in toluene:benzene could be due to some variance in the emission ratio between the mix of cigarette types/brands or a small additional toluene source. For Wendy, two of five showings fell within or near the cigarette smoke toluene:benzene ratios, although these showings had much smaller benzene and toluene emission rates.
Chemical composition of other simultaneous VOC emissions are consistent with THS composition
Besides the compounds shown in Fig. 1, we also observed emissions of compounds that (i) are known to be in fresh tobacco smoke and THS, (ii) trended with 2,5-dimethylfuran and benzene in the high-time resolution data with simultaneous increases during THS emission events (fig. S2), (iii) often had well-correlated emission rates with other THS-related compounds, and (iv) had significantly higher emission rates during R-rated films, according to the t test (Table 1). Notable compounds satisfying these criteria include furanoids (e.g., furfural and furfuryl alcohol), aromatics (e.g., phenol and cresols), isoprene, and oxygenates (e.g., acetone, acetic acid, and 2,3-butanedione) (fig. S2) (26–28). Although monoterpenes and some aromatics (e.g., benzaldehyde) have greater uncertainty in their emission rates and statistical measures because of non-THS sources, they are observed and included here because previous literature highlights them as prominent tobacco smoke components (22).
Terpenes are well-known components of cigarette emissions (22). Sharp increases in monoterpenes (measured via PTR-TOF MS) were observed with THS tracers at the start of films (fig. S2B), but other major emissions of monoterpenes were occasionally present, notably during family films unaccompanied by THS tracers. We calculated the THS monoterpene (primarily limonene) emission rate using 9 of 10 R-rated films, excluding the third film on day 1 because of a large non-THS spike during that showing. The resulting ratio of monoterpene to 2,5-dimethylfuran emission rates was 2.6 ± 1.1, comparable to the ratio of 2.3 found in cigarette emissions (6). Isoprene is a known emission from cigarettes (6, 26, 27) and is exhaled by humans in appreciable quantities. The observed emission ratio for isoprene to 2,5-dimethylfuran was 6.8 ± 1.2, which is similar but slightly lower than the single literature value of 9.4 in fresh tobacco smoke (26).
The most abundant of these THS-related compounds were acetone, acetic acid, and acetaldehyde, which made up 51% of the emissions profile (Fig. 2D). They have other known and common indoor sources including humans and cleaning products (29), but these emission rates were calculated using only the THS events from R-rated films to minimize contributions from other sources. We recognize that other human-related sources could comprise some fraction of these, and potentially other, emission rates. However, we do not attempt any such subtraction of these other sources because of the large uncertainties associated with applying highly variable per-person emission factors to the diverse demographics studied here. Therefore, we present the overall observed emission rates during the THS emission events in R-rated films, which are statistically significantly larger than G-rated films (t test results; Table 1). With the exception of day 4, which was affected by large cleaning-related emission events before business hours, emission rates are well correlated with THS tracers (i.e., r = 0.68 to 0.90 with 2,5-dimethylfuran; Table 1), and real-time concentration enhancements are aligned with THS tracers (Fig. 1B and fig. S3). Although these species have been previously studied as components of cigarette emissions and THS (6, 27), emission factor data for them remains limited (i.e., one sample in one paper). The ratios to 2,5-dimethylfuran compared to literature were 5.8 ± 2.0 versus 6.0 mg/mg for acetic acid, 15.4 ± 2.2 versus 10.5 mg/mg for acetaldehyde, and 19.5 ± 6.4 versus 5.6 mg/mg for acetone, respectively (27). The higher ratios of acetaldehyde and acetone in this study may be indicative of audience-related non-THS sources or variations in compound-dependent THS off-gassing rates.
Offline electron ionization (EI)–MS analysis of adsorbent tube samples, collected according to Sheu et al. (30), was used to identify and confirm single compounds (e.g., C5H4O2 as furfural) and determine the distribution of isomers (e.g., C8 aromatics) detected in the online MS data. The diversity in C8–9 aromatics was similar to that observed in past studies (22), and we report a much more extensive speciation of the C9–10 aromatics than previous work (Fig. 2D). In addition to those compounds measured via online MS and summarized in Fig. 2D, we also observed a wide range of VOCs-IVOCs in the offline samples that have been reported in prior cigarette emissions or THS studies, including n-alkanes (C7 to C18), aromatics (e.g., 1- and 2-methylnaphthalene), and nitrogen-containing compounds (e.g., pyridine and 2-methylpyridine) (6, 21, 22, 25, 28, 31). Previous papers have noted the presence of alkylbenzenes up to C11, but alkylbenzenes up to C19 (i.e., 1-methyldodecylbenzene) were observed in our samples. As in this study, most nitrogen-containing THS compounds have been previously detected almost exclusively in the aerosol phase, with the prominent exception of acetonitrile (6).
Large concentrations of nicotine and associated nitrogen-containing compounds in aerosol samples
The aerosol phase provides further evidence for elevated levels of THS in the movie theater environment. Multihour samples of particulate matter were collected on Teflon filters throughout each day of films (11 days total) and analyzed offline via liquid chromatography with electrospray ionization and high-resolution MS (LC-ESI-TOF) for molecular-level speciation of the functionalized organic aerosol, using methods detailed by Ditto et al. (32). Consistent with previous work (32), the definition of functionalized organic aerosol here excludes hydrocarbons (i.e., CH) and sulfur-containing compounds without oxygen (i.e., CHS), which do not ionize well in LC-ESI-TOF. The inclusion of CH compounds typically present in OA would add additional observed analytes and thus decrease the reported ion abundance fractions for functionalized compounds. The scope of functionalized organic aerosol includes both functionalized primary and secondary organic aerosol (SOA), although SOA (including recondensed IVOCs from THS) will dominate the functionalized organic aerosol signal, and outdoor contributions will be minimized because of the filtering of particles in the building’s air intake. A comparison of observed functionalized compound class distributions to outdoor samples from Atlanta, GA (32) and Mainz, Germany (33) showed major differences (Fig. 3). The outdoor Mainz data provided an indoor-outdoor intercomparison, while the Atlanta data, representative of another urban center, were analyzed using the same instrument and methods.
Fig. 3. Average chemical composition of functionalized organic aerosol collected on daily filters throughout the campaign during business hours.
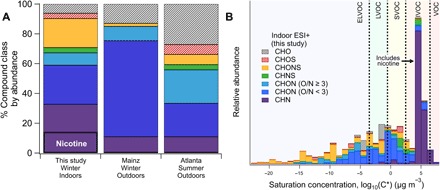
(A) Compound class distribution compared between three sites, including outdoor comparisons to Mainz and Atlanta (32, 33). Nicotine (labeled) made up 15% of functionalized aerosol abundance measured via high-resolution LC-ESI-TOF in positive mode, and nitrogen-containing compounds made up 88% of all identified compounds (fig. S6A and table S3). (B) The volatility distribution for the positive mode data displays the dominance of CHN compounds in the IVOC range, with the remaining compounds populating the SVOC, low volatility organic compound (LVOC), and extremely low volatility organic compound (ELVOC) ranges. Typical volatility distributions can be found in the work by Ditto et al. for Atlanta and other sites (32). Additional figures and numerical data for positive- and negative-mode ESI can be found in fig. S6 and table S3.
Reduced nitrogen compounds (i.e., CHN), many of which are associated with tobacco smoke (9, 10), comprised a large fraction (34%) of the functionalized organic aerosol by ion abundance, present in electrospray positive mode. Nicotine (C10H14N2) alone made up to 15% of the total abundance of positive-mode functionalized organic aerosol (Fig. 3A), consistent with previous research that showed that clothing can retain and expose its wearer to significant quantities of nicotine (15). Along with nicotine, which was confirmed using an authentic standard, we also observed multiple other prominent nitrogen-containing compounds, including C10H12N2 (i.e., anatabine, confirmed with standard), C9H12N2 (e.g., nornicotine), C9H9N (e.g., skatole, a tobacco additive), C7H7N [e.g., THS tracer 3-ethenylpyridine (3-EP)], and C10H12N2O (i.e., cotinine, a nicotine metabolite, confirmed with standard) (11, 14, 15).
By ion abundance, 88% of total functionalized organic aerosol contained nitrogen. A considerable proportion of observed compounds contained nitrogen, oxygen, and sulfur (CHONS): 25% in positive mode and 43% in negative mode by abundance (Fig. 3 and fig. S6). The prominence of CHON is similar to the other outdoor sites, but CHONS dominates the remainder of the aerosol phase as opposed to CHO (Fig. 3A and fig. S6). Nitrosamine standards were run to confirm a lack of nitrosamines detected in the aerosol-phase samples, but this test does not rule out their presence on surfaces. We hypothesize that the lack of TSNAs detected is due to an inability of these nitrosamines to repartition from surfaces to indoor aerosols and/or insufficient kinetics for formation on indoor aerosols over these time scales (i.e., lack of HONO or insufficient relative humidity) (10, 13).
DISCUSSION
Observed gas- and aerosol-phase organic compounds are characteristic of THS originating from tobacco smoke
In summary, we identify key, prevalent gas-phase tracers of tobacco smoke (i.e., 2,5-dimethylfuran, 2-methylfuran, and acetonitrile). Concentrations of these tracers and coemitted compounds (e.g., aromatics, aldehydes, and furanoids) show sharp, well-correlated increases during THS emission events that occur upon the arrival of new audiences (i.e., not randomly) as shown by both their consistent timing and co-occurrence with changes in non-THS markers of occupancy, specifically with CO2 and the siloxane D5 (Fig. 1 and fig. S5). These VOC emissions (Table 1) are known components of tobacco smoke, and their composition is consistent with THS (fig. S4).
This study also includes the first high-resolution, nontargeted, comprehensive speciation of complex indoor aerosol mixtures. Reduced nitrogen species (CHN), mostly in the intermediate-volatility range, comprise 34% of the total abundance of functionalized organic aerosol, and nicotine was the most abundant single compound by nearly an order of magnitude. The prevalence of THS tracers that partitioned to the aerosol phase implies substantial THS contamination of other surfaces in this indoor nonsmoking environment and ones with similar THS emission events (12). Formulas for compounds associated with nicotine and purported CHN oxidation products (e.g., CHONS) were present in substantial quantities as well.
Elevated concentrations of hazardous VOCs and IVOCs from human transport of THS into indoor nonsmoking environments
Because of the strict no-smoking policy enforced by the theater, the occurrence of emission events immediately after audience arrival, the fact that emission events are significantly larger for those attending R-rated films and for later showings despite much smaller attendance numbers (Table 1), known adsorption/desorption of THS from materials and surfaces (9, 10, 15, 26), and the other statistical analyses presented, we conclude that humans transport THS into the theater via their clothing and bodies, which represent multiple different but currently inseparable pathways. This observation is in line with what has been theorized in the past but until now had yet to be proven empirically (1, 17). Nicotine and other I/SVOCs from THS have been previously observed on surfaces, dust, and aerosol (via partitioning), but human transport of hazardous VOC concentrations into nonsmoking environments has not been shown.
In this case study, THS off-gassing from humans is a noticeable indoor source of hazardous VOCs. Table 1 shows the emission rates of a variety of THS compounds, many of which are designated by the U.S. Environmental Protection Agency (EPA) as hazardous air pollutants (HAPs) or have shown significant health effects in other studies (labeled in Table 1) (6). Among them, benzene is of particular importance as a carcinogen, but acrolein, formaldehyde, and other coemitted species (e.g., 1,3-butadiene) also contribute to stochastic mortality (1). Furthermore, many of the Table 1 compounds are reactive gas-phase compounds (e.g., aromatics and monoterpenes) that can contribute to indoor SOA formation (34). So, gas-phase THS emissions are important precursors to indoor chemistry and poor indoor air quality—even when particulate matter is filtered from supply air such as in the case study location.
The results demonstrate that human THS transport also results in persistent THS contamination, and indoor nonsmoking environments can accumulate long-term THS VOC contamination from the human transport of THS, even without any direct SHS contributions. Persistent VOC contamination resulting from THS transport and repartitioning to surfaces/materials is directly supported by observed baseline concentrations at the start of each day and the relative ratios of THS tracer furanoids (Fig. 1 and fig. S4A). Repeated THS emission events and their repartitioning to surfaces led to increasing concentrations over the course of each day and the weekend (Fig. 1), and reduced theater ventilation rates while closed (e.g., 0.25 hour−1) kept concentrations even higher overnight. So, this THS accumulation is especially important for indoor environments with lower AERs, such as 0.14 hour−1 typical of many tight homes (35), considerably lower than the 1.5 hour−1 in the theater presented here. This persistence of VOCs is consistent with and helps explain past observations of nicotine and other lower-volatility THS compounds on surfaces in nonsmoking environments entered by smokers (e.g., neonatal intensive care units) (1).
Human transport of THS leads to uptake of reduced nitrogen species by indoor organic aerosol
The percentage of reduced nitrogen compounds (CHN) in the theater (34%) was substantial higher than that of outdoors in Mainz (11%), which was likely a consequence of sampling from a confined indoor space with substantial CHN emissions. Using volatilities calculated as described by Li et al. (36), most of these CHN compounds, including nicotine, fell in the IVOC range (Fig. 3B), which means that they can readily partition between the gas phase and aerosol phase. While IVOCs are normally prone to partitioning into the gas phase at equilibrium, past cigarette smoke research has shown that nitrogen-containing IVOCs (e.g., nicotine and 3-EP) primarily end up in the condensed phase at equilibrium, largely because of their functionality and likely also because of higher-organic loadings indoors (6, 12).
Although the aerosol-phase MS measurements are not available as real-time data, the large abundances of the reduced nitrogen compounds provide strong evidence of gas-phase THS emissions into the theater, considering that the theater was supplied with fresh filtered air during the study. This observation is consistent with past work showing that surfaces were contaminated with nicotine and associated lower-volatility THS compounds in nonsmoking environments (1). As less volatile compounds, they have been shown to be persistent in indoor environments and are likely to contribute to background THS contamination over time (1, 7).
The prominence of THS-related CHN compounds in this study matches the results from the work of DeCarlo et al., who identified a THS factor consisting of primarily reduced nitrogen species (contributing an average of 0.85 μg m−3 PM1) (9). They also proposed a pathway that would explain the transport of clothing/bodily borne I/SVOCs to aerosol, in which reduced nitrogen compounds can volatilize from clothing or bodies into the gas phase and then partition irreversibly to acidic aqueous indoor aerosols (9). Since outdoor aerosol has been estimated to have a pH between 0 and 2 due to its inorganic composition (i.e., sulfate, ammonium, and nitrate levels) (37), the aerosol would protonate these reduced nitrogen compounds (e.g., nicotine pKa,1 = 8.02, pKa,2 = 3.12), which would sequester them in the aerosol phase (9). Previous studies for acidity have modeled pH based solely on inorganic species, but considering how prominent the organic fraction is indoors, the indoor modeled aerosol pH may be higher because of the prevalence of these hydrogen-accepting reduced nitrogen compounds (9).
The prominence of THS-based CHN in the aerosol phase is the most likely explanation for the correspondingly high levels of CHONS, which may come as a result of CHN reactions with sulfate/sulfuric acid. Another possible contributor could be the absence of photolysis indoors. Because the theater’s relative humidity is fairly low (approximately 30%) during the winter months, we expect less aerosol hydrolysis and aqueous processing, which would preserve these nitrogen and sulfur functional groups.
Other considerations and sources
The magnitude of each THS emission event is dependent on (i) the number of smokers and SHS-exposed nonsmokers, (ii) the smoking habits and SHS exposure routes of individual audience members, and (iii) time since exposure; but the emission rates calculated in this study are intended to be independent of those factors (i.e., the total mass of emissions released per hour) because this information was unavailable. For some compounds, these emission rates may well be lower limits since deposition will act as a sink (12) or may include minor contributions from other sources—pointing to the need for isolated THS emissions studies from people.
One consideration in this and other future studies is the impact of electronic cigarettes (e-cigarettes), either that some fraction of the THS contamination transported into the theater could originate from e-cigarettes in addition to tobacco smoking or that e-cigarettes were illegally used indoors. E-cigarettes emit negligible amounts of 2,5-dimethylfuran, 2-methylfuran, and other nonoxygenated aromatics (e.g., benzene) (27, 38). While some compounds (e.g., nicotine and acetophenone) are also observed in e-cigarette emissions (27, 38, 39), the full spectrum and ratios of observed emissions to major THS tracers here are consistent with the source profile of THS from cigarette smoke (6, 22) (Fig. 1, Table 1, and fig. S4). Thus, we conclude that e-cigarettes did not noticeably contribute to the THS VOC emission events in this study. However, e-cigarettes could still have contributed to the observed THS nicotine abundances in Fig. 3, given the presence of nicotine in some vaping liquids (27, 38).
To explore possible contributions from “thirdhand vapor” or direct emissions, studies should examine the trends of prominent e-cigarette VOCs glycerol (C3H8O3) and 1,3-propanediol (C3H8O2). However, they share major PTR-TOF MS ions (C3H6O2H+ and C3H6OH+, respectively) with several other VOCs (e.g., acetone for C3H6OH+), which provide challenges in isolating their signal. The online PTR-TOF measurements do not suggest emissions from illegal indoor e-cigarette use since concentration spikes occur at the start of films soon after entry and the vast majority of increases in glycerol’s PTR mass coincided with increases in tobacco cigarette THS tracers, which is consistent with glycerol’s use as a cigarette additive (40).
We acknowledge that, for several of the compounds reported in Table 1, there are potential contributions from sources other than THS. For instance, noticeable toluene emissions throughout the first film on day 1 are not matched in magnitude by the other aromatics, indicating the presence of other sources (Fig. 1). However, to minimize any bias for all compounds, emissions rate averages in Table 1 only include the THS emission events occurring during R-rated films. The resulting regression of toluene versus 2,5-dimethylfuran has a slope of 6.1 ± 1.5 in the R-rated films, which is consistent with the THS ratio by Sleiman et al. (6.7 ± 0.9) (fig. S4B) (6). In addition, its THS emissions were well correlated with benzene throughout the measurements (Table 1, Fig. 1, and fig. S5).
Comparison of emissions and exposure to SHS
To contextualize and compare potential exposure to gas-phase HAPs from THS emission events to SHS exposure, we determined the equivalent amount of SHS exposure for VOCs with established emission factors in SHS studies (22, 26, 27) (table S1). Depending on the VOC, audiences in R-rated films were exposed to 1 to 10 cigarette equivalents of SHS on average during THS emission events (Fig. 4A and table S1). During the last showing on day 2 (Resident Evil), in a 1-hour period (±30 min around the movie start time), 38 audience members emitted and were exposed to the equivalent amount of 2,5-dimethylfuran emitted from 5.6 cigarettes and 8 to 15 SHS cigarette equivalents of benzene, C8 aromatics, and C9 aromatics. For this particular movie showing, each occupant in the movie theater inhaled 10.7 μg of benzene, providing an estimated breathing rate of 0.8 m3/hour (9). For the small THS emission events observed in 2,5-dimethylfuran and other tracers in some of the family movie showings, the SHS exposure equivalents averaged only 0.25 cigarettes in these fresh emission events compared with an average of 2.5 SHS cigarette equivalents for the R-rated action movies.
Fig. 4. Exposure to hazardous gas-phase THS VOCs corresponds to high levels of SHS and will be exacerbated by smaller room volumes and ventilation rates at other sites.
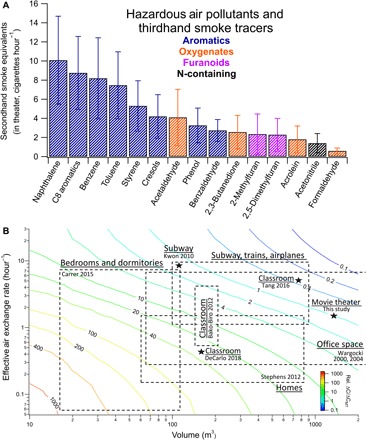
(A) Average SHS cigarette equivalents (± SD) for the R-rated THS emission events were calculated using literature emission factors per combusted cigarette (μg/cig) and Table 1 emission rates (table S1 and fig. S7) (22, 26, 27). Variability in the SHS equivalents between VOCs is primarily due to the variance in the rate of THS uptake and off-gassing, the THS contamination age, and the cigarette types/brands used in literature data. (B) Variations in relative concentration enhancements for THS VOCs as a function of room volume and effective AER for the same emissions profile as the theater. Concentration enhancement ratios (ΔC/ΔCMT, marked by the contour lines) represent the expected concentration increase in other environments from fresh THS emissions (ΔC) compared to the observed change in the large movie theater (ΔCMT) (1300 m3, 1.5 hour−1). A range of typical room volumes and effective AER (dashed boxes and stars) are shown as examples of these parameters for other indoor environments (9, 42–48). Stars were used to mark single locations, while boxes outline these parameters for a range of test sites.
Considerable differences in SHS equivalents exist between compounds, in large part because of the variability in off-gassing rates. From the available studies, THS aromatics are known to more rapidly off-gas from clothing and breath when compared with functionalized compounds such as acetonitrile (17, 19). Functionalized species, especially nitrogen-containing compounds (9, 10, 12), have shown much more rapid uptake and stronger adsorption to materials. Some oxygen-containing compounds [e.g., glyoxal (41)] behave similarly, albeit to a lesser extent. The results in Fig. 4A are largely consistent with this but are also affected by the uncertainties in the available emission factor data; the lack of information on cigarette brands and types smoked in this study; and the potential contributions from other non-THS sources (i.e., acetaldehyde), although the strong correlations of these compounds with THS tracers during THS emission events minimize this bias.
The SHS equivalents calculations for “fresh” emission events above do not include the exposure to persistent background contamination present at the site from past THS emission events (over the prior days, weeks, or months), which have an impact on the background VOC concentrations at this site. Estimates of occupant exposure to SHS cigarette equivalents would be notably elevated relative to those shown in Fig. 4A if persistent THS contamination were included. In addition, considering that the measurements taken were of bulk air exiting via the theater’s ceiling vents, spatial variations within the theater could lead to even higher exposures for those sitting near the source individuals.
These SHS equivalents could originate from cigarettes smoked by smokers themselves and/or from exposure to the smoke from nearby smokers in outdoor or indoor environments. The uptake (i.e., mass loading) of this original SHS contamination (for later THS off-gassing) would be enhanced if it were to occur in a more concentrated indoor environment with less ventilation or if a greater number of cigarettes were smoked, especially in the case of high-occupancy public places without an explicit ban on indoor smoking.
It is an important distinction that THS is not a source of primary PM, unlike SHS. Sleiman et al. estimated the relative health effects of THS and SHS (see Introduction) and found that the total PM2.5 contributed a substantial fraction (>90%) of overall tobacco smoke harm for those exposed to both SHS and THS, although a more complete survey of all THS-related emissions, reaction by-products, and their health effects is needed and represents an important area for future work (6). However, the uptake of THS I/SVOCs by preexisting aerosol will contribute to PM enhancement in nonsmoking locations. As demonstrated in a prior study, THS uptake contributed ~29% of the indoor PM1 in a nonsmoking environment (9).
Implications for other indoor environments
The movie theater environment is used as a case study, but the conclusions derived here are generalizable to other locations. The theater’s size and ventilation rate dilute an individual’s exposure to potentially hazardous VOCs. However, even in this well-ventilated environment, THS emission events were evident and led to persistent THS. Other locations may have a greater diversity of non–THS VOC sources, but THS emission events remain important for a diversity of sites. THS contamination is known to be very prevalent (1), and the emission events that transport THS contamination described here will have greater ramifications in spaces with smaller volumes or poorer ventilation.
The observed emission rates of VOC HAPs into a more confined or less well-ventilated space (e.g., a motor vehicle, bar, train, or a small room in a home) would lead to much higher concentrations and occupant exposure. Classrooms, office spaces, and public transit (e.g., trains, buses, aircraft, and subway cars) are prominent examples of nonsmoking environments with smaller rooms and a cyclic occupancy of 20 to 100 people. Subway cars are an extreme case of a confined indoor environment (150 m3) with high human density (up to ~150 to 180 per car), along with variable ventilation depending on car model and operation. Given that the theater studied had a large volume (1300 m3) and high effective exchange rate (1.5 hour−1), similar emissions entering a much smaller space and a considerably lower effective AER would exacerbate the accumulation and persistence of HAPs. Since the observations from this movie theater represent emissions from groups of people cycling in and out of a space, the emission profiles for the movie theater can be extended to other similar public spaces.
Using the same emission profile as that observed in the theater, we modeled the expected concentration increase for a range of volumes (10 to 2000 m3) and effective AERs (0.05 to 30 hour−1). We report the volumes and measured AERs for indoor spaces from previous studies (9, 42–48) (Fig. 4B). For instance, in a home with approximately 140 m3 of space and an effective AER of 0.5 hour−1, the average enhancement above baseline is expected to be 40 times that observed in the movie theater, assuming the same emission profile (Fig. 4B). If the VOC of interest were benzene, then the increase of approximately 0.75 parts per billion (ppb) on day 1 would instead be an increase by 0.75 × 40 = 36 ppb by the end of the day.
These concentration enhancements ratios are specifically for VOCs and describe the indoor environment as a whole, so concentrations in some parts of the room could be higher because of heterogeneity in indoor air circulation. They also do not include contributions from persistent THS or the increased role of repartitioning to other surfaces over longer time scales and decreased ventilation (see section S1). This estimate is sensitive to variation in the magnitude and temporal dynamics of THS emissions with occupant demographics and behavior, resulting in cumulative emissions that could be higher or lower than those in the movie theater. The frequency and magnitude of emission events and room volume will determine maximum concentrations. The room ventilation rate will determine the residence time of the emitted VOCs. Multiplying the magnitude of the emission profile by a positive scalar c is equivalent to scaling the emission rate and also scales the concentration enhancement ratio (ΔC/ΔCMT) by that same scalar c. Doing so therefore scales up or down the final concentration enhancement by that factor c.
Future work
Further experimentation is necessary to enable better modeling of THS emissions, chemistry, and exposure. Controlled studies focused on the chemical composition and chemical processing of the gas phase, aerosol phase, and surface phase would contribute to a better mechanistic understanding of how individuals contaminated with THS contribute to a room’s organic compound concentrations and how those dynamics affect occupants in return. To expand on the existing state of THS emissions research, well-constrained, high-time resolution studies of tobacco smoke and THS in a chamber or a similarly controlled and monitored test environment would isolate THS emissions from smokers; differentiate breath versus body/clothing emissions as part of a much-needed mass balance; examine residual repartitioned THS on occupants and surfaces; and provide insight into the magnitude and composition of THS contamination as a function of the number of cigarettes smoked and their evolution over time. These results would clarify the THS contribution of compounds with other major indoor sources (e.g., acetone, acetaldehyde, acetic acid, and monoterpenes). Experiments for determining THS off-gassing rates from contaminated materials after their removal from an SHS-rich environment should investigate variance across tobacco brands/types, furnishing materials, temperature-dependent variance in sorption/off-gassing, and volume/ventilation rates. These laboratory studies will also enable a more detailed and isolated chemical characterization of gas-phase emissions across a full range of volatilities.
Outside of laboratory tests, conducting more real-world measurements would help quantify the magnitude of THS transport to other indoor environments. Current smoking laws and indoor air quality standards in the United States and Germany do not address THS transport of HAPs, and applying a variety of sampling and analytical approaches, along with continued monitoring of a diversity of environments, would provide crucial information for policy refinement. To enable indoor mass balances for THS compounds, field studies should consider all sources/sinks, including THS partitioned to materials and surfaces. Refining and using “wipe”-based methods may reveal TSNAs and other surface-bound THS-related compounds not found in the air sampling for this manuscript. Quantifying TSNA levels both in the aerosol phase and on surfaces would inform potential exposure to this suite of highly carcinogenic compounds. In addition, considering the rapid increase in e-cigarette usage, field and laboratory studies are also needed to measure the emissions of THS or thirdhand vapor from adsorbed e-cigarette vapors.
MATERIALS AND METHODS
Experimental design
Measurements were collected at Cinestar Cinema Complex (Mainz, Germany) in collaboration with the Max Planck-Institute for Chemistry (MPIC) during January and February 2017. The viewing room was 1300 m3, and the nominal air supply rate was 6500 m3/hour (5 hour−1), with fresh ventilation air consisting 100% of outdoor air. Because of heterogeneous mixing, the effective AER was 1.5 hour−1 (23). Previous papers sampling from the same facility contain more details about sample collection (23, 24).
Audience members could only be exposed to tobacco smoke before entering the large theater building, either as smokers or in the presence of smokers. This exposure to direct or secondhand tobacco smoke could occur before arrival from an indoor or outdoor location up until just outside the theater building (smoking at which was observed), thus resulting in a variety of THS ages. Movie-going smokers are required to finish smoking their cigarettes before entering the building and have to wait 5 to 10 min to purchase tickets and walk to their screening room, which is sufficient time for complete smoke exhalation (18 to 90 s) based on clinical tests (20, 49). Since our goal is to characterize the dynamics of THS emissions without SHS interference, this movie theater presents a very useful test facility because of its compliance with a strict no-smoking policy, along with no recent history of indoor smoking. It also minimizes other common indoor sources (e.g., cooking) and isolates a group of people for multihour periods in a closed room, which they enter at similar times before the start of the film.
The campaign took place from 26 January to 7 February, coinciding with gas and aerosol sample collection for offline spectrometric analysis. Measurements via online PTR-TOF MS were collected from 27 to 30 January. During the sampling period, the cinema room displayed either four or five movies a day. Movie showtimes listed by the theater were provided, although they indicate the start of premovie previews, which last approximately 15 min on average.
Wendy (1 hour and 31 min), Resident Evil (1 hour and 47 min), and Irre Helden (1 hour and 53 min) are the movies featured in the online MS data. For this manuscript, Wendy is considered as a G-rated family movie, while Resident Evil and Irre Helden are R-rated action movies. No appreciable difference in the audience sizes throughout the 4 days of sampling was observed when comparing the G-rated to the R-rated films. R-rated films had an average of 78 ± 84 (n = 10) audience members, while Wendy showings had an average of 87 ± 80 (n = 5) moviegoers. An accompanying CO2 measurement was collected using a LI-COR LI-7000 (1 Hz), which was cross-referenced with the CO2 signal (mass/charge ratio = 45.0015) obtained from the PTR-TOF MS (Fig. 1A).
Real-time VOC measurements via online MS
Online, real-time sampling was conducted via a proton transfer mass spectrometer (PTR-TOF-MS-800, IONICON Analytik GmbH). Data originally collected in counts per second (cps) were converted to normalized cps (ncps) with the calculation from Williams et al. (24). The ncps values were converted to ppb by volume (ppbv) using an authentic standard cylinder with 14 components (Apel Riemer Environmental) or by calculating the compound-specific sensitivity (ncps/ppbv) (table S1). Additional detail and data processing methodology are in the Supplementary Materials and cited literature (23, 24).
Offline VOC speciation
Adsorbent tubes containing quartz wool, glass beads, and Tenax TA were prepared and handled as described by Sheu et al. (30). Samples were collected at 120 SCCM (standard cubic centimeters per minute) for 1 hour (7.2 liters). Field and sampling blanks were collected for comparison. All tubes were stored in a −80°C freezer after collection. The tubes were desorbed via a modified thermal desorption system (Markes TD-100) into a gas chromatograph (Agilent 7890B) containing an HP-5ms Ultra Inert column (30 m by 250 μm by 0.5 μm), which led into a vacuum EI mass spectrometer (Agilent 5977A). For more information on the adsorbent tube preparation, sampling, and desorption and GC methods, refer to Sheu et al. (30).
Offline speciation of organic aerosol
Daily PM samples were collected on polytetrafluoroethylene filters seated in a modified 316L stainless steel (passivated) filter housing (Pall) at 20 SLPM (standard liters per minute) (see Sheu et al. for a diagram) with sampling durations between 4.5– and 10 hours depending on the total duration of movie screenings on a given day (30). Filters were stored in a −80°C freezer after collection. Filter collection and analysis followed the procedure outlined in the Methods section by Ditto et al. (32). Each sample (5 μl) was run via high-performance liquid chromatography (HPLC; Agilent, 1260 Infinity with Thermo Scientific Hypercarb porous graphitic carbon reverse-phase column, 3-μm particle size, 2.1-mm column diameter, 30-mm column length), ionized with electrospray ionization, and analyzed using MS (TOF) for both positive and negative modes on an Agilent 6550 Q-TOF. Liquid chromatography started with a mobile phase of 5%:95% methanol:water and increased in methanol for 20 min until the mobile phase reached 90%:10%. Authentic standards of nicotine, myosmine, nornicotine, cotinine, anatabine, and anabasine were run for confirmation. HPLC data from other studies featured in this paper were collected and analyzed via the same system with minor differences (Atlanta PM10) (32) or with UHPLC-Orbitrap (Mainz PM2.5) (33). Calibrations were not possible for the entire observed complex mixture of functionalized compounds, so the results are reported in ion abundance here, consistent with common practice (e.g., Ditto et al. and Wang et al.) (32, 33).
Filter sample data were analyzed using Agilent MassHunter Qualitative Software to extract analyte peaks and provide high–mass accuracy molecular formulas (mass accuracy, <1 to 2 parts per million), leveraging isotope pattern and spacing to improve identifications. Compound identifications from MassHunter were imported into Igor Pro for data quality assurance and quality control (QC/QA) according to procedures by Ditto et al. (32), using strict exclusion criteria for LC peak quality and molecular formula assignment. The calculation of volatility was done via the parameterization presented by Li et al., which depends solely on the molecular formula (36). For extensive detail on filter extraction, LC-ESI parameters, and data processing, consult Ditto et al. (32).
Statistical analysis summary
All data analysis was done in Igor Pro 8. Emission rates were generated via a box model used previously (23) using 2-min resolution PTR-TOF MS data, with terms for emissions and ventilation contributing to changes in concentration (eq. S5). Integration of the emissions profile 30 min before and after movie start times produced the emission rates (Table 1) for each 1-hour THS emission event. This 1-hour time window was chosen to isolate the THS-related emissions occurring before/during previews and at the beginning of the film, to decrease the effect of persistent THS off-gassing, and to minimize influence from other sources. These emission rates represent effective gas-phase emission rates because of the wide variety of noncontaminated surfaces that interact with the gas phase. Sorption rates and tendencies to the diverse, uncertain mixture of surfaces/materials (related to either occupants or the room) will vary by material and compound volatility/functionality. Given these uncertainties, compound-dependent surface uptake of emitted THS compounds was not explicitly separated here. Hence, we acknowledge that the presented emission rates are potentially lower limits for some compounds. By 30 min after showtime, because of ventilation, concentrations decreased, and any remaining emissions contributed considerably less mass to the derived emission rate term for the THS emission event.
An unpaired, two-sample t test was run to compare emission rates from R-rated (n = 10) versus family films (n = 5). It was evaluated for α = 0.05 with heteroscedaticity (i.e., unequal variances). To test the hypothesis that R-rated movies experienced higher emission rates, a one-tailed test was used. Least-squares regressions of these emission rates for HAPs and other known THS components were executed to derive correlation coefficients and slopes (and uncertainties) relative to 2,5-dimethylfuran and benzene. Literature on VOC emission factors from cigarettes (and other sources for Fig. 2B; see table S2 for references) and laboratory chamber studies on the chemical composition of THS were used for comparison to the relative composition of THS VOCs observed in this study (6, 22, 26, 27). Per-cigarette emission factors (table S1) were used to compare emission rates to equivalent SHS emissions per cigarette and contextualize exposure (Fig. 4A).
The same box model was used to evaluate the potential impact of similar THS emission events on individual VOC concentrations in smaller or less ventilated spaces than the theater environment. Test cases were run for a matrix of room volumes and ventilation rates, and the overall concentration increase was compared to that of the movie theater to produce a concentration enhancement ratio (ΔC/ΔCMT). The contour plot in Fig. 4B was produced via interpolation of the gridded matrix of results in Igor Pro.
Supplementary Material
Acknowledgments
We thank Cinestar Mainz for providing a venue for data collection and N. Wang (MPIC) for assisting with deployment at the theater, as well as U. Pöschl for hosting D.R.G. at MPIC. We would also like to thank K. Wang and T. Hoffmann of Johannes Gutenberg-Universität Mainz for providing some relevant Mainz outdoor data from their 2018 paper. We thank P. Misztal for assistance with some of the steps needed to fine-tune the accuracy of the PTR data calibration. Last, we also thank J. Peccia for feedback and helpful discussions. Funding: R.S. would like to acknowledge financial support from NSF GRFP (DGE1122492 and DGE1752134). D.R.G. is grateful for support from the Alexander von Humboldt Fellowship, which made this collaboration possible, as well as the Sloan Foundation (G-2015-14134) and the U.S. EPA. This publication was developed under Assistance Agreement no. RD835871 awarded by the U.S. EPA to Yale University. It has not been formally reviewed by EPA. The views expressed in this document are solely those of the authors and do not necessarily reflect those of the EPA. EPA does not endorse any products or commercial services mentioned in this publication. Author contributions: C.S., J.W., and D.R.G. conceived the study. C.S., T.K., and J.W. collected and provided preprocessing of the PTR-TOF MS data. R.S. (tubes) and J.C.D. (filters) prepared the offline samples. D.R.G. and C.S. collected the offline samples. R.S. (tubes) and J.C.D. (filters) analyzed the offline samples. R.S. conducted subsequent analysis across all of the PTR-TOF MS, adsorbent tube, and filter data and compiled the results. R.S. and D.R.G. interpreted the data and wrote the paper. All authors commented on and discussed the manuscript to help refine the interpretation and presentation of results. Competing interests: The authors declare that they have no competing interests. Data and materials availability: All data needed to evaluate the conclusions in the paper are present in the paper and/or the Supplementary Materials. Additional data related to this paper may be requested from the authors.
SUPPLEMENTARY MATERIALS
Supplementary material for this article is available at http://advances.sciencemag.org/cgi/content/full/6/10/eaay4109/DC1
Section S1. Data analysis methods
Fig. S1. Acetonitrile decay rate during a movie and examples of late arrival THS events.
Fig. S2. Concentration profiles for other compounds present in THS not shown in Fig. 1.
Fig. S3. Acetone, acetic acid, and acetaldehyde spike simultaneously with known THS tracers.
Fig. S4. Ratio of 2-methylfuran to 2,5-dimethylfuran throughout real-time data collection and a literature tobacco smoke versus THS ratio to 2,5-dimethylfuran comparison.
Fig. S5. Concentration profile for THS compounds in Fig. 1 with Day 4 included.
Fig. S6. Compound class distribution and volatility distributions of functionalized organic aerosol.
Fig. S7. Average SHS cigarette equivalents and SDs during 10 R-rated THS emission events including all non-HAPs.
Table S1. Summary of compounds, literature emission factors, and PTR-TOF MS parameters for known THS compounds.
Table S2. Benzene and toluene ratios from literature.
Table S3. Compound class composition for functionalized aerosol.
REFERENCES AND NOTES
- 1.Jacob P. III, Benowitz N. L., Destaillats H., Gundel L., Hang B., Martins-Green M., Matt G. E., Quintana P. J. E., Samet J. M., Schick S. F., Talbot P., Aquilina N. J., Hovell M. F., Mao J.-H., Whitehead T. P., Thirdhand smoke: New evidence, challenges, and future directions. Chem. Res. Toxicol. 30, 270–294 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nazaroff W. W., Singer B. C., Inhalation of hazardous air pollutants from environmental tobacco smoke in US residences. J. Expo. Anal. Environ. Epidemiol. 14, S71–S77 (2004). [DOI] [PubMed] [Google Scholar]
- 3.Waring M. S., Siegel J. A., An evaluation of the indoor air quality in bars before and after a smoking ban in Austin, Texas. J. Expo. Sci. Environ. Epidemiol. 17, 260–268 (2007). [DOI] [PubMed] [Google Scholar]
- 4.World Health Organization (WHO), WHO Report on the Global Tobacco Epidemic,2017 (2017).
- 5.Winickoff J. P., Friebely J., Tanski S. E., Sherrod C., Matt G. E., Hovell M. F., McMillen R. C., Beliefs about the health effects of “thirdhand” smoke and home smoking bans. Pediatrics 123, e74–e79 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sleiman M., Logue J. M., Luo W., Pankow J. F., Gundel L. A., Destaillats H., Inhalable constituents of thirdhand tobacco smoke: Chemical characterization and health impact considerations. Environ. Sci. Technol. 48, 13093–13101 (2014). [DOI] [PubMed] [Google Scholar]
- 7.Matt G. E., Quintana P. J. E., Zakarian J. M., Fortmann A. L., Chatfield D. A., Hoh E., Uribe A. M., Hovell M. F., When smokers move out and non-smokers move in: Residential thirdhand smoke pollution and exposure. Tob. Control 20, e1 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Weschler C. J., Nazaroff W. W., Semivolatile organic compounds in indoor environments. Atmos. Environ. 42, 9018–9040 (2008). [Google Scholar]
- 9.DeCarlo P. F., Avery A. M., Waring M. S., Thirdhand smoke uptake to aerosol particles in the indoor environment. Sci. Adv. 4, eaap8368 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Collins D. B., Wang C., Abbatt J. P. D., Selective uptake of third-hand tobacco smoke components to inorganic and organic aerosol particles. Environ. Sci. Technol. 52, 13195–13201 (2018). [DOI] [PubMed] [Google Scholar]
- 11.Whitehead T. P., Havel C., Metayer C., Benowitz N. L., Jacob P., Tobacco alkaloids and tobacco-specific nitrosamines in dust from homes of smokeless tobacco users, active smokers, and nontobacco users. Chem. Res. Toxicol. 28, 1007–1014 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Singer B. C., Revzan K. L., Hotchi T., Hodgson A. T., Brown N. J., Sorption of organic gases in a furnished room. Atmos. Environ. 38, 2483–2494 (2004). [Google Scholar]
- 13.Sleiman M., Gundel L. A., Pankow J. F., Jacob P. III, Singer B. C., Destaillats H., Formation of carcinogens indoors by surface-mediated reactions of nicotine with nitrous acid, leading to potential thirdhand smoke hazards. Proc. Natl. Acad. Sci. U.S.A. 107, 6576–6581 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Matt G. E., Quintana P. J. E., Hovell M. F., Bernert J. T., Song S., Novianti N., Juarez T., Floro J., Gehrman C., Garcia M., Larson S., Households contaminated by environmental tobacco smoke: Sources of infant exposures. Tob. Control 13, 29–37 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bekö G., Morrison G., Weschler C. J., Koch H. M., Pälmke C., Salthammer T., Schripp T., Toftum J., Clausen G., Measurements of dermal uptake of nicotine directly from air and clothing. Indoor Air 27, 427–433 (2017). [DOI] [PubMed] [Google Scholar]
- 16.Hang B., Snijders A. M., Huang Y., Schick S. F., Wang P., Xia Y., Havel C., Jacob P. III, Benowitz N., Destaillats H., Gundel L. A., Mao J.-H., Early exposure to thirdhand cigarette smoke affects body mass and the development of immunity in mice. Sci. Rep. 7, 41915 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ueta I., Saito Y., Teraoka K., Miura T., Jinno K., Determination of volatile organic compounds for a systematic evaluation of third-hand smoking. Anal. Sci. 26, 569–574 (2010). [DOI] [PubMed] [Google Scholar]
- 18.Chien Y.-C., Chang C.-P., Liu Z.-Z., Volatile organics off-gassed among tobacco-exposed clothing fabrics. J. Hazard. Mater. 193, 139–148 (2011). [DOI] [PubMed] [Google Scholar]
- 19.Jordan A., Hansel A., Holzinger R., Lindinger W., Acetonitrile and benzene in the breath of smokers and non-smokers investigated by proton transfer reaction mass spectrometry (PTR-MS). Int. J. Mass Spectrom. Ion Process. 148, L1–L3 (1995). [Google Scholar]
- 20.Invernizzi G., Ruprecht A., De Marco C., Paredi P., Boffi R., Residual tobacco smoke: Measurement of its washout time in the lung and of its contribution to environmental tobacco smoke. Tob. Control 16, 29–33 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Martin P., Heavner D. L., Nelson P. R., Maiolo K. C., Risner C. H., Simmons P. S., Morgan W. T., Ogden M. W., Environmental tobacco smoke (ETS): A market cigarette study. Environ. Int. 23, 75–90 (1997). [Google Scholar]
- 22.Charles S. M., Jia C., Batterman S. A., Godwin C., VOC and particulate emissions from commercial cigarettes: Analysis of 2,5-DMF as an ETS tracer. Environ. Sci. Technol. 42, 1324–1331 (2008). [DOI] [PubMed] [Google Scholar]
- 23.Stönner C., Edtbauer A., Williams J., Real-world volatile organic compound emission rates from seated adults and children for use in indoor air studies. Indoor Air 28, 164–172 (2018). [DOI] [PubMed] [Google Scholar]
- 24.Williams J., Stönner C., Wicker J., Krauter N., Derstroff B., Bourtsoukidis E., Klüpfel T., Kramer S., Cinema audiences reproducibly vary the chemical composition of air during films, by broadcasting scene specific emissions on breath. Sci. Rep. 6, 25464 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Alonso M., Godayol A., Anticó E., Sanchez J. M., Assessment of environmental tobacco smoke contamination in public premises: Significance of 2,5-dimethylfuran as an effective marker. Environ. Sci. Technol. 44, 8289–8294 (2010). [DOI] [PubMed] [Google Scholar]
- 26.Singer B. C., Hodgson A. T., Guevarra K. S., Hawley E. L., Nazaroff W. W., Gas-phase organics in environmental tobacco smoke. 1. Effects of smoking rate, ventilation, and furnishing level on emission factors. Environ. Sci. Technol. 36, 846–853 (2002). [DOI] [PubMed] [Google Scholar]
- 27.Schripp T., Markewitz D., Uhde E., Salthammer T., Does e-cigarette consumption cause passive vaping? Indoor Air 23, 25–31 (2013). [DOI] [PubMed] [Google Scholar]
- 28.Grob K., Voellmin J. A., GC-MS analysis of the “semi-volatiles” of cigarette smoke. J. Chromatogr. Sci. 8, 218–220 (1970). [Google Scholar]
- 29.Khare P., Gentner D. R., Considering the future of anthropogenic gas-phase organic compound emissions and the increasing influence of non-combustion sources on urban air quality. Atmos. Chem. Phys. 18, 5391–5413 (2018). [Google Scholar]
- 30.Sheu R., Marcotte A., Khare P., Charan S., Ditto J. C., Gentner D. R., Advances in offline approaches for chemically speciated measurements of trace gas-phase organic compounds via adsorbent tubes in an integrated sampling-to-analysis system. J. Chromatogr. A 1575, 80–90 (2018). [DOI] [PubMed] [Google Scholar]
- 31.Kim Y. M., Harrad S., Harrison R. M., Concentrations and sources of volatile organic compounds in urban domestic and public microenvironments. Indoor Built Environ. 10, 147–153 (2001). [DOI] [PubMed] [Google Scholar]
- 32.Ditto J. C., Barnes E. B., Khare P., Takeuchi M., Joo T., Bui A. A. T., Lee-taylor J., Eris G., Chen Y., Aumont B., Jimenez J. L., Ng N. L., Griffin R. J., Gentner D. R., An omnipresent diversity and variability in the chemical composition of atmospheric functionalized organic aerosol. Commun. Chem. 1, 75 (2018). [Google Scholar]
- 33.Wang K., Zhang Y., Huang R.-J., Cao J., Hoffmann T., UHPLC-Orbitrap mass spectrometric characterization of organic aerosol from a central European city (Mainz, Germany) and a Chinese megacity (Beijing). Atmos. Environ. 189, 22–29 (2018). [Google Scholar]
- 34.Destaillats H., Lunden M. M., Singer B. C., Coleman B. K., Hodgson A. T., Weschler C. J., Nazaroff W. W., Indoor secondary pollutants from household product emissions in the presence of ozone: A bench-scale chamber study. Environ. Sci. Technol. 40, 4421–4428 (2006). [DOI] [PubMed] [Google Scholar]
- 35.Persily A., Musser A., Emmerich S. J., Modeled infiltration rate distributions for U.S. housing. Indoor Air 20, 473–485 (2010). [DOI] [PubMed] [Google Scholar]
- 36.Li Y., Pöschl U., Shiraiwa M., Molecular corridors and parameterizations of volatility in the chemical evolution of organic aerosols. Atmos. Chem. Phys. 16, 3327–3344 (2016). [Google Scholar]
- 37.Weber R. J., Guo H., Russell A. G., Nenes A., High aerosol acidity despite declining atmospheric sulfate concentrations over the past 15 years. Nat. Geosci. 9, 282–285 (2016). [Google Scholar]
- 38.Schober W., Szendrei K., Matzen W., Osiander-Fuchs H., Heitmann D., Schettgen T., Jörres R. A., Fromme H., Use of electronic cigarettes (e-cigarettes) impairs indoor air quality and increases FeNO levels of e-cigarette consumers. Int. J. Hyg. Environ. Health 217, 628–637 (2014). [DOI] [PubMed] [Google Scholar]
- 39.Sleiman M., Logue J. M., Montesinos V. N., Russell M. L., Litter M. I., Gundel L. A., Destaillats H., Emissions from electronic cigarettes: Key parameters affecting the release of harmful chemicals. Environ. Sci. Technol. 50, 9644–9651 (2016). [DOI] [PubMed] [Google Scholar]
- 40.Borgerding M. F., Bodnar J. A., Chung H. L., Mangan P. P., Morrison C. C., Risner C. H., Rogers J. C., Simmons D. F., Uhrig M. S., Wendelboe F. N., Wingate D. E., Winkler L. S., Chemical and biological studies of a new cigarette that primarily heats tobacco. Part 1. Chemical composition of mainstream smoke. Food Chem. Toxicol. 36, 169–182 (1998). [PubMed] [Google Scholar]
- 41.Kroll J. H., Ng N. L., Murphy S. M., Varutbangkul V., Flagan R. C., Seinfeld J. H., Chamber studies of secondary organic aerosol growth by reactive uptake of simple carbonyl compounds. J. Geophys. Res. Atmos. 110, D23207 (2005). [Google Scholar]
- 42.Carrer P., Wargocki P., Fanetti A., Bischof W., De Oliveira Fernandes E., Hartmann T., Kephalopoulos S., Palkonen S., Seppänen O., What does the scientific literature tell us about the ventilation–health relationship in public and residential buildings? Build. Environ. 94, 273–286 (2015). [Google Scholar]
- 43.Wargocki P., Wyon D. P., Fanger P. O., The performance and subjective responses of call-center operators with new and used supply air filters at two outdoor air supply rates. Indoor Air 14, 7–16 (2004). [DOI] [PubMed] [Google Scholar]
- 44.Wargocki P., Wyon D. P., Sundell J., Clausen G., Fanger P. O., The effects of outdoor air supply rate in an office on perceived air quality, sick building syndrome (SBS) symptoms and productivity. Indoor Air 10, 222–236 (2000). [DOI] [PubMed] [Google Scholar]
- 45.Kwon S.-B., Cho Y., Park D., Park E.-Y., Study on the indoor air quality of Seoul metropolitan subway during the rush hour. Indoor Built Environ. 17, 361–369 (2008). [Google Scholar]
- 46.Stephens B., Siegel J. A., Penetration of ambient submicron particles into single-family residences and associations with building characteristics. Indoor Air 22, 501–513 (2012). [DOI] [PubMed] [Google Scholar]
- 47.Bakó-Biró Z., Clements-Croome D. J., Kochhar N., Awbi H. B., Williams M. J., Ventilation rates in schools and pupils’ performance. Build. Environ. 48, 215–223 (2012). [Google Scholar]
- 48.Tang X., Misztal P. K., Nazaroff W. W., Goldstein A. H., Volatile organic compound emissions from humans indoors. Environ. Sci. Technol. 50, 12686–12694 (2016). [DOI] [PubMed] [Google Scholar]
- 49.Gordon S. M., Wallace L. A., Brinkman M. C., Callahan P. J., Kenny D. V., Volatile organic compounds as breath biomarkers for active and passive smoking. Environ. Health Perspect. 110, 689–698 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pagonis D., Sekimoto K., de Gouw J., A library of proton-transfer reactions of H3O+ ions used for trace gas detection. J. Am. Soc. Mass Spectrom. 30, 1330–1335 (2019). [DOI] [PubMed] [Google Scholar]
- 51.Warneke C., De Gouw J. A., Kuster W. C., Goldan P. D., Fall R., Validation of atmospheric VOC measurements by proton-transfer-reaction mass spectrometry using a gas-chromatographic preseparation method. Environ. Sci. Technol. 37, 2494–2501 (2003). [DOI] [PubMed] [Google Scholar]
- 52.Gueneron M., Erickson M. H., Vanderschelden G. S., Jobson B. T., PTR-MS fragmentation patterns of gasoline hydrocarbons. Int. J. Mass Spectrom. 379, 97–109 (2015). [Google Scholar]
- 53.Lindinger W., Hansel A., Jordan A., Proton-transfer-reaction mass spectrometry (PTR–MS): On-line monitoring of volatile organic compounds at pptv levels. Chem. Soc. Rev. 27, 347–375 (1998). [Google Scholar]
- 54.Cappellin L., Karl T., Probst M., Ismailova O., Winkler P. M., Soukoulis C., Aprea E., Märk T. D., Gasperi F., Biasioli F., On quantitative determination of volatile organic compound concentrations using proton transfer reaction time-of-flight mass spectrometry. Environ. Sci. Technol. 46, 2283–2290 (2012). [DOI] [PubMed] [Google Scholar]
- 55.Zhao J., Zhang R., Proton transfer reaction rate constants between hydronium ion (H3O+) and volatile organic compounds. Atmos. Environ. 38, 2177–2185 (2004). [Google Scholar]
- 56.Tani A., Hayward S., Hewitt C. N., Measurement of monoterpenes and related compounds by proton transfer reaction-mass spectrometry (PTR-MS). Int. J. Mass Spectrom. 223–224, 561–578 (2003). [Google Scholar]
- 57.Hodgson A. T., Daisey J. M., Mahanama K. R. R., Ten Brinke J., Alevantis L. E., Use of volatile tracers to determine the contribution of environmental tobacco smoke to concentrations of volatile organic compounds in smoking environments. Environ. Int. 22, 295–307 (1996). [Google Scholar]
- 58.Tang X., Misztal P. K., Nazaroff W. W., Goldstein A. H., Siloxanes are the most abundant volatile organic compound emitted from engineering students in a classroom. Environ. Sci. Technol. Lett. 2, 303–307 (2015). [Google Scholar]
- 59.Moldoveanu S., Coleman W. III, Wilkins J., Determination of benzene and toluene in exhaled cigarette smoke. Beitr. Tab. Int. Contrib. Tob. Res. 23, 107–114 (2008). [Google Scholar]
- 60.Darrall K. G., Figgins J. A., Brown R. D., Phillips G. F., Determination of benzene and associated volatile compounds in mainstream cigarette smoke. Analyst 123, 1095–1101 (1998). [DOI] [PubMed] [Google Scholar]
- 61.McDonald B. C., de Gouw J. A., Gilman J. B., Jathar S. H., Akherati A., Cappa C. D., Jimenez J. L., Lee-Taylor J., Hayes P. L., McKeen S. A., Cui Y. Y., Kim S.-W., Gentner D. R., Isaacman-VanWertz G., Goldstein A. H., Harley R. A., Frost G. J., Roberts J. M., Ryerson T. B., Trainer M., Volatile chemical products emerging as largest petrochemical source of urban organic emissions. Science 359, 760–764 (2018). [DOI] [PubMed] [Google Scholar]
- 62.Abd Hamid H. H., Jumah N. S., Talib Latif M., Kannan N., BTEXs in indoor and outdoor air samples: Source apportionment and health risk assessment of benzene. J. Environ. Sci. Public Heal. 01, 49–56 (2017). [Google Scholar]
- 63.Gentner D. R., Worton D. R., Isaacman G., Davis L. C., Dallmann T. R., Wood E. C., Herndon S. C., Goldstein A. H., Harley R. A., Chemical composition of gas-phase organic carbon emissions from motor vehicles and implications for ozone production. Environ. Sci. Technol. 47, 11837–11848 (2013). [DOI] [PubMed] [Google Scholar]
- 64.Lan T. T. N., Binh N. T. T., Daily roadside BTEX concentrations in East Asia measured by the Lanwatsu, Radiello and Ultra I SKS passive samplers. Sci. Total Environ. 441, 248–257 (2012). [DOI] [PubMed] [Google Scholar]
- 65.Monod A., Sive B. C., Avino P., Chen T., Blake D. R., Sherwood Rowland F., Monoaromatic compounds in ambient air of various cities: A focus on correlations between the xylenes and ethylbenzene. Atmos. Environ. 35, 135–149 (2001). [Google Scholar]
- 66.Gelencsér A., Siszler K., Hlavay J., Toluene–benzene concentration ratio as a tool for characterizing the distance from vehicular emission sources. Environ. Sci. Technol. 31, 2869–2872 (1997). [Google Scholar]
- 67.Warneke C., McKeen S. A., de Gouw J. A., Goldan P. D., Kuster W. C., Holloway J. S., Williams E. J., Lerner B. M., Parrish D. D., Trainer M., Fehsenfeld F. C., Kato S., Atlas E. L., Baker A., Blake D. R., Determination of urban volatile organic compound emission ratios and comparison with an emissions database. J. Geophys. Res. Atmos. 112, D10S47 (2007). [Google Scholar]
- 68.Du L., Batterman S., Godwin C., Rowe Z., Chin J.-Y., Air exchange rates and migration of VOCs in basements and residences. Indoor Air 25, 598–609 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Jia C., Batterman S., Godwin C., VOCs in industrial, urban and suburban neighborhoods, Part 1: Indoor and outdoor concentrations, variation, and risk drivers. Atmos. Environ. 42, 2083–2100 (2008). [Google Scholar]
- 70.Loh M. M., Houseman E. A., Gray G. M., Levy J. I., Spengler J. D., Bennett D. H., Measured concentrations of VOCs in several non-residential microenvironments in the United States. Environ. Sci. Technol. 40, 6903–6911 (2006). [DOI] [PubMed] [Google Scholar]
- 71.Raw G. J., Coward S. K. D., Brown V. M., Crump D. R., Exposure to air pollutants in English homes. J. Expo. Anal. Environ. Epidemiol. 14, S85–S94 (2004). [DOI] [PubMed] [Google Scholar]
- 72.Schneider P., Gebefügi I., Richter K., Wölke G., Schnelle J., Wichmann H.-E., Heinrich J., Indoor and outdoor BTX levels in German cities. Sci. Total Environ. 267, 41–51 (2001). [DOI] [PubMed] [Google Scholar]
- 73.Miri M., Rostami Aghdam Shendi M., Ghaffari H. R., Ebrahimi Aval H., Ahmadi E., Taban E., Gholizadeh A., Yazdani Aval M., Mohammadi A., Azari A., Investigation of outdoor BTEX: Concentration, variations, sources, spatial distribution, and risk assessment. Chemosphere 163, 601–609 (2016). [DOI] [PubMed] [Google Scholar]
- 74.Du L., Prasauskas T., Leivo V., Turunen M., Pekkonen M., Kiviste M., Aaltonen A., Martuzevicius D., Haverinen-Shaughnessy U., Assessment of indoor environmental quality in existing multi-family buildings in North–East Europe. Environ. Int. 79, 74–84 (2015). [DOI] [PubMed] [Google Scholar]
- 75.Wagner P., Kuttler W., Biogenic and anthropogenic isoprene in the near-surface urban atmosphere — A case study in Essen, Germany. Sci. Total Environ. 475, 104–115 (2014). [DOI] [PubMed] [Google Scholar]
- 76.von Schneidemesser E., Monks P. S., Plass-Duelmer C., Global comparison of VOC and CO observations in urban areas. Atmos. Environ. 44, 5053–5064 (2010). [Google Scholar]
- 77.Akagi S. K., Yokelson R. J., Wiedinmyer C., Alvarado M. J., Reid J. S., Karl T., Crounse J. D., Wennberg P. O., Emission factors for open and domestic biomass burning for use in atmospheric models. Atmos. Chem. Phys. 11, 4039–4072 (2011). [Google Scholar]
- 78.Christian T. J., Kleiss B., Yokelson R. J., Holzinger R., Crutzen P. J., Hao W. M., Saharjo B. H., Ward D. E., Comprehensive laboratory measurements of biomass-burning emissions: 1. Emissions from Indonesian, African, and other fuels. J. Geophys. Res. 108, 4719 (2003). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material for this article is available at http://advances.sciencemag.org/cgi/content/full/6/10/eaay4109/DC1
Section S1. Data analysis methods
Fig. S1. Acetonitrile decay rate during a movie and examples of late arrival THS events.
Fig. S2. Concentration profiles for other compounds present in THS not shown in Fig. 1.
Fig. S3. Acetone, acetic acid, and acetaldehyde spike simultaneously with known THS tracers.
Fig. S4. Ratio of 2-methylfuran to 2,5-dimethylfuran throughout real-time data collection and a literature tobacco smoke versus THS ratio to 2,5-dimethylfuran comparison.
Fig. S5. Concentration profile for THS compounds in Fig. 1 with Day 4 included.
Fig. S6. Compound class distribution and volatility distributions of functionalized organic aerosol.
Fig. S7. Average SHS cigarette equivalents and SDs during 10 R-rated THS emission events including all non-HAPs.
Table S1. Summary of compounds, literature emission factors, and PTR-TOF MS parameters for known THS compounds.
Table S2. Benzene and toluene ratios from literature.
Table S3. Compound class composition for functionalized aerosol.


