Abstract.
Point-of-care urine-lipoarabinomannan (LAM) Alere Determine TB-LAM assay has shown utility diagnosing tuberculosis (TB) in HIV-positive, severely immunocompromised, TB-symptomatic patients. We assessed LAM results in severely immunocompromised patients, who had LAM systematically performed at new or follow-up HIV consultations. This was a prospective, observational study on consecutive ambulatory, > 15-year-old HIV-positive patients with CD4 < 100 cells/µL in Mozambique. Clinical assessments and LAM were performed for all and microscopy, Xpert, sputum culture, and chest X-ray for LAM-positive participants. Patients were followed up for 6 months. Of 360 patients, half were ART-naive. Lipoarabinomannan positivity was 11.9% (43/360), higher among symptomatic patients compared with asymptomatic: 18.5% (30/162), and 6.6% (13/198), respectively, P = 0.001. Tuberculosis was bacteriologically confirmed in 6/35 LAM-positive patients (2 of them asymptomatic). Lipoarabinomannan positivity was associated with higher risk of mortality (adjusted odds ratio [aOR]: 4.6, 95% CI: 1.3–15.6, P = 0.015). Systematic urine-LAM allows for rapid TB treatment initiation in severely immunocompromised HIV ambulatory patients and identifies patients at a higher risk of death.
In 2017, there were nearly a million cases of tuberculosis (TB) among people living with HIV (PLHIV), causing 300,000 deaths.1 The WHO recommends symptom-based TB screening for PLHIV and further evaluation of symptomatic patients using GeneXpert MTB/RIF.2,3 Yet, symptom-based TB screening is not routinely performed in many HIV programs, sputum samples can be difficult to produce, and this molecular technology may not be available in low-resource peripheral health facilities. Point-of-care urine lateral flow lipoarabinomannan assay Alere Determine TB-LAM (LAM) has shown utility diagnosing TB and reducing mortality in severely immunocompromised, TB-symptomatic HIV-positive patients.4,5 Diagnostic accuracy studies of LAM for TB diagnosis of HIV-positive patients irrespective of signs and symptoms have provided inconsistent results.6–13 In a recent systematic review, the sensitivity and specificity of LAM in outpatients, irrespective of their TB symptoms, was 31% and 95%, respectively,14 and the just published WHO TB-LAM revised guidelines recommend the use of LAM in HIV-positive patients with CD4 count les than 100 cells/µL, irrespective of signs and symptoms of TB15 We assessed LAM results and TB diagnosis in ambulatory, severely immunosuppressed HIV patients, who had LAM systematically performed at new or regular HIV consultations. We also assessed the association between LAM results and mortality at 6 months.
This prospective, observational study included consecutively > 15-year-old, ambulatory HIV-positive patients with CD4 < 100 cells/µL presenting for new or follow-up HIV consultations at the Centro de Referência de Alto-Maé (CRAM), in Maputo, Mozambique, from December 2014 to November 2016. Patients taking TB treatment were not eligible. The non-provision of sputum sample was not an exclusion criterion. The CRAM is an outpatients HIV referral facility dually supported by the Mozambican Ministry of Health and Médecins Sans Frontières (MSF). The main reasons for referral to the CRAM were advanced HIV with CD4 less than 100 cells/µL, suspicion of antiretroviral therapy (ART) failure, Kaposi Sarcoma, HIV-positive drug users, hepatitis C coinfection, opportunistic infections, and adverse events associated with ART.
At the initial consultation, a clinician performed a full clinical examination that included an anamnesis and a physical examination, and a urine-LAM test (Alere Determine TB LAM Ag, Abbott, Palatine, IL [formerly Alere, Waltham, MA]). The LAM test was used at the CRAM before the study initiation. The medical staff had received a 4-hour training before using the test, and short refreshment training was given before the study initiation. During the study period, the LAM test was performed by the clinician in the same consultation room or by a nurse in a separate room. Lipoarabinomannan-positive patients were asked to provide two sputum samples (one “on-spot” and one early morning) for auramine staining and light emitting diode fluorescence microscopy, GeneXpert MTB/RIF using regular cartridges (Xpert; Cepheid, Sunnyvale, CA), and culture (BACTEC MGIT 960; BD, Franklin Lakes, NJ) GenoType Mycobacterium CM and GenoType Mycobacterium AS based on DNA STRIP technology were used to identify non-tuberculosis mycobacterium (NTM). These qualitative tests can identify the Mycobacterium tuberculosis complex and 27 clinically relevant NTM from liquid or solid medium culture. Chest X-ray was also requested for LAM-positive patients. Lipoarabinomannan positivity was defined as grade ≥ 1 on a 4-grade reading card. Negative LAM results required no further evaluation as per the study protocol. However, clinicians could request further TB investigations based on their clinical judgment. Lipoarabinomannan result was used to guide TB treatment. Patients were followed up for 6 months regardless of their TB treatment status.
We assessed LAM positivity, TB diagnosis, and TB treatment in the overall population. In addition, we assessed the proportion of patients with bacteriologically confirmed TB among LAM-positive patients. Finally, we assessed the proportion of patients with at least one symptom among cough, fever, weight loss, or night sweats reported in the clinical examination. The presence of any symptom was recorded, regardless of the duration and the severity. We calculated the LAM positivity among patients with at least one symptom and among patients with none of the symptoms. We also assessed the proportion of patients initiating anti-TB treatment at their first consultation, stratified by LAM result and the presence of symptoms. Finally, we assessed the association between LAM positivity and mortality at 6 months. Bacteriologically confirmed TB was defined as Mycobacterium tuberculosis detected by culture or Xpert MTB/RIF. Patients were considered “seriously ill” if one of the following danger signs was present: temperature > 39°C, respiratory rate > 30 respirations/minute, heart rate > 120 beats/minute, or if the patient was unable to walk without help.
Continuous variables are presented as medians with interquartile ranges (IQRs), categorical variables as counts, and percentages. We used univariate and multivariate logistic regression models with a penalized likelihood estimation to account for rare events to assess the association between LAM positivity and mortality in the first 6 months after enrollment, building age, gender, body mass index (BMI), ART, CD4 count, and the presence of TB symptoms into the models.16 Using a backward elimination, stepwise approach, only variables with a P-value < 0.2 in univariate analyses were included in the final multivariate model. Crude and adjusted odds ratios with 95% CIs were assessed, and an alpha level of 5% was used for all statistical tests. Kaplan–Meier estimates and log-rank test were used to explore difference in mortality according to LAM results. Data were analyzed using Stata v.13 (College Station, TX).
The National Ethical Review Committee of Mozambique and the MSF Ethical Review Board approved the study. Written informed consent was obtained for all patients. For those aged 16 and 17 years, consent was obtained from a parent/legal guardian and assent was obtained from the patient.
In total, 360 HIV-positive patients were included (median age 36 years, 51.1% women) (Table 1). Half were ART naive, 72.4% had CD4 < 50 cells/µL, 4.4% had a BMI < 16 kg/m2, and 3.6% were seriously ill. The median CD4 was 31 cells/µL [IQR: 13–53]. Overall, 198 (55.5%) did not present any symptom, 132 (36.7%) presented one symptom, and 30 (8.3%) presented two symptoms. All symptoms were mild and of short duration.
Table 1.
Demographic and clinical characteristics at first consultation
| All, N = 360 n (%) | LAM positive, N = 43 n (%) | LAM negative, N = 317 n (%) | P-value | |
|---|---|---|---|---|
| Women | 184 (51.1) | 20 (46.5) | 164 (51.7) | 0.769 |
| Age (years), median [IQR] | 36 [31–43] | 37 [29–42] | 36 [32–43] | 0.722 |
| BMI (kg/m2), median [IQR] | 21 [19–24] | 21 [19–23] | 21 [19–24] | 0.159 |
| BMI < 16 (kg/m2) | 16 (4.4) | 5 (11.6) | 11 (3.5) | 0.014 |
| Antiretroviral treatment-naïve | 188 (52.2) | 22 (51.2) | 166 (52.4) | 0.921 |
| Seriously ill | 13 (3.6) | 7 (16.3) | 6 (1.9) | < 0.001 |
| Seriously ill or body mass index < 16 | 26 (7.2) | 11 (25.6) | 15 (4.7) | < 0.001 |
| CD4 count (cells/µL) | ||||
| Median [IQR] | 31 [13–53] | 24 [13–46] | 33 [13–56] | 0.280 |
| CD4 < 50 | 259 (72.4) | 33 (76.7) | 226 (71.8) | 0.492 |
| Reported symptoms | ||||
| Cough | 64 (17.8) | 12 (27.9) | 52 (16.5) | 0.171 |
| Fever | 32 (8.9) | 25 (7.9) | 7 (16.3) | 0.070 |
| Chest pain | 12 (3.3) | 11 (3.5) | 1 (2.3) | 0.695 |
| Haemoptysis | 0 | 0 | 0 | – |
| Difficulty to breath | 4 (1.1) | 2 (0.6) | 2 (0.7) | 0.018 |
| Night sweats | 2 (0.6) | 2 (0.6) | 0 | 0.818 |
| Weight loss | 57 (15.9) | 45 (14.2) | 12 (28.6) | 0.017 |
| At least one of the WHO symptoms for tuberculosis screening | 162 (45.0) | 30 (69.8) | 132 (41.6) | 0.001 |
| Clinical examination findings | ||||
| Temperature > 37.4°C | 16 (4.4) | 6 (13.9) | 10 (3.2) | 0.001 |
| Respiratory rate > 20/minutes | 146 (40.6) | 28 (65.1) | 118 (37.3) | < 0.001 |
| Heart rate > 100/minutes | 52 (14.4) | 13 (30.2) | 39 (12.3) | 0.002 |
| LAM result | ||||
| No line | 289 (80.3) | – | 289 (91.2) | – |
| Fainter than grade 1 | 28 (7.8) | – | 28 (8.8) | – |
| Grade 1 | 20 (5.6) | 20 (46.5) | – | – |
| Grade 2 | 12 (3.3) | 12 (27.9) | – | – |
| Grade 3 | 6 (1.7) | 6 (14.0) | – | – |
| Grade 4 | 5 (1.4) | 5 (11.6) | – | – |
IQR = interquartile range; LAM = lipoarabinomannan. The symptoms for TB screening include cough, fever, night sweats, and weight loss. If the patient is seriously ill, the temperature is > 39°C and/or respiratory rate is > 30/minutes and/or heart rate is > 120/minutes.
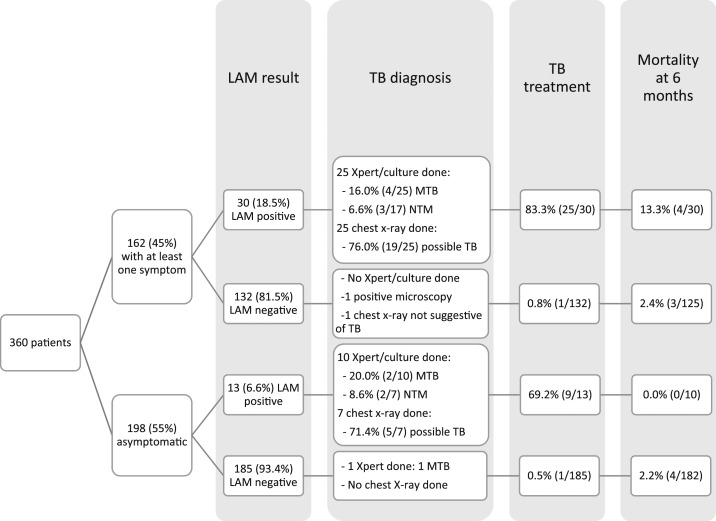
Lipoarabinomannan positivity was 11.9% (43/360). Patients with CD4 < 50 cells/µL and CD4 50–99 cells/µL had similar LAM positivity rates (P = 0.646). Lipoarabinomannan positivity was higher in seriously ill patients than in those not seriously ill, 53.9% (7/13) and 10.4% (36/347), respectively, P < 0.001, and in those with a BMI < 16 kg/m2 than those with higher BMI, 31.3% (5/16) and 11.0% (37/337), respectively, P = 0.014. Of the seriously ill, or BMI < 16 kg/m2 LAM-positive patients, 72.7% had a LAM grade ≥ 2. Among 317 LAM-negative patients, 28 (8.8%) had a line fainter than grade 1. Lipoarabinomannan positivity was 18.5% (30/162) among patients with symptoms and 6.6% (13/198) among asymptomatic participants (P = 0.001) (Figure 1). Lipoarabinomannan positivity was similar in patients with one symptom (18.9%, 25/132) and in patients with two symptoms (16.7%, 5/30), P = 0.772. Of the symptomatic and asymptomatic LAM-positive patients, 25/30 and 10/13 received Xpert or culture results and four and two had bacteriologically confirmed TB. Overall, among 35 LAM-positive patients with Xpert or culture results, six (17.1%) had bacteriologically confirmed TB, and among 24 LAM-positive patients with culture results, five (20.8%) had NTM isolated (none with Mycobacterium tuberculosis mixed infection detected). However, speciation was not possible in any of these cases (isolates were not stored or did not grow on secondary culture). No cases of rifampicin resistance were detected. Of the 43 LAM-positive patients, 34 (79.1%) initiated anti-TB treatment at the first consultation.
Figure 1.
Patient flow diagram.
Overall, mortality was 3.2% (11/347). Vital status at 6 months could not be ascertained for 13 patients. Mortality was significantly higher in LAM-positive patients than in LAM-negative patients: 10.0% (4/40) versus 2.3% (7/307), log-rank test P = 0.009. All deceased LAM-positive patients had at least one symptom. After adjustment for CD4 count, LAM-positive results were associated with higher mortality risk (aOR: 4.6, 95% CI: 1.3–15.6, P = 0.015). The increased mortality risk in LAM-positive patients occurred mainly after 2 months of follow-up (Figure 2). Among LAM-negative patients, mortality was 7.1% (2/28) in patients with a visible LAM test line (fainter than grade 1) compared with 1.8% (5/278) in patients with no line, P = 0.071.
Figure 2.
Mortality according to the lipoarabinomannan result in the first 6 months of follow-up after initial consultation among 347 patients with vital status ascertained at 6 months (log-rank test P = 0.009). This figure appears in color at www.ajtmh.org.
In this study, LAM positivity was relatively high among severely immunocompromised patients at new or follow-up HIV consultations. Although the large majority of the symptomatic patients had an isolated symptom and all symptoms were mild and of short duration, LAM positivity was higher in this group than the asymptomatic group. Minor symptoms may be an indication of incipient TB which underlines the importance of performing symptomatic TB screening followed by TB investigations in patients with symptoms. Systematically, using LAM allowed the immediate initiation of anti-TB treatment in 18.5% of these symptomatic patients and in 6.6% of the asymptomatic patients. These patients may have otherwise been missed.
In our study, some LAM-positive patients had negative culture results or NTM isolated. It is difficult to know whether those were false-positive LAM results or patients with TB in whom Mycobacterium tuberculosis did not grow in culture. Cross-reactions between LAM and NTM in patients with NTM disease have been previously reported.17,18 On the other hand, a positive LAM may have also been the result of an extrapulmonary or disseminated TB that the study did not assess for. Systematic LAM testing may result in the treatment of some patients who do not have TB, although LAM also identifies patients at higher risk of mortality, and the high mortality risk in severely immunosuppressed and LAM-positive patients may make this compromise worthwhile in high TB prevalence groups. In our study, the overall proportion of confirmed TB cases was low. However, some TB cases may have been missed among LAM-positive patients who were only investigated for pulmonary TB and among LAM-negative who did not receive Xpert or culture. The higher mortality among LAM-positive patients may be due to disseminated TB or could reflect immune-altered responses in these patients.19,20 Systematic LAM testing should not replace TB symptom screening or discourage molecular or microscopic TB investigation (especially because Xpert has higher sensitivity and detects rifampicin resistance). Rather, our results show that the combination of approaches could be an effective TB diagnosis strategy in severely immunocompromised HIV-positive patients, diagnosing those who would have otherwise been missed, allowing rapid TB treatment initiation and identifying those at higher risk of death.
Some studies of pre-ART patients have found LAM useful for diagnosing TB.7,8,11,13 In our study, half of the LAM-positive patients were on ART, and ART-naive patients did not have a higher likelihood of being LAM positive than those on ART, suggesting systematic LAM’s utility for diagnosing TB in severely immunosuppressed patients, regardless of their ART status. A South African study of patients under HIV care found very low LAM positivity,6 although high degrees of immunosuppression in our study population (median CD4 was 31 cells/µL compared with 111 cells/µL in Hanifa et al. study) may partially explain this difference.
Tuberculosis-lipoarabinomannan has practical advantages: it can be used at the point of care, it uses urine (which can be produced by almost all patients), and results are rapidly available (within 25 minutes).21 Implementing systematic LAM in our setting required a short training time, minimal logistical input, little extra workload for users, and was considered simple to perform.22 Tuberculosis is difficult to diagnose in patients with minor or subclinical symptoms, or those with extrapulmonary disease.23–25 Lipoarabinomannan could also help identifying TB in these patients. However, systematic urine-LAM irrespective of TB symptoms, in severely immunocompromised patients, may be complicated to implement by its dependence on CD4 measurement to select patients. Now that ART is recommended for all HIV patients and VL testing is replacing CD4 monitoring for ART patients, these cell count measures may be less available. A possible solution could be LAM-testing only patients with a higher likelihood of LAM positivity (in this study, they were those seriously ill or with low BMI), although this would mean missing a proportion of the LAM-positive patients.
This study has some limitations. It was conducted in operational research conditions where Xpert (using regular cartridge) and culture were systematically requested only for LAM-positive patients. For others, tests were performed only if requested by study or clinic staff. Therefore, we were unable to assess TB prevalence in the overall population. However, this also shows that, in practice, clinic-level TB symptom screening is often not performed properly, and symptomatic patients are often not further investigated for TB.
Systematic urine-LAM allows for rapid TB treatment initiation in severely immunocompromised, HIV-positive patients and identifies those at a higher risk of death. A diagnostic approach combining TB symptom screening and parallel LAM testing, followed by Xpert in patients with symptoms, could improve TB diagnosis in HIV-positive, severely immunocompromised, ambulatory patients. Immediate TB treatment and close medical monitoring should be ensured for LAM-positive patients. Further research is necessary to determine if this diagnostic and treatment strategy can reduce mortality in this group of patients.
Acknowledgments:
We thank all patients, care providers, and data managers at the study site. We are grateful to the Médecins Sans Frontières staff team and the Ministry of Health and National TB Control Program in Mozambique for their support of this study. Thanks to Emily Lynch and Janet Ousley for editing the manuscript.
REFERENCES
- 1.World Health Organization , 2018. Global Tuberculosis Report 2018. Geneva, Switzerland: WHO. [Google Scholar]
- 2.World Health Organization , 2011. Guidelines for Intensified Tuberculosis Case-Finding and Isoniazid Preventive Therapy for People Living with HIV in Resource-Constrained Settings. Geneva, Switzerland: WHO. [Google Scholar]
- 3.World Health Organization , 2017. Guidelines for Managing Advanced HIV Disease and Rapid Initiation of Antiretroviral Therapy, July 2017. Geneva, Switzerland: WHO. [PubMed] [Google Scholar]
- 4.Peter JG, et al. 2016. Effect on mortality of point-of-care, urine-based lipoarabinomannan testing to guide tuberculosis treatment initiation in HIV-positive hospital inpatients: a pragmatic, parallel-group, multicountry, open-label, randomised controlled trial. Lancet 387: 1187–1197. [DOI] [PubMed] [Google Scholar]
- 5.Gupta-Wright A, et al. 2018. Rapid urine-based screening for tuberculosis in HIV-positive patients admitted to hospital in Africa (STAMP): a pragmatic, multicentre, parallel-group, double-blind, randomised controlled trial. Lancet 392: 292–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hanifa Y, et al. 2016. Diagnostic accuracy of lateral flow urine LAM assay for TB screening of adults with advanced immunosuppression attending routine HIV care in South Africa. PLoS One 11: e0156866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yoon C, et al. 2019. Yield and efficiency of novel intensified tuberculosis case-finding algorithms for people living with HIV. Am J Respir Crit Care Med 199: 643–650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Floridia M, et al. 2017. Tuberculosis case finding with combined rapid point-of-care assays (Xpert MTB/RIF and determine TB LAM) in HIV-positive individuals starting antiretroviral therapy in Mozambique. Clin Infect Dis 65: 1878–1883. [DOI] [PubMed] [Google Scholar]
- 9.Drain PK, Losina E, Coleman SM, Giddy J, Ross D, Katz JN, Bassett IV, 2016. Rapid urine lipoarabinomannan assay as a clinic-based screening test for active tuberculosis at HIV diagnosis. BMC Pulm Med 16: 147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bjerrum S, Kenu E, Lartey M, Newman MJ, Addo KK, Andersen AB, Johansen IS, 2015. Diagnostic accuracy of the rapid urine lipoarabinomannan test for pulmonary tuberculosis among HIV-infected adults in Ghana-findings from the DETECT HIV-TB study. BMC Infect Dis 15: 407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Drain PK, Losina E, Coleman SM, Giddy J, Ross D, Katz JN, Walensky RP, Freedberg KA, Bassett IV, 2014. Diagnostic accuracy of a point-of-care urine test for tuberculosis screening among newly-diagnosed HIV-infected adults: a prospective, clinic-based study. BMC Infect Dis 14: 110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Balcha TT, Winqvist N, Sturegård E, Skogmar S, Reepalu A, Jemal ZH, Tibesso G, Schön T, Björkman P, 2014. Detection of lipoarabinomannan in urine for identification of active tuberculosis among HIV-positive adults in Ethiopian health centres. Trop Med Int Health 19: 734–742. [DOI] [PubMed] [Google Scholar]
- 13.Lawn SD, Kerkhoff AD, Vogt M, Wood R, 2012. Diagnostic accuracy of a low-cost, urine antigen, point-of-care screening assay for HIV-associated pulmonary tuberculosis before antiretroviral therapy: a descriptive study. Lancet Infect Dis 12: 201–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bjerrum S, Schiller I, Dendukuri N, Kohli M, Nathavitharana RR, Zwerling AA, Denkinger CM, Steingart KR, Shah M, 2019. Lateral flow urine lipoarabinomannan assay for detecting active tuberculosis in people living with HIV. Cochrane Database Syst Rev 10: CD011420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.World Health Organization , 2019. Lateral Flow Urine Lipoarabinomannan Assay (LF-LAM) for the Diagnosis of Active Tuberculosis in People Living with HIV–Policy Update (2019). Available at: http://apps.who.int/bookorders. Accessed November 14, 2019. [Google Scholar]
- 16.Firth D, 1993. Bias reduction of maximum likelihood estimates. Biometrika 80: 27–38. [Google Scholar]
- 17.Nel JS, Lippincott CK, Berhanu R, Spencer DC, Sanne IM, Ive P, 2017. Does disseminated nontuberculous mycobacterial disease cause false-positive determine TB-LAM lateral flow assay results? A retrospective review. Clin Infect Dis 65: 1226–1228. [DOI] [PubMed] [Google Scholar]
- 18.Gupta-Wright A, Kerkhoff AD, Meintjes G, Corbett EL, 2018. Urinary lipoarabinomannan detection and disseminated nontuberculous mycobacterial disease. Clin Infect Dis 66: 158. [DOI] [PubMed] [Google Scholar]
- 19.Gupta-Wright A, Peters JA, Flach C, Lawn SD, 2016. Detection of lipoarabinomannan (LAM) in urine is an independent predictor of mortality risk in patients receiving treatment for HIV-associated tuberculosis in sub-Saharan Africa: a systematic review and meta-analysis. BMC Med 14: 53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lawn SD, Gupta-Wright A, 2015. Detection of lipoarabinomannan (LAM) in urine is indicative of disseminated TB with renal involvement in patients living with hiv and advanced immunodeficiency: evidence and implications. Trans R Soc Trop Med Hyg 110: 180–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lawn SD, 2012. Point-of-care detection of lipoarabinomannan (LAM) in urine for diagnosis of HIV-associated tuberculosis: a state of the art review. BMC Infect Dis 12: 103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mathabire Rucker SC, et al. 2019. Feasibility of using Determine TB-LAM to diagnose tuberculosis in HIV-positive patients in programmatic conditions: a multisite study. Glob Health Action 12: 1672366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kranzer K, Houben RM, Glynn JR, Bekker L-G, Wood R, Lawn SD, 2010. Yield of HIV-associated tuberculosis during intensified case finding in resource-limited settings: a systematic review and meta-analysis. Lancet Infect Dis 10: 93–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mtei L, et al. 2005. High rates of clinical and subclinical tuberculosis among HIV-infected ambulatory subjects in Tanzania. Clin Infect Dis 40: 1500–1507. [DOI] [PubMed] [Google Scholar]
- 25.Wood R, Middelkoop K, Myer L, Grant AD, Whitelaw A, Lawn SD, Kaplan G, Huebner R, McIntyre J, Bekker L-G, 2007. Undiagnosed tuberculosis in a community with high HIV prevalence: implications for tuberculosis control. Am J Respir Crit Care Med 175: 87–93. [DOI] [PMC free article] [PubMed] [Google Scholar]