Abstract
Pyonephrosis is gross accumulation of pus within an obstructed renal collecting system that, if left untreated, can lead to potentially fatal septic shock. Treatment requires urgent decompression coupled with systemic antibiotics. Percutaneous nephrostomy (PCN) placement, first described in 1976 for the treatment of pyonephrosis, is now widely utilized for emergent decompression in these patients. When performed by an experienced interventional radiologist, PCN is a safe procedure with technical success rates of over 96 to 99%. This article will address the clinical presentation of pyonephrosis, and will discuss the indications, technique, complications, and outcomes of emergent PCN placement. Additionally, the expanded indications for PCN placement in nonemergent scenarios will also be described.
Keywords: pyonephrosis, nephrostomy, hydronephrosis, nephrolithiasis, interventional radiology
Percutaneous nephrostomy (PCN) placement, first described in 1976 for the treatment of pyonephrosis, 1 is now widely utilized for emergent decompression in these patients. When performed by an experienced interventional radiologist, PCN is a safe procedure with technical success rates of over 96 to 99%. 2 3 PCN placement for decompression of the obstructed renal collecting system for any indication was first described in 1955. 4 It is now widely accepted as a safe and effective procedure for decompression of both infected and sterile collecting systems. 3 5 6 Additionally, nonemergent indications for PCN placement have expanded, and now outnumber those placed for emergencies.
Often referred to as “pus under pressure,” pyonephrosis is superinfection of an obstructed renal collecting system. Emergent decompression with intensive postprocedural monitoring is paramount due to the risk of sepsis and associated morbidity and mortality in these patients. Pyonephrosis can occur in any age range. In adults, nephrolithiasis accounts for 50 to 70% of cases, 2 7 and the inciting bacteria is most commonly a gram-negative organism, specifically Escherichia coli . 8 In children, a congenital obstructing lesion is usually present, and a variety of gram-negative bacteria are common. 9 10 Patients will sometimes present with the classic triad of fevers, flank pain, and hydronephrosis, but manifestations of urosepsis also include hemodynamic instability, leukocytosis, renal failure, and lactic acidosis. 2 7 While imaging alone cannot always differentiate pyonephrosis from simple hydronephrosis, dependent echogenic urine-debris levels seen under ultrasound cinch the diagnosis ( Fig. 1 ).
Fig. 1.
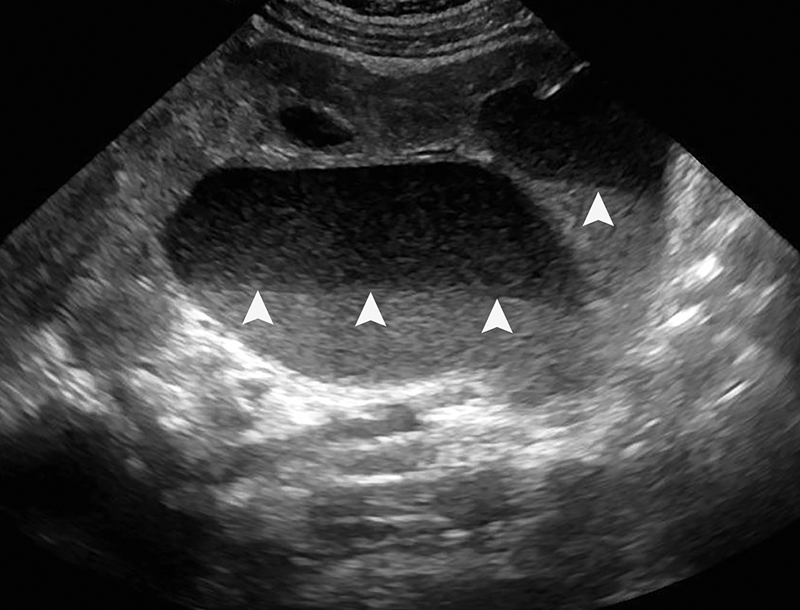
Renal ultrasound in a 9-month-old child with a congenital ureteral pelvic junction (UPJ) obstruction demonstrates marked hydronephrosis of the left kidney with dependent echoes ( white arrowheads ) layering within the pelvis and calyces. The fluid-debris interface represents pus within an obstructed collecting system, which is diagnostic of pyonephrosis.
Indications
While this review focuses specifically on emergent PCN placement for pyonephrosis, it is important to recognize the expanded, nonemergent indications for which PCN is most frequently performed. The broad categories for PCN placement include urinary drainage for obstruction, urinary diversion, and provision of access to the collecting system.
Urinary obstruction may result from intrinsic or extrinsic compression caused by stones, malignancy, or iatrogenic conditions. Noninfected urinary tract obstruction accounts for 70 to 90% of nephrostomies. 2 3 11 It is important to recognize nonobstructive hydronephrosis related to pregnancy, overhydration, diabetes insipidus, or diuretic administration, as PCN is not necessary in these scenarios. 2 Infected urinary obstruction, or pyonephrosis, may account for 3 to 19% of nephrostomies, 2 11 12 is most commonly a result of stone disease, and requires emergent decompression.
In cases of urinary leak, urinary fistulae, and hemorrhagic cystitis, urinary diversion can be achieved with bilateral PCN placement. Finally, PCN placement may be performed to provide access to the proximal collecting system to perform percutaneous or other endoscopic procedures. Such procedures include percutaneous nephrolithotomy or nephrostolithotomy, antegrade ureteral stent placement, cooling pyeloperfusion, foreign body retrieval, and other diagnostic procedures. 2
Preprocedure Assessment
Before traveling to the interventional radiology suite, any hemodynamically compromised patient must first be resuscitated with appropriate administration of fluids and, if necessary, vasoactive medications.
In the setting of pyonephrosis or urosepsis, all patients should already be receiving intravenous (IV) antibiotics. Broad-spectrum, gram-negative coverage with ceftriaxone or ampicillin/sulbactam should be used. 13 It is noteworthy that, even in the absence of infection, antibiotic prophylaxis in high-risk patients may decrease postprocedural complications from 50 to 9%. 13 Risk factors include advanced age, diabetes, bladder dysfunction, neurogenic bladder, or prior ureteral manipulation. While the data are less clear for patients without risk factors, 14 all patients undergoing PCN placement at the authors' institution receive preprocedural antibiotics. Per Society of Interventional Radiology (SIR) consensus guidelines, antibiotics include 1 g cefazolin IV, 1 g ceftriaxone IV, 1.5 to 3 g ampicillin/sulbactam IV, 2 g ampicillin IV combined with 1.5 mg/kg gentamicin IV, and vancomycin or clindamycin and an aminoglycoside if allergic to penicillin. 13
Coagulopathies should be corrected prior to the procedure, and routine laboratory evaluation should therefore include a complete cell blood count (CBC) and international normalized ratio (INR). For those patients receiving IV unfractionated heparin, activated partial thromboplastin time (aPTT) should also be evaluated. Ideally, platelet count should be greater than 50,000, INR should be less than or equal to 1.5, and aPTT should be no greater than 1.5 times control. 15 Fresh frozen plasma (FFP) should be administered to achieve INR levels below 1.5, platelets should be transfused to raise the platelet count above 50,000, and heparin should be held or reversed until the aPTT is normalized. Due to the risk of arrhythmia, severe hyperkalemia and metabolic acidosis should also be corrected.
In certain cases, PCN placement can be done under local anesthesia to avoid respiratory depression and blood pressure lability associated with moderate sedation. This is particularly prudent during procedures requiring prone positioning. If sedation is required, consultation with the department of anesthesiology prior to the procedure is wise in the emergent setting, as urosepsis may rapidly progress during or shortly after the procedure.
Finally, cross-sectional imaging should be reviewed to determine the underlying etiology of obstruction, and to plan the best trajectory for access. Important anatomical considerations include the presence of renal masses or cysts, the relationship of the involved kidney with adjacent solid organs (namely, the liver and spleen), interposition of colon posteriorly along the intended PCN trajectory, and level of the kidney with respect to the ribs.
Description of Access and Technique
Patient Preparation
After informed consent is obtained, the patient is brought to the interventional radiology suite and placed in the prone position on the fluoroscopy table. A pillow can be placed under the ipsilateral abdomen to elevate the flank 20 to 30 degrees, optimizing the trajectory into a posterior calyx. 16 17 This posterolateral trajectory theoretically reduces the risk of bleeding because the needle passes through the avascular plane of Brodel, a relatively avascular area between the terminal branches of the anterior and posterior segmental renal arteries, demarcated by the junction of the posterior one-third and anterior two-thirds of the renal cortex ( Fig. 2 ). In reality, accessing this avascular plane is impractical from a bleeding standpoint. However, it does provide the most direct pathway to the renal pelvis, in contrast to the acute entry angle resulting from anterior calyceal puncture ( Fig. 3 ). 17 Additionally, this trajectory results in a more lateral catheter skin exit site, which provides better comfort for the patient when lying supine. 18
Fig. 2.
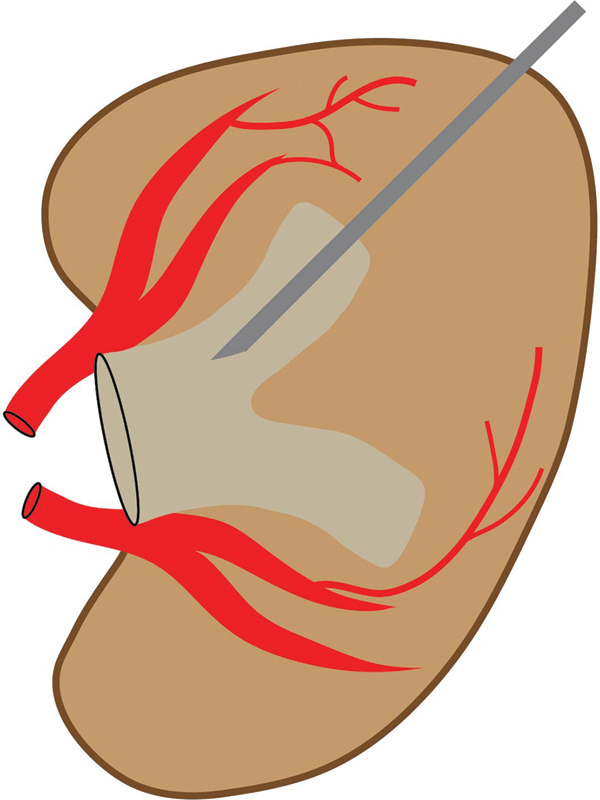
Schematic of the kidney demonstrates ideal posterior calyceal access through Brodel's avascular plane along a posterolateral trajectory of ∼20–30 degrees. The avascular plane of Brodel is a relatively avascular area between the terminal branches of the anterior and posterior segmental renal arteries, at the junction of the anterior two-thirds and posterior one-third of the renal contour.
Fig. 3.
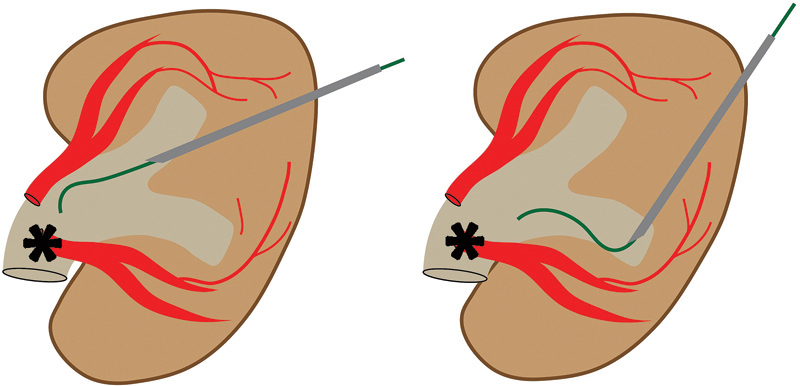
A posterior calyceal puncture ( black arrowhead ) provides the most direct pathway for wire passage into the renal pelvis ( asterisk ). In contrast, anterior calyceal puncture ( white arrowhead ) results an acute entry angle into the renal pelvis ( asterisk ).
Standard Single-Stick Access
After the patient is prepped and draped using standard aseptic technique, the skin access site is anesthetized with 1% lidocaine. In the setting of pyonephrosis, a single-stick access technique with ultrasound guidance is preferred, as there is usually hydronephrosis making dilated calyces readily accessible ( Fig. 4 ). A 22-gauge Chiba needle (Cook Medical, Bloomington, IN) is advanced into the posterior calyx, preferably along a subcostal trajectory to avoid thoracic complications. 17 19 20 Return of urine from the needle hub either spontaneously or with gentle aspiration confirms appropriate positioning. A urine sample should be sent for microbiology analysis to help tailor appropriate antimicrobial therapy.
Fig. 4.
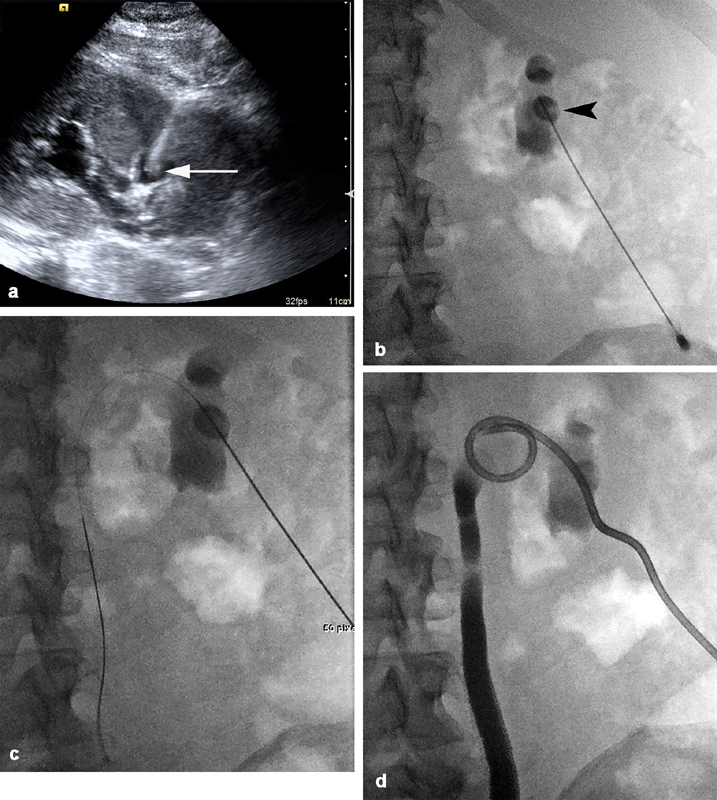
A 54-year-old woman with bilateral hydronephrosis and progressive renal failure secondary to an obstructing bladder mass requires bilateral PCN. ( a ) Ultrasound guidance is utilized to access a posterior calyx in the right kidney using a 22-gauge needle. The needle tip is visualized within the posterior calyx ( white arrow ), and there is moderate hydronephrosis. ( b ) After aspiration of urine to confirm position within the collecting system, a small and equal volume of dilute contrast is injected to opacify the posterior calyx ( black arrowhead ) and collecting system. ( c ) A 0.018-inch wire passes without resistance into the renal pelvis and down the ureter. ( d ) Final fluoroscopic image demonstrates an 8-Fr catheter with Cope loop appropriately positioned within the renal pelvis. The collecting system has been decompressed, and contrast opacifies the ureter and proximal collecting system.
It is essential to minimize any unnecessary manipulation within the collecting system. Therefore, as long as the renal pelvis or renal hilum is not punctured directly, most other access to the collecting system is sufficient. Contrast injection should be avoided or at least minimized, as further distention of an already pressurized and infected collecting system could result in intravasation of bacteria and acute urosepsis. If contrast is needed to confirm appropriate calyceal access, an equal amount of urine should first be aspirated.
Catheter Placement
Once acceptable needle access is established, a 0.018-inch nitinol guidewire (Boston Scientific, Marlborough, MA) is advanced through the 22-gauge Chiba needle. Passage of the wire into the ureter serves to further confirm needle position and may minimize subsequent urothelial dissection or calyceal perforation. This step is preferred in nonemergencies, but in pyonephrosis the additional manipulation is unnecessary and may increase the risk of urosepsis. There should only be enough wire looped in an adjacent calyx to allow adequate support for exchange of the coaxial introducer.
The wire should easily advance into the collecting system without resistance. Recognition of false wire passage is important, as the needle may withdraw after access, may be positioned within the intimal plane of the urothelium, or may be back-walled against the urothelium. Tactile resistance and “pretzeling” of the wire (demonstrated under fluoroscopy) both indicate the wire is not within the collecting system. 18 When encountered, the wire should be withdrawn under fluoroscopic guidance to avoid shearing off the wire tip, and if any resistance occurs during wire removal, both wire and needle should be removed together. If contrast is injected with the needle mispositioned, infiltration of the subintimal space and perirenal fat with air and contrast will obscure the kidney on ultrasound and fluoroscopy, making subsequent access much more challenging ( Fig. 5 ). This should be avoided.
Fig. 5.
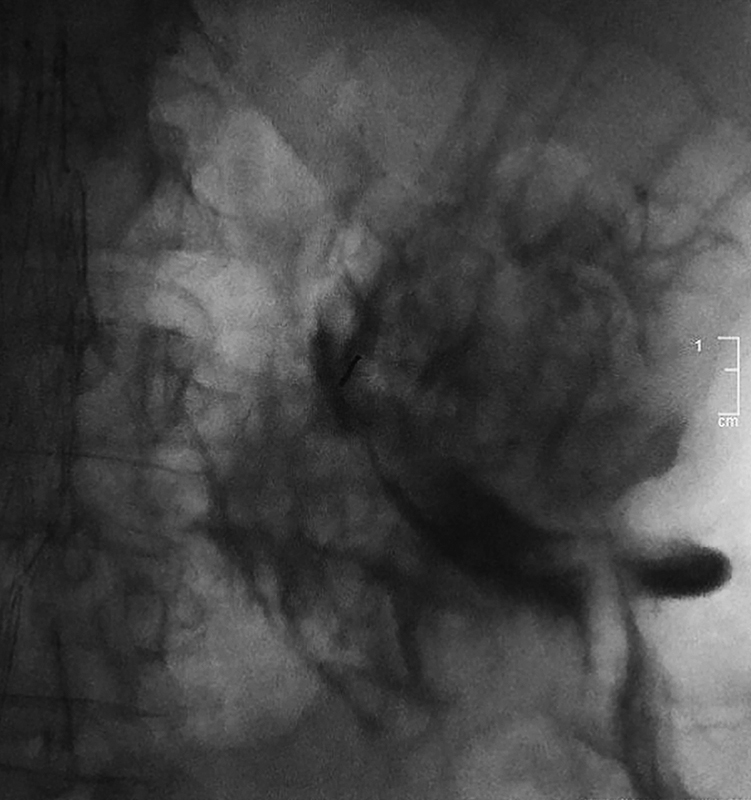
A 49-year-old woman with a vesicovaginal fistula requires bilateral diverting nephrostomies. After ultrasound-guided access into a nondilated collecting system is attempted, urine cannot be aspirated, and wire passage is met with resistance. Contrast injection is performed, resulting in subintimal and perinephric extravasation of both contrast and air. Further attempts at access are unsuccessful because the collecting system is obscured under both ultrasound and fluoroscopy, and the procedure is aborted.
With the 0.018-inch wire in place, the needle is removed and a coaxial 4 and 6 Fr dilator/sheath assembly (AccuStick II Introducer System; Boston Scientific) is advanced. The direction of force as the coaxial system is advanced should be directly along the trajectory of the wire to avoid kinking the wire as the coaxial system is advanced through the subcutaneous tissues, and as it enters the renal parenchyma. Once the 6-Fr dilator/sheath is in the collecting system, a stiff 0.035-inch wire (Amplatz Super Stiff guidewire, Boston Scientific) is advanced into either the ureter or coiled in the renal pelvis. The tract is serially dilated from the skin to the calyx with 8-, 10-, and 12-Fr dilators, and a 12-Fr drainage pigtail catheter (Flexima ADPL, Boston Scientific) is advanced over the wire and into the collecting system. In the setting of pyonephrosis, a larger bore catheter is always used to minimize premature catheter occlusion by purulent debris. The wire and inner stiffener are removed, and Cope loop formed within the renal pelvis. The catheter is secured to the skin with either a Roman sandal suture or adhesive fixation device (StayFIX Catheter Fixation Device; Merit Medical, South Jordan, UT). The catheter is attached to a gravity drainage bag, which allows urine output to be monitored and recorded.
Alternative Methods of Access and Troubleshooting
18-Gauge Needle Access
Occasionally, scarred renal parenchyma makes passage of a coaxial introducer over a 0.018-inch wire difficult. This step can be avoided by using a larger 18-gauge needle, which facilitates immediate placement of an 0.035-inch wire into the ureter or renal pelvis, and has been shown to be as safe as access with a smaller 22-gauge needle. 21
Two-Stick Needle Access
If initial access is inadequate or too central, a two-stick method can be utilized ( Fig. 6 ). 22 Notably, this technique is quite useful when the collecting system is nondilated. First, a 22-gauge Chiba needle is advanced into the collecting system. This can be done under ultrasound by directly accessing the renal pelvis. Alternatively, if a radiopaque stone or ureteral stent is present, it can be targeted under fluoroscopy with a “down the barrel approach.” 22 A small amount of contrast (less than or equal to the amount of aspirated urine) is injected to confirm positioning within the collecting system, followed by a small amount of air, which will rise and fill the nondependent posterior calyces. Via a posterolateral approach, a second 22-gauge Chiba needle is then advanced under “down the barrel” fluoroscopic guidance, and into the desired air-filled posterior calyx.
Fig. 6.
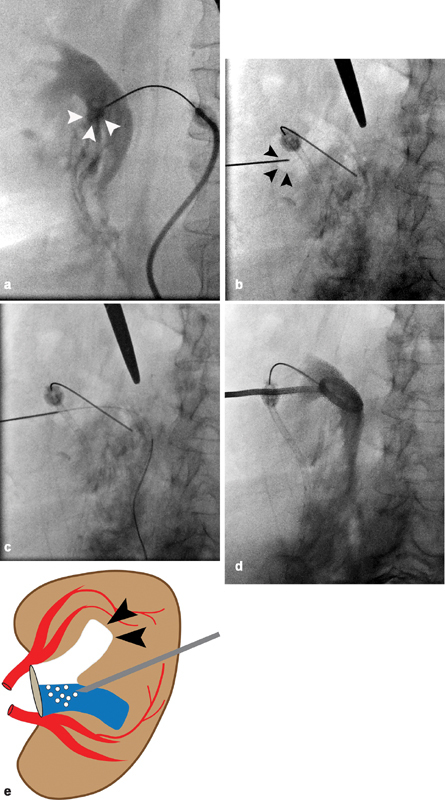
A 56-year-old woman with an obstructing proximal left ureteral stone underwent failed retrograde ureteral stent placement, which was complicated by ureteral perforation, and now requires PCN. ( a ) Initial 22-gauge needle placement performed under ultrasound results in central access of an anterior calyx ( white arrowheads ). ( b ) A small amount of air is injected, which non-dependently fills multiple posterior calyces. The posterior inferior calyx ( black arrowheads ) is targeted with a second 22-gauge needle. ( c ) A 0.018-inch wire passes without resistance into the collecting system via the posterior calyceal access and into the ureter. ( d ) Final image demonstrates placement of an 8-Fr PCN with its cope loop appropriately positioned in the renal pelvis. ( e ) A simplified illustration demonstrates air filling the nondependent posterior calyx ( black arrowheads ) as it is injected via initial needle access, which is positioned in the infundibulum near the anterior calyx.
Intravenous Contrast Injection
If access cannot be established with the single-stick or two-stick techniques, and if the patient's renal function permits, 50 mL of IV contrast can be administered. This will opacify the renal calyces in the excretory phase and provide a fluoroscopic target. CT guidance can also be utilized as a last resort.
Intercostal Access
Inferior calyceal access via a subcostal approach mitigates the risks of thoracic complications and is generally preferred in the emergent setting for decompression. However, a subcostal approach is not always possible, and, in some nonemergent scenarios, is less favorable. Specifically, when provision of access is planned prior to lithotripsy, superior calyceal access is indicated for staghorn calculi, large upper caliceal calculi, calculi associated with ureteropelvic junction (UPJ) pathology, and large upper-ureteral calculi. 23 In these scenarios, upper pole access may necessitate an intercostal approach. An understanding of thoracic anatomy is crucial to minimize complications associated with transpleural access, which include pneumothorax, pleural effusion, urothorax, and intercostal arterial injury.
The parietal pleura reflect at the level of the 10th rib in the midaxillary line, and variably along the 12th rib posteriorly. The lateral half of the 12th rib lies below the parietal pleura as it courses superiorly. The diaphragm usually inserts more inferiorly, along the lower margin of the 12th rib, the transverse process of the first lumbar vertebral body, and the anterior surface of the upper lumbar vertebral bodies. During quiet respiration, the lung normally does not fill the costophrenic sulcus, lying approximately at the 10th thoracic vertebral body posteriorly ( Fig. 7 ), but can descend two vertebral levels with deep inspiration. 24 By accessing above the lateral half of the 12th rib during expiration, injury to the pleura can be minimized. 23 Access above the 11th rib significantly increases the risk of pleural injury, 25 and is therefore avoided in some institutions. 24
Fig. 7.
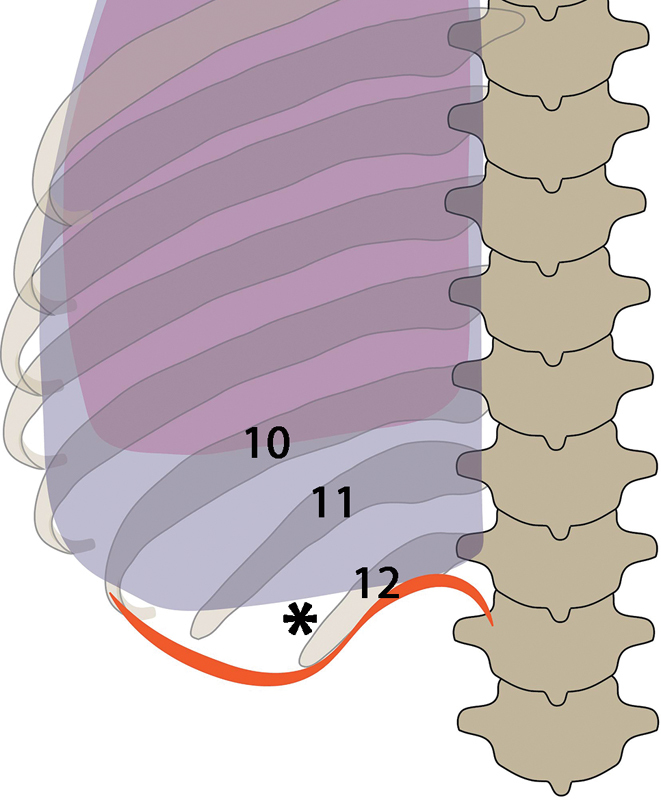
The parietal pleura ( black arrows ) reflects at the level of the 10th rib in the midaxillary line, and variably along the 12th rib posteriorly. The lateral half of the 12th rib lies below the parietal pleura as it courses superiorly. The diaphragm ( black arrowheads ) usually inserts more inferiorly, along the lower margin of the 12th rib, the transverse process of the first lumbar vertebral body, and the anterior surface of the upper lumbar vertebral bodies. During quiet respiration, the lung normally does not fill the costophrenic sulcus, lying approximately at the 10th thoracic vertebral body posteriorly ( white arrowheads ), but can descend two vertebral levels with deep inspiration. Access above the lateral half of the 12th rib ( asterisk ) during expiration minimizes the risk of thoracic injury.
Retrograde Ureteral Stent Placement
While a mainstay for the treatment of pyonephrosis, PCN is not the only method to emergently decompress the collecting system. Urologists can place retrograde ureteric stents, which are equally effective for managing pyonephrosis. 12 26 The technical success rates for retrograde ureteric stenting are reported at 98%, 26 with no difference in overall complication rates compared with PCN. 12 Currently, there is no consensus regarding the choice of first-line therapy. Rather, the preferred method is determined by the urologist/interventional radiologist's experience and the available local resources.
Each technique has advantages and disadvantages that should be considered. PCN can be done with local or moderate anesthesia, while retrograde stenting usually requires general anesthesia and a fully staffed operating room. Retrograde stent failure is more likely if there are multiple calculi, larger calculi, or calculi located in the upper ureter. 27 If the ureteral orifice is obscured by tumor, stricture, or extrinsic compression, retrograde stenting is probably less successful. 26 It seems logical that retrograde stenting should carry an increased risk of postprocedural sepsis because of the increased ureteral instrumentation, and smaller catheter sizes. However, this has not been demonstrated in multiple studies. 12 26 In contrast, retrograde stenting provides an internal means of decompression through a natural orifice, which results in better patient comfort and a lower risk of hemorrhage when compared with PCN, which requires external drainage and a renal parenchymal puncture.
Ultimately, the priority in patients with pyonephrosis is urgent decompression. Close collaboration with the urological service is essential to ensure expeditious treatment with either PCN or retrograde stent placement. And PCN should be immediately performed as a bailout if retrograde stenting fails.
Postprocedure Care
Due to the risk of transient bacteremia and sepsis immediately after PCN placement, all patients remain hospitalized and closely monitored, not infrequently in the intensive care unit (ICU). 6 17 These patients are seen on morning rounds by the interventional radiologist. Vital signs and laboratory values (e.g., white blood cell count, lactic acid, creatinine, and potassium) are monitored to ensure resolution of infection and recovery of renal function. Daily urine output from each PCN is monitored. Hematuria is almost universal after PCN placement and should clear within 72 hours. If gross hematuria persists and hemoglobin levels downtrend, a vascular injury should be suspected. Urokinase, a proteolytic enzyme found in urine, breaks down blood clots, and so rarely cause catheter obstruction. However, purulent debris may cause temporary occlusion, or the catheter may become kinked or inadvertently displaced. Therefore, the catheter insertion site should be evaluated every day. If output decreases, the catheter should be flushed with 10 mL of sterile saline and inspected for kinks. If urine output subsequently remains low, a fluoroscopic evaluation is necessary, because the catheter may need urgent repositioning or replacement.
In 1 to 2 weeks, once the patient is stable and the urinary system is decompressed, an antegrade nephrostogram is performed to further evaluate the obstructing lesion. In close collaboration with urology, a follow-up plan is devised. If long-term stenting is necessary, internalization with a ureteral stent is attempted. For those patients requiring long-term PCN catheters, routine catheter exchange is scheduled every 6 to 12 weeks.
Results
In the setting of obstruction, technical success for PCN approaches 99%, and is slightly lower (approximately 82–96%) for nondilated collecting systems. 2 3 16 Left untreated, pyonephrosis carries a mortality rate of 19.2%. Urgent decompression dramatically reduces mortality to 0.04%. 1 28 29 Two mechanisms contribute to this effect. 30 Following decompression, there is an immediate and significant increase in renal plasma flow rates, which increases antimicrobial concentration in both the renal parenchyma and in the urine. Additionally, mechanical decompression immediately reduces the bacteria and debris burden within the collecting system. The inability to reach therapeutic antibiotic levels in an obstructed collecting system is demonstrated by the frequent failure of medical therapy alone. 30 In the absence of infection, decompression helps protect against deteriorating renal perfusion and function. 17
Complications and Management
PCN is a very safe and well-tolerated procedure with mortality rates between 0.04 and 0.3%. 6 22 The overall complication rate for PCN is approximately 10%, 2 and the major complication rate is approximately 3 to 4%. 6 31 These complications are stratified based on patient outcome and per SIR standard of practice guidelines. Minor complications have no consequence and require no therapy, nominal therapy, or overnight observation. Major complications progress in severity when they result in minor therapy or hospitalization less than 48 hours; major therapy, an unplanned increase in level of care, or prolonged hospitalization over 48 hours; a permanent adverse sequela; or death. 32
Major complications related to PCN include sepsis, hemorrhage requiring transfusion, visceral organ injury, and pleural injury. Not surprisingly, major complications were reported at higher rates in the setting of pyonephrosis (6%), 5 and when performed emergently after hours (5.7%) compared with normal working hours (1.8%). 6
Sepsis
Sepsis is the most common complication following PCN placement and is also the most likely cause of escalation of care and patient death, thus emphasizing the importance of intensive postprocedural monitoring. Sepsis is defined by the SIR Standards of Practice Committee as fever and chills with hypotension requiring a major increase in level of care. Such an escalation of care may include emergent resuscitation with fluids, vasoactive medications, and intubation. Reported sepsis rates in the setting of routine PCN placement are between 1.8 and 2.2%. 6 31 The SIR guideline threshold in nonemergent scenarios is less than 4%. In the setting of pyonephrosis, the threshold guideline is higher at less than 10%, reflecting the increased risk in these patients. 2 Lee et al reported an increased rate of 3.6% in patients with pyonephrosis. 5 It was also notable that all patients in this study developed a transient increase in body temperature after the procedure. In the event that patients develop fevers with rigors, 25 to 50 mg of IV meperidine may be administered.
Theoretically, sepsis should be the most controllable of the major complications, as the risk factors are identifiable. 6 31 In the setting of pyonephrosis, it is essential that broad-spectrum antibiotics are initiated preprocedurally. Rapid decompression in these patients is essential, as is good procedural technique to prevent overmanipulation and overdistention of the collecting system. These factors were all contributory to postprocedure sepsis in multiple retrospective studies. 30 31
Hemorrhage
While transient hematuria is almost universal after PCN, hemorrhage requiring transfusion is rare. It often manifests as persistent or recurrent hematuria with downtrending hemoglobin and may be associated with hemodynamic instability. Reported rates of hemorrhage requiring transfusion after PCN range from 0.5 to 1.5%, 6 16 31 with higher rates (2.4%) reported in the emergent setting. 5 The SIR guideline threshold for this complication is less than 4%. 2 Venous oozing may result if a freshly placed catheter becomes partially retracted and has sideholes transgressing the renal parenchyma. This can be addressed by repositioning the catheter or upsizing the catheter to tamponade the tract. 22 33
While less frequently encountered, pulsatile arterial bleeding is the most severe cause of hemorrhage. The threshold guideline for arterial bleeding is less than 1%. 2 It is associated with more central access into the pelvis or infundibulum, which are adjacent to the larger segmental and intralobular renal arteries. 6 When arterial bleeding is suspected in a hemodynamically stable patient, computed tomographic angiography (CTA) of the abdomen should be performed. If IV contrast is contraindicated, a Doppler ultrasound (DUS) may be sufficient to demonstrate a pseudoaneurysm or arteriovenous fistula. After imaging confirmation of arterial injury, or in the instance of a hemodynamically unstable patient, conventional angiography is performed to identify and embolize the culprit vessel ( Fig. 8 ). In contradistinction to the liver and bowel, in which collateral supply is rich, the renal arteries are end arteries. Therefore, one needs to only coil embolize the artery proximal to the site of injury. In the liver and bowel, coiling must be performed both proximal and distal to the site of injury to prevent “back door” bleeding from collateral arcades.
Fig. 8.
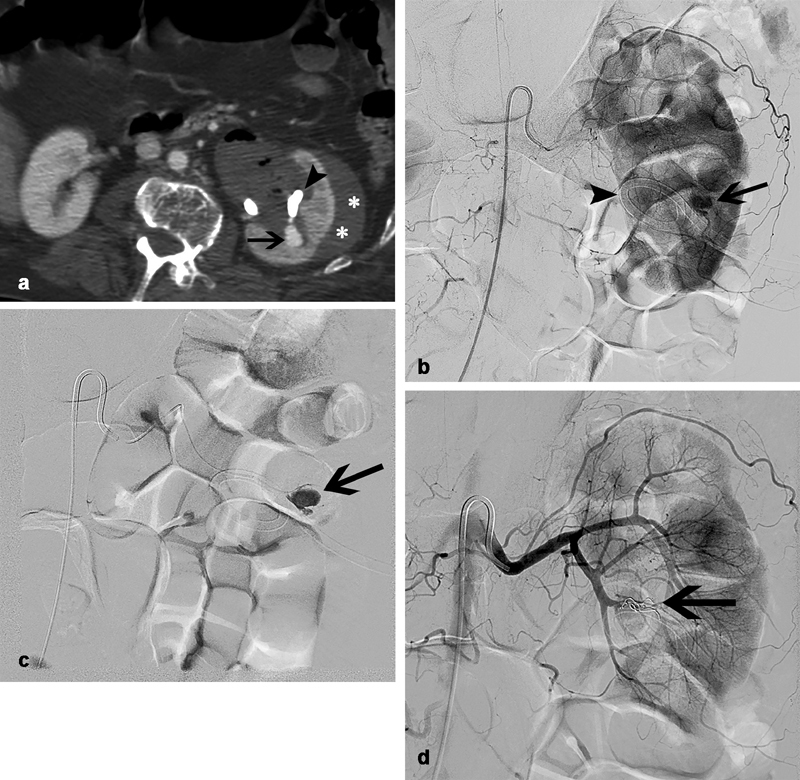
A 72-year-old with obstructing nephrolithiasis underwent left-sided PCN and had persistent hematuria with a significant drop in hemoglobin 3 days after placement. ( a ) Computed tomographic angiography demonstrates a subcapsular hematoma ( white asterisks ) and pseudoaneurysm ( black arrow ) immediately adjacent to the catheter ( black arrowhead ). ( b ) Digital subtraction angiography (DSA) with reverse curve catheter positioned in the main left renal artery ( c ) and a superselective DSA with microcatheter positioned in a posterior segmental branch confirm the presence of an iatrogenic pseudoaneurysm ( black arrow ) related to catheter placement ( black arrowhead ). ( d ) After microcoils are deployed through the microcatheter proximal to the site of vessel injury, DSA from the main left renal artery demonstrates successful occlusion of the culprit artery ( black arrow ) and no further filling of the pseudoaneurysm.
In the event of intermittent hematuria or pulsatile bleeding during catheter exchange, it is important to consider the tamponade effect by the catheter, which may be masking an arterial injury. If CTA or DUS fails to demonstrate a pseudoaneurysm despite a high clinical suspicion for an arterial injury, conventional angiography should be performed first with the catheter in place, and then after the catheter is removed over a secure wire. If an arterial injury is present, removing the catheter will often unmask it, and immediate coil embolization can then be performed.
Transcolonic Injury
In general, the prescribed posterolateral subcostal approach is free of interposed colon at risk of transgression during catheter placement. The SIR threshold guideline for bowel transgression is less than 1%, with a very low reported rates between 0.2 and 0.5%. 2 This risk is further mitigated by the review of cross-sectional imaging prior to the procedure, and with the use of ultrasound guidance which may allow for real-time visualization and avoidance of interposed colon during needle placement.
A small number of patients have posterior interposition of colon which puts them at risk for bowel transgression during catheter placement. Multiple studies have also demonstrated that prone positioning increases the incidence of interposed colon identified on CT compared with supine positioning ( Fig. 9 ). 34 35
Fig. 9.
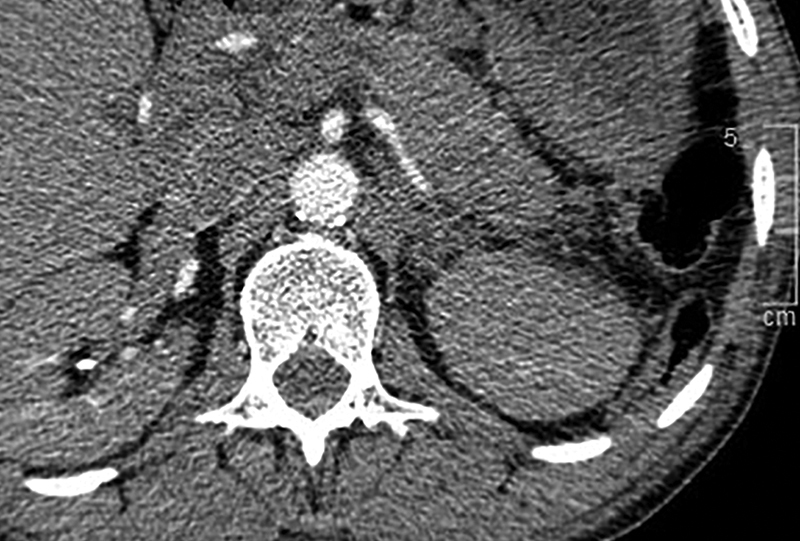
Computed tomography of the chest in a patient undergoing evaluation for pulmonary embolus incidentally demonstrates interposition of colon ( white arrowheads ) posterior to the left kidney.
If colonic interposition is identified prospectively, a more medial trajectory will reduce the risk of bowel transgression. If there is concern for colonic transgression at the time of needle access, a 0.018-inch wire can be advanced through the 22-gauge needle into the collecting system. Using a 4-Fr angled catheter (Glidecath; Terumo Interventional Systems, Somerset, NJ) attached to a Tuohy-Borst sidearm adaptor (Cook Medical, Bloomington, IN), a pullback tractogram over the wire can be performed prior to dilation and catheter placement. If transgression is identified, this can generally be managed conservatively with antibiotics.
In the event that a catheter inadvertently transgresses colon, conservative management is an appropriate first step for asymptomatic patients. However, it is also important to consult surgical colleagues so that escalation of therapy can be anticipated and planned if surgical intervention becomes necessary. The principles of management are similar to management of other entero-enteral or entero-cutaneous fistula: minimize flow through the fistula with diversion, suppression of secretions, and minimization exogenous flow. 36 To accomplish this, the catheter is retracted into the colon to allow for tract maturation while the patient is placed on bowel rest with total parenteral nutrition. After 2 weeks, the catheter can be withdrawn into the adjacent retroperitoneum, and subsequently removed after the fistula has healed.
Pleural Injury
Although possible for the lung to inflate below the 12th rib with deep inspiration, it is unlikely, making intrathoracic injury during subcostal PCN rare. 20 Pleural complication rates (pneumothorax, empyema, hydrothorax, and hemothorax) are between 0.1 and 0.6% during routine PCN. 2 However, when intercostal access is performed, complications increase more than 10-fold.
The theoretical risk calculated for pleural transgression in the 11th intercostal space during expiration was 29% in the right lung and 16% in the left lung. 20 This risk was based on the projected needle path on sagittal reconstructed computed tomography. In practice, reported pleural complication rates are lower. In a study by Picus et al, 8% of patients who underwent intercostal PCN for stone removal developed pleural effusions and 4% developed pneumothoraxes. 24 Munver et al reported an intrathoracic complication rate of 1.4 and 23% for access in the 11th and 10th intercostal spaces, respectively, demonstrating a significant increase in risk for access above the 11th rib. 25 Despite these risks, many advocate for intercostal access in the setting of stone removal because of a more optimal approach to the intrarenal collecting system, shorter operating time, less bleeding, and higher stone clearance rates. 23
Conclusion
Pyonephrosis can rapidly progress into life-threatening septic shock, and requires urgent decompression coupled with systemic antibiotics. PCN is a widely utilized, safe, and effective method to decompress the collecting system. Indications for PCN have expanded to nonemergent scenarios and include urinary drainage, urinary diversion, and access provision. Complication rates are slightly higher in the emergent setting, with sepsis being the most common and most likely complication to result in escalation of care and death. Therefore, good procedural technique to prevent overmanipulation and overdistention of the collecting system is essential and is best accomplished using a single-stick technique under ultrasound guidance. While retrograde stenting is as effective, there are some advantages of PCN over retrograde stenting, and the ultimate choice of treatment requires close collaboration with urology.
Footnotes
Conflict of Interest None declared.
References
- 1.Barbaric Z L, Davis R S, Frank I N, Linke C A, Lipchik E O, Cockett A T. Percutaneous nephropyelostomy in the management of acute pyohydronephrosis. Radiology. 1976;118(03):567–573. doi: 10.1148/118.3.567. [DOI] [PubMed] [Google Scholar]
- 2.Pabon-Ramos W M, Dariushnia S R, Walker T G et al. Quality improvement guidelines for percutaneous nephrostomy. J Vasc Interv Radiol. 2016;27(03):410–414. doi: 10.1016/j.jvir.2015.11.045. [DOI] [PubMed] [Google Scholar]
- 3.Farrell T A, Hicks M E. A review of radiologically guided percutaneous nephrostomies in 303 patients. J Vasc Interv Radiol. 1997;8(05):769–774. doi: 10.1016/s1051-0443(97)70658-4. [DOI] [PubMed] [Google Scholar]
- 4.Goodwin W E, Casey W C, Woolf W. Percutaneous trocar (needle) nephrostomy in hydronephrosis. J Am Med Assoc. 1955;157(11):891–894. doi: 10.1001/jama.1955.02950280015005. [DOI] [PubMed] [Google Scholar]
- 5.Lee W J, Patel U, Patel S, Pillari G P. Emergency percutaneous nephrostomy: results and complications. J Vasc Interv Radiol. 1994;5(01):135–139. doi: 10.1016/s1051-0443(94)71470-6. [DOI] [PubMed] [Google Scholar]
- 6.Lewis S, Patel U. Major complications after percutaneous nephrostomy-lessons from a department audit. Clin Radiol. 2004;59(02):171–179. doi: 10.1016/s0009-9260(03)00336-2. [DOI] [PubMed] [Google Scholar]
- 7.Ng C K, Yip S K, Sim L S et al. Outcome of percutaneous nephrostomy for the management of pyonephrosis. Asian J Surg. 2002;25(03):215–219. doi: 10.1016/S1015-9584(09)60178-0. [DOI] [PubMed] [Google Scholar]
- 8.Yoder I C, Lindfors K K, Pfister R C. Diagnosis and treatment of pyonephrosis. Radiol Clin North Am. 1984;22(02):407–414. [PubMed] [Google Scholar]
- 9.Bitsori M, Raissaki M, Maraki S, Galanakis E. Acute focal bacterial nephritis, pyonephrosis and renal abscess in children. Pediatr Nephrol. 2015;30(11):1987–1993. doi: 10.1007/s00467-015-3141-3. [DOI] [PubMed] [Google Scholar]
- 10.Uehling D T, Hahnfeld L E, Scanlan K A. Urinary tract abnormalities in children with acute focal bacterial nephritis. BJU Int. 2000;85(07):885–888. doi: 10.1046/j.1464-410x.2000.00622.x. [DOI] [PubMed] [Google Scholar]
- 11.Radecka E, Magnusson A. Complications associated with percutaneous nephrostomies. A retrospective study. Acta Radiol. 2004;45(02):184–188. doi: 10.1080/02841850410003671. [DOI] [PubMed] [Google Scholar]
- 12.Pearle M S, Pierce H L, Miller G L et al. Optimal method of urgent decompression of the collecting system for obstruction and infection due to ureteral calculi. J Urol. 1998;160(04):1260–1264. [PubMed] [Google Scholar]
- 13.Chehab M A, Thakor A S, Tulin-Silver S et al. Adult and pediatric antibiotic prophylaxis during vascular and IR procedures: a Society of Interventional Radiology Practice Parameter Update endorsed by the Cardiovascular and Interventional Radiological Society of Europe and the Canadian Association for Interventional Radiology. J Vasc Interv Radiol. 2018;29(11):1483–150100. doi: 10.1016/j.jvir.2018.06.007. [DOI] [PubMed] [Google Scholar]
- 14.Cochran S T, Barbaric Z L, Lee J J, Kashfian P. Percutaneous nephrostomy tube placement: an outpatient procedure? Radiology. 1991;179(03):843–847. doi: 10.1148/radiology.179.3.2028003. [DOI] [PubMed] [Google Scholar]
- 15.Patel I J, Davidson J C, Nikolic B et al. Consensus guidelines for periprocedural management of coagulation status and hemostasis risk in percutaneous image-guided interventions. J Vasc Interv Radiol. 2012;23(06):727–736. doi: 10.1016/j.jvir.2012.02.012. [DOI] [PubMed] [Google Scholar]
- 16.Montvilas P, Solvig J, Johansen T E. Single-centre review of radiologically guided percutaneous nephrostomy using “mixed” technique: success and complication rates. Eur J Radiol. 2011;80(02):553–558. doi: 10.1016/j.ejrad.2011.01.109. [DOI] [PubMed] [Google Scholar]
- 17.Uppot R N. Emergent nephrostomy tube placement for acute urinary obstruction. Tech Vasc Interv Radiol. 2009;12(02):154–161. doi: 10.1053/j.tvir.2009.08.010. [DOI] [PubMed] [Google Scholar]
- 18.Thornton R H, Covey A M. Urinary drainage procedures in interventional radiology. Tech Vasc Interv Radiol. 2016;19(03):170–181. doi: 10.1053/j.tvir.2016.06.002. [DOI] [PubMed] [Google Scholar]
- 19.Krohmer S J, Pillai A K, Guevara C J, Bones B L, Dickey K W. Image-guided nephrostomy interventions: how to recognize, avoid, or get out of trouble. Tech Vasc Interv Radiol. 2018;21(04):261–266. doi: 10.1053/j.tvir.2018.07.008. [DOI] [PubMed] [Google Scholar]
- 20.Hopper K D, Yakes W F. The posterior intercostal approach for percutaneous renal procedures: risk of puncturing the lung, spleen, and liver as determined by CT. AJR Am J Roentgenol. 1990;154(01):115–117. doi: 10.2214/ajr.154.1.2104692. [DOI] [PubMed] [Google Scholar]
- 21.Clark T W, Abraham R J, Flemming B K. Is routine micropuncture access necessary for percutaneous nephrostomy? A randomized trial. Can Assoc Radiol J. 2002;53(02):87–91. [PubMed] [Google Scholar]
- 22.Dyer R B, Regan J D, Kavanagh P V, Khatod E G, Chen M Y, Zagoria R J. Percutaneous nephrostomy with extensions of the technique: step by step. Radiographics. 2002;22(03):503–525. doi: 10.1148/radiographics.22.3.g02ma19503. [DOI] [PubMed] [Google Scholar]
- 23.Ajib K M, Matta I F, Zgheib J T, Jabbour M E. Non-angled intercostal percutaneous access under full expiration: safety is not an issue anymore. J Endourol. 2017;31(08):736–741. doi: 10.1089/end.2017.0078. [DOI] [PubMed] [Google Scholar]
- 24.Picus D, Weyman P J, Clayman R V, McClennan B L. Intercostal-space nephrostomy for percutaneous stone removal. AJR Am J Roentgenol. 1986;147(02):393–397. doi: 10.2214/ajr.147.2.393. [DOI] [PubMed] [Google Scholar]
- 25.Munver R, Delvecchio F C, Newman G E, Preminger G M. Critical analysis of supracostal access for percutaneous renal surgery. J Urol. 2001;166(04):1242–1246. [PubMed] [Google Scholar]
- 26.Flukes S, Hayne D, Kuan M, Wallace M, McMillan K, Rukin N J. Retrograde ureteric stent insertion in the management of infected obstructed kidneys. BJU Int. 2015;115 05:31–34. doi: 10.1111/bju.12918. [DOI] [PubMed] [Google Scholar]
- 27.Pandey S, Sharma D, Sankhwar S et al. Are there any predictive risk factors for failure of ureteric stent in patients with obstructive urolithiasis with sepsis? Investig Clin Urol. 2018;59(06):371–375. doi: 10.4111/icu.2018.59.6.371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zagoria R J, Dyer R B. Do's and don't's of percutaneous nephrostomy. Acad Radiol. 1999;6(06):370–377. doi: 10.1016/s1076-6332(99)80233-5. [DOI] [PubMed] [Google Scholar]
- 29.Borofsky M S, Walter D, Shah O, Goldfarb D S, Mues A C, Makarov D V. Surgical decompression is associated with decreased mortality in patients with sepsis and ureteral calculi. J Urol. 2013;189(03):946–951. doi: 10.1016/j.juro.2012.09.088. [DOI] [PubMed] [Google Scholar]
- 30.Lang E K, Price E T. Redefinitions of indications for percutaneous nephrostomy. Radiology. 1983;147(02):419–426. doi: 10.1148/radiology.147.2.6836120. [DOI] [PubMed] [Google Scholar]
- 31.Rana A M, Zaidi Z, El-Khalid S. Single-center review of fluoroscopy-guided percutaneous nephrostomy performed by urologic surgeons. J Endourol. 2007;21(07):688–691. doi: 10.1089/end.2006.0281. [DOI] [PubMed] [Google Scholar]
- 32.Khalilzadeh O, Baerlocher M O, Shyn P B et al. Proposal of a new adverse event classification by the Society of Interventional Radiology Standards of Practice Committee. J Vasc Interv Radiol. 2017;28(10):1432–1.437E6. doi: 10.1016/j.jvir.2017.06.019. [DOI] [PubMed] [Google Scholar]
- 33.Li A C, Regalado S P. Emergent percutaneous nephrostomy for the diagnosis and management of pyonephrosis. Semin Intervent Radiol. 2012;29(03):218–225. doi: 10.1055/s-0032-1326932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tuttle D N, Yeh B M, Meng M V, Breiman R S, Stoller M L, Coakley F V. Risk of injury to adjacent organs with lower-pole fluoroscopically guided percutaneous nephrostomy: evaluation with prone, supine, and multiplanar reformatted CT. J Vasc Interv Radiol. 2005;16(11):1489–1492. doi: 10.1097/01.RVI.0000175331.93499.44. [DOI] [PubMed] [Google Scholar]
- 35.Prassopoulos P, Gourtsoyiannis N, Cavouras D, Pantelidis N. A study of the variation of colonic positioning in the pararenal space as shown by computed tomography. Eur J Radiol. 1990;10(01):44–47. doi: 10.1016/0720-048x(90)90086-q. [DOI] [PubMed] [Google Scholar]
- 36.Miller G L, Summa J. Transcolonic placement of a percutaneous nephrostomy tube: recognition and treatment. J Vasc Interv Radiol. 1997;8(03):401–403. doi: 10.1016/s1051-0443(97)70580-3. [DOI] [PubMed] [Google Scholar]


