Abstract
A 70-year-old man with insulinoma-associated antigen-2 autoantibodies developed diabetes mellitus (DM) without ketoacidosis after starting nivolumab to treat advanced gastric cancer. He subsequently exhibited preserved insulin-secretion capacity for over one year. Immune checkpoint inhibitors (ICIs) infrequently cause type 1 DM associated with the rapid loss of insulin secretion and ketoacidosis as an immune-related adverse event. ICIs may also cause non-insulin-dependent DM by inducing insulin resistance if there is islet autoantibody-related latent beta-cell dysfunction. The present case highlights the importance of testing blood glucose levels regularly to diagnose DM in patients treated with ICIs, even if they do not have diabetic ketoacidosis.
Keywords: diabetes mellitus, insulinoma-associated antigen-2 autoantibody, nivolumab, C-peptide, thyroid peroxidase autoantibody, gastric cancer
Introduction
Immune checkpoint inhibitors (ICIs) are a promising new class of anticancer drug (1). The clinical benefits afforded by ICIs can be accompanied by immune-related adverse events (IRAEs) that affect multiple organs, mainly the skin, gut, liver, lung, and endocrine tissues (2). Common endocrine IRAEs include thyroiditis and hypophysitis, while uncommon IRAEs include adrenalitis and type 1 diabetes mellitus (T1D) (3).
T1D is a heterogeneous, metabolic disease characterized by an immune-mediated progressive destruction of pancreatic beta cells, usually leading to insulin deficiency (4). Patients with T1D, including those with ICI-related T1D, often exhibit circulating autoantibodies against islet antigens, such as glutamic acid decarboxylase autoantibodies (GADA) and insulinoma-associated antigen-2 autoantibodies (IA-2Ab) (5, 6). The rate of beta-cell destruction in patients with spontaneous T1D varies widely from case to case (4), whereas almost all previously reported patients with ICI-related T1D exhibited rapid loss of insulin secretion within days or weeks and then presented with acute severe hyperglycemia and diabetic ketoacidosis (DKA) (6-15). The time from ICI administration to the T1D onset varies from case to case, ranging from within two weeks to greater than one year.
In addition to inducing rapid-onset T1D, ICI therapy may impair glucose metabolism by inducing insulin resistance associated with increased levels of circulating inflammatory markers (16). ICI therapy compromises glycemic control in patients with pre-existing type 2 diabetes mellitus (T2D) (6) and induces an increase in HbA1c levels in non-diabetic individuals (17).
We herein report an unusual case of an IA-2Ab-positive patient who developed non-insulin dependent DM without DKA shortly after starting ICI therapy with a programmed death-1 inhibitor nivolumab.
Case Report
A 70-year-old Japanese man with advanced gastric cancer was admitted to our hospital in February 2018 because of hyperglycemia 2 weeks after initiation of nivolumab therapy. He had a family history of T2D in his mother and his maternal aunt and uncle. The patient had never smoked and had no regular drinking habit. He had been diagnosed with essential hypertension and dyslipidemia at 64 years of age and started antihypertensive and anti-lipid medication. He had never been obese. His body weight (BW) had been 55 kg when he was 20 years old and remained around 58 kg from the age of 25 to 67 years old, until May 2015, when he developed fatigue and loss of appetite.
The patient visited his primary care doctor in August 2015, presenting with a marked BW loss (10 kg), low blood pressure, low serum cholesterol levels, and anemia. He discontinued antihypertensive and anti-lipid medications and was examined by the Department of Gastroenterology and Hepatology at our hospital the next day. An endoscopic examination revealed type 2 advanced cancer (18) at the antrum of the stomach. Computed tomography (CT) showed multiple swollen lymph nodes at the pyloric region, hepatoduodenal ligament, common hepatic artery, and the left gastric artery. The patient underwent distal gastrectomy with extended (D2) lymph-node dissection and Billroth II reconstruction (19) in September 2015. The histopathological features of the resected gastric cancer were consistent with those of differentiated tubular adenocarcinoma. His postoperative disease status was classified as stage IIIC (T4aN3M0) according to a gastric cancer staging system (18).
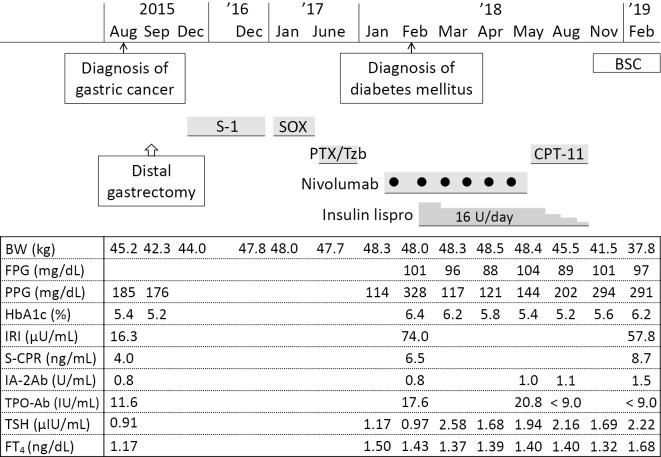
The patient had no disease progression during 9 courses (4 weeks each) of postoperative adjuvant therapy with 100 mg/day of an oral fluoropyrimidine anti-cancer agent, S-1, from December 2015 to November 2016 (Figure). However, CT performed in December 2016 revealed the appearance of hepatic hilar lymph node metastasis. He therefore underwent 7 courses of chemotherapy (100 mg/day S-1 for 2 weeks in combination with 150 mg oxaliplatin on day 1, every 3 weeks) (SOX regimen) from January 2017 to May 2017, which controlled his disease but was discontinued because of bone marrow suppression. As the resected tumor had overexpressed human epidermal growth factor receptor 2 (HER2), he underwent combination chemotherapy with weekly application of 80 mg paclitaxel (on days 1, 8, and 15, every 4 weeks) and tri-weekly application of 290 mg trastuzumab from June 2017 to December 2017.
Figure.
Clinical course of the patient. Blank columns indicate that the laboratory parameters were not measured. Fasting plasma glucose (FPG) and postprandial plasma glucose (PPG) levels were measured using either a blood test or a blood glucose self-monitoring device. All measurements of serum C-peptide immunoreactivity (S-CPR) and immunoreactive insulin (IRI) levels were performed on postprandial blood samples. BSC: best supportive care, BW: body weight, CPT-11: irinotecan, FT4: free thyroxine, HbA1c: glycated hemoglobin, IA-2Ab: insulinoma-associated antigen-2 autoantibody, PTX: paclitaxel, SOX: chemotherapy with oxaliplatin combined with S-1, TPO-Ab: thyroid peroxidase autoantibody, TSH: thyroid-stimulating hormone, Tzb: trastuzumab
CT performed in December 2017 showed enlarged hepatic hilar lymph node metastasis. The patient began third-line chemotherapy with 3 mg/kg nivolumab (145 mg) every 2 weeks in late January 2018. However, a blood test of samples obtained 2 hours after breakfast 2 weeks after the first administration of nivolumab revealed high postprandial plasma glucose (PPG) level (328 mg/dL) and glycated hemoglobin (HbA1c) (6.4%) (Table 1). The postprandial levels of serum C-peptide immunoreactivity (S-CPR) (6.5 ng/mL) and immunoreactive insulin (IRI) (74.0 μU/mL) were also high. The serum levels of acetoacetate (4 μmol/L) and 3-hydroxybutyrate (10 μmol/L) were normal, and a urinalysis was negative for ketone bodies. The patient was referred and admitted to the Department of Endocrinology and Metabolism of our hospital the same day.
Table 1.
Laboratory Findings (February 2018).
| Hematology | ||||
| Red blood cells | 418×104 | /μL | (435-555) | |
| Hemoglobin | 12.5 | g/dL | (13.7-16.8) | |
| Hematocrit | 39.3 | % | (40.7-50.1) | |
| White blood cells | 5,300 | /μL | (3,300-8,600) | |
| Platelets | 13.9×104 | /μL | (15.8-34.8) | |
| Blood chemistry | ||||
| Postprandial plasma glucose | 328 | mg/dL | (70-139) | |
| Postprandial S-CPR | 6.5 | ng/mL | ||
| Postprandial serum IRI | 74.0 | μU/mL | ||
| Glycated hemoglobin (HbA1c) | 6.4 | % | (4.6-6.2) | |
| Acetoacetate | 4 | μmol/L | (<55) | |
| 3-Hydroxybutyrate | 10 | μmol/L | (<85) | |
| Total protein | 7.1 | g/dL | (6.6-8.1) | |
| Albumin | 3.9 | g/dL | (4.1-5.1) | |
| Total cholesterol | 155 | mg/dL | (150-219) | |
| Triglycerides | 96 | mg/dL | (50-149) | |
| Aspartate aminotransferase | 22 | IU/L | (13-30) | |
| Alanine aminotransferase | 24 | IU/L | (10-42) | |
| Amylase | 101 | IU/L | (44-132) | |
| Creatine kinase | 44 | IU/L | (59-248) | |
| Urea nitrogen | 10.0 | mg/dL | (8.0-20.0) | |
| Creatinine | 0.63 | mg/dL | (0.65-1.07) | |
| Uric acid | 3.9 | mg/dL | (3.7-7.8) | |
| Sodium | 143 | mEq/L | (138-145) | |
| Potassium | 3.5 | mEq/L | (3.6-4.8) | |
| Chloride | 106 | mEq/L | (101-108) | |
| C-reactive protein | 0.15 | mg/dL | (0-0.14) | |
| Thyroid-stimulating hormone | 1.17 | μIU/mL | (0.5-5.0) | |
| Free triiodothyronine | 2.77 | pg/mL | (2.30-4.00) | |
| Free thyroxine | 1.50 | ng/dL | (0.90-1.70) | |
| Urinalysis | ||||
| Specific gravity | 1.027 | (1.005-1.020) | ||
| Glucose | Positive | |||
| Ketone bodies | Negative | |||
| Protein | Negative | |||
| Occult blood | Negative | |||
The reference range for each parameter is shown in parentheses.
The blood and urine samples were taken at 9 AM (2 h after breakfast).
IRI: immunoreactive insulin, S-CPR: serum C-peptide immunoreactivity
Upon admission, the patient had no noticeable hyperglycemic symptoms, such as thirst or polydipsia. The patient's height and BW were 168 cm and 48 kg, respectively, for a body mass index of 17.0 kg/m2. His body temperature, blood pressure, and pulse rate were 36.4℃, 126/67 mmHg, and 108 beats/min, respectively. The patient did not exhibit thyromegaly, superficial swollen lymph nodes in the neck or inguinal region, chest rales, heart murmurs, abdominal tenderness, or peripheral edema. Funduscopy detected no diabetic retinopathy.
The 75-g oral glucose tolerance test (OGTT) (Table 2) indicated a diagnosis of DM associated with impaired first-phase insulin secretion and postprandial hyperglycemia. The patient tested negative for islet autoantibodies, such as GADA (<5.0 IU/mL), islet cell antibodies (ICA) (a negative qualitative test result), insulin autoantibodies (<125.0 nU/mL), and zinc transporter 8 autoantibodies (<15.0 U/mL), but tested positive for IA-2Ab (0.8 U/mL; reference range, <0.4 U/mL) (Cosmic, Tokyo, Japan).
Table 2.
The 75-g Oral Glucose Tolerance Test Results (February 2018).
| Time (min) | |||||
|---|---|---|---|---|---|
| 0 | 30 | 60 | 120 | 180 | |
| Plasma glucose (mg/dL) | 101 | 256 | 336 | 228 | 117 |
| Serum immunoreactive insulin (μU/mL) | 3.7 | 22.0 | 77.2 | 37.8 | 12.4 |
| Serum C-peptide immunoreactivity (ng/mL) | 0.9 | 3.3 | 6.3 | 6.7 | 3.9 |
The patient had a low insulinogenic index (0.12; reference range: >0.40).
The patient tested negative for other organ-specific autoantibodies, such as anti-nuclear antibody, pituitary autoantibody, thyroglobulin autoantibody (Tg-Ab), thyroid-stimulating hormone binding inhibitor immunoglobulin (TBII), gastric parietal cell autoantibody, intrinsic factor autoantibody, and adrenocortical autoantibody, but tested positive for thyroid peroxidase autoantibody (TPO-Ab) (17.6 IU/mL; reference range, <16.0 IU/mL) (Roche Diagnostics, Tokyo, Japan). Blood chemistry revealed normal levels of serum free thyroxine (1.50 ng/dL) and thyroid-stimulating hormone (1.17 μIU/mL) (Table 1). Ultrasonography of the thyroid gland showed a normal-sized thyroid gland with no abnormal echogenicity or a tumor.
Human leukocyte antigen (HLA) typing revealed the presence of A*02:07/31:01, B*35:01/46:01, and C*01:02/03:03 class I genes and DRB1*04:03/08:03, DQB1*03:02/06:01, DQA1*01:03/03:01, and DPB1*02:01/02:02 class II genes.
The patient began insulin therapy (insulin lispro shortly before meals, 16 U/day) to correct postprandial hyperglycemia and was discharged on day 8 after admission.
A retrospective laboratory examination using frozen-stored blood samples obtained from the patient in August 2015 showed a negative result on the TPO-Ab test (9.0 IU/mL) but a positive result on the IA-2Ab test (0.8 U/mL; Figure).
Abdominal CT performed in April 2018 after the sixth administration of nivolumab detected no abnormalities in the spleen, pancreas, or kidneys but revealed metastasis to the liver. The patient discontinued nivolumab therapy. He subsequently received 4 courses of chemotherapy with weekly irinotecan 150 mg (on days 1, 8, and 15, every 4 weeks) from May 2018 to September 2018.
The metastatic cancer progressed, and his appetite and body weight decreased; the amount of insulin required was reduced, and insulin therapy was discontinued in September 2018.
The patient did not consent to continuing the anticancer therapy after he reviewed the potential benefits and side effects and instead chose best supportive care. In terms of his DM, self-monitoring of blood glucose levels revealed fasting and 2-h PPG levels of approximately 100 and 290 mg/dL, respectively. A blood test of samples obtained 2 hours after breakfast in February 2019 revealed high levels of PPG (291 mg/dL), postprandial S-CPR (8.7 ng/mL), and postprandial serum IRI (57.8 μU/mL) (Figure), indicating preservation of the endogenous insulin secretion capacity.
Discussion
An IA-2Ab-positive Japanese patient with advanced gastric cancer developed DM without DKA two weeks after initiation of nivolumab therapy. The 75-g OGTT revealed non-insulin-dependent DM associated with impaired first-phase insulin secretion and mild to moderate postprandial hyperglycemia (Table 2). Insulin therapy was initiated to correct the hyperglycemia. He subsequently exhibited a preserved insulin secretion capacity for more than one year (Figure). To our knowledge, this is the first reported case of an islet autoantibody-positive patient with non-insulin-dependent DM induced by ICI therapy.
Islet autoantibodies are commonly present at the onset of T1D and persist for varying durations after onset (5). Importantly, they precede the development of T1D by months to years. Several studies have also shown that the pre-existence of islet autoantibodies, including IA-2Ab, is predictive of ICI-related T1D (7-11). In the present case, the patient had exhibited circulating IA-2Ab 2.5 years earlier, and he developed DM shortly after initiation of nivolumab therapy, which he continued for 4 months (Figure). However, thereafter, he did not exhibit insulin deficiency for more than one year. It is therefore unlikely that our patient exhibited typical ICI-related T1D associated with the rapid loss of insulin secretion.
Slowly progressive T1D (SPT1D) is a subtype of T1D recognized in Japan (20) and is related to latent autoimmune diabetes in adults (LADA) in other countries (21). SPT1D is characterized by a gradual decrease in endogenous insulin secretion, the presence of islet autoantibodies, the absence of DKA at diabetes onset, and clinical features similar to those of T2D. The rate of decrease in insulin secretion varies from case to case, and many patients progress to insulin deficiency within 6 years after diabetes onset (20); however, some patients do not exhibit insulin deficiency even more than 10 years after DM onset because of extremely gradual or no decrease in insulin secretion capacity (22). Most SPT1D cases are GADA-positive, but GADA-negative/IA-2Ab-positive patients have been reported (23, 24); these patients tended to exhibit better-preserved insulin secretion than did GADA-positive patients. On the other hand, it has been suggested that in addition to inducing rapid-onset T1D as an IRAE, ICI therapy impairs glucose metabolism by inducing insulin resistance associated with inflammatory responses (16). ICI therapy induces an increase in HbA1c levels in non-diabetic individuals (17) but may not have any marked short-term impact on plasma glucose levels in such individuals (16). In the present case, our patient exhibited IA-2Ab and had clinical features of T2D, such as a medical history of essential hypertension and dyslipidemia and a family history of T2D. He developed DM with postprandial hyperglycemia without DKA shortly after initiation of nivolumab, with no decrease in the endogenous insulin secretion capacity as observed by the lack of any reduction in S-CPR or IRI levels compare to those values 2.5 years earlier (Figure). He subsequently exhibited a preserved insulin secretion capacity for more than one year. Thus, our patient basically exhibited SPT1D triggered by ICI-induced insulin resistance against a background of pre-existing, IA-2Ab-related subclinical beta-cell dysfunction.
ICI-related thyroiditis usually develops within weeks or months after starting ICI therapy, with or without the appearance of thyroid autoantibodies, such as Tg-Ab and TPO-Ab (25). Patients often present with painless thyroiditis associated with transient thyrotoxicosis, followed by a rapid transition to hypothyroidism that requires long-term levothyroxine replacement therapy. Our patient developed TPO-Ab positivity shortly after initiation of nivolumab therapy. However, he did not exhibit abnormal thyroid hormone levels nor ultrasonographic findings suggestive of thyroiditis, and his TPO titers normalized after discontinuation of nivolumab (Figure). These findings suggest that nivolumab generated transient thyroid autoimmunity without causing a clinically evident thyroid disorder.
In conclusion, we describe an IA-2Ab-positive patient who developed non-insulin-dependent DM associated with mild to moderate postprandial hyperglycemia shortly after initiation of nivolumab. Nivolumab therapy probably induced his DM by inducing insulin resistance against a background of pre-existing, IA-2Ab-related latent beta-cell dysfunction. The present case highlights the importance of testing plasma glucose levels (especially PPG levels) regularly to diagnose possible DM in patients treated with ICIs, even when such patients do not present with symptoms suggestive of severe hyperglycemia or DKA.
The authors state that they have no Conflict of Interest (COI).
Acknowledgement
The authors thank the clinical laboratory technicians of Uonuma Institute of Community Medicine, Niigata University Medical and Dental Hospital, for their valuable technical support.
References
- 1. Pennock GK, Chow LQ. The evolving role of immune checkpoint inhibitors in cancer treatment. Oncologist 20: 812-822, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Michot JM, Bigenwald C, Champiat S, et al. Immune-related adverse events with immune checkpoint blockade: a comprehensive review. Eur J Cancer 54: 139-148, 2016. [DOI] [PubMed] [Google Scholar]
- 3. Barroso-Sousa R, Barry WT, Garrido-Castro AC, et al. Incidence of endocrine dysfunction following the use of different immune checkpoint inhibitor regimens: a systematic review and meta-analysis. JAMA Oncol 4: 173-182, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. American Diabetes Association Diagnosis and classification of diabetes mellitus. Diabetes Care 37 (Suppl 1): S81-S90, 2014. [DOI] [PubMed] [Google Scholar]
- 5. Winter WE, Schatz DA. Autoimmune markers in diabetes. Clin Chem 57: 168-175, 2011. [DOI] [PubMed] [Google Scholar]
- 6. Kotwal A, Haddox C, Block M, Kudva YC. Immune checkpoint inhibitors: an emerging cause of insulin-dependent diabetes. BMJ Open Diabetes Res Care 7: e000591, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Gauci ML, Laly P, Vidal-Trecan T, et al. Autoimmune diabetes induced by PD-1 inhibitor-retrospective analysis and pathogenesis: a case report and literature review. Cancer Immunol Immunother 66: 1399-1410, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Godwin JL, Jaggi S, Sirisena I, et al. Nivolumab-induced autoimmune diabetes mellitus presenting as diabetic ketoacidosis in a patient with metastatic lung cancer. J Immunother Cancer 5: 40, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Clotman K, Janssens K, Specenier P, Weets I, De Block CEM. Programmed cell death-1 (PD-1) inhibitor induced type 1 diabetes mellitus: mini-review. J Clin Endocrinol Metab 103: 3144-3154, 2018. [DOI] [PubMed] [Google Scholar]
- 10. Stamatouli AM, Quandt Z, Perdigoto AL, et al. Collateral damage: insulin-dependent diabetes induced with checkpoint inhibitors. Diabetes 67: 1471-1480, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Galligan A, Xu W, Fourlanos S, et al. Diabetes associated with immune checkpoint inhibition: presentation and management challenges. Diabet Med 8: 1283-1290, 2018. [DOI] [PubMed] [Google Scholar]
- 12. Baden MY, Imagawa A, Abiru N, et al. Characteristics and clinical course of type 1 diabetes mellitus related to anti-programmed cell death-1 therapy. Diabetol Int 10: 58-66, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Okamoto M, Okamoto M, Gotoh K, et al. Fulminant type 1 diabetes mellitus with anti-programmed cell death-1 therapy. J Diabetes Investig 7: 915-918, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Usui Y, Udagawa H, Matsumoto S, et al. Association of serum anti-GAD antibody and HLA haplotypes with type 1 diabetes mellitus triggered by nivolumab in patients with non-small cell lung cancer. J Thorac Oncol 12: e41-e43, 2017. [DOI] [PubMed] [Google Scholar]
- 15. Sakurai K, Niitsuma S, Sato R, Takahashi K, Arihara Z. Painless thyroiditis and fulminant type 1 diabetes mellitus in a patient treated with an immune checkpoint inhibitor, nivolumab. Tohoku J Exp Med 244: 33-40, 2018. [DOI] [PubMed] [Google Scholar]
- 16. Gauci ML, Boudou P, Baroudjian B, et al. Occurrence of type 1 and type 2 diabetes in patients treated with immunotherapy (anti-PD-1 and/or anti-CTLA-4) for metastatic melanoma: a retrospective study. Cancer Immunol Immunother 67: 1197-1208, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Gauci ML, Boudou P, Squara PA, PATIO group, et al. Checkpoint inhibitor treatment induces an increase in HbA1c in nondiabetic patients. Melanoma Res 29: 328-332, 2019. [DOI] [PubMed] [Google Scholar]
- 18. Japanese Gastric Cancer Association Japanese classification of gastric carcinoma: 3rd English edition. Gastric Cancer 14: 101-112, 2011. [DOI] [PubMed] [Google Scholar]
- 19. Japanese Gastric Cancer Association Japanese gastric cancer treatment guidelines 2014 (ver. 4). Gastric Cancer 20: 1-19, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Tanaka S, Ohmori M, Awata T, et al. Diagnostic criteria for slowly progressive insulin-dependent (type 1) diabetes mellitus (SPIDDM) (2012): report by the Committee on Slowly Progressive Insulin-Dependent (Type 1) Diabetes Mellitus of the Japan Diabetes Society. Diabetol Int 6: 1-7, 2015. [Google Scholar]
- 21. Buzzetti R, Zampetti S, Maddaloni E. Adult-onset autoimmune diabetes: current knowledge and implications for management. Nat Rev Endocrinol 13: 674-686, 2017. [DOI] [PubMed] [Google Scholar]
- 22. Hoshina S, Miura J, Sugizawa E, Shimura K, Uchigata Y. Clinical features of slowly progressive type 1 (insulin-dependent) diabetes mellitus: a comparative study based on degree of obesity at diagnosis of diabetes. Diabetol Int 6: 91-97, 2015. [Google Scholar]
- 23. Miura J, Tei R, Sorimachi E, et al. A case of slowly progressive type 1 diabetes mellitus with past history of obesity and presence of IA-2 antibody but not GAD antibody. J Japan Diab Soc 51: 507-511, 2008. [Google Scholar]
- 24. Borg H, Gottsäter A, Fernlund P, Sundkvist G. A 12-year prospective study of the relationship between islet antibodies and beta-cell function at and after the diagnosis in patients with adult-onset diabetes. Diabetes 51: 1754-1762, 2002. [DOI] [PubMed] [Google Scholar]
- 25. Iyer PC, Cabanillas M, Waguespack SG, et al. Immune-related thyroiditis with immune checkpoint inhibitors. Thyroid 28: 1243-1251, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]