Abstract.
We describe hypoxemic pneumonia prevalence in outpatient and inpatient settings, in-hospital mortality, and clinical guideline performance for identifying hypoxemia in young infants in Malawi. In this retrospective analysis of a prospective cohort study, we investigate infants younger than 2 months participating in pneumonia surveillance at seven hospitals and 18 outpatient health centers in Malawi between 2011 and 2014. Logistic regression, multiple imputation with chained equations, and pattern mixture modeling were used to determine the association between peripheral capillary oxyhemoglobin saturation (SpO2) levels and hospital mortality. We describe outpatient clinician hospital referral recommendations based on clinical characteristics and SpO2 distributions. Among 1,879 analyzed cases, SpO2 < 90% was more prevalent among outpatient health center cases compared with hospitalized cases (22.6% versus 13.5%, 95% CI: 17.6–28.4% and 12.0–15.3%, respectively). A larger proportion of hospitalized infants had signs of respiratory distress compared with infants at health centers (67.7% versus 56.6%, P < 0.001) and most hospitalized infants were boys (56.7% versus 40.6%, P < 0.001). An SpO2 of 90–92% and < 90% was associated with similarly increased odds of in-hospital mortality (adjusted odds ratio [aOR]: 4.3 and 4.4, 95% CI: 1.7–11.1 and 1.8–10.5, respectively). Unrecorded, or unobtainable, SpO2 was highly associated with mortality (n = 127, aOR: 18.1; 95% CI: 7.6–42.8). Four of 22 (18%) infants at health centers who did not meet clinical referral criteria had an SpO2 ≤ 92%. Clinicians should consider hospital referral in young infants with a SpO2 ≤ 92%. Infants with unobtainable SpO2 readings should be considered a high-risk group, and hospital referral of these cases may be appropriate.
INTRODUCTION
In 2015 lower respiratory tract infections were the leading cause of death in children younger than 5 years.1 Despite an overall reduction in pneumonia-related mortality, infants younger than 2 months continue to have high case-fatality rates.1 The 2014 WHO Integrated Management of Childhood Illness (IMCI) guidelines used in outpatient health facilities in low- and middle-income countries (LMICs) state that in children aged 2–59 months, a peripheral capillary oxyhemoglobin saturation (SpO2) < 90% warrants hospital referral.2 The IMCI guidelines provide no referral guidance when pulse oximetry is used in infants younger than 2 months. Studies suggest that moderate hypoxemia (SpO2 90–92%) independently confers an increased risk of mortality in children aged 2–59 months.3,4 Most frontline facilities in LMICs do not have pulse oximeters, devices considered the standard for noninvasively measuring SpO2,5,6 and physical examination findings alone do not reliably detect hypoxemia.7–11 Previously, we reported that 68.7% of Malawian children aged 2–59 months with hypoxemia were misidentified as appropriate for outpatient care when screened using the 2014 WHO IMCI guidelines without pulse oximetry.12
We describe the epidemiology of hypoxemia in Malawian infants younger than 2 months with pneumonia using data collected prospectively during a pneumococcal conjugate vaccine effectiveness study.13 We determined 1) hypoxemia prevalence and 2) the association between inpatient mortality and different thresholds of hypoxemia, and 3) if clinical guidelines alone, without pulse oximetry, identified hypoxemic young infants requiring hospital referral.
MATERIALS AND METHODS
Setting.
Malawi is a landlocked country in sub-Saharan Africa with 17.6 million people, 51.5% of whom live on less than $1.25 per day.14,15 Community health workers, nurses, and nonphysician clinicians deliver health center services. More than 90% of hospital-based care is provided at government-operated district hospitals free of charge, and church-operated rural hospitals charge small fees and have limited inpatient capacity.16 Our study sites included 18 health centers, four rural hospitals, two district hospitals, and one regional tertiary referral hospital in Mchinji and Lilongwe districts at an altitude of 1,000–1,200 meters above sea level.
Data collection.
This is a retrospective secondary analysis of a prospective cohort study evaluating pneumococcal vaccine effectiveness. Full details of training methodology and quality assessment are published elsewhere.12,13 Using procedures that had been in place and embedded within routine clinical care since 2001, we conducted active pneumonia surveillance. Government healthcare providers were trained to use pulse oximeters using a standardized protocol and collected data during routine care of children aged 0–59 months with cough and/or difficulty breathing from October 2011 to June 2014. At district and referral hospitals, lay health workers, called vital sign assistants, were trained to ensure that vital signs, including SpO2, were recorded for each patient.17
Pulse oximeters outfitted with adult clip probes (Acare Technology, Xinzhuang, Taiwan, China), validated in Malawian infants younger than 2 months, were supplied by the Lifebox Foundation (London, England).18 Providers applied the probe to the great toe of children weighing < 10 kg or aged 2 years or younger. Hospital staff completed case report forms on all patients.19 All participating healthcare providers were retrained at the study’s midpoint (early 2013) by a pediatric pulmonologist and Malawi Ministry of Health (MoH) staff. Monthly supervisory visits by study staff consisted of direct observation of patient care, including pulse oximetry use, and review of case classification, management, and data record quality. Supervisory visits emphasized case identification and remediation of poorly performing sites. Weekly death audits at district and rural hospitals were performed to ensure case report forms agreed with facility death registers. Using this same dataset, we previously published a description of the epidemiology of hypoxemia and the performance of the 2014 WHO IMCI guidelines in identifying hypoxemia among Malawian children aged 2–59 months.12
Definitions.
Table 1 describes hospital referral criteria for pneumonia according to the 2000 Malawi MoH and 2014 WHO IMCI guidelines
Table 1.
Hospital referral criteria for infants aged 0–2 months with pneumonia
| 2000 Malawi Ministry of Health | 2014 WHO Integrated Management of Childhood Illness |
|---|---|
| Fast breathing* or chest wall indrawing or danger signs† | Fast breathing* or chest wall indrawing or poor feeding or convulsions or temperature ≥ 37.5°C or < 35.5°C or decreased movement |
* Fast breathing defined as a respiratory rate > 60 breaths per minute.
† Danger signs include central cyanosis, severe respiratory distress (grunting, head nodding, and nasal flaring), stridor in a calm child, a general WHO danger sign (inability to drink and/or breastfeed, lethargy or unconscious, or convulsions), and apnea.
Inclusion and exclusion criteria.
In the original vaccine effectiveness study, all children aged 0–59 months with cough and/or shortness of breath met the study inclusion criteria.13 In this study, only infants younger than 2 months were included. Those who received supplemental oxygen at the time pulse oximetry was measured were excluded. Cases with documented clinical findings of pneumonia (Table 1) were included in the primary analysis except when we evaluated if clinical guidelines identify all hypoxemic infants at outpatient health centers as hospital referral eligible.
Analysis.
Hypoxemia prevalence.
We compared the clinical characteristics and prevalence of hypoxemia at different thresholds among eligible infants at hospitals and outpatient health centers. Data were described using Student’s t-test for normally distributed data, Mann–Whitney U for nonparametric data, and chi-squared and Fisher’s exact tests for proportions.
Association between in-hospital mortality and hypoxemia.
Deaths were recorded only in the hospital dataset. If outcome data were missing, we assumed the child did not die in the hospital as we conducted weekly intensive death audits. We used multivariable logistic regression to measure the association between inpatient mortality and SpO2 at different thresholds. We defined the following as potential confounders a priori as they are parameters included in the WHO IMCI guidelines and based on our clinical experience: gender, degree of malnutrition defined by WHO weight-for-age z-score, lower chest wall indrawing (retractions), audible wheezing, severe respiratory distress (grunting, head nodding, or nasal flaring), tachypnea (respiratory rate ≥ 60, 70, or 80 breaths per minute), tachycardia (heart rate ≥ 160, 180, or 190 beats per minute), bradycardia (heart rate < 110 or 100 beats per minute), fever (temperature ≥ 37.5°C, as per the WHO guidelines), hypothermia (temperature < 35.5°C), and general WHO danger signs (central cyanosis, stridor, inability to drink, lethargy, or convulsions).20 We chose tachypnea, tachycardia, and bradycardia cutoffs based on population normative values and international guidelines published by the American Heart Association Pediatric Advanced Life Support program and the WHO.20 All potential confounders were investigated in bivariate analysis, and those with a P-value < 0.2 were included in the final model. In the primary analysis (Model 1), missing values for confounders were imputed, using multiple (10) imputations with chained equations (MICEs), and unrecorded SpO2 was included as a nominal group.21
We observed staff unsuccessfully attempt to obtain pulse oximetry measurements, with SpO2 left unrecorded in these cases, suggesting these data may be missing not at random (MNAR). To test our assumption, we conducted three sensitivity analyses. In Model 2, we created a composite binary pattern mixture variable to identify cases that had a high degree of missing confounders.22 First, we compared missing confounders by survival outcomes. Peripheral oxyhemoglobin saturation was excluded from consideration, as our goal was to determine if pulse oximetry readings were MNAR. Tachycardia and bradycardia were excluded, given that SpO2 and heart rates were both measured using pulse oximetry. Confounders were included in the composite binary pattern mixture variable if they were missing in more than 25% of the deceased or surviving cases. Cases were identified as having high instance of missing confounders if they were missing one of the identified confounders. We treated unrecorded SpO2 as a nominal group and used MICE to account for missing confounders but included a binary pattern mixture variable in the multivariable logistic regression model. In Model 3, unrecorded SpO2 and missing confounders were imputed using MICE. Model 4 was the same as Model 3 but included the pattern mixture variable.
Referral guidelines and pulse oximetry.
We evaluated the added value of using pulse oximetry among young infants at outpatient health centers in two ways. First, we determined the proportion of cases classified as appropriate for outpatient management based on Malawi MoH clinical guidelines alone (Table 1). Second, we retrospectively applied the 2014 WHO IMCI guidelines to compare the number of infants meeting the referral criteria, assuming perfect application of these guidelines, with and without the availability of pulse oximetry.
Stata software version 14.2. (StataCorp. 2015, College Station, TX) was used to perform all analyses.
Ethical approval.
The National Health Sciences Research Committee of Malawi (protocol: 941) and the Ethics Committee of University College London (protocol: 2006/002) provided ethical approval. Given that the study collected routine clinical data, the ethical boards did not require informed consent.
RESULTS
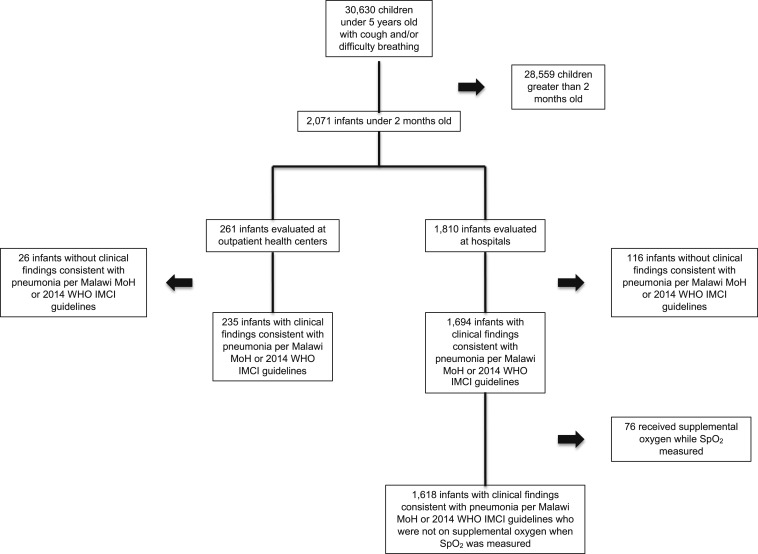
A total of 2,071 encounters were evaluated for study inclusion. Documented clinical findings warranting either referral or hospitalization as per the Malawi MoH guidelines were noted in 235 (90.0%) encounters at health centers and 1,694 (93.6%) encounters at hospitals. Of the hospitalized patients, 76 (4.5%) were excluded, leaving 1,618 hospitalized cases in the analysis (Figure 1).
Figure 1.
Cases included in analysis.
Hypoxemia prevalence.
A larger proportion of cases at outpatient health centers, compared with hospitals, had an SpO2 < 90% or had an unrecorded SpO2. Hospitalized infants, compared with those evaluated at health centers, had a higher prevalence of chest wall indrawing and severe respiratory distress. A larger proportion of hospitalized infants were male versus female (56.7% versus 40.6%, P < 0.001) (Table 2).
Table 2.
Observed clinical characteristics of infants admitted to hospitals or evaluated at health centers diagnosed with pneumonia based on the 2000 Malawi Ministry of Health guidelines
| Patient and clinical characteristics | Hospitals, N = 1,618 | Health centers, N = 235 | P-value |
|---|---|---|---|
| SpO2, median value, % (IQR) | 96 (92–98) | 94 (89–97) | < 0.001 |
| SpO2 > 92%, n (%, 95% CI) | 1,088 (67.2, 64.9–69.5) | 123 (52.3, 45.9–58.7) | < 0.001 |
| SpO2 90–92%, n (%, 95% CI) | 184 (11.4, 9.9–13.0) | 30 (12.8, 9.1–17.7) | |
| SpO2 < 90%, n (%, 95% CI) | 219 (13.5, 12.0–15.3) | 53 (22.6, 17.6–28.4) | |
| Unrecorded SpO2, n (%, 95% CI) | 127 (7.9, 6.6–9.3) | 29 (12.3, 8.7–17.2) | |
| Respiratory rate, median (IQR) | 64 (59–69) | 63 (61–67) | 0.50 |
| Missing, n (%) | 110 (6.8) | 16 (6.8) | |
| Weight (kg), mean (SD) | 4.4 (1.6) | 4.5 (1.5) | 0.23 |
| Missing, n (%) | 69 (4.3) | 12 (5.1) | |
| Male, n (%, 95% CI) | 918 (56.7, 54.3–59.1) | 113 (48.1, 41.7–54.5) | 0.07 |
| Female, n (%, 95% CI) | 657 (40.6, 38.2–43.0) | 105 (44.7, 38.4–51.1) | |
| Missing, n (%) | 43 (2.7) | 17 (7.2) | |
| WAZ > −2, n (%, 95% CI) | 1,256 (77.6, 75.5–79.6) | 175 (74.5, 68.5–79.6) | 0.37 |
| −3 < WAZ ≤ −2, n (%, 95% CI) | 172 (10.6, 9.2–12.2) | 26 (11.1, 7.6–15.8) | |
| WAZ ≤ −3, n (%, 95% CI) | 85 (5.3, 4.3–6.5) | 7 (3.0, 1.4–6.1) | |
| Missing, n (%) | 105 (6.5) | 27 (11.5) | |
| Chest wall indrawing, n (%, 95% CI) | 1,471 (90.9, 89.4–92.2) | 165 (70.2, 64.0–75.7) | < 0.001 |
| Missing, n (%) | 67 (4.1) | 7 (3.0) | |
| WHO danger signs,* n (%, 95% CI) | 377 (23.3, 21.3–25.4) | 63 (26.8, 21.5–32.9) | 0.64 |
| Missing, n (%) | 196 (12.1) | 10 (4.3) | |
| Severe respiratory distress,† n (%, 95% CI) | 1,096 (67.7, 65.4–70.0) | 133 (56.6, 50.2–62.8) | < 0.001 |
| Missing, n (%) | 147 (9.1) | 8 (3.4) |
IQR = interquartile range; SpO2 = peripheral capillary oxyhemoglobin saturation; WAZ = WHO weight-for-age z-score.
* Danger signs include central cyanosis, stridor in a calm child, apnea, or a general danger sign (inability to drink and/or breastfeed, lethargy or unconscious, or convulsions).
† Severe respiratory distress: grunting, head nodding, or nasal flaring.
At outpatient health centers, infants with an unrecorded SpO2 had a lower mean weight (3.9 versus 4.6 kg, P = 0.03) and a higher prevalence of WHO danger signs (44.8% versus 24.3%, P = 0.02) compared with those with a recorded SpO2 (Table 3). A larger proportion of hospitalized infants with an unrecorded SpO2 measurement, compared with hospitalized infants with a recorded SpO2, had WHO danger signs (33.1% versus 22.5%, P < 0.001), and a smaller proportion had fever (26.0% versus 32.7%, P = 0.02). Otherwise, clinical characteristics of patients with recorded versus unrecorded SpO2 measurements were not statistically different. Notably, a large proportion of cases with unrecorded SpO2 measurements were missing other covariates (Table 3).
Table 3.
Clinical characteristics of infants with pneumonia categorized by successful pulse oximetry measurement and clinical setting
| Variable | Hospitals (n = 1,618) | Health centers (n = 235) | ||||
|---|---|---|---|---|---|---|
| Successful, n (%) | Unsuccessful, n (%) | P | Successful, n (%) | Unsuccessful, n (%) | P | |
| Peripheral capillary oxyhemoglobin saturation measurement | 1,491 (92.2) | 127 (7.8) | – | 206 (87.7) | 29 (12.3) | – |
| Male | 855 (57.3, 54.8–59.8) | 63 (49.6, 41.0–58.3) | 0.31 | 103 (50.0, 43.2–56.8) | 10 (34.5, 19.4–53.5) | 0.07 |
| Female | 603 (40.4, 38.0–43.0) | 54 (42.5, 34.2–51.3) | 87 (42.2, 35.6–49.1) | 18 (62.1, 43.2–77.9) | ||
| Missing | 33 (2.2) | 10 (7.9) | 16 (7.8) | 1 (3.5) | ||
| Weight in kg, mean (SD) | 4.4 (1.7) | 4.3 (1.3) | 0.80 | 4.6 (1.5) | 3.9 (0.9) | 0.03 |
| Missing | 33 (2.2) | 36 (28.4) | 6 (2.9) | 6 (20.7) | ||
| Respiratory rate, median (interquartile range) | 64 (59–68) | 65 (59–71) | 0.61 | 63 (60–67) | 64 (62–67) | 0.65 |
| Missing | 39 (2.6) | 71 (55.9) | 8 (3.9) | 8 (27.6) | ||
| Heart rate ≥ 190 | 89 (6.0, 4.9–7.3) | 0 | 0.16 | 2 (1.0, 0.2–3.8) | 0 | 0.66 |
| Heart rate < 110 | 67 (4.5, 3.6–5.7) | 2 (1.6, 0.4–6.1) | 96 (46.6, 39.8–53.5) | 1 (3.5, 0.5–21.6) | ||
| Missing | 24 (1.6) | 113 (89.0) | 1 (0.5) | 25 (86.2) | ||
| Fever | 488 (32.7, 30.4–35.2) | 33 (26.0, 19.1–34.3) | 0.02 | 84 (40.8, 34.2–47.7) | 9 (31.3, 16.8–50.2) | 0.29 |
| Hypothermia | 29 (2.0, 1.4–2.8) | 4 (3.2, 1.2–8.1) | 5 (2.4, 1.0–5.7) | 2 (6.9, 1.7–24.4) | ||
| Missing | 26 (1.7) | 50 (39.4) | 12 (5.8) | 6 (20.7) | ||
| WHO danger signs* | 335 (22.5, 20.4–24.7) | 42 (33.1, 25.4–41.7) | < 0.001 | 50 (24.3, 18.9–30.6) | 13 (44.8, 27.8–63.2) | 0.02 |
| Missing | 163 (10.9) | 33 (26.0) | 9 (4.4) | 1 (3.5) | ||
| Severe respiratory distress† | 1,015 (68.1, 65.7–70.4) | 81 (63.8, 55.0–71.7) | 0.68 | 113 (54.9, 48.0–61.6) | 20 (69.0, 49.8–83.2) | 0.23 |
| Missing | 117 (7.8) | 20 (15.8) | 8 (3.9) | 0 | ||
| Chest wall indrawing | 1,349 (90.5, 88.9–91.9) | 122 (96.1, 90.9–98.4) | 0.40 | 142 (68.9, 62.2–74.9) | 23 (79.3, 60.5–90.6) | 0.37 |
| Missing | 66 (4.4) | 1 (0.8) | 7 (3.4) | 0 | ||
| 2014 integrated management of childhood illness referral eligible‡ | 1,486 (99.7) | 127 (100.0) | 1.00 | 203 (98.5) | 28 (96.6) | 0.77 |
| Missing | 5 (0.3) | 0 | 3 (1.5) | 1 (3.5) | ||
* WHO danger signs: central cyanosis, stridor in a calm child, a general danger sign (inability to drink and/or breastfeed, lethargy or unconscious, and convulsions), apnea.
† Severe respiratory distress: grunting, head nodding, and nasal flaring.
‡ WHO Integrated Management of Childhood Illness guidelines.
Association between in-hospital mortality and hypoxemia.
The overall hospital case fatality rate was 3.6% (n = 59). Generally, death cases had a higher prevalence of missing data (Supplemental Material 1). Table 4 describes the bivariate analysis for confounder selection and the adjusted odds (aOR) for mortality using logistic regression with MICE and pattern mixture modeling. In Model 1, infants with an SpO2 90–92% or an SpO2 < 90% had a similarly increased aOR for in-hospital mortality at 4.3 (95% CI: 1.7–11.1) and 4.4 (95% CI: 1.8–10.5), respectively. Children with an unrecorded SpO2 had the highest case fatality rate at 18.9% (95% CI: 13.0–26.7%) and an aOR for in-hospital mortality of 18.1 (95% CI: 7.6, 42.8). Model 2, using a binary pattern mixture composite variable for missing danger signs or malnutrition, produced similar results to Model 1, noting the wide CIs for each. Models 3 and 4, imputing unrecorded SpO2 using MICE, produced aOR for mortality associated with an SpO2 90–92% and an SpO2 < 90% similar to those of Models 1 and 2. There was a modest increase in the associated odds for mortality attributed to the pattern mixture variable in Model 4, compared with Model 2, at 3.7 versus 2.4 (aOR 95% CI: 1.2, 4.7 and 1.9, 7.2, respectively).
Table 4.
Predictors of in-hospital mortality of young infants aged 0–2 months with pneumonia defined as per the 2000 Malawi Ministry of Health guidelines
| Variable | Bivariate | Model 1† (n = 1,618), aOR (95% CI) | Model 2‡, (n = 1,618), aOR (95% CI) | Model 3§ (n = 1,618), aOR (95% CI) | Model 4‖ (n = 1,618), aOR (95% CI) | |||
|---|---|---|---|---|---|---|---|---|
| Total cases, N = 1,618 | Died, n (%), 59 (3.6) | Survived, n (%), 1,559 (96.4) | OR (95% CI) | P | ||||
| SpO2 93–100% | 10 (0.9) | 1,078 (99.1) | 1.0 | – | 1.0 | 1.0 | 1.0 | 1.0 |
| SpO2 90–92% | 10 (5.4) | 174 (94.6) | 6.2 (2.5–15.1) | < 0.001 | 4.3 (1.7–11.1) | 4.4 (1.7–11.3) | 3.3 (1.4–7.8) | 3.3 (1.4–7.7) |
| SpO2 < 90% | 15 (6.8) | 204 (93.2) | 7.9 (3.5–17.9) | – | 4.4 (1.8–10.5) | 4.4 (1.8–10.7) | 4.2 (1.8–9.8) | 4.5 (2.0–10.2) |
| Unrecorded SpO2 | 24 (18.9) | 103 (81.1) | 25.1 (11.7–54.0) | – | 18.1 (7.6–42.8) | 12.9 (4.8–34.5) | – | – |
| Male | 27 (2.9) | 891 (97.1) | 1.0 | – | 1.0 | 1.0 | 1.0 | 1.0 |
| Female | 28 (4.3) | 629 (95.7) | 1.5 (0.9–2.5) | 0.16 | 1.2 (0.7–2.5) | 1.3 (0.7–2.3) | 1.2 (0.7–2.3) | 1.2 (0.6–2.3) |
| Missing | 4 (9.3) | 39 (90.7) | – | – | – | – | – | – |
| WAZ > −2 | 24 (1.9) | 1,232 (98.1) | 1.0 | – | 1.0 | 1.0 | 1.0 | 1.0 |
| −3 < WAZ ≤ −2 | 8 (4.7) | 164 (95.3) | 2.5 (1.1–5.7) | < 0.001 | 2.3 (1.0–5.4) | 2.2 (1.0–5.2) | 2.5 (1.1–5.6) | 2.3 (1.0–5.6) |
| WAZ ≤ −3 | 12 (14.1) | 73 (85.9) | 8.4 (4.1–17.5) | – | 6.6 (2.6–16.7) | 6.3 (2.6–15.4) | 6.4 (2.5–16.0) | 6.5 (2.3–18.6) |
| Missing | 15 (14.3) | 90 (85.7) | – | – | – | – | – | – |
| Respiratory rate < 60 | 11 (2.8) | 379 (97.2) | 1.0 | – | – | – | – | – |
| Respiratory rate ≥ 60 | 26 (2.3) | 1,092 (97.7) | 0.8 (0.4–1.7) | 0.59 | – | – | – | – |
| Missing | 22 (20.0) | 88 (80.0) | – | – | ||||
| Respiratory rate < 70 | 28 (2.4) | 1,152 (97.6) | 1.0 | – | – | |||
| Respiratory rate ≥ 70 | 9 (2.7) | 319 (97.3) | 1.2 (0.5–2.5) | 0.70 | – | |||
| Missing | 22 (20.0) | 88 (80.0) | – | – | – | – | – | – |
| Respiratory rate < 80 | 32 (2.3) | 1,352 (97.7) | 1.0 | – | ||||
| Respiratory rate ≥ 80 | 5 (4.0) | 119 (96.0) | 1.8 (0.7–4.6) | 0.24 | ||||
| Missing | 22 (20.0) | 88 (80.0) | – | – | ||||
| Heart rate > 110 and < 190 | 27 (2.0) | 1,297 (98.0) | 1.0 | – | 1.0 | 1.0 | 1.0 | 1.0 |
| Heart rate ≥ 190 | 5 (5.6) | 84 (94.4) | 32.5 (0.9–6.4) | 0.06 | 3.1 (1.1–8.9) | 3.0 (0.9–10.0) | 3.5 (1.2–10.9) | 3.4 (1.2–9.3) |
| Heart rate < 110 | 7 (10.1) | 62 (89.9) | 5.0 (2.1–11.9) | < 0.001 | 3.6 (1.4–9.7) | 4.2 (1.710.6) | 3.5 (1.2–10.5) | 4.5 (1.6–12.8) |
| Missing | 21 (15.3) | 116 (84.7) | – | – | – | – | – | – |
| Normal temperature | 20 (2.0) | 968 (98.0) | 1.0 | – | 1.0 | 1.0 | 1.0 | 1.0 |
| Fever | 19 (3.6) | 502 (96.4) | 1.8 (1.0–3.5) | 0.06 | 1.6 (0.9–3.1) | 1.6 (0.8–3.2) | 1.7 (0.8–3.3) | 1.7 (0.83.7) |
| Hypothermia | 8 (24.2) | 25 (75.8) | 15.5 (6.2–38.5 | < 0.001 | 6.1 (1.8–20.8) | 5.4 (1.717.4) | 6.8 (2.3–20.4) | 6.1 (1.9-19.2 |
| Missing | 12 (15.8) | 64 (84.2) | – | – | – | – | – | – |
| No danger signs | 15 (1.4) | 1,030 (98.6) | 1.0 | – | 1.0 | 1.0 | 1.0 | 1.0 |
| WHO danger signs | 26 (6.9) | 351 (93.1) | 5.1 (2.7–9.7) | < 0.001 | 2.7 (1.3–5.5) | 2.4 (1.2–4.7) | 2.8 (1.4–5.7) | 2.4 (1.2–4.9) |
| Cyanosis | 3 (8.3) | 33 (91.7) | 3.4 (1.0–11.6) | 0.07 | – | – | – | – |
| Stridor | 2 (3.5) | 55 (96.5) | 1.3 (0.3–5.7) | 0.46 | – | – | – | – |
| Apnea | 7 (5.9) | 111 (94.1) | 2.5 (1.1–5.9) | 0.03 | – | – | – | – |
| Inability to drink | 17 (8.6) | 180 (91.4) | 5.2 (2.7–10.0) | < 0.001 | – | – | – | – |
| Convulsions | 8 (23.5) | 26 (76.5) | 13.2 (5.5–31.5) | < 0.001 | – | – | – | – |
| Lethargy | 4 (5.6) | 67 (94.4) | 2.2 (0.7–6.2) | 0.14 | – | – | – | – |
| Missing | 18 (9.2) | 178 (90.8) | – | – | – | – | – | – |
| No severe respiratory distress | 4 (1.1) | 371 (98.9) | 1.0 | – | 1.0 | 1.0 | 1.0 | 1.0 |
| Severe respiratory distress | 42 (3.8) | 1,054 (96.2) | 3.0 (1.2–7.7) | 0.008 | 3.7 (1.2–11.7) | 2.9 (0.9–9.4) | 2.3 (0.7–7.3) | 2.4 (0.8–7.4) |
| Grunting | 24 (5.1) | 446 (94.9) | 2.8 (1.5–5.2) | 0.001 | – | – | – | – |
| Nasal flaring | 33 (3.6) | 872 (96.4) | 1.7 (0.9–3.3) | 0.12 | – | – | – | – |
| Head nodding | 13 (3.1) | 412 (96.9) | 1.2 (0.6–2.3) | 0.67 | – | – | – | – |
| Missing | 13 (8.8) | 134 (91.2) | – | – | – | – | – | – |
| Chest wall indrawing | 55 (3.7) | 1,416 (96.3) | 1.0 (0.3–3.3) | 0.59 | – | – | – | – |
| Missing | 1 (1.5) | 66 (98.5) | – | – | – | – | – | – |
| Wheezing | 8 (3.0 | 260 (97.0) | 1.1 (0.5–2.4) | 0.81 | ||||
| Missing | 21 (8.5) | 226 (91.5) | – | – | – | – | – | – |
| Low amount of missing data* | 32 (2.4) | 1,327 (97.6) | – | – | – | 1.0 | – | 1.0 |
| High amount of missing data | 27 (10.4) | 32 (89.6) | – | – | – | 2.4 (1.2–4.7) | – | 3.7 (1.9–7.2) |
aOR = adjusted odds ratio; SpO2 = peripheral capillary oxyhemoglobin saturation; WAZ = weight-for-age z-score. Statistically significant findings are in bold.
* Pattern mixture variable, binary variable in which either the degree of malnutrition or the presence of danger signs was not recorded.
† Logistic regression, unrecorded pulse oximetry reading treated as a nominal category.
‡ Logistic regression, unrecorded pulse oximetry reading treated as a nominal category, analysis includes a binary missing data variable (bottom of the table).
§ Logistic regression, unrecorded pulse oximetry reading treated as missing data and imputed using MICE.
‖ Logistic regression, unrecorded pulse oximetry reading treated as missing data and imputed using MICE, analysis includes a binary missing data variable (bottom of the table).
Referral guidelines and pulse oximetry.
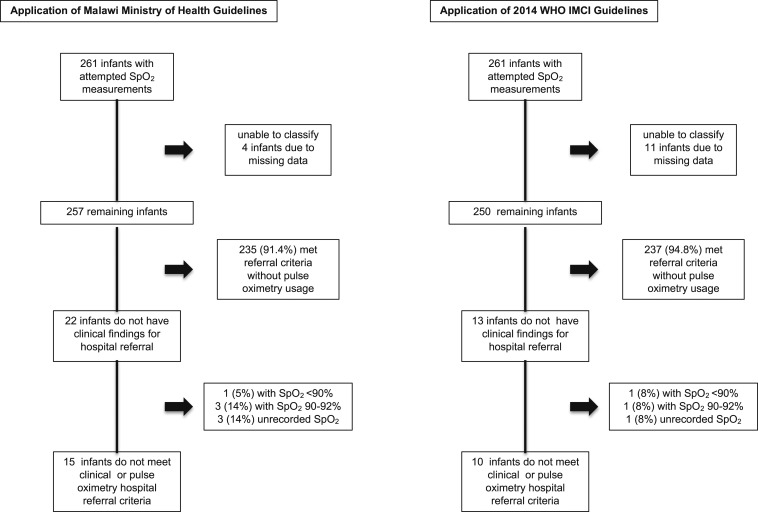
At outpatient health centers, a similar proportion of infants were eligible for hospital referral based on the Malawi MoH (n = 235/261) and 2014 WHO IMCI (n = 237/261) guidelines when retrospectively assuming pulse oximetry was unavailable (90.0% versus 90.8%, P = 0.88). Four cases had too much missing data to determine if they met the Malawi MoH referral guidelines and were excluded from the rest of the analyses. The Malawi MoH clinical guidelines, without pulse oximetry, classified 22/257 (8.6%) infants as appropriate for outpatient management. One (5%) of these 22 infants had an SpO2 < 90%, three (14%) had an SpO2 of 90–92%, and pulse oximetry was unrecorded for three (14%) (Figure 2). Application of the Malawi MoH guidelines without pulse oximetry misclassified 2% (1/54) with an SpO2 < 90% and 5% (4/87) of infants with an SpO2 ≤ 92% as appropriate for outpatient management (Supplemental Material 2). Comparatively, the 2014 WHO IMCI guidelines misclassified 2% (1/54) with an SpO2 < 90% and 1/87 (1%) of infants with an SpO2 ≤ 92% (Supplemental Material 3). Of the 13 infants who did not meet hospital referral clinical criteria, one (8%) had an SpO2 < 90% and one (8%) had an SpO2 ≤ 92%.
Figure 2.
Performance of the Malawi Ministry of Health and 2014 WHO Integrated Management of Childhood Illness guidelines at identifying hypoxemic infants with cough and/or shortness of breath.
DISCUSSION
We analyzed 1,879 cases of Malawian infants younger than 2 months presenting to outpatient health centers and hospitals with WHO-defined pneumonia and found that infants with an SpO2 of 90–92% had a near-equal aOR for inpatient mortality compared with those with an SpO2 < 90%. This is not surprising as SpO2 in some cases may overestimate arterial blood oxygen saturation (SaO2).23 An SpO2 of 89–92% on average correlates with an SaO2 of 90% and partial pressure of oxygen of 60 mmHg in an otherwise healthy child.24 The 2013 WHO Pocket Book of Hospital Care, used in hospital settings, recommends starting supplemental oxygen in young infants with an SpO2 < 90% or those with central cyanosis or severe respiratory distress.25 We found a high (24.9–35.4%) prevalence of clinically meaningful hypoxemia (SpO2 ≤ 92%) similar to other studies that defined hypoxemia as an SpO2 < 90%.26–28 Among the small proportion of infants with upper respiratory tract infection symptoms who did not meet the 2014 WHO IMCI hospital referral criteria, 15% were still hypoxemic (SpO2 ≤ 92%) and, therefore, at high risk of mortality. The WHO IMCI guidelines, used by health workers at outpatient health centers, do not provide any referral guidance when SpO2 is measured in infants younger than 2 months but state that older children aged 2–59 months with an SpO2 < 90%, independent of other examination findings, should be referred to hospitals.2 Without more specific guidance from the WHO on the application of SpO2 measurements among young infants, clinicians may or may not apply these recommendations to infants younger than 2 months. Our findings generate uncertainty around the generalizability of the current WHO guidelines that lack pulse oximetry recommendations for young infants in Malawi and potentially other LMICs.
Notably, we found a markedly elevated aOR of mortality associated with an unrecorded SpO2 which was robust to multiple modeling approaches. These infants comprised 8% of hospitalized cases and accounted for 41% of all deaths. Both health center outpatients and hospital inpatients had a higher prevalence of WHO danger signs among infants with an unrecorded SpO2. One explanation for the associated increased mortality, based on direct observations, is that SpO2 was not documented after attempted but failed measurements. A high proportion of these cases were likely infants with circulatory compromise due to shock and poor peripheral perfusion that rendered obtaining pulse oximetry readings difficult. Indicators of poor perfusion such as capillary refill time, cool extremities, or history of reduced frequency of urination, which are included in the WHO’s Emergency Triage and Treatment guidelines,29 are not included in the WHO IMCI guidelines. A study conducted in the United States found that the capillary refill time and cool extremities had moderate to poor inter-rater reliability.30 We recommend further exploration on the feasibility and reliability of healthcare providers collecting these signs, especially in the context of the inability to obtain SpO2 measurement.
Like many LMICs, pulse oximetry and supplemental oxygen availability and uptake are poor in Malawi, especially in outpatient health centers.5,6,31 This situation is particularly disturbing, as the prevalence of hypoxemia, regardless of the threshold used, was higher among outpatients. A retrospective analysis of the Malawi Child Lung Health Program from 2001 to 2012 found that among infants younger than 2 months, referral from a health center was an independent mortality risk factor.16 This finding may reflect discrepancies among care-seeking behaviors of communities living near hospitals, challenges in the referral pathway, or lack of oxygen and antibiotic availability at health centers. Some of these children may have died en route to the hospital or families may not go to the hospital despite referral. The use of pulse oximetry in outpatient settings has the potential to strengthen multiple aspects of young infant primary care, including improving the identification of true high-risk cases, strengthen referral recommendations by healthcare providers currently relying solely on nonspecific clinical signs such as respiratory rate, increase the uptake of referral recommendations by families, and ultimately improve clinical care through refinement of hospital transfer processes and supplemental oxygen prescribing practices. Further research will be needed to more fully evaluate pulse oximetry effects on the primary care health system, especially as it pertains to young infants.
A large proportion of infants evaluated at health centers met the hospital referral criteria, even without pulse oximetry usage because of the high prevalence of tachypnea. Young infants are at high risk of rapid decompensation from serious bacterial infections including pneumonia. Given the relative high prevalence of serious bacterial infections in this age-group, the WHO guidelines set a very low threshold for hospital referral for expert evaluation or, if indicated, inpatient admission. The Malawi MoH clinical guidelines misclassified 5% of infants with cough and/or shortness of breath with an SpO2 ≤ 92% as appropriate for outpatient management. Implementation of the 2014 WHO IMCI guidelines would reduce this proportion by half. If healthcare workers were to adhere to the IMCI guidelines, very few infants with an SpO2 ≤ 92% would be discharged home. This finding is in contrast with our previous finding among Malawian children aged 2–59 months that 68.7% with an SpO2 < 90% were misclassified by the clinical guidelines as appropriate for outpatient care.12 Our dataset does not explicitly indicate how healthcare workers may have been influenced by either a “normal” or “abnormal” SpO2, and it remains unclear whether busy healthcare workers may over-rely on SpO2 reading, compared with the clinical criteria, when making referral decisions. Further research on whether “normal” SpO2 measurements among children otherwise clinically eligible for referral may detrimentally affect referral decision-making.
Limitations.
Understanding practices and outcomes in routine clinical care is essential to informing guideline and program development. Although a primary strength of our study is that these data reflect real-life clinical practices in Malawi, programmatic data have some limitations, most notably missing data. Death cases had a high degree of missing data on other variables, limiting the certainty of our conclusions. It is possible that the need to stabilize these critically ill patients precluded hospital staff’s recording of clinical data at the time of admission. We found evidence that many of these measurements were MNAR, as inclusion of the pattern mixture variable in the logistic regression model still resulted in an unrecorded SpO2 associated with an increased aOR for in-hospital mortality. We did not conduct home-based follow-up to assess if families followed referral recommendations, and infants were later taken to higher level health facilities or returned for re-evaluation, developed more severe disease, or died. Hemoglobin and malaria testing were not routinely documented as part of the pneumonia surveillance program. Both would be useful in assessing illness etiology and the former to determine inherently low oxygen-carrying capacity. Finally, our conclusions regarding the proportion of hypoxemic infants with upper respiratory infection symptoms missed by clinical guidelines alone are limited by the small sample size and should be interpreted within this context.
CONCLUSION
This observational study reflects real-world pneumonia care practices for young infants at outpatient health centers and hospitals by healthcare providers in a nonclinical trial setting in Malawi. Our findings pose several important questions for further research and on areas of focus for guideline revisions with regard to use of pulse oximetry and its feasibility of implementation and operationalization. Given that this was an observational study, with missing data, caution should be taken in the interpretation of these results. Nonetheless, our findings remain compelling, particularly when considering the strong association of an SpO2 of 90–92% and < 90% with hospital mortality, along with the high prevalence of hypoxemia in outpatient settings. Importantly, our findings, if replicated elsewhere, provide evidence supporting revision of the WHO guidelines to recommend hospital referral of young infants at outpatient centers with an SpO2 ≤ 92%, or for whom a pulse oximetry reading is unobtainable.
Supplemental materials
Acknowledgments:
We thank Rashid Deula for leading efforts in data collection and curation of the original study. We thank the Republic of Malawi Ministry of Health and the communities and traditional authorities of Lilongwe and Mchinji districts of Malawi. This work is the direct result of the dedication and hard work of our field and data collection staff. We also thank the editorial assistance by B. Lee Ligon and internal review by Sue Torrey, Andrea Cruz, and Manish Shah of the Department of Pediatrics, Baylor College of Medicine, Houston, Texas. A previous version of this work was presented at the 2018 Consortium of Universities for Global Health meeting in New York, New York.
Note: Supplemental materials appear at www.ajtmh.org.
REFERENCES
- 1.Liu L, Oza S, Hogan D, Chu Y, Perin J, Zhu J, Lawn JE, Cousens S, Mathers C, Black RE, 2016. Global, regional, and national causes of under-5 mortality in 2000–15: an updated systematic analysis with implications for the sustainable development goals. Lancet Lond Engl 388: 3027–3035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.World Health Organization , 2014. IMCI Chart Booklet. Available at: http://www.who.int/maternal_child_adolescent/documents/IMCI_chartbooklet/en/. Accessed December 15, 2017. [Google Scholar]
- 3.Lazzerini M, Sonego M, Pellegrin MC, 2015. Hypoxaemia as a mortality risk factor in acute lower respiratory infections in children in low and middle-income countries: systematic review and meta-analysis. PLoS One 10: e0136166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hooli S, Colbourn T, Lufesi N, Costello A, Nambiar B, Thammasitboon S, Makwenda C, Mwansambo C, McCollum ED, King C, 2016. Predicting hospitalised paediatric pneumonia mortality risk: an external validation of RISC and mRISC, and local tool development (RISC-Malawi) from Malawi. PLoS One 11: e0168126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ginsburg AS, Van Cleve WC, Thompson MI, English M, 2012. Oxygen and pulse oximetry in childhood pneumonia: a survey of healthcare providers in resource-limited settings. J Trop Pediatr 58: 389–393. [DOI] [PubMed] [Google Scholar]
- 6.English M, et al. 2014. Adoption of recommended practices and basic technologies in a low-income setting. Arch Dis Child 99: 452–456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bassat Q, et al. 2011. Distinguishing malaria from severe pneumonia among hospitalized children who fulfilled integrated management of childhood illness criteria for both diseases: a hospital-based study in Mozambique. Am J Trop Med Hyg 85: 626–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mwaniki MK, Nokes DJ, Ignas J, Munywoki P, Ngama M, Newton CR, Maitland K, Berkley JA, 2009. Emergency triage assessment for hypoxaemia in neonates and young children in a Kenyan hospital: an observational study. Bull World Health Organ 87: 263–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Duke T, Mgone J, Frank D, 2001. Hypoxaemia in Children with Severe Pneumonia in Papua New Guinea [Oxygen Therapy in Children]. Available at: https://www.ingentaconnect.com/content/iuatld/ijtld/2001/00000005/00000006/art00004#. Accessed November 19, 2018. [PubMed] [Google Scholar]
- 10.Usen S, Weber M, 2001. Clinical signs of hypoxaemia in children with acute lower respiratory infection: indicators of oxygen therapy [oxygen therapy in children]. Int J Tuberc Lung Dis 5: 505–510. [PubMed] [Google Scholar]
- 11.Rao YK, Midha T, Kumar P, Tripathi VN, Rai OP, 2012. Clinical predictors of hypoxemia in Indian children with acute respiratory tract infection presenting to pediatric emergency department. World J Pediatr 8: 247–251. [DOI] [PubMed] [Google Scholar]
- 12.McCollum ED, et al. 2016. Pulse oximetry for children with pneumonia treated as outpatients in rural Malawi. Bull World Health Organ 94: 893–902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McCollum ED, et al. 2017. Impact of the 13-valent pneumococcal conjugate vaccine on clinical and hypoxemic childhood pneumonia over three years in Central Malawi: an observational study. PLoS One 12: e0168209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.National Statistical Office , 2018. 2018 Malawi Population and Housing Census Preliminary Report. Zomba: National Statistical Office. [Google Scholar]
- 15.The World Bank , 2018. Malawi Data. Available at: https://data.worldbank.org/country/malawi. Accessed February 14, 2019. [Google Scholar]
- 16.Lazzerini M, et al. 2016. Mortality and its risk factors in Malawian children admitted to hospital with clinical pneumonia, 2001–12: a retrospective observational study. Lancet Glob Health 4: e57–e68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Olson D, Preidis GA, Milazi R, Spinler JK, Lufesi N, Mwansambo C, Hosseinipour MC, McCollum ED, 2013. Task shifting an inpatient triage, assessment and treatment programme improves the quality of care for hospitalised Malawian children. Trop Med Int Health 18: 879–886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.King C, et al. 2019. Performance of a novel reusable pediatric pulse oximeter probe. Pediatr Pulmonol 54: 1052–1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Enarson PM, Gie R, Enarson DA, Mwansambo C, 2009. Development and implementation of a national programme for the management of severe and very severe pneumonia in children in Malawi. PLoS Med 6: e1000137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fleming S, Thompson M, Stevens R, Heneghan C, Plüddemann A, Maconochie I, Tarassenko L, Mant D, 2011. Normal ranges of heart rate and respiratory rate in children from birth to 18 years of age: a systematic review of observational studies. Lancet Lond Engl 377: 1011–1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.White IR, Royston P, Wood AM, 2011. Multiple imputation using chained equations: issues and guidance for practice. Stat Med 30: 377–399. [DOI] [PubMed] [Google Scholar]
- 22.Bell ML, Fairclough DL, 2014. Practical and statistical issues in missing data for longitudinal patient-reported outcomes. Stat Methods Med Res 23: 440–459. [DOI] [PubMed] [Google Scholar]
- 23.West JB, 1973. Respiratory Physiology: the Essentials. Baltimore, MD: Williams & Wilkins. [Google Scholar]
- 24.Lee WW, Mayberry K, Crapo R, Jensen RL, 2000. The accuracy of pulse oximetry in the emergency department. Am J Emerg Med 18: 427–431. [DOI] [PubMed] [Google Scholar]
- 25.World Health Organization , 2013. Pocket Book of Hospital Care for Children: Guidelines for the Management of Common Childhood Illnesses, 2nd edition Geneva, Switzerland: WHO. [PubMed] [Google Scholar]
- 26.Subhi R, Adamson M, Campbell H, Weber M, Smith K, Duke T, 2009. The prevalence of hypoxaemia among ill children in developing countries: a systematic review. Lancet Infect Dis 9: 219–227. [DOI] [PubMed] [Google Scholar]
- 27.English M, et al. 2003. Causes and outcome of young infant admissions to a Kenyan district hospital. Arch Dis Child 88: 438–443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Onyango FE, Steinhoff MC, Wafula EM, Wariua S, Musia J, Kitonyi J, 1993. Hypoxaemia in young Kenyan children with acute lower respiratory infection. BMJ 306: 612–615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.WHO , 2016. Paediatric Emergency Triage, Assessment and Treatment: care of Critically-Ill Children. Available at: http://www.who.int/maternal_child_adolescent/documents/paediatric-emergency-triage-update/en/. Accessed November 20, 2017. [PubMed] [Google Scholar]
- 30.Florin TA, Ambroggio L, Brokamp C, Rattan MS, Crotty EJ, Kachelmeyer A, Ruddy RM, Shah SS, 2017. Reliability of examination findings in suspected community-acquired pneumonia. Pediatrics 140: e20170310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McCollum ED, Bjornstad E, Preidis GA, Hosseinipour MC, Lufesi N, 2013. Multicenter study of hypoxemia prevalence and quality of oxygen treatment for hospitalized Malawian children. Trans R Soc Trop Med Hyg 107: 285–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.