Abstract
A 64-year-old Japanese man suffered cardiopulmonary arrest, which may have resulted from sepsis and/or hyperosmolar hyperglycemic non-ketonic coma, and was admitted after successful resuscitation. He had watery diarrhea on day 18 and was diagnosed with cytomegalovirus enterocolitis. In addition, computed tomography performed on day 27 and colonoscopy revealed gastric emphysema and intestinal pseudolipomatosis, respectively. This report is the first to describe a patient with cytomegalovirus enterocolitis and subsequent gastric emphysema and pseudolipomatosis. Gastrointestinal cytomegalovirus infection may underlie gastric emphysema and intestinal pseudolipomatosis, particularly in patients with relative or obvious immune dysfunction.
Keywords: cytomegalovirus colitis, pseudolipomatosis, gastric emphysema, post-resuscitation, diabetes mellitus
Introduction
Gastric emphysema and emphysematous gastritis are the two principal conditions characterized by gas within the gastric wall evident on a radiological examination. Emphysematous gastritis is an infection of the gastric wall by gas-forming bacteria, which is often life-threatening, with an estimated mortality rate approaching 60% (1, 2). In contrast, gastric emphysema is the collection of gas within the gastric wall with no associated bacterial infection (3, 4). It generally has a relatively benign course with the spontaneous resolution of gas collection.
Based on the pathogenesis, gastric emphysema can be classified into three types: traumatic, obstructive, and pulmonary (5). In traumatic gastric emphysema, there is direct transmural diffusion of gas from an air-distended stomach following mucosal damage, e.g. due to a gastric biopsy or gastrostomy. Obstructive gastric emphysema is caused by gastric outlet obstruction due to cancer or a peptic ulcer. The increased intragastric pressure and diseased mucosa associated with outlet obstruction lead to gas diffusion into the gastric wall. In pulmonary gastric emphysema, gas that is considered to originate from pulmonary bullae travels via the esophagus to the stomach. Although various diseases have been reported as underlying factors, to our knowledge, cytomegalovirus enterocolitis has not been reported to underlie gastric emphysema or intestinal pseudolipomatosis.
We herein report a case of a post-resuscitation patient who was found to have gastric emphysema during the course of cytomegalovirus enterocolitis. Of note, pseudolipomatosis and cytomegalovirus infection were found in the ileum at the same time. The possible correlation between cytomegalovirus enterocolitis, intestinal pseudolipomatosis, and gastric emphysema is discussed later in this report.
Case Report
A 63-year-old Japanese man was brought to the hospital in an ambulance because of loss of consciousness. Although he had previously been diagnosed with nonalcoholic fatty liver disease, he did not visit the hospital to receive medical checkups or medical care following his retirement at 60 years of age. He had no history of diabetes or glucose intolerance.
He suffered cardiopulmonary arrest with pulseless electrical activity in the ambulance. External cardiac massage and manual ventilation were immediately started after endotracheal intubation on arrival at the hospital, and he was successfully resuscitated with adrenaline.
Laboratory tests showed high values for the white blood cell count (16,800 /μL), red blood cell count (5,570,000 /μL), and hemoglobin concentration (17.8 g/dL). Blood glucose (513 mg/dL), aspartate aminotransferase (236 U/L), alanine aminotransferase (183 U/L), sodium (167 mmol/L), potassium (4.9 mmol/L), blood urea nitrogen (121.4 mg/dL), creatinine (5.04 mg/dL), uric acid (24.6 mg/dL), amylase (433 U/L), triglyceride (575 mg/dL), and creatine phosphokinase (1,422 U/L) levels were also elevated. The C-reactive protein level was elevated to 23.79 mg/dL on day 3 and gradually improved subsequently. A urinalysis did not reveal glucose or ketone bodies in his urine. He tested negative for human immunodeficiency virus. Computed tomography performed on days 1 and 6 did not reveal emphysema or wall thickening of the gastrointestinal tract. Lactobacillus fermentum and Lactobacillus paracasei were isolated from the blood culture. Based on the results of the laboratory test and blood culture, we considered sepsis and/or hyperosmolar hyperglycemic non-ketonic coma due to uncontrolled diabetes to have caused the cardiopulmonary arrest.
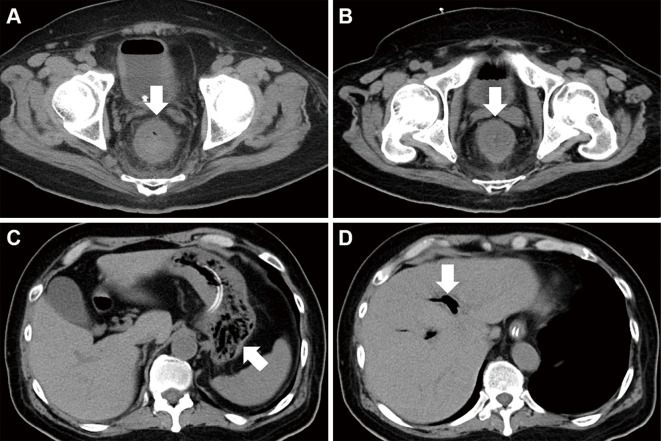
We started treating the patient with intravenous injection of antibiotics and insulin, continuous hemodiafiltration, and mechanical ventilation (Fig. 1). Ceftriaxone was administered on day 1 and switched to tazobactam/piperacillin on day 3. The antibiotic was changed to sulbactam/ampicillin based on the results of the antibiotic susceptibility testing for Lactobacillus fermentum and Lactobacillus paracasei. The administration of antibiotics was stopped on day 15. The general condition of the patient gradually improved, and he was extubated on day 15. However, he had watery diarrhea on day 18, and computed tomography performed on day 21 showed circumferential wall thickening of the rectum (Fig. 2A). No organisms of significance were isolated from the stool culture, and Clostridium difficile toxin was not detected in the stool. The watery diarrhea persisted despite antibiotic treatment, and rectal wall thickening was observed on computed tomography on day 27 (Fig. 2B). In addition, gastric emphysema (Fig. 2C) and hepatic portal venous gas (Fig. 2D) were noted. The white blood cell count was 14,040 /μL, and the C-reactive protein level was 2.96 mg/dL on day 27.
Figure 1.
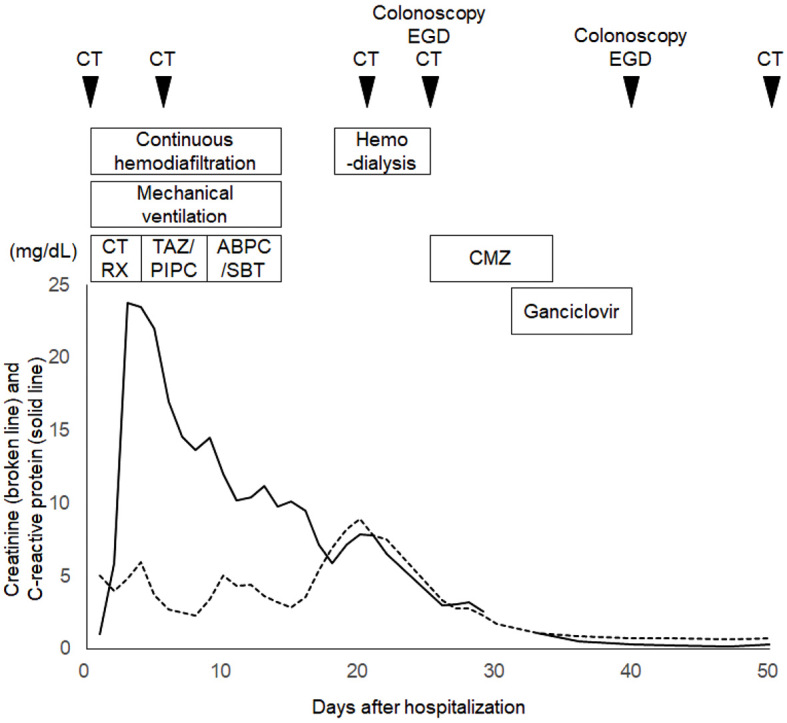
Clinical course of the patient. CT: computed tomography, EGD: esophagogastroduodenoscopy, CTRX: ceftriaxone, TAZ/PIPC: tazobactam/piperacillin, ABPC/SBT: sulbactam/ampicillin, CMZ: cefmetazole
Figure 2.
Computed tomography images. Circumferential wall thickening of the rectum is observed on day 20 (A). Rectal wall thickening is also seen on day 26 (B). Gastric emphysema (C) and hepatic portal venous gas (D) are also seen.
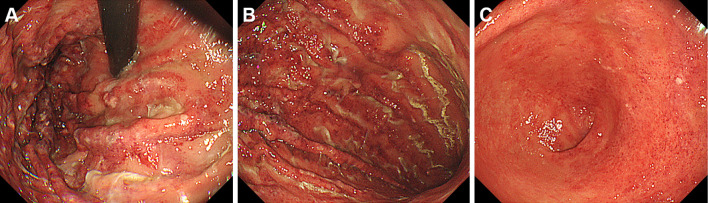
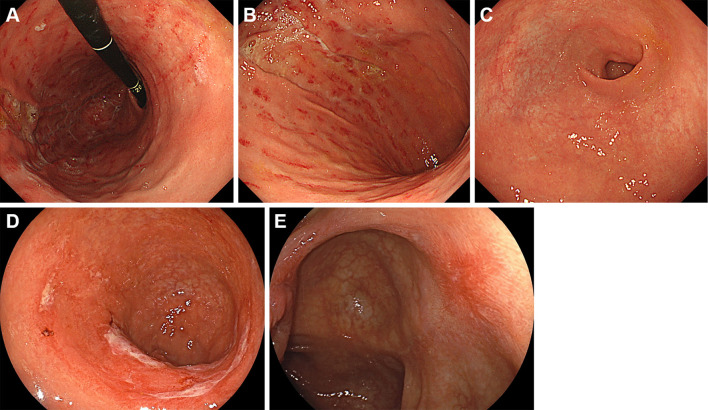
Esophagogastroduodenoscopy performed on day 27 revealed diffuse edema and multiple longitudinal areas of redness and erosion in the gastric fundus and gastric body (Fig. 3A, B). The gastric antrum was also affected, but the redness was less than that in the gastric fundus and body (Fig. 3C). These endoscopic findings were consistent with gastric emphysema, and biopsy sampling of the gastric lesions was not performed.
Figure 3.
Esophagogastroduodenoscopy images of gastric emphysema. Diffuse edema and multiple longitudinal areas of redness and erosion are seen in the gastric fundus and gastric body (A, B). The gastric antrum is also involved (C).
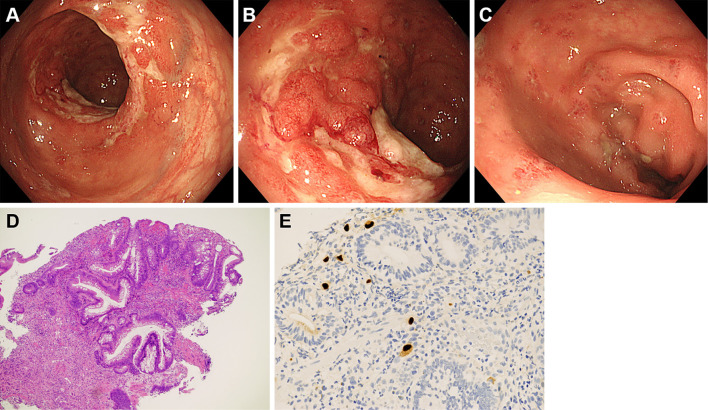
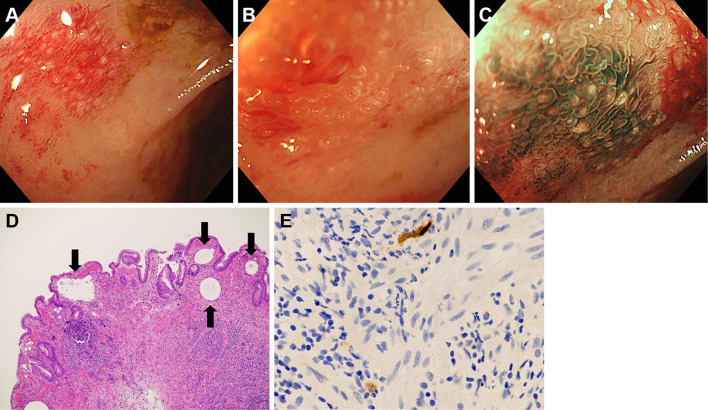
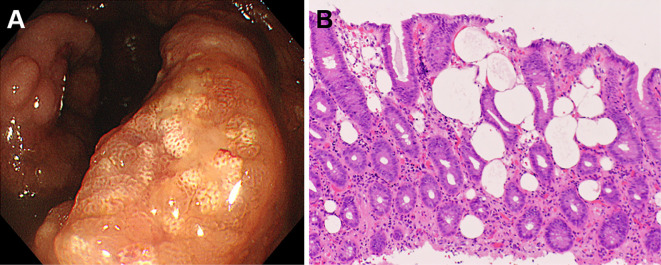
The patient underwent colonoscopy on the same day, which revealed large ulcers in the rectum (Fig. 4A, B). Aphthous lesions were also found in the rectum (Fig. 4C). A biopsy of the ulcer showed infiltration of neutrophils and lymphocytes (Fig. 4D), and immunostaining revealed cytomegalovirus-positive cells (Fig. 4E). Reddish lesions were found in the terminal ileum during colonoscopy (Fig. 5A), and observations at higher magnifications revealed microbubbles within the reddish mucosa (Fig. 5B). Narrow-band imaging showed the microbubbles as round whitish deposits (Fig. 5C). A histopathological examination revealed optically empty coalescent vacuoles within the lamina propria of the ileum and infiltration of neutrophils and lymphocytes (Fig. 5D). Cytomegalovirus-positive cells were also found in the ileum (Fig. 5E). White lesions were found in the ascending colon (Fig. 6A), and a biopsy examination of the white lesions revealed small vacuoles within the mucosa (Fig. 6B).
Figure 4.
Colonoscopy images of the rectum. Large ulcers (A, B) and aphthous lesions (C) are found in the rectum. Infiltration of neutrophils and lymphocytes is seen in the biopsy specimen (D). Cytomegalovirus-positive cells are also seen (E).
Figure 5.
Colonoscopy images of the ileum. Reddish lesions with microbubbles are seen in the ileum (A, B). Narrow-band imaging shows the microbubbles as round, whitish deposits (C). Optically empty coalescent vacuoles are seen in the biopsy specimen (D). Cytomegalovirus-positive cells are also present (E).
Figure 6.
Colonoscopy images of the ascending colon. White lesions are seen (A), and the biopsy specimens show small vacuoles within the mucosa (B), indicating pseudolipomatosis.
A cytomegalovirus pp65 antigenemia (C7-HRP) test of the peripheral blood was positive in 1/50,000 cells. Therefore, we diagnosed the patient with cytomegalovirus enterocolitis and subsequent pseudolipomatosis of the ileum and ascending colon, gastric emphysema, and hepatic portal venous gas.
Cefmetazole was administered on days 28 to 36, and ganciclovir was administered on days 33 to 40. The administration of ganciclovir resulted in the resolution of the watery diarrhea. His biochemistry results were almost normalized on day 36. Esophagogastroduodenoscopy and colonoscopy were repeated on day 40, and they showed improvements in the gastric (Fig. 7A-C), rectal (Fig. 7D), ileal, and colonic (Fig. 7E) lesions. Improvements in proctitis, gastric emphysema, and hepatic portal venous gas were confirmed by computed tomography. The patient was transferred to another hospital for rehabilitation on day 54. Alpha-glucosidase inhibitors were not administered throughout the treatment in our hospital.
Figure 7.
Images from esophagogastroduodenoscopy and colonoscopy repeated after two weeks. Improvements are noted in the gastric (A-C), rectal (D), and colonic (E) lesions.
Discussion
In the patient presented in this case report, gastric emphysema and intestinal pseudolipomatosis occurred during cytomegalovirus enterocolitis. To our knowledge, this is the first report of a patient presenting with these three diseases.
Pseudolipomatosis is diagnosed based on histopathological evidence of variable-sized cystic spaces within the lamina propria (6-8). Pseudolipomatosis of the large intestine typically appears as whitish or yellowish lesions with slight elevations (8-11), as found in the ascending colon of our patient (Fig. 6). We also previously reported that microbubbles can be seen within the mucosa at high endoscopic magnifications (8). Therefore, we promptly considered the lesion to be pseudolipomatosis, since microbubbles were found within the ileal mucosa (Fig. 5). Of note, cytomegalovirus-positive cells were found in the ileal mucosa in addition to pseudolipomatosis. Although several mechanisms, such as chemical injury by a disinfectant, particularly hydrogen peroxide, and mechanical injury during an endoscopic procedure, have been proposed for the pathogenesis of colonic pseudolipomatosis (10, 12, 13), underlying cytomegalovirus infection has never been proposed. This case report suggests that cytomegalovirus infection may be the cause of intestinal pseudolipomatosis, at least in some cases.
The endoscopic features of gastric emphysema reportedly include gastric mucosal alterations, such as edema, redness, erosion, and ulcers, which correspond to the area of the emphysematous mucosa (3, 14). In the present patient, diffuse edema and multiple longitudinal areas of redness and erosion were predominantly observed in the gastric fundus and gastric body, a finding that was consistent with the typical endoscopic features of gastric emphysema. We considered bacterial emphysematous gastritis an unlikely diagnosis of the gastric lesions because the C-reactive protein level had not been elevated when the gas collection in the stomach was detected.
As described above, gastric emphysema can be classified into three types: traumatic, obstructive, and pulmonary emphysema, depending on the cause of gas accumulation in the stomach. However, based on the clinical and histological features of the present patient, we hypothesize another mechanism in addition to these three: gas originating from the intestine that travels to the stomach. Small vacuoles, which indicate pseudolipomatosis, are formed in the lamina propria of ileal and colonic mucosae. These microbubbles may then migrate and accumulate in the gastric wall, resulting in large empty spaces in the submucosal and muscular layers of the stomach (i.e. gastric emphysema). We also previously reported a patient with pneumatosis intestinalis, which is another condition characterized by gas accumulation in the gastrointestinal tract that may occur secondary to pseudolipomatosis (8). Therefore, we hypothesize that cytomegalovirus infection in the small and large intestines triggered intestinal pseudolipomatosis and subsequently caused gastric emphysema.
Another hypothesis regarding the pathogenesis of gastric emphysema in our patient is that cytomegalovirus infects and damages the gastric mucosa and/or wall, ultimately leading to gas accumulation in the gastric wall. Infection-induced gastric pneumatosis is generally caused by gas-forming bacteria and is termed emphysematous gastritis (15, 16), which differs from gastric emphysema. There are no reports linking emphysematous gastritis or gastric emphysema to cytomegalovirus infection, but Balasuriya et al. reported a patient with severe cytomegalovirus colitis who had portal venous gas and pneumatosis coli (17). Thus, cytomegalovirus may have caused direct gastric injury in our patient. Unfortunately, we cannot confirm the cytomegalovirus infection in the stomach because we did not perform a biopsy of the gastric lesions.
Another hypothesis is that mechanical ventilation traumatized the respiratory tract and induced pulmonary gas collection, which traveled via the esophagus to the stomach (18). As described earlier, in pulmonary-type gastric emphysema, gas originating from the pulmonary bullae move to the stomach. However, this mechanism is unlikely in the present patient, as he was extubated on day 15, and gastric emphysema was diagnosed on day 27, with no signs of gas collection in the gastric mucosa noted by computed tomography on days 1, 6, or 21.
Most reports of cytomegalovirus infection of the gastrointestinal tract are of immunosuppressed patients, such as those on immunosuppressive therapy or chemotherapy and those with malignant diseases, inflammatory bowel diseases, or human immunodeficiency virus infection (19, 20). Furthermore, several authors have suggested that diabetes or glucose intolerance may cause cytomegalovirus infection (21-23). Our patient was neither immunodeficient nor on immunosuppressive therapy, but uncontrolled diabetes may have caused immune dysfunction, leading to cytomegalovirus enterocolitis. Furthermore, it is likely that his mucosal integrity had been compromised by ischemia, since the patient was admitted after resuscitation. Overall, this case report emphasizes that cytomegalovirus enterocolitis can occur in immunosuppressed patients and patients with relative immune dysfunction caused by diabetes and/or a resuscitated status.
In conclusion, in our post-resuscitation patient, gastric emphysema and intestinal pseudolipomatosis occurred during cytomegalovirus enterocolitis. Although such a disease combination is considered to be rare, the clinical course of our patient shows that gastrointestinal cytomegalovirus infection may underlie gastric emphysema and intestinal pseudolipomatosis, particularly in patients with relative or obvious immune dysfunction.
The authors state that they have no Conflict of Interest (COI).
References
- 1. Hadas-Halpren I, Hiller N, Guberman D. Emphysematous gastritis secondary to ingestion of large amounts of Coca Cola. Am J Gastroenterol 88: 127-129, 1993. [PubMed] [Google Scholar]
- 2. Singh K. Emphysematous gastritis associated with Sarcina ventriculi. Case Rep Gastroenterol 13: 207-213, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ihoriya H, Yumoto T, Iwamuro M, et al. . Gastric emphysema in a critically ill patient successfully treated without surgery. Case Rep Crit Care 2019: 1824101, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Tomoda Y, Kagawa S, Kurata S, Tanaka K. Gas within the stomach wall and hepatic portal vein. BMJ Case Rep. Forthcoming. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Agha FP. Gastric emphysema: an etiologic classification. Australas Radiol 28: 346-352, 1984. [DOI] [PubMed] [Google Scholar]
- 6. Snover DC, Sandstad J, Hutton S. Mucosal pseudolipomatosis of the colon. Am J Clin Pathol 84: 575-580, 1985. [DOI] [PubMed] [Google Scholar]
- 7. Kaassis M, Croue A, Carpentier S, Burtin P, Boyer J. A case of colonic pseudolipomatosis: a rare complication of colonoscopy? Endoscopy 29: 325-327, 1997. [DOI] [PubMed] [Google Scholar]
- 8. Iwamuro M, Tanaka T, Kawabata T, et al. . Pseudolipomatosis of the colon and cecum followed by pneumatosis intestinalis. Intern Med 57: 2501-2504, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Jonas G, Mahoney A, Murray J, Gertler S. Chemical colitis due to endoscope cleaning solutions: a mimic of pseudomembranous colitis. Gastroenterology 95: 1403-1408, 1988. [DOI] [PubMed] [Google Scholar]
- 10. Kim SJ, Baek IH. Colonic mucosal pseudolipomatosis: disinfectant colitis? Gastroenterol Nurs 35: 208-213, 2012. [DOI] [PubMed] [Google Scholar]
- 11. Brevet M, Chatelain D, Bartoli E, et al. . Colonic pseudolipomatosis: clinical, endoscopical and pathological features in nine cases. Gastroenterol Clin Biol 30: 9-13, 2006. [DOI] [PubMed] [Google Scholar]
- 12. Sheehan JF, Brynjolfsson G. Ulcerative colitis following hydrogen peroxide enema: case report and experimental production with transient emphysema of colonic wall and gas embolism. Lab Invest 9: 150-168, 1960. [PubMed] [Google Scholar]
- 13. Waring JP, Manne RK, Wadas DD, Sanowski RA. Mucosal pseudolipomatosis: an air pressure-related colonoscopy complication. Gastrointest Endosc 35: 93-94, 1989. [DOI] [PubMed] [Google Scholar]
- 14. Pineda Bonilla JJ, Diehl DL, Babameto GP, Smith RE. Massive hepatic portal venous gas and gastric pneumatosis secondary to gastric ischemia. Gastrointest Endosc 78: 540, 2013. [DOI] [PubMed] [Google Scholar]
- 15. Nehme F, Rowe K, Nassif I. Emphysematous gastritis with hepatic portal venous gas: a shift towards conservative management. BMJ Case Rep. Forthcoming. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Alvin M, Al Jalbout N. Emphysematous gastritis secondary to Sarcina ventriculi. BMJ Case Rep. Forthcoming. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Balasuriya HD, Abeysinghe J, Cocco N. Portal venous gas and pneumatosis coli in severe cytomegalovirus colitis. ANZ J Surg 88: 113-114, 2018. [DOI] [PubMed] [Google Scholar]
- 18. Angelino V, Volpicelli G, Cardinale L. Gastric emphysema after intubation. J Belg Soc Radiol 101: 3, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Griffiths PD. Burden of disease associated with human cytomegalovirus and prospects for elimination by universal immunisation. Lancet Infect Dis 12: 790-798, 2012. [DOI] [PubMed] [Google Scholar]
- 20. Maiorana A, Baccarini P, Foroni M, Bellini N, Giusti F. Human cytomegalovirus infection of the gastrointestinal tract in apparently immunocompetent patients. Hum Pathol 34: 1331-1336, 2003. [DOI] [PubMed] [Google Scholar]
- 21. Lancini D, Faddy HM, Flower R, Hogan C. Cytomegalovirus disease in immunocompetent adults. Med J Aust 201: 578-580, 2014. [DOI] [PubMed] [Google Scholar]
- 22. Mendy A, Gasana J, Vieira ER, Diallo H. Prospective study of cytomegalovirus seropositivity and risk of mortality from diabetes. Acta Diabetol 51: 723-729, 2014. [DOI] [PubMed] [Google Scholar]
- 23. Lee CY, Chen YH, Lu PL. Reactivated cytomegalovirus proctitis in an immunocompetent patient presenting as nosocomial diarrhea: a case report and literature review. BMC Infect Dis 17: 113, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]