Abstract
The spleen is the most commonly injured organ after blunt abdominal trauma. Nonoperative management with splenic arterial embolization (SAE) is the current standard of care for hemodynamically stable patients. Current data favor the use of proximal and coil embolization techniques in adults, while observation is suggested in the pediatric population. In this review, the authors describe the most recent evidence informing the clinical indications, techniques, and complications for SAE.
Keywords: splenic arterial embolization, blunt splenic injury, interventional radiology, trauma
The spleen is the most commonly injured solid organ following blunt abdominal trauma. 1 Arteriographic hemostasis of the spleen was initially described in 1981 by Sclafani as an adjunctive procedure to improve the success of surgical repair and/or salvage. 2 The high rate of mortality associated with postoperative sepsis and recognition of the immunologic role of the spleen compelled the need for splenic preservation techniques. 3 Accordingly, nonoperative management (NOM) is the current standard of care in hemodynamically stable patients with blunt splenic injury (BSI). While some aspects remain controversial, splenic arterial embolization (SAE) is an integral adjunct to NOM.
In this review, the authors describe the most recent evidence informing the clinical indications, techniques, and complications for SAE.
Clinical Indication
A high index of suspicion is required for the timely diagnosis and triage of splenic injury. Most commonly, splenic injury is a result of blunt force following a motor vehicle collision. Hemodynamically unstable patients are predominantly treated with operative management and may be evaluated with focused assessment with sonography in trauma exam and/or diagnostic peritoneal lavage to confirm the presence of intraperitoneal hemorrhage.
Hemodynamically stable patients are routinely offered computed tomography (CT) for the assessment of BSI severity. Anatomical classification of injury severity by CT findings is widely determined by the American Association for the Surgery of Trauma (AAST) scale 4 ( Table 1 ). The most recently revised 2018 AAST guidelines incorporate vascular injury (i.e., active extravasation, pseudoaneurysm, arteriovenous fistula) into the imaging criteria for grade IV visceral injury. 5 The sensitivity, specificity, and accuracy of CT have been reported to be as high as 81, 90, and 83%, respectively, predicting the need for SAE. 6 7 As not all actively bleeding injuries are detected by CT, splenic angiography may be indicated in high-grade visceral injury—however, this decision must be weighed against complications of angiography.
Table 1. American Association for the Surgery of Trauma classification scheme for splenic trauma.
| Grade I | - Subcapsular hematoma <10% surface area - Parenchymal laceration <1 cm depth - Capsular tear |
| Grade II | - Subcapsular hematoma <10–50% surface area - Intraparenchymal hematoma <5 cm - Parenchymal laceration 1–3 cm depth |
| Grade III | - Subcapsular hematoma >50% surface area - Intraparenchymal hematoma ≥5 cm - Parenchymal laceration >3 cm depth |
| Grade IV | - Vascular injury or active bleeding confined within splenic capsule - Parenchymal laceration involving segmental or hilar vessels producing >25% devascularization |
| Grade V | - Shattered spleen - Vascular injury or active bleeding extending beyond splenic capsule |
The World Society of Emergency Surgery (WSES) has recently proposed a new classification system that considers both hemodynamic status and AAST score as a more comprehensive assessment of injury severity 8 ( Table 2 ). However, the clinical impact of either classification system has yet to be definitively demonstrated.
Table 2. WSES classification scheme for splenic trauma.
| WSES I (minor) | Stable | AAST I–II |
| WSES II (moderate) | Stable | AAST III |
| WSES III (moderate) | Stable | AAST IV–V |
| WSES IV (severe) | Unstable | I–V |
Abbreviations: AAST, American Association for the Surgery of Trauma; WSES, World Society of Emergency Surgery.
In addition to severity of BSI, CT is important in revealing additional injuries that may necessitate operative management as well as in serving as a baseline study for follow-up imaging. This is especially important, as delayed splenic vascular complications after NOM of BSI are common, with a reported incidence as high as 23%. 9
Banerjee et al demonstrated that despite wide practice variation at level I trauma centers, centers with higher rates of SAE use have higher spleen salvage and lower NOM failure rates. 10 In general, AAST grade I–II injuries are observed and grade IV and V injuries are managed with SAE. The management of grade III injuries is debated. A systematic review of 23 studies published in 2017 by Crichton et al demonstrated that while SAE significantly reduced the failure of NOM in patients with grade IV and V BSI, it has minimal effect in those with grade I to III injuries. 11
Techniques and Controversies
Standard angiographic technique is used. Femoral or radial access is obtained and the celiac artery is selected with a curved (Cobra C2 or Rosch Celiac RC2) or reverse-curved catheter (Simmons, SOS, or visceral selective). Celiac angiography is performed to evaluate for collateral flow to the spleen. Subsequently, the splenic artery is catheterized for embolization. Depending on the level of tortuosity and angle of origin, a microcatheter may be required for secure access, but may limit the ability to obtain diagnostic angiography. Position of the catheter tip for embolization may be in the main splenic artery (proximal) or in distal branches (distal). The evidence comparing distal versus primary embolization is limited and remains controversial.
Angiographic Findings
Vascular injury presents as a variety of angiographic findings on splenic angiography. For good quality diagnostic digital subtraction angiography, the arterial, parenchymal, and venous phases of contrast should be imaged and correlated with available cross-sectional imaging to confirm location of injury. Angiography is best performed through a 5-Fr catheter, as the contrast volume limitations of a microcatheter reduce the quality of angiography in this high-flow territory. Focal pseudoaneurysms are most commonly seen, with free extravasation more rarely observed. Other findings include arteriovenous fistulae, which are associated with a high rate of failure of embolization, vessel truncation, and widespread, multifocal petechial contrast pooling associated with parenchymal defects ( Fig. 1 ).
Fig. 1.
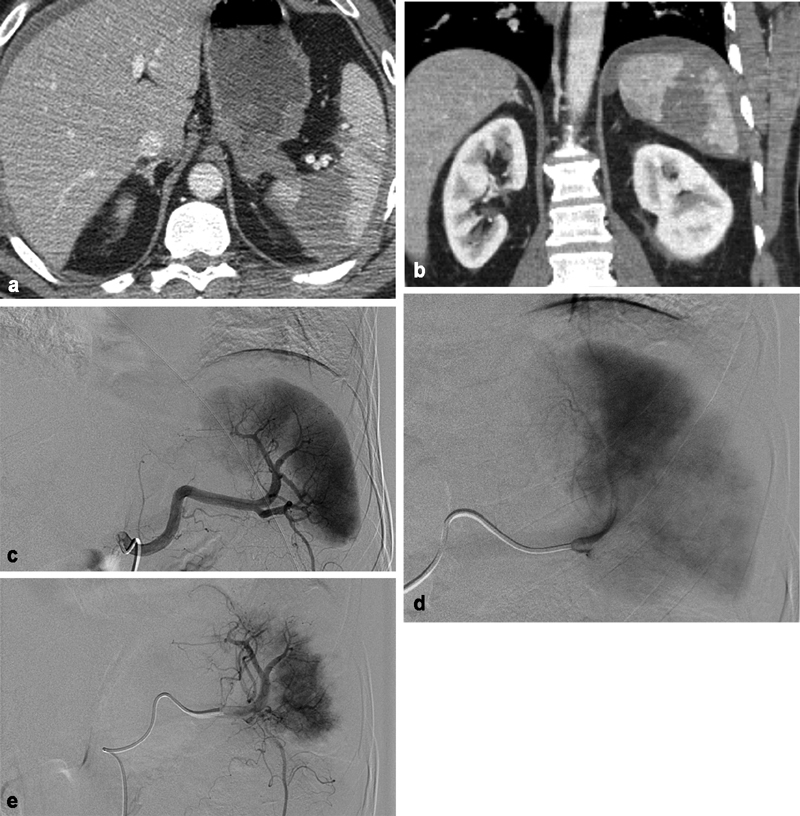
A 50-year-old man who fell from 20 feet. ( a ) Axial CT of the abdomen and ( b ) coronal CT of the abdomen showing extensive splenic contusion/laceration with perisplenic hematoma consistent with a grade IV injury. ( c ) Early-phase splenic angiography. ( d ) Late-phase splenic angiography demonstrating multiple segmental perfusion defects, correlating to known splenic lacerations. Faint petechial contrast pooling in the region of the parenchymal defect. ( e ) Postembolization angiography following Gelfoam embolization from the distal main splenic artery with resultant sluggish flow and peripheral vascular pruning.
Embolization Technique
Proximal embolization is performed by occluding the midportion of the main splenic artery with the goal of decreasing perfusion pressure within the spleen while allowing for continued blood supply via collateral vessels ( Fig. 2 ). The dorsal pancreatic artery and the pancreatica magna should be identified on angiography, and embolization performed between these two branches to avoid devascularization of the pancreas and ischemic pancreatitis. Distal embolization is performed by occluding flow as distally and close to the injury as possible ( Fig. 3 ). The goal of distal embolization is to focally devascularize at the location of the injury while preserving splenic artery patency.
Fig. 2.
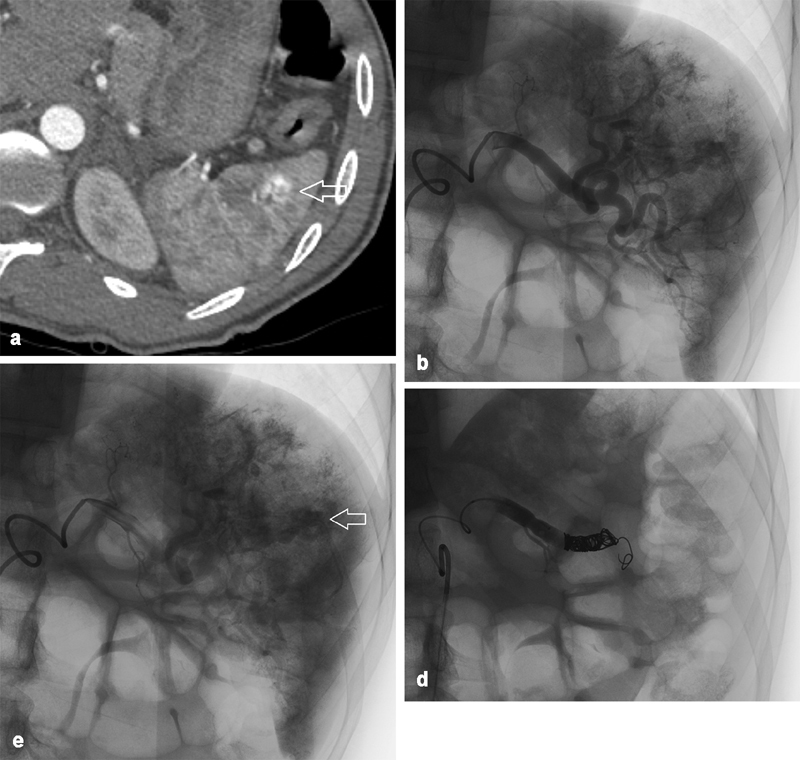
A 24-year-old individual involved in pedestrian–automobile collision. ( a ) Axial CT showing an arterial blush in the lateral spleen ( arrow ) adjacent to multiple vessels suggestive of a pseudoaneurysm. ( b ) Splenic angiography showing extravascular contrast at multiple sites. ( c ) Delayed imaging on angiography shows contrast pooling ( arrow on one such collection), representative of multifocal pseudoaneurysms. ( d ) Angiography posttreatment. Given the multifocal findings, it was determined to embolize the spleen proximally using multiple coils beyond the dorsal pancreatic artery. Complete vascular occlusion demonstrated.
Fig. 3.
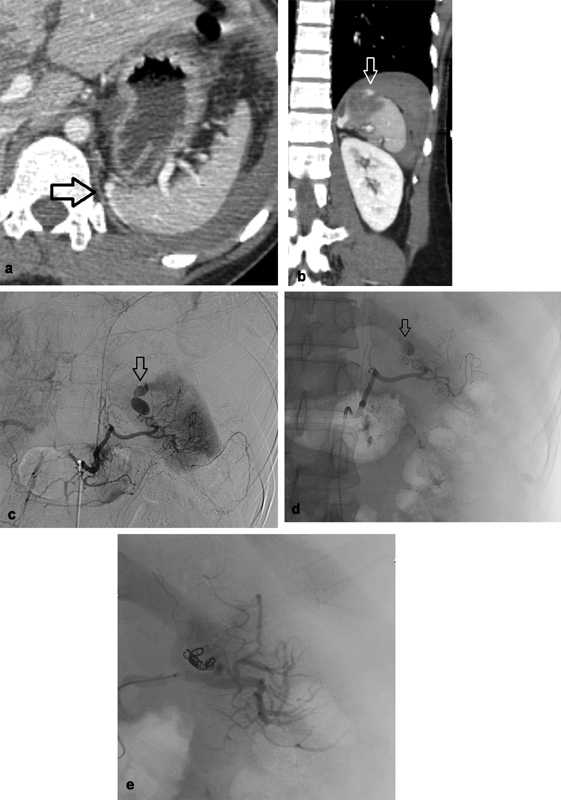
A 29-year-old man involved in motor vehicle crash. ( a ) Axial CT abdomen and ( b ) coronal CT abdomen showing small focal laceration along the superior medial aspect of the spleen with extravasation of contrast ( arrow ). ( c ) Celiac angiography. ( d ) Splenic angiography demonstrating a pseudoaneurysm along the medial aspect of the spleen with active extravasation into the peritoneum ( arrow ). ( e ) Embolization of a distal splenic artery branch supplying the pseudoaneurysm was performed with multiple coils and small aliquots of Gelfoam slurry resulting in no flow seen past the area of embolization, no evidence of remaining pseudoaneurysm or extravasation.
Coils and Gelfoam are the most common agents employed in SAE. Gelfoam traps platelets as it travels with blood within vasculature to both physically occlude flow and promote thrombus formation. It is subsequently absorbed by macrophages and allows for restoration of vessel patency. 12 Alternatively, coils are permanent but can be placed more accurately into a predetermined location, though they have been criticized for migrating when undersized to the vessel. Although maintaining vessel patency is an appealing feature, data suggest that Gelfoam is associated with a higher rate of life-threatening complications compared with coil embolization. Gelfoam is reported to increase the risk of rebleeding and infarction, as it often occludes distal and collateral vessels. 13 Furthermore, air injected with Gelfoam can increase the risk of aerobic infection. 12
A meta-analysis of 15 retrospective cohort studies published in 2011 by Schnüriger et al reported that both proximal and distal techniques had similar rates of infarctions and infection requiring splenectomy, but distal embolization resulted in higher rate of segmental infarctions not requiring splenectomy. 13 A more recent meta-analysis of 23 studies published in 2017 by Rong et al suggested that proximal embolization reduced severe complications (defined as any complication meeting the Clavien-Dindo grade IV classification, i.e., life-threatening complication requiring intensive care) and complications requiring surgical management. 14 Proximal embolization has also been considered by some authors to be more favorable as it often requires a shorter procedure time, decreasing the chance of patients becoming unstable in the interventional suite and reducing radiation exposure in these patients, who tend to be younger. 4 However, proximal embolization is also criticized as it leads to permanent occlusion of the splenic artery and precludes further angiographic management if rebleeding occurs. For many operators, distal embolization is reserved for cases in which there is focal, limited injury to the spleen that is readily identifiable on angiography and readily accessible in a relatively stable patient.
Complications
A recent 9-year retrospective study by Corn et al compared the complication rate of BSI between laparotomy, embolization, and observation. The operative group had the highest complication and mortality rates of 50.7 and 26.3%, respectively. The embolization group had the lowest complication and mortality rates of 5.3 and 2.6%, respectively. Of note, the patients in the operative and embolization groups had similar splenic injury grades, but patients in the operative group had more severe hemoperitoneum. 15
Postprocedure Management
Patients should be actively followed up by the interventional radiology team to anticipate and manage potential complications in collaboration with surgical and intensive care teams. Postembolization patients remain at risk for rebleeding, necessitating hemodynamic monitoring and observation for signs and symptoms of bleeding. Immunization of postembolization patients is controversial. However, literature does suggest that immune function of the spleen is preserved following embolization. 16
Special Considerations in Pediatric Trauma
Recent data demonstrate high splenic salvage rates with the use of SAE as an adjunct to NOE in adult patients. 17 A retrospective study of children with BSI over a 10-year period at a level I trauma center published in 2015 by Bansal et al suggests that there were no statistical differences in the need for splenectomy, transfusion, or length in stay between SAE and NOM even in the presence of contrast blush. 18
Conclusions
Cumulative evidence suggests that NOM with SAE should be prioritized and widely accepted as the standard of care, with the caveat that children may be more likely to benefit from watchful waiting. Proximal embolization is the preferred approach with multifocal or large territory parenchymal injury, or in patients with hemodynamic instability that precludes longer procedures. Distal embolization may be beneficial in patients with focal, angiographically evident injury to preserve access for future intervention and minimize global splenic ischemia.
Footnotes
Conflict of Interest None of the authors have conflicts.
References
- 1.Ahuja C, Farsad K, Chadha M. An overview of splenic embolization. AJR Am J Roentgenol. 2015;205(04):720–725. doi: 10.2214/AJR.15.14637. [DOI] [PubMed] [Google Scholar]
- 2.Sclafani S J. The role of angiographic hemostasis in salvage of the injured spleen. Radiology. 1981;141(03):645–650. doi: 10.1148/radiology.141.3.7029619. [DOI] [PubMed] [Google Scholar]
- 3.Bisharat N, Omari H, Lavi I, Raz R. Risk of infection and death among post-splenectomy patients. J Infect. 2001;43(03):182–186. doi: 10.1053/jinf.2001.0904. [DOI] [PubMed] [Google Scholar]
- 4.Imbrogno B F, Ray C E. Splenic artery embolization in blunt trauma. Semin Intervent Radiol. 2012;29(02):147–149. doi: 10.1055/s-0032-1312577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Organ injury scaling 2018 update: spleen, liver, and kidney: erratum. J Trauma Acute Care Surg. 2019;87(02):512. doi: 10.1097/TA.0000000000002419. [DOI] [PubMed] [Google Scholar]
- 6.Shanmuganathan K, Mirvis S E, Boyd-Kranis R, Takada T, Scalea T M. Nonsurgical management of blunt splenic injury: use of CT criteria to select patients for splenic arteriography and potential endovascular therapy. Radiology. 2000;217(01):75–82. doi: 10.1148/radiology.217.1.r00oc0875. [DOI] [PubMed] [Google Scholar]
- 7.Marmery H, Shanmuganathan K, Mirvis S E et al. Correlation of multidetector CT findings with splenic arteriography and surgery: prospective study in 392 patients. J Am Coll Surg. 2008;206(04):685–693. doi: 10.1016/j.jamcollsurg.2007.11.024. [DOI] [PubMed] [Google Scholar]
- 8.Coccolini F, Montori G, Catena F et al. Splenic trauma: WSES classification and guidelines for adult and pediatric patients. World J Emerg Surg. 2017;12:40. doi: 10.1186/s13017-017-0151-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Furlan A, Tublin M E, Rees M A, Nicholas D H, Sperry J L, Alarcon L H. Delayed splenic vascular injury after nonoperative management of blunt splenic trauma. J Surg Res. 2017;211:87–94. doi: 10.1016/j.jss.2016.11.062. [DOI] [PubMed] [Google Scholar]
- 10.Banerjee A, Duane T M, Wilson S Pet al. Trauma center variation in splenic artery embolization and spleen salvage: a multicenter analysis J Trauma Acute Care Surg 2013750169–74., discussion 74–75 [DOI] [PubMed] [Google Scholar]
- 11.Crichton J, Naidoo K, Yet B, Brundage S, Perkins Z. The role of splenic angioembolization as an adjunct to nonoperative management of blunt splenic injuries: A systematic review and meta-analysis. J Trauma Acute Care Surg. 2017;83(05):934–943. doi: 10.1097/TA.0000000000001649. [DOI] [PubMed] [Google Scholar]
- 12.Abada H T, Golzarian J. Gelatine sponge particles: handling characteristics for endovascular use. Tech Vasc Interv Radiol. 2007;10(04):257–260. doi: 10.1053/j.tvir.2008.03.002. [DOI] [PubMed] [Google Scholar]
- 13.Schnüriger B, Inaba K, Konstantinidis A, Lustenberger T, Chan L S, Demetriades D. Outcomes of proximal versus distal splenic artery embolization after trauma: a systematic review and meta-analysis. J Trauma. 2011;70(01):252–260. doi: 10.1097/TA.0b013e3181f2a92e. [DOI] [PubMed] [Google Scholar]
- 14.Rong J J, Liu D, Liang M et al. The impacts of different embolization techniques on splenic artery embolization for blunt splenic injury: a systematic review and meta-analysis. Mil Med Res. 2017;4:17. doi: 10.1186/s40779-017-0125-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Corn S, Reyes J, Helmer S D, Haan J M. Outcomes following blunt traumatic splenic injury treated with conservative or operative management. Kans J Med. 2019;12(03):83–88. [PMC free article] [PubMed] [Google Scholar]
- 16.Schimmer J AG, van der Steeg A F, Zuidema W P. Splenic function after angioembolization for splenic trauma in children and adults: a systematic review. Injury. 2016;47(03):525–530. doi: 10.1016/j.injury.2015.10.047. [DOI] [PubMed] [Google Scholar]
- 17.Cinquantini F, Simonini E, Di Saverio S et al. Non-surgical management of blunt splenic trauma: a comparative analysis of non-operative management and splenic artery embolization-experience from a European trauma center. Cardiovasc Intervent Radiol. 2018;41(09):1324–1332. doi: 10.1007/s00270-018-1953-9. [DOI] [PubMed] [Google Scholar]
- 18.Bansal S, Karrer F M, Hansen K, Partrick D A. Contrast blush in pediatric blunt splenic trauma does not warrant the routine use of angiography and embolization. Am J Surg. 2015;210(02):345–350. doi: 10.1016/j.amjsurg.2014.09.028. [DOI] [PubMed] [Google Scholar]


