Abstract
Inflammatory myofibroblastic tumor is a rare intermediate-grade tumor. We herein report the case of an 81-year-old man with rectal ulceration and abnormal retroperitoneal soft tissue with a high serum level of IgG4. The administration of prednisolone reduced the retroperitoneal lesion; however, the rectal ulceration expanded. Surgical resection was performed. A histopathological examination revealed proliferating spindle cells accompanied by inflammatory cells and plasma cells. Liver metastasis emerged two months after surgical resection, and the histology of the proliferating spindle cells sampled by a fine-needle biopsy was similar to that of the rectal tissue. The patient ultimately died of inflammatory myofibroblastic tumor.
Keywords: ALK, IgG4, inflammatory myofibroblastic tumor, liver metastasis, rectum
Introduction
Inflammatory myofibroblastic tumor (IMT) is a rare intermediate-grade soft tissue neoplasm. IMT mostly arises in the lung, mesentery, and omentum in children and young adults and is characterized by spindle-shaped myofibroblasts and the inflammatory infiltration of eosinophils, lymphocytes, and plasma cells. The majority of IMT cases show rearrangement of the anaplastic lymphoma kinase (ALK) gene and ALK-positive cells. Most IMT cases are cured by surgical resection, and recurrence and metastasis are extremely rare.
We herein report the case of 81-year-old man with IMT affecting the rectum and retroperitoneum and subsequently the liver. Immunohistochemistry did not show ALK-positive cells, and fluorescence in situ hybridization did not show ALK rearrangement in the lesions.
Case Report
The patient was an 81-year-old man who had undergone distal gastrectomy for advanced gastric cancer at 71 years of age and complained of a 2-month history of anal pain. Sigmoid colonoscopy revealed type 2-like ulceration in the rectum up to the pectinate line (Fig. 1).
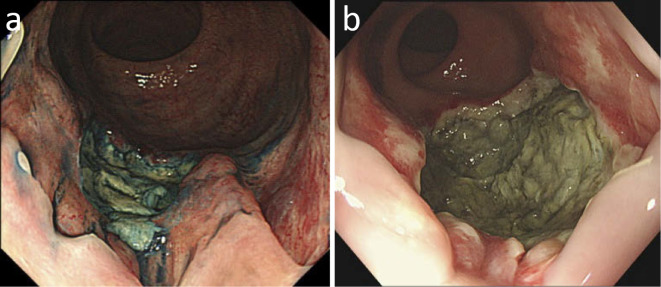
Figure 1.
(a) Colonoscopy with spraying of indigocarmine dye revealed an ulcerative lesion with an embankment located over the pectinate line. (b) A re-examination by endoscopy at three months after the initial examination in (a) demonstrated expanded and deepened ulceration.
Contrast-enhanced computed tomography (CE-CT) showed a mass 22 mm in size on the posterior rectal wall with an enhanced rim and central necrosis and slightly enhanced soft tissue around the abdominal, common iliac, and external iliac arteries, which was associated with right hydronephrosis (Fig. 2). Magnetic resonance imaging (MRI) demonstrated a rectal mass with low intensity on T1-weighted imaging (WI), intermediate intensity on T2-WI and high intensity on diffusion WI. 2-[18F]fluoro-2-deoxy-D-glucose (FDG)-positron emission tomography (PET)/CT revealed that the maximum standardized uptake value (SUVmax) of the rectal mass was 12.03, while that of the para-aortic soft tissue was 6.8 (Fig. 3).
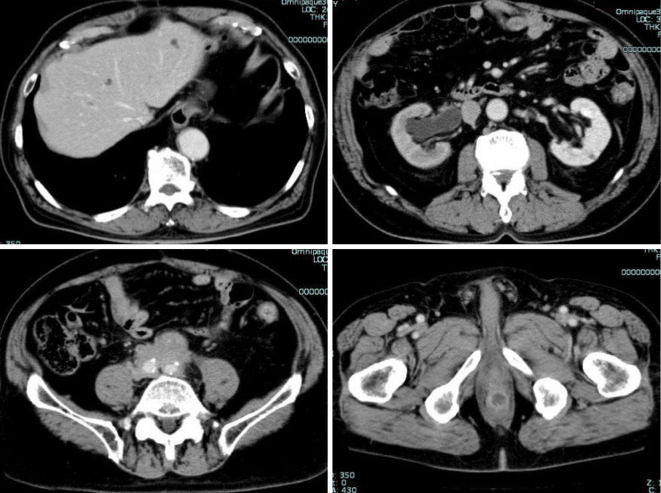
Figure 2.
CE-CT revealed cystic lesions in the liver (top left), hydronephrosis (top right), abnormal soft tissue around the common iliac artery (bottom left), and a mass 22 mm in size on the posterior rectal wall with an enhanced rim and central necrosis (bottom right).
Figure 3.
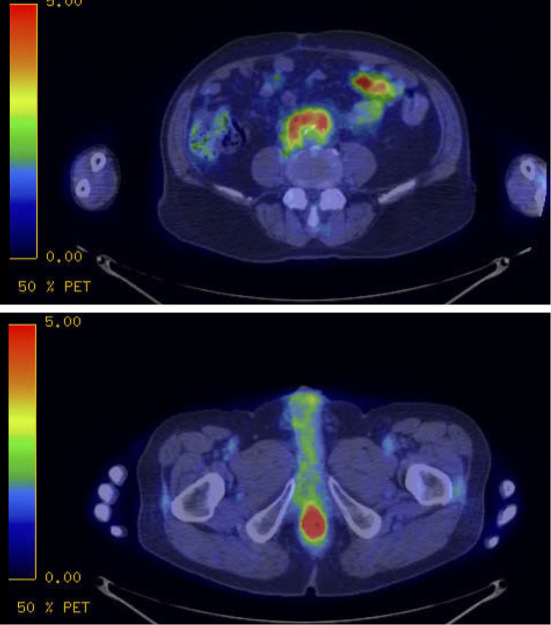
FDG-PET/CT revealed an increased uptake in the para-aortic soft tissue (top) and the rectal mass (bottom).
A fecal culture examination did not reveal any specific pathogenic bacteria. A laboratory examination showed a high serum level of IgG4 (309 mg/dL). Multiple tissue specimens of the rectal ulceration, which were obtained several times by an endoscopic biopsy, did not show any malignant findings. A few specimens showed spindle cells in the lamina propria mucosa to the muscularis mucosa that were immunohistochemically negative for c-kit, S-100, and CD34, while a few plasma cells were positive for IgG4. Although endoscopy showed that the ulceration had a slight expanding tendency over a few months, IgG4-related lesions of the rectum and retroperitoneal region were considered the most likely diagnosis (1, 2).
Prednisolone (40 mg per day) was administered as a diagnostic therapy and tapered by 10 mg and 5 mg once the dose had been reduced to 20 mg every 2 weeks. After tapering off predonisolone, CE-CT showed that the soft tissue lesion around the abdominal, common iliac and external iliac arteries had been reduced to half its initial size, while there was no change in the size of the rectal mass. Antibiotic treatment without oral food intake did not improve the ulceration, and as the patient was still suffering from uncontrolled anal pain, he decided to undergo surgical resection of the rectum and anus in order to receive a definitive diagnosis.
Miles' operation was performed, but the mass removal was incomplete because necrotic tissue was attached to the bladder. A histological examination of the resected specimen revealed proliferating spindle cells that were positive for vimentin, SMA, CD10 and β-catenin; slightly positive for cytokeratin AE1/AE3; and negative for EMA, c-kit, CD34, desmin, S100, ALK, HMB, STAT6, and WT-1, with positive staining with a Ki-67 of 30% at hot spots and 20-30 IgG4+ plasma cells per high-powered field (Fig. 4). The patient had an uncured wound site and underwent drainage of necrotic tissue after the operation.
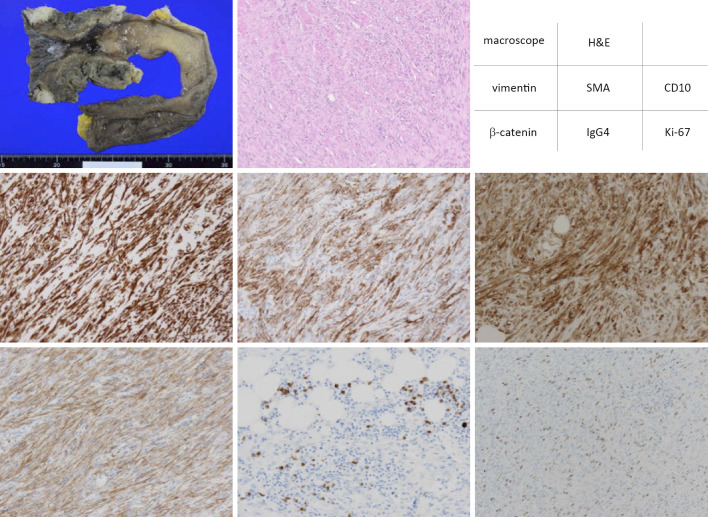
Figure 4.
Hematoxylin and Eosin (H&E) staining and immunohistochemistry staining were performed for the rectal ulceration in the resected specimen. The proliferating spindle cells were positively stained with vimentin, SMA, CD10 and β-catenin. Focal infiltration of IgG4-positive plasma cells was observed. About 30% of proliferating spindle cells were positively stained with Ki-67 in the hot spot. Vasculitis was not observed.
At two months after surgical resection, CE-CT demonstrated the emergence of a new cystic mass in the liver (Fig. 5). Primovist-enhanced MRI revealed that the mass had a low intensity on T1-WI and high intensity on T2-WI with an enhanced rim in the early period. Ultrasonography (US) showed no flow in the space-occupying lesion, and CE-US exhibited no enhancement in the mass.
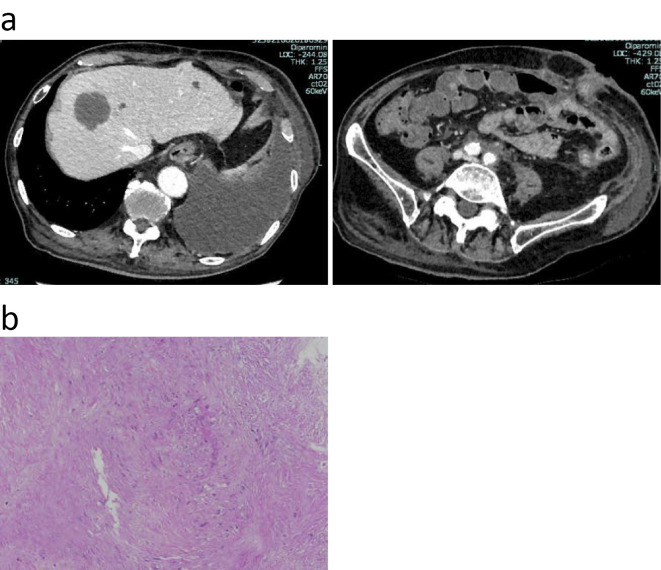
Figure 5.
(a) CE-CT demonstrated a new space-occupying lesion in the liver two months after surgery (top left). Soft tissue around the common iliac artery was significantly reduced (top right). (b) FNA was performed to obtain a tissue specimen of the space-occupying lesion in the liver. Hematoxylin and Eosin staining revealed proliferating spindle cells.
Since the lesion was presumed to represent a metastatic tumor or liver abscess, a fine-needle biopsy was performed. A histological examination revealed proliferating spindle cells similar to the tissue in the rectum. The final diagnosis of these lesions was IMT with liver metastasis. Fluorescence in situ hybridization (FISH) did not show any ALK rearrangement in this tumor (Fig. 6). The patient died of IMT at one month after the diagnosis of liver metastasis. The macroscopic findings at the autopsy showed that the remaining tumor had expanded into the bladder, prostate and pelvic floor as well as around the common iliac arteries. There were multiple nodules in the liver. The left lung had adhered to the parietal pleura and diaphragm, and the visceral pleura was thickened. Microscopically, spindle cells with mild to moderate nuclear atypia proliferated in storiform, and fascicular cells spread along with fibrosis as well as focal infiltration of lymphocytes, plasma cells, and neutrophils.
Figure 6.
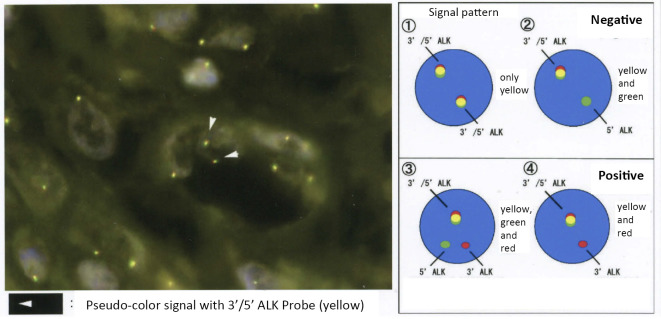
Fluorescence in situ hybridization (FISH) did not demonstrate ALK rearrangement in the tumor.
Discussion
The World Health Organization (WHO) defines IMT as an intermediate fibrocytic neoplasm that is histologically characterized by the presence of myofibroblastic spindle cells accompanied by chronic inflammatory infiltration with eosinophils, lymphocytes, and plasma cells. IMT predominantly affects the lung in children and young adults, and metastasis is rare. Several cases of adult-onset IMT in the large intestine have been reported (3-6), and there are few reports of patients with metastasis. Given the relatively old age of the present patient, this case was in the minority among IMT cases. To our knowledge, no case of IMT developing in the intestine with metastasis resulting in death due to the disease has been reported.
Since the radiological findings of IMT vary due to the presence of fibrosis, histological findings are needed to diagnose IMT (7). Although the biopsy for the rectal ulceration obtained more than 40 pieces in total over several sessions in our patient, only a few tissue specimens showed spindle cells. Therefore, since it was difficult to diagnose IMT of the rectum by endoscopy, surgical resection was necessary for its diagnosis.
The details concerning the tumorigenesis of IMT remain unclear; however, clonal rearrangement of the ALK gene on the short arm of chromosome 2 at 2p23 is frequently observed (8), with 50-60% of IMTs immunohistochemically positive for ALK (9). Coffin et al. reported that old age was likely to be associated with ALK negativity (10). The present case was also older with an ALK-negative status. Surgical resection is the most important first-line treatment for IMT and usually achieves a radical cure. However, in unresectable cases of ALK-translocated IMT, the ALK inhibitor crizotinib may be a useful treatment (11).
IgG4-related disease is a steroid-responsive multiorgan system disorder characterized by IgG4-positive plasma cell infiltration and fibrosis as well as high serum levels of IgG4 in many cases (1). IMT often shows IgG4-positive cells (12), and due to their similar histologies regarding plasma cell infiltration, IMT and IgG4-related disease need to be carefully differentiated. Zhu et al. reported that IMT showed less infiltration of lymphocytes and plasma cells, more cytological atypia, and fewer IgG4-positive cells than IgG4-related inflammatory pseudotumor (IPT) and that ALK-positive cells were specific to IMT (13). Further investigations will be needed in order to better diagnose IMT and IgG4-related disease.
We encountered an elderly man with IMT who initially presented with rectal ulceration and abnormal retroperitoneal soft tissue that required differentiation from IgG4-related disease. An endoscopic biopsy was insufficient to diagnose IMT by a histological examination. These lesions did not respond to steroid therapy, and radical resection was not completed. He was unable to undergo post-surgical therapy for the tumor due to tumor-associated necrosis. It was concluded that the newly emerged liver tumor was metastatic, given its histological similarity to the rectal mass. The patient died of IMT one month after the diagnosis of liver metastasis. We reported a rare case of IMT with an aggressive course.
The authors state that they have no Conflict of Interest (COI).
References
- 1. Kamisawa T, Zen Y, Pillai S, Stone JH. IgG4-related disease. Lancet 385: 1460-1471, 2015. [DOI] [PubMed] [Google Scholar]
- 2. Choi SB, Lim CH, Cha MG, Kang WK. IgG4-related disease of the rectum. Ann Surg Treat Res 90: 292-295, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Gurzu S, Bara T, Jung I. Inflammatory myofibroblastic tumor of the colon. J Clin Oncol 31: e155-e158, 2013. [DOI] [PubMed] [Google Scholar]
- 4. Kim EY, Lee IK, Lee YS, et al. Inflammatory myofibroblastic tumor in colon. J Korean Surg Soc 82: 45-49, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Spyrou I, Davakis S, Moris D, et al. Inflammatory pseudotumour of the colon. Ann R Coll Surg Engl 99: e151-e153, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Tanaka A, Hirabayashi K, Sadahiro S, et al. Inflammatory myofibroblastic tumor of the ascending colon in adults manifested by positive fecal occult blood test. Gastrointest Endosc 71: 214-216, 2010. [DOI] [PubMed] [Google Scholar]
- 7. Cantera JE, Alfaro MP, Rafart DC, et al. Inflammatory myofibroblastic tumours: a pictorial review. Insights Imaging 6: 85-96, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lovly CM, Gupta A, Lipson D, et al. Inflammatory myofibroblastic tumors harbor multiple potentially actionable kinase fusions. Cancer Discov 4: 889-895, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Cook JR, Dehner LP, Collins MH, et al. Anaplastic lymphoma kinase (ALK) expression in the inflammatory myofibroblastic tumor: a comparative immunohistochemical study. Am J Surg Pathol 25: 1364-1371, 2001. [DOI] [PubMed] [Google Scholar]
- 10. Coffin CM, Hornick JL, Fletcher CD. Inflammatory myofibroblastic tumor: comparison of clinicopathologic, histologic, and immunohistochemical features including ALK expression in atypical and aggressive cases. Am J Surg Pathol 31: 509-520, 2007. [DOI] [PubMed] [Google Scholar]
- 11. Butrynski JE, D'Adamo DR, Hornick JL, et al. Crizotinib in ALK-rearranged inflammatory myofibroblastic tumor. N Engl J Med 363: 1727-1733, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Saab ST, Hornick JL, Fletcher CD, Olson SJ, Coffin CM. IgG4 plasma cells in inflammatory myofibroblastic tumor: inflammatory marker or pathogenic link? Mod Pathol 24: 606-612, 2011. [DOI] [PubMed] [Google Scholar]
- 13. Zhu L, Li J, Liu C, et al. Pulmonary inflammatory myofibroblastic tumor versus IgG4-related inflammatory pseudotumor: differential diagnosis based on a case series. J Thorac Dis 9: 598-609, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]