Abstract.
The emergence of artemisinin-resistant Plasmodium falciparum in the Greater Mekong Subregion threatens both the efficacy of artemisinin-based combination therapy (ACT), the first-line treatment for malaria, and prospects for malaria elimination. Monitoring of ACT efficacy is essential for ensuring timely updates to elimination policies and treatment recommendations. In 2014–2015, we assessed the therapeutic efficacies of artemether–lumefantrine (AL) and dihydroartemisinin–piperaquine (DP) for the treatment of uncomplicated P. falciparum at three study sites in Rakhine, Shan, and Kachin states in Myanmar. Patients presenting with uncomplicated P. falciparum malaria were enrolled, treated, and followed up for 28 days for AL or 42 days for DP. Both AL and DP demonstrated good therapeutic efficacy at all three study sites. The 28-day cure rate for AL was > 96% across all study sites, and the 42-day cure rate for DP was 100%. Parasitemia on day 3 was detected in 0%, 3.3%, and 3.6% of participants treated with AL at the Rakhine, Shan, and Kachin sites, respectively. No participants treated with DP were parasitemic on day 3. No evidence of P. falciparum k13 mutations was found at the Rakhine study site. A high prevalence of k13 mutations associated with artemisinin resistance was observed at the Kachin and Shan state study sites. These results confirm that ACT efficacy has been resilient in therapeutic efficacy study (TES) sentinel sites in Myanmar, despite the presence at some sites of k13 mutations associated with resistance. Studies are ongoing to assess whether this resilience persists.
INTRODUCTION
In 2015, Myanmar committed to eliminate malaria by 2030. Despite historically having the highest malaria burden in the Greater Mekong Subregion (GMS),1,2 Myanmar has made impressive progress toward malaria elimination.3 Between 2005 and 2014, the annual reported malaria cases and deaths decreased by 81.1% and 93.5%, respectively.3 Myanmar now has fewer malaria cases than Cambodia.3,4 This decline in malaria mortality and incidence coincided with an increase in support for malaria control programs by the national government and international partners as well as expanded access to artemisinin combination therapies (ACTs) as first-line treatments for Plasmodium falciparum malaria.3
The emergence of artemisinin resistance in the GMS, including Myanmar, poses a serious threat to continued progress toward malaria elimination.5,6 The Myanmar Ministry of Health and Sports recommends three ACTs as first-line treatments for P. falciparum infections: artesunate–mefloquine (AM), artemether–lumefantrine (AL), or dihydroartemisinin–piperaquine (DP).7 Several groups have reported an alarming decrease in efficacy of AM along the Thailand–Myanmar border,8–10 with 42-day cure rates as low as 42%.9 The efficacy of AL remains high in Myanmar,11 although studies in Cambodia have found evidence of resistance in the GMS.12 Similarly, DP has had high therapeutic efficacy in Myanmar,13,14 but evidence of DP resistance has emerged in other parts of the GMS.15,16 Some worry that the high efficacies of AL and DP may be the “calm before the storm” in Myanmar.17 Indeed, a recent study at two sites in northern and central Myanmar demonstrated good therapeutic efficacy of DP but found parasite clearance half-lives corresponding to an “intermediate resistance” phenotype.14
Regularly updated data on ACT efficacy are critical for informing national programs on changing P. falciparum response to ACTs and ensuring effective treatment of P. falciparum malaria. For this reason, the WHO recommends routine monitoring of ACT efficacy at sentinel sites at least once every 2 years.
In this study, we assessed the therapeutic efficacy of AL and DP for treatment of uncomplicated P. falciparum malaria at three sites in Myanmar in 2014 and 2015 using the standard WHO protocol.18 Efficacy results were shared with the WHO and the Myanmar National Malaria Control Programme (NMCP) in real time. Here, we present efficacy results and results of genotyping molecular markers of artemisinin resistance.
METHODS
Study sites and population.
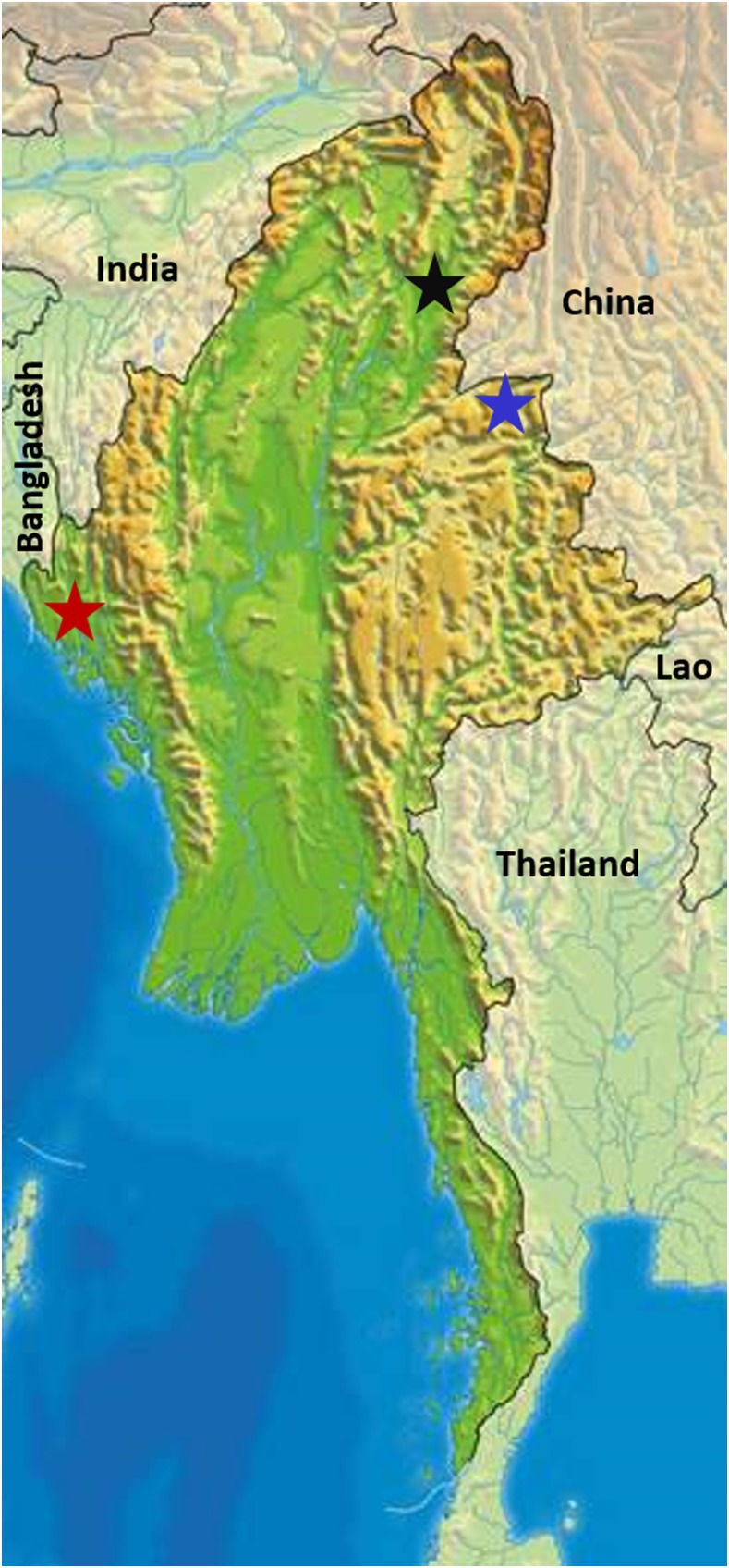
Therapeutic efficacy evaluations of AL and DP were performed in three of 14 states/regions of Myanmar: Rakhine (western coastal region of Myanmar, near Bangladesh border), Shan, and Kachin (northeastern regions of Myanmar, bordering the Yunnan Province of China) states. Locations of the study sites are depicted in Figure 1. The study drugs were evaluated in Buthidaung township of the Rakhine site between August 2014 and January 2015. Studies in Mu Se township of the Shan site and Myitkyina township of Kachin site were conducted between July and December 2015.
Figure 1.
Locations of the three study sites in Myanmar are indicated by red, blue, and black stars for Rakhine, Shan and Kachin study sites, respectively. This figure appears in color at www.ajtmh.org.
Individuals with acute uncomplicated malaria were screened and eligible participants enrolled at study clinics after obtaining written informed consent from adults or assent/adult guardian consent from children. Eligibility criteria included age of at least 6 years, P. falciparum monoinfections with parasitemias of 500–100,000 parasites/µL as determined by microscopy, and fever, defined as oral temperature ≥ 37.5°C or reported history of fever in the last 24 hours. Pregnant women, patients with evidence of severe malaria or severe clinical manifestations requiring immediate medical attention, and patients with fever of other or unknown etiologies were excluded from the studies. Any individuals with severe clinical signs and symptoms were referred to the local hospital.
Study design.
This was a prospective, open-label, one-arm community clinical study to evaluate clinical and parasitological responses to AL and DP treatments for uncomplicated falciparum malaria. The standard WHO therapeutic efficacy protocol was followed.18 At each of the three study sites, the efficacies of AL and DP were evaluated sequentially. On enrollment, finger-prick blood samples were collected for preparing blood smears as well as dried blood spot (DBS) samples on Whatman 3 mm filter paper. The DBS samples were air dried and stored in plastic bags with desiccant. Enrolled patients were treated with AL (Coartem®, Novartis, Switzerland) at a target dose of 2 mg/kg artemether and 12 mg/kg lumefantrine twice daily for 3 days, or with DP (Duo-cotecxin®, Zhejiang Holley Nanhu Pharmaceutical Co. Ltd, Jiaxing City, P. R. China; quality controlled by SGS Netherlands, Spijkenisse, The Netherlands) at a target dose of 2.0–2.4 mg/kg dihydroartemisinin and 16.0–19.2 mg/kg piperaquine once daily for 3 days. All drug administrations were directly observed. All study drugs were provided by the WHO malaria programme via the NMCP.
The day of study enrollment and drug treatment initiation was designated as day 0. Study participants were monitored on days 1, 2, 3, 7, 14, 21, and 28 for AL and additionally on days 35 and 42 for DP. At each follow-up visit, scheduled or unscheduled, a standard physical examination, including oral temperature measurement, was performed. Thick and thin blood films for parasite counts were obtained at each follow-up visit, and DBS samples were collected on days 0, 7, 14, 28, 35, and 42, or any unscheduled visit for suspected malaria. Hemoglobin concentrations were measured on days 0, 3, 7, 14, 21, 28, 35, and 42 using a HemoCue analyzer (HemoCue America, Brea, CA). In the event of treatment failure, patients in the AL study were treated with DP and patients in the DP study were treated with AL, following the national malaria treatment guidelines.
This study was independently reviewed and approved by the institutional review boards of the Department of Medical Research (DMR), Myanmar, and the WHO, and registered on the Australian New Zealand Clinical Trials Registry (ACTRN12614000216617).
Laboratory methods.
Microscopy.
Light microscopy was performed independently by two trained microscopists. Slides were stained with fresh Giemsa and read at ×1000 magnification. For determining study eligibility, a rapid staining procedure (10% Giemsa for 10–15 minutes) was used. Parasite density was subsequently determined using a slower stain (2.5–3% Giemsa for 45–60 minutes) and counting the number of asexual parasites against 200 white blood cells (WBCs). When 500 parasites were counted before 200 WBCs were counted, the counting was stopped and parasite density was determined against the number of WBCs counted. If fewer than 10 parasites were counted per 200 WBCs, counting was continued to at least 500 WBCs. Parasite densities were calculated based on the assumption of 6,000 WBCs/µL. Parasite densities were averaged between the two readers. A slide was categorized as negative only when examination of 1,000 WBCs did not reveal any asexual parasites. In the event of discordant results between the two readers (parasite species, positivity, or > 50% difference in parasite densities), a third independent microscopist examined the slides, and the parasite density was taken as the average of the two most concordant results.
Parasite genotyping.
Genotyping was performed to differentiate between recrudescent infections and reinfections for patients who had positive parasite densities on day 7 or later. Polymerase chain reaction (PCR) genotyping was performed on the DBS samples collected on day 0 and the days when parasitemia was detected during the follow-up period. The PCR targets were three polymorphic antigen loci: merozoite surface protein 1 (msp1), merozoite surface protein 2 (msp2), and glutamate-rich protein (glurp). The DBS sample extraction and PCR were performed at the DMR, Yangon, Myanmar, according to previously published methods.19,20 An infection was determined to be a new infection if all alleles in the posttreatment sample were different from those in the day 0 sample. An infection was recrudescent if at least one allele at each locus was common between day 0 and posttreatment samples.
k13 sequencing.
Dried blood spot samples collected on day 0 at the time of malaria diagnosis by microscopy were examined for polymorphisms in the k13 propeller domain, known to be associated with artemisinin resistance, using previously published protocols.6 In brief, DBS samples were eluted into PBS with saponin (0.5%). Then, DNA was extracted using a QIAamp Mini Kit (Qiagen, Hilden, Germany), and the k13 propeller domain was amplified using previously described K13_PCR_F, K13_PCR_R, K13_N1_F, and K13_N1_R primers.6 The PCR products were purified using filter plates (Edge Biosystems, Gaithersburg, MD). The product was labeled with a BigDye Terminator v. 3.1 Reaction Kit (Applied Biosystems, Torrance, CA) before ethanol precipitation and capillary electrophoresis on an ABI 3730XL automated sequencer as recommended by the manufacturer’s instructions.
Outcome evaluation and data analysis.
Descriptive analyses were used to compare baseline demographic (i.e., age and gender) and clinical (i.e., weight and parasitemia) characteristics across studies. Treatment outcomes for each participant patient were classified according to WHO guidelines as early treatment failure, late clinical failure (LCF), late parasitological failure (LPF), or adequate clinical and parasitological response (ACPR).18 Occurrence of each class of the treatment outcome, prevalence of parasitemia at day 3, and patients lost to follow-up were evaluated descriptively for each study.
The primary outcome measures were the efficacy of AL and DP, defined as the proportion of patients with PCR-corrected ACPR at day 28 for AL and day 42 for DP. Because few failures were expected, inferential methods appropriate for proportions close to one were used: the Wilson score method was used to construct 95% CIs for each proportion, and exact binomial tests were used to test the null hypothesis that the proportions were less than or equal to 0.90. This null hypothesis corresponds to the WHO-recommended threshold for making treatment policy decisions in response to TES findings.18 Thus, failure to reject the null hypothesis would indicate a lack of statistical evidence for therapeutic efficacy.
The primary outcomes were assessed using a per-protocol analysis: we excluded patients lost to follow-up before days 28/42 for AL/DP, patients without valid PCR results at days 28/42 for AL/DP, and patients with a PCR-confirmed reinfection. The secondary outcome measures were the parasite clearance rate (for AL and DP, separately) and the prevalence of k13 mutations. For the parasite clearance rate, an exploratory analysis of individual patient parasitemia over time was conducted. An exponential decay model was fit to find an estimate of the half-life (with 95% CI) of an infection after treatment with AL or DP. Finally, the overall and type-specific prevalences of different k13 mutations in our patient population were calculated.
RESULTS
A total of 370 patients with suspected malaria were screened, and 359 met the inclusion criteria and enrolled. The major reasons for exclusion were negative blood smear, positive non-falciparum blood smear, and inability to follow-up for 4 or 6 weeks. Two participants each from the Shan and Kachin sites were lost to follow-up before day 28 in the AL evaluation. The remaining 355 completed the study. No severe or unexpected adverse events were reported. Two participants were excluded from final data analysis: one (AL treated in Kachin site) because of PCR-confirmed reinfection, and one (DP treated in the Rakhine site) because of an indeterminate result on PCR differentiation of recrudescence versus reinfection. Thus, a total of 353 individuals were included in the final analysis.
Baseline characteristics.
Baseline demographic and clinical characteristics are shown in Table 1. For all studies, most of the participants were young adult males. The overall mean age was 32.5 years (range: 10–60 years). The geometric mean of parasite densities at enrollment varied from 5,794 to 10,217 parasites/µL (range: 480–104,040 parasites/µL).
Table 1.
Baseline characteristics of study participants
| Site | Year | Drug | Enrolled, n | Male, n (%) | Age | Weight in kg mean (range) | Parasitemia geometric mean (range) | |
|---|---|---|---|---|---|---|---|---|
| Mean years (range) | > 15 years, n (%) | |||||||
| Kachin | 2015 | AL | 56 | 37 (66.1) | 34.6 (12–56) | 49 (87.5) | 50.2 (28–65) | 5,794 (744–83,353) |
| Kachin | 2015 | DP | 56 | 41 (73.2) | 38.2 (12–60) | 51 (91.1) | 54 (34–67) | 8,372 (1,188–83,353) |
| Rakhine | 2014 | AL | 70 | 62 (88.6) | 26.6 (18–57) | 70 (100) | 52.6 (25–65) | 10,217 (960–86,520) |
| Rakhine | 2014–15 | DP | 57 | 57 (100) | 27 (12–50) | 54 (94.7) | 54.7 (33–63) | 4,080 (480–104,040) |
| Shan | 2015 | AL | 60 | 43 (71.7) | 35.9 (10–60) | 53 (88.3) | 51.8 (29–61) | 7,392 (550–89,025) |
| Shan | 2015 | DP | 60 | 43 (71.7) | 33.6 (12–58) | 56 (93.3) | 53.3 (30–65) | 7,511 (1,002–98,000) |
AL = artemether–lumefantrine; DP = dihydroartemisinin–piperaquine; n = sample size.
Clinical and parasitological responses.
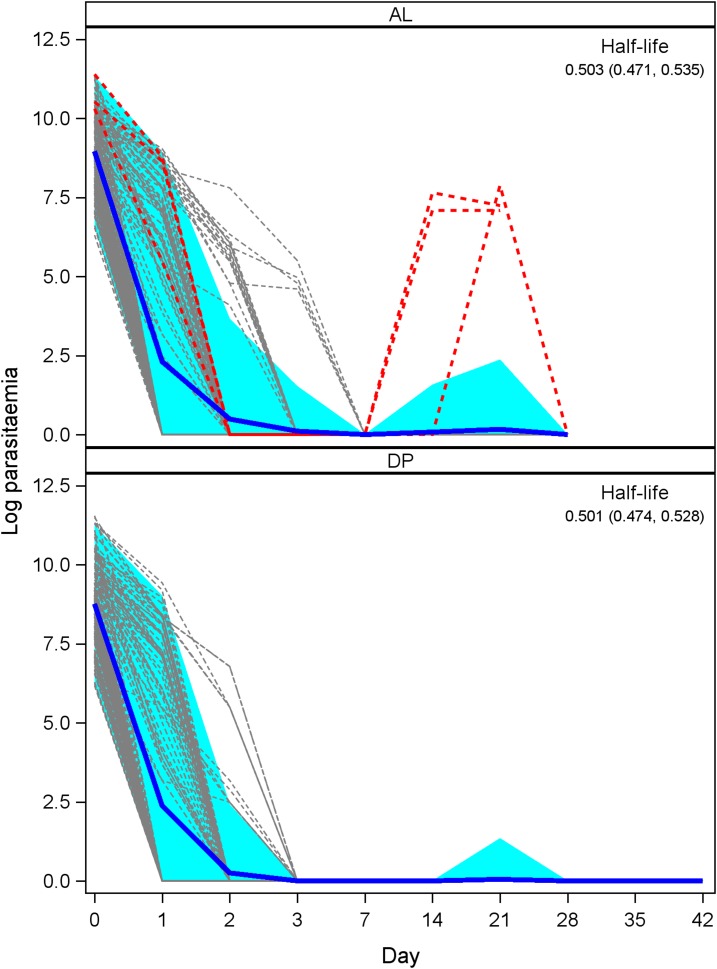
Clinical and parasitological responses are shown in Table 2, and individual clearance patterns are shown in Figure 2.
Table 2.
Treatment response by the study site and study drug
| Site/drug/number enrolled | Day 3 positive, n (%) | Non-PCR corrected | PCR corrected | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Loss | LCF | LPF | ACPR, n (%) | Loss | LCF | LPF | ACPR, n (%) | ||
| Kachin/AL/56 | 2 (3.6) | 2 | 0 | 2 | 52 (96.3) | 3 | 0 | 1 | 52 (98.1) |
| Kachin/DP/56 | 0 (0) | 0 | 0 | 0 | 56 (100) | 0 | 0 | 0 | 56 (100) |
| Rakhine/AL/70 | 0 (0) | 0 | 0 | 0 | 70 (100) | 0 | 0 | 0 | 70 (100) |
| Rakhine/DP/57 | 0 (0) | 0 | 1 | 0 | 56 (98.2) | 1 | 0 | 0 | 56 (100) |
| Shan/AL/60 | 2 (3.3) | 2 | 0 | 2 | 56 (96.6) | 2 | 0 | 2 | 56 (96.6) |
| Shan/DP/60 | 0 (0) | 0 | 0 | 0 | 60 (100) | 0 | 0 | 0 | 60 (100) |
ACPR = adequate clinical and parasitological response; AL = artemether–lumefantrine; DP = dihydroartemisinin–piperaquine; LCF = late clinical failure; loss = lost to follow-up or excluded; LPF = late parasitological failure.
Figure 2.
Parasite density overtime for patients enrolled in the artemether–lumefantrine studies (top) and dihydroartemisinin–piperaquine studies (bottom). Individual parasite clearance curves are in gray, with individuals identified as late parasitological failure highlighted in red. The blue curves represent the average clearance patterns across all patients for each drug, with 95% CIs shaded in light blue. This figure appears in color at www.ajtmh.org.
Day 3 parasitemia.
Of the 359 participants enrolled in the study, four treated with AL were parasitemic on day 3, two each from the Kachin and Shan sites, corresponding to 3.6% and 3.3% of site participants, respectively. The geometric mean of their parasite density was 22,572 parasites/µL on day 0 and 144 parasites/µL on day 3. No participants treated with DP were parasite positive on day 3.
Artemether–lumefantrine treatment response.
The PCR-uncorrected analysis showed 28-day cure rates of 96.3–100% for AL. Before PCR correction, four participants, two each from the Kachin and Shan sites, were characterized as LPF (positive on day 21). None of them were parasite positive on day 3. Of these four LPF, one from the Kachin and two from the Shan sites were confirmed to be recrudescent infection by PCR, whereas the other was reinfected and, thus, excluded from the PCR-corrected analysis.
Dihydroartemisinin–piperaquine treatment response.
All participants treated with DP reached ACPR, except one participant at the Rakhine site who presented with fever and parasitemia on day 21 and was, therefore, classified as LCF. This individual was later excluded from the final analysis because PCR genotyping was unable to determine whether this infection was new or recrudescent. Therefore, PCR-corrected ACPR for DP was 100%.
k13 genotyping and treatment response.
A summary of k13 genotypes is shown in Table 3. Sequencing of the k13 gene was successful for 292 of 359 (81.6%) day 0 samples. Most failures to sequence were because of insufficient sample material. The prevalence of k13 mutations across all sites was 46.2% (135/292). One individual at the Kachin site had two distinct k13 mutations. No k13 mutations were detected at the Rakhine site. In the Kachin and Shan sites, the most common mutation was F446I, making up 83.1% (113/136) of all observed mutations. Participants in the DP efficacy evaluation at the Kachin site had the highest prevalence of F446I mutations, with 81.8% (36/44) of samples that were sequenced displaying the mutation. The next most prevalent k13 mutation was A676D, making up 5.1% (7/136) of all observed mutations.
Table 3.
k13 mutations at enrollment
| Site/drug/number enrolled | k13 genotyped, n (%) | Wild type n (%) | k13 mutation, n (%) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| F446I | G453D | N458Y | S485N | R561H | C580Y | Q661R | A676D | V692L | |||
| Kachin/AL/56 | 51 (91.1) | 23 (45.1) | 22 (43.1) | 0 (0) | 0 (0) | 0 (0) | 1 (2.0) | 0 (0) | 0 (0) | 1 (2.0) | 4 (7.8) |
| Kachin/DP/56 | 44 (78.6) | 7 (15.9) | 36 (81.8) | 0 (0) | 1 (2.3) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Rakhine/AL/70 | 58 (82.9) | 58 (100) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Rakhine/DP/57 | 43 (75.4) | 43 (100) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Shan/AL/60 | 59 (98.3) | 11 (18.6) | 38 (64.4) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 5 (8.5) | 0 (0) | 5 (8.5) | 0 (0) |
| Shan/DP/60 | 38 (63.3) | 15 (38.5) | 17 (44.7) | 1 (2.6) | 0 (0) | 2 (5.1) | 0 (0) | 0 (0) | 1 (2.6) | 1 (2.6) | 1 (2.6) |
AL = artemether–lumefantrine; DP = dihydroartemisinin–piperaquine; n = sample size.
All three individuals with recrudescent infections had an F446I mutation. Two of the 4 (50%) participants positive on day 3 had the F446I mutation, whereas the remaining two had R561H and A676D mutations. Of the 135 participants with k13 mutations, 128 (94.8%) achieved ACPR, 3 (2.2%) were lost to follow-up, one (0.7%) had a reinfection, and three (2.2%) had LPF.
DISCUSSION
Both AL and DP demonstrated high therapeutic efficacy (> 96% cure rate) in all three study sites in Myanmar, with treatment failure rates < 4% for AL and 0% for DP, across all study sites, well below the WHO policy threshold of 10% for changing treatment.21 The highest prevalence of persistent parasitemia on day 3 after AL or DP, a surrogate marker for artemisinin resistance, was 3.6%, which may signal a need for additional monitoring.21 Taken together, these data do not show any evidence of artemisinin resistance and suggest that ACT treatment remained effective in the study sites in Myanmar at the time the studies were completed.
The results of this study, conducted in 2014–2015, are consistent with findings from previous therapeutic efficacy studies in the same region. A 2010 study in Rakhine found the ACPR of AL and DP to be 96.3% and 98.7%, respectively.22 Our results, showing 100% ACPR for both AL and DP at the Rakhine site, demonstrate that both drugs remain efficacious in the study sites. In 2011–2012, at the same study site in Shan State, the day-3 parasitemia prevalence and the 28-day cure rate for AL were 7.3% and 100%, respectively.11 These rates are similar to our findings in 2014–2015, indicating that there was no apparent decline in AL efficacy in the intervening time period. Similarly, a previous TES study of DP efficacy in the same study site in Kachin State in 2013–2014 reported the same 42-day cure rate of 100%.14 A day-3 parasite prevalence in a 2013 study by Tun et al.14 was 30%, notably higher than that in our current report and may be explained by significantly higher day 0 parasite densities (geometric mean: 36,779 parasites/µL) than those in our study (geometric mean: 8,372 parasites/µL), as day-3 parasitemia is strongly and positively associated with day 0 parasite density. However, these differences in efficacy at sites within the same geographic region highlight the importance of assessing ACT efficacy longitudinally at the same sites, ideally every 1–2 years. As was the case with the present study, TES data should be shared with NMCPs and other policy makers in real time, before publication.
Mutations in the k13 propeller domain are known to be associated with artemisinin resistance.23 We did not detect any k13 mutations at the Rakhine study site, a reassuring observation in this region known to have the highest rate of falciparum malaria in Myanmar (unpublished data, Myanmar NMCP). However, 70.3% (135/192) of participants enrolled in the Kachin and Shan study sites for which sequences were obtained had k13 mutations, a prevalence similar to or higher than previously reported for these regions.24–28 Consistent with previous studies in this region, the vast majority (83.1%) of these mutations were F446I.24–28 The WHO designates this mutation as a validated marker of artemisinin resistance.21 In in vitro studies, the F446I mutation has been associated with increased ring stage survival of clinical isolates in the presence of dihydroartemisinin.29 The F446I mutation has been associated in vivo with prolonged parasite clearance times after treatment with DP,14,24,27,30 corresponding to an intermediate resistance phenotype. Although the observations in this study of high ACT treatment efficacy were reassuring, an increasing prevalence of k13 mutations highlights the importance of monitoring ACT efficacy despite a declining malaria burden. Slowing of P. falciparum clearance by artemisinins in western Cambodia was first reported in 2008,31,32 and progressive declines in ACT efficacy followed over several years.33–35 Rapidly declining ACT efficacy in Cambodia and Vietnam was attributed to genetic polymorphisms in P. falciparum associated with resistance to multiple drugs (copy number variations in pfmdr1 and in plasmepsin 2/3), in the context of high rates of k13 mutations.36 Currently, the most commonly used ACT partner drug in Myanmar is lumefantrine, and the use of others such as mefloquine and piperaquine is limited. Data on these genetic polymorphisms, in association with clinical outcomes, are limited in Myanmar. A 2013 study at sites across Myanmar found an overall low proportion (5.5%) of P. falciparum parasites with multiple pfmdr1 copies, which has been associated with decreased sensitivity to mefloquine and lumefantrine; however, the proportion of parasites with multiple pfmdr1 copies varied greatly by site, with proportions up to 30.8% at the northern Shan State site.28 Similar proportions of parasites with multiple pfmdr1 copies were observed in a 2011–2013 study throughout Myanmar.37 Data on plasmepsin 2/3 copy numbers, a marker of piperaquine resistance, in Myanmar are sparse. A 2014–2017 study on the border areas between eastern Myanmar and Thailand found no parasites with multiple plasmepsin 2 copies.38 There are no data, to our knowledge, from other parts of Myanmar. More comprehensive monitoring is needed to understand trends in molecular markers, such as k13, pfmdr1, pfcrt, and plasmepsin 2/3, known or suspected to be associated with resistance to ACT partner drugs in Myanmar. The WHO-sponsored TES study network provides a suitable platform for these evaluations because these well-coordinated studies use the same design for multiple studies in different sites and at different times, providing an opportunity to monitor changes over space and time and to make valid comparisons of data from multiple studies.
Despite the high prevalence of k13 mutations observed in our study, the therapeutic efficacies of AL and DP were high. Infrequent observations (in days) in this study did not allow for precise determination of parasite clearance times or parasite clearance half-lives, which provide a more sensitive measure of early effects of artemisinin resistance on parasite survival. Even if more frequent observation is not possible, recording the exact times of blood collection could be helpful for determining a better estimate of parasite clearance. Thus, we do not know if the observed k13 genotypes were associated with prolonged parasite survival in these studies. However, k13 mutations were found in all participants with day-3 parasitemia and LPF, suggesting selection of mutant, resistant parasites that were subsequently eliminated by the longer-acting ACT partner drugs. The finding that F446I mutation was present in all three participants with recrudescent infections, and the fact that F446I is highly prevalent in Myanmar and the Myanmar–China border, underlines the importance of this mutation in this region.
Strengths of this study include active participation from the local communities (with very low rates of loss to follow-up) and ability to adhere to the standard WHO protocol for therapeutic efficacy studies over years, allowing direct comparison with other studies that follow the same design performed overtime and across geographic areas. Limitations include sparse study sites that may limit generalizability of the results to the rest of Myanmar, and low malaria burden, precluding a large sample size in a given time. Continued monitoring of efficacy with contemporaneous sharing of results with policymakers is essential for timely updates to national policies and recommendations for treatment of P. falciparum malaria.
CONCLUSION
Two commonly used artemisinin-based combination therapies, AL and dihydroartemisinin–piperaquine, demonstrated a high therapeutic efficacy in all three study sites, affirming their continued use as first-line treatments for uncomplicated P. falciparum malaria in Myanmar. No evidence of k13 mutations was found at the Rakhine study site, but high prevalences of k13 mutations, known or suspected to be associated with artemisinin resistance, were observed at Kachin and Shan study sites. These results, along with evidence of progressive ACT treatment failure in other areas of the GMS, highlight the importance of continued monitoring ACT therapeutic efficacy, as well as investigating the changing pattern of partner drug resistance, to ensure rapid and appropriate response to potential emergence of resistance.
Acknowledgments:
We thank all study participants and the parents/guardians of the children who participated in the study for their time and effort, the Myanmar National Malaria Control Program and township and village community leadership for active participation and programmatic support, and the DMR and Duke laboratory crews for training logistic and technical support.
REFERENCES
- 1.World Health Organization , 2017. World Malaria Report 2017. Geneva, Switzerland: WHO. [Google Scholar]
- 2.Hay SI, Okiro EA, Gething PW, Patil AP, Tatem AJ, Guerra CA, Snow RW, 2010. Estimating the global clinical burden of Plasmodium falciparum malaria in 2007. PLoS Med 7: e1000290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mu TT, Sein AA, Kyi TT, Min M, Aung NM, Anstey NM, Kyaw MP, Soe C, Kyi MM, Hanson J, 2016. Malaria incidence in Myanmar 2005–2014: steady but fragile progress towards elimination. Malar J 15: 503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.World Health Organization , 2018. World Malaria Report 2018. Geneva, Switzerland: WHO. [Google Scholar]
- 5.Takala-Harrison S, et al. 2015. Independent emergence of artemisinin resistance mutations among Plasmodium falciparum in southeast Asia. J Infect Dis 211: 670–679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ménard D, et al. 2016. A worldwide map of Plasmodium falciparum K13-propeller polymorphisms. N Engl J Med 374: 2453–2464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Myanmar Ministry of Health , 2014. National Strategic Plan - Malaria Prevention and Control 2010–2016. Nay Pyi Taw, Myanmar: Myanmar Ministry of Health. [Google Scholar]
- 8.Phyo AP, et al. 2016. Declining efficacy of artemisinin combination therapy against P. Falciparum malaria on the Thai–Myanmar border (2003–2013): the role of parasite genetic factors. Clin Infect Dis 63: 784–791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Carrara VI, et al. 2013. Malaria burden and artemisinin resistance in the mobile and migrant population on the Thai–Myanmar border, 1999–2011: an observational study. PLoS Med 10: e1001398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Na-Bangchang K, Ruengweerayut R, Mahamad P, Ruengweerayut K, Chaijaroenkul W, 2010. Declining in efficacy of a three-day combination regimen of mefloquine-artesunate in a multi-drug resistance area along the Thai-Myanmar border. Malar J 9: 273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Myint MK, Rasmussen C, Thi A, Bustos D, Ringwald P, Lin K, 2017. Therapeutic efficacy and artemisinin resistance in northern Myanmar: evidence from in vivo and molecular marker studies. Malar J 16: 143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Denis MB, et al. 2006. Efficacy of artemether–lumefantrine for the treatment of uncomplicated falciparum malaria in northwest Cambodia. Trop Med Int Health 11: 1800–1807. [DOI] [PubMed] [Google Scholar]
- 13.Wang Y, et al. 2015. Clinical efficacy of dihydroartemisinin–piperaquine for the treatment of uncomplicated Plasmodium falciparum malaria at the China–Myanmar border. Am J Trop Med Hyg 93: 577–583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tun KM, et al. 2016. Parasite clearance rates in upper Myanmar indicate a distinctive artemisinin resistance phenotype: a therapeutic efficacy study. Malar J 15: 185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Amaratunga C, et al. 2016. Dihydroartemisinin–piperaquine resistance in Plasmodium falciparum malaria in Cambodia: a multisite prospective cohort study. Lancet Infect Dis 16: 357–365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Thriemer K, et al. 2014. Delayed parasite clearance after treatment with dihydroartemisinin-piperaquine in Plasmodium falciparum malaria patients in central Vietnam. Antimicrob Agents Chemother 58: 7049–7055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fairhurst RM, 2015. High antimalarial efficacy of dihydroartemisinin–piperaquine on the China–Myanmar border: the calm before the storm. Am J Trop Med Hyg 93: 436–437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.World Health Organization , 2009. Methods for Surveillance of Antimalarial Drug Efficacy. Geneva, Switzerland: WHO. [Google Scholar]
- 19.Ranford-Cartwright LC, Taylor J, Umasunthar T, Taylor LH, Babiker HA, Lell B, Schmidt-Ott JR, Lehman LG, Walliker D, Kremsner PG, 1997. Molecular analysis of recrudescent parasites in a Plasmodium falciparum drug efficacy trial in Gabon. Trans R Soc Trop Med Hyg 91: 719–724. [DOI] [PubMed] [Google Scholar]
- 20.World Health Organization , 2008. Methods and Techniques for Clinical Trials on Antimalarial Drug Efficacy: Genotyping to Identify Parasite Populations. Geneva, Switzerland: WHO. [Google Scholar]
- 21.World Health Organization , 2018. Status Report on Artemisinin Resistance and ACT Efficacy (August 2018). Geneva, Switzerland: WHO. [Google Scholar]
- 22.Nyunt MH, et al. 2017. Clinical and molecular surveillance of artemisinin resistant falciparum malaria in Myanmar (2009–2013). Malar J 116: 333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ariey F, et al. 2014. A molecular marker of artemisinin-resistant Plasmodium falciparum malaria. Nature 505: 50–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Huang F, et al. 2015. A single mutation in K13 predominates in southern China and is associated with delayed clearance of Plasmodium falciparum following artemisinin treatment. J Infect Dis 212: 1629–1635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang Z, Shrestha S, Li X, Miao J, Yuan L, Cabrera M, Grube C, Yang Z, Cui L, 2015. Prevalence of K13-propeller polymorphisms in Plasmodium falciparum from China-Myanmar border in 2007–2012. Malar J 14: 168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zeng W, Bai Y, Wang M, Wang Z, Deng S, Ruan Y, Feng S, Yang Z, Cui L, 2017. Significant divergence in sensitivity to antimalarial drugs between neighboring Plasmodium falciparum populations along the eastern border of Myanmar. Antimicrob Agents Chemother 61: e01689-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang Z, et al. 2015. Artemisinin resistance at the China-Myanmar border and association with mutations in the K13 propeller gene. Antimicrob Agents Chemother 59: 6952–6959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Win AA, Imwong M, Kyaw MP, Woodrow CJ, Chotivanich K, Hanboonkunupakarn B, Pukrittayakamee S, 2016. K13 mutations and pfmdr1 copy number variation in Plasmodium falciparum malaria in Myanmar. Malar J 15: 110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang J, Huang Y, Zhao Y, Ye R, Zhang D, Pan W, 2018. Introduction of F446I mutation in the K13 propeller gene leads to increased ring survival rates in Plasmodium falciparum isolates. Malar J 17: 248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liu H, et al. 2015. In vivo monitoring of dihydroartemisinin-piperaquine sensitivity in Plasmodium falciparum along the China-Myanmar border of Yunnan province, China from 2007 to 2013. Malar J 14: 47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Noedl H, Se Y, Schaecher K, Smith BL, Socheat D, Fukuda MM; Artemisinin Resistance in Cambodia 1 (ARC1) Study Consortium , 2008. Evidence of artemisinin-resistant malaria in western Cambodia. N Engl J Med 359: 2619–2620. [DOI] [PubMed] [Google Scholar]
- 32.Dondorp AM, et al. 2009. Artemisinin resistance in Plasmodium falciparum malaria. N Engl J Med 361: 455–467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bustos MD, Wongsrichanalai C, Delacollette C, Burkholder B, 2013. Monitoring antimalarial drug efficacy in the Greater Mekong Subregion: an overview of in vivo results from 2008 to 2010. Southeast Asian J Trop Med Public Health 44: 201–230; discussion 306–307. [PubMed] [Google Scholar]
- 34.World Health Organization , 2019. Available at: https://www.who.int/malaria/areas/drug_resistance/drug_efficacy_database/en/. Accessed September 2019.
- 35.World Health Organization , 2019. Available at: https:www.who.int/malaria/maps/threats/. Accessed September 2019.
- 36.van der Pluijm RW, et al. 2019. Determinants of dihydroartemisinin-piperaquine treatment failure in Plasmodium falciparum malaria in Cambodia, Thailand, and Vietnam: a prospective clinical, pharmacological, and genetic study. Lancet Infect Dis 19: 952–961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Srimuang K, Miotto O, Lim P, Fairhurst RM, Kwiatkowski DP, Woodrow CJ, Imwong M; Tracking Resistance to Artemisinin Collaboration , 2016. Analysis of anti-malarial resistance markers in pfmdr1 and pfcrt across southeast asia in the tracking resistance to artemisinin collaboration. Malar J 15: 541 Erratum in: Malar J 2018;17(1):325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Landier J, Parker DM, Thu AM, Lwin KM, Delmas G, Nosten FH; Malaria Elimination Task Force Group , 2018. Effect of generalised access to early diagnosis and treatment and targeted mass drug administration on Plasmodium falciparum malaria in eastern Myanmar: an observational study of a regional elimination programme. Lancet 391: 1916–1926. [DOI] [PMC free article] [PubMed] [Google Scholar]