Abstract
Antiphospholipid autoantibodies (aPLs), a major maternal risk factor for preeclampsia, are taken into the syncytiotrophoblast where they bind intracellular vesicles and mitochondria. Subsequently, large quantities of extracellular vesicles (EVs) extruded from syncytiotrophoblast into the maternal circulation are altered such that they cause maternal endothelial cell activation. However, the mechanism driving this change is unknown. First trimester placental explants were treated with aPL for 18 h. The EVs were then collected by different centrifugation. The levels of HSP 70, misfolded proteins, caspase 8 activity, and Mixed Lineage Kinase domain-Like (MLKL) were measured in placental explants and EVs. In addition, the levels of TNF-α and CD95 in conditioned medium were also measured. Treating placental explants with aPL caused an increase in levels of HSP 70, misfolded proteins and MLKL in placental explants and EVs. Increased activity of caspase 8 was also seen in placental explants. Higher levels of TNF-α were seen conditioned medium from aPL-treated placental explant cultures. aPLs appear to induce endoplasmic reticulum stress in the syncytiotrophoblast in a manner that involved caspase 8 and TNF-α. To avoid accumulation of the associated misfolded proteins and MLKL, the syncytiotrophoblast exports these potentially dangerous proteins in EVs. It is likely that the dangerous proteins that are loaded into placental EVs in preeclampsia contribute to dysfunction of the maternal cells.
Keywords: antiphospholipid antibodies, endoplasmic reticulum stress, extracellular vesicle, placenta, preeclampsia
Introduction
Antiphospholipid antibodies are autoantibodies that have been reported to increase a woman’s risk of developing preeclampsia, a human pregnancy specific disorder, up to 10-fold [1]. These antibodies also cause recurrent miscarriage and stillbirth [2]. However, the underlying mechanisms by which these autoantibodies contribute to the pathologies of pregnancy diseases are still not fully understood. During pregnancy, large quantities of placental extracellular vesicles (EVs) are extruded from placental syncytiotrophoblast into the maternal circulation [3,4]. These EVs contain proteins and nucleic acids and can transfer their cargo to recipient cells/tissues and affect the function of those recipient cells [5–8]. We have previously shown that antiphospholipid antibodies are internalized into the syncytiotrophoblast resulting in the production of toxic EVs [9]. We do not understand how antiphospholipid antibodies induce the production of toxic EVs. However, we have previously shown that once internalized into the syncytiotrophoblast, the antibodies interacted with both mitochondria and unidentified intracellular vesicular structures to consequently alter the proteome of extruded EVs [10]. Whether there is a relationship between these intracellular vesicles and the increased extrusion of EVs in response to antiphospholipid antibodies is not known. Mitochondria are involved in controlling cell death and coordinate and communicate with the endoplasmic reticulum. Increased levels of heat shock protein 70 (HSP70) (also known as GRP78 or HSP5A), a marker of endoplasmic reticulum stress were reported in placentae from women with preeclampsia [11] as well as, in the circulation of women with preeclampsia [12]. We have also recently shown that aggregated transthyretin is specifically increased in EVs released from placentae of women with preeclampsia [13]. Protein aggregation, due to misfolding, is another feature of endoplasmic reticulum stress. Increased endoplasmic reticulum stress may also result from the production of pro-inflammatory molecules, such as TNF-a [14,15]. We have previously showed that TNF-a induced the production of toxic placental EVs, consequently induced maternal endothelial cell activation.
Since the syncytiotrophoblast is a single multinucleated cell that covers the entire placenta, its death would likely result in loss of the pregnancy and the production of dangerous/toxic EVs by the syncytiotrophoblast has been suggested to be a mechanism that allows this massive cell to eliminate toxins. We hypothesized that production of toxic placental EVs induced by antiphospholipid antibodies due to a change in the pathways associated with EV biogenesis in the syncytiotrophoblast.
Methods
This investigation received the approval of the Northern X Health and Disabilities Ethics Committee, New Zealand (NTX/12/06/057/AM06) and conforms to the principles outlined in the Declaration of Helsinki. All patient-derived tissues were obtained following written informed consent.
Collection of placentae
First trimester placentae (total n = 45) were collected from elective surgical terminations of on-going pregnancies ranging from 8 to 12 weeks of gestation from Epsom Day Unit, Auckland City Hospital. The placenta from a woman with Antiphospholipid Syndrome and a healthy term placenta were collected from National Women’s Health, Auckland City Hospital, New Zealand.
Antiphospholipid and control antibodies
The murine monoclonal antiphospholipid antibodies, ID2 and IIC5, were produced in our laboratory. The hybridomas were cultured and the monoclonal antibodies purified on HiTrap Protein G columns (GE Healthcare) as previously described [16]. These monoclonal antiphospholipid antibodies have been extensively characterized and have anticardiolipin, anti β2GPI and lupus anticoagulant activities, and are thus triple positive antiphospholipid antibodies [10]. A murine monoclonal IgG1 isotype antibody (Life Technologies, Auckland) was used as isotype-matched treatment control antibody in experiments involving ID2 and IIC5. The concentration of ID2 or IIC5 or antibody control IgG used in the present study was 25 μg/ml. This level of antiphospholipid antibodies in a patient would be considered to be a low positive [17].
Culture of placental explants and preparation of placental EVs
Placental explants (approximately 400 mg) collected from elective surgical terminations (intrauterine suction) were dissected and cultured in Netwell™ culture inserts (400 μm mesh) in 12-well culture plates (tissue culture treated, Corning) for 18 h at 37°C in Advanced DMEM/F12 containing 2% FBS in an ambient oxygen atmosphere containing 5% CO2, in the presence or absence of ID2 or IIC5 or IgG as described previously [18–20]. In some experiments, placental explants were cultured with ID2 or IIC5 or IgG in the presence of the caspase 8 Inhibitor (Z-IETD-FMK) (10 ng/ml) or the MLKL inhibitor, necrosulfonamide (NSA) (1 μM). After 18 h, the Netwell™ inserts (containing the explants) were then removed and the tissues were collected and stored at −20°C. Half of the tissue was used for staining and half of the issue was lysed with RIPA buffer (50 mM Tris, 150 mM NaCl, 1% sodium deoxycholate, 0.1% SDS, 1% Nonident P40 substitute, protease inhibitor and 1 mM phenylmethanesulfonylfluoride) for Western blotting.
The conditioned media from above cultures were aspirated and macrovesicles were removed by centrifuging at 2000 g for 5 min. The supernatant was then centrifuged at 20,000 g for 1 h for collection of micro-EVs. The supernatant was further centrifuged at 100,000 g for 1 h for collection of nano-EVs (Avanti J30I Ultracentrifuge, JA 30.50 fixed angle rotor, Beckman Coulter, New Zealand).
Measurement of caspase 8 activity
The activity of caspase 8 in placental explants that had been treated with ID2 or IIC5 or IgG was measured by Fluorescent FLICA kit according to the manufacturer’s instructions (Bio-Rad, Auckland).
Measurement of soluble TNF-α and CD95 in conditioned media
The levels of TNF-α and CD95 (Fas-ligand) in conditioned media from first placental explants that had been treated with ID2 or IIC5 or IgG were measured by ELISA according to the manufacturer's instructions (R & D, New Zealand). All the samples were examined in duplicate and the investigators were not blinded to the treatment. The coefficient of variation (CV%) for the ELISAs measuring TNF-α or CD95 was less than 10%.
Measurement of misfolded proteins
Misfolded proteins in placental explants that had been treated with ID2 or IIC5 or IgG were measured using the fluorescent compound, Thioflavin-T (ThT), which has been suggested to use for detection endoplasmic reticulum stress (Sigma-Aldrich, Australia) as described previously [21]. Briefly, frozen placental explants were sectioned and fixed with 4% paraformaldehyde (PFA) for 5 min in room temperature and washed with PBS. Sections were then stained with ThT (500 μM) for 3 min at room temperature. After PBS washing, sections were then counter-stained with DAPI for 1 min. In some experiments, a term placenta from a woman with Antiphospholipid Antibody Syndrome and a healthy term placenta were collected and sectioned. Sections were then also stained with ThT. The sections were then examined by fluorescent microscope under the same setting (Nikon, ECLISPE Ni-E motorized fluorescent microscope Japan). Placental explants that were treated with Thapsigargin (1 μM) were used as positive control for endoplasmic reticulum stress [22]
Misfolded proteins in EVs collected from placental explant cultures described above were stained with ThT (5 μM) for 10 min and read in a fluorescent plate reader at 485 nm (Synergie 2, BioTek, Auckland, New Zealand), following a previous report [21].
Measurement of HSP 70 and MLKL by Western blotting
The relative levels of HSP70 and Mixed Lineage Kinase domain-Like (MLKL) in placental explants or in placental EVs from first trimester placental explants that had been treated with ID2 or IIC5 were measured by Western blotting. Proteins from placental explants or placental EVs were extracted with RIPA buffer and all samples (20 μg of total protein) were loaded on 10% SDS-PAGE gels and electrophoresed then transferred to nitrocellulose membranes. Non-specific binding was blocked by incubating membranes in 3% BSA in PBST for 1 h at room temperature and then membranes were incubated with rabbit polyclonal anti human HSP70 (Abcam, Auckland, 1: 250) or MLKL antibody (ThermoFisher, Auckland, 1:250) in blocking solution for 2 h at room temperature. After washing with PBS-T three times, the membranes were incubated with goat anti rabbit secondary antibody (1:2000) for 1 h at room temperature. After washing with PBS-T, the membranes were incubated with Amersham™ ECL™ Prime Western blotting detection reagent. Chemiluminescence of the membranes was detected by Image Quant LAS3000.
Protein levels of HSP70 and MLKL were analyzed relative to the β-actin loading control. β-Actin was detected on the same membranes as follows. Membranes were stripped with stripping buffer (62.5 mM Tris-Hcl, pH 6.7, 2%SDS and 100 mM 2ME) at 50°C for 30 min then non-specific binding was blocked by incubation in blocking solution for 1 h. Then, the membranes were incubated with mouse anti-human β-actin (1:5000, Abcam, Sapphire Biosciences, Sydney) for 1 h at room temperature. After washing three times with PBST the membranes were incubated with HRP-conjugated goat anti-mouse antibody (1:5000, Jackson Immunoresearch Laboratories, Pennsylvania) for 1 h at room temperature. After washing with PBST, the membranes were incubated with Amersham™ ECL™ Prime Western blotting detection reagent. Chemiluminescence from the membranes was detected using an Image Quant LAS3000 (ThermoFisher, Auckland).
Statistical analysis
The levels of TNF-α or CD95 and the concentration of micro-EVs or nano-EVs were expressed as median and range. The statistical significance in the levels of TNF-α or CD95 and fluorescent intensity of Th T in placental EVs were assessed with Mann–Whitney test or a Kruskal–Wallis test (ANOVA) as appropriate using Prism software package. Analysis of the semi-quantification of Western blots was employed a Mann–Whitney U test using the Prism software package. All the experiments were repeated at least three times. P < 0.05 was considered as statistically significant.
Results
The levels of HSP70 were significantly increased in placental explants and placental EVs following treatment with antiphospholipid antibodies
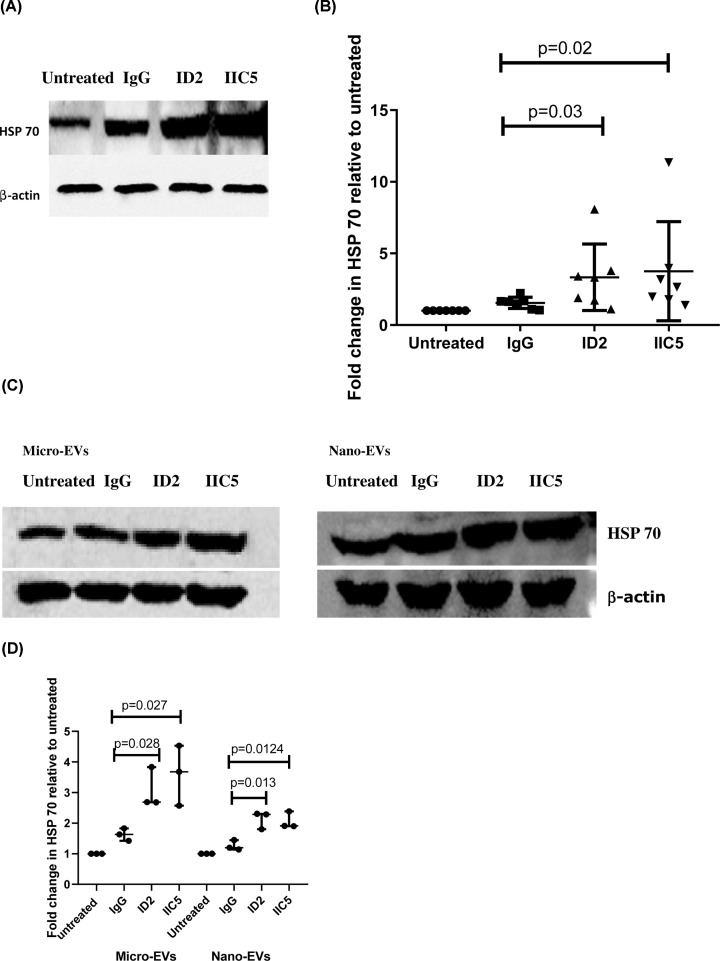
To investigate if antiphospholipid antibodies are one of the factors that lead to increased endoplasmic reticulum stress in preeclampsia, we measured the levels of HSP 70, a sensor of endoplasmic reticulum stress in placental explants that had been treated with antiphospholipid antibodies. Compared with control IgG-treated or untreated explants, the levels of HSP70 were significantly increased in placental explants that had been treated with either ID2 or IIC5 (Figure 1A,B, P = 0.02 or P = 0.03, respectively; n = 7). In addition, the levels of HSP70 in micro-EVs or nano-EVs from placental explants that had been treated with either ID2 or IIC5 were significantly (P < 0.028) increased compared with EVs from control IgG-treated explants (Figure 1C,D).
Figure 1. The increased levels of HSP70 in placental explants or placental EVs follwoing treatment with antiphospholipid antibbodies.
(A) Representative Western blot of placental explants that had been treated with ID2, IIC5 or control isotype-matched IgG or an untreated explant probed with an antibody recognising HSP70. (B) Graph showing the semi-quantitative analysis of the Western blots for HSP70 in treated placental explants following normalization to the levels of β-actin (n = 7). (C) Representative Western blot of EVs from placental explants that had been treated with ID2, IIC5 or control isotype-matched IgG or an untreated explant. (D) Semi-quantitative analysis of the Western blots in C after normalization to levels of β-actin (n = 3). Data show median and range.
Antiphospholipid antibodies induced the production of misfolded proteins in placental explants and EVs extruded from placental explants
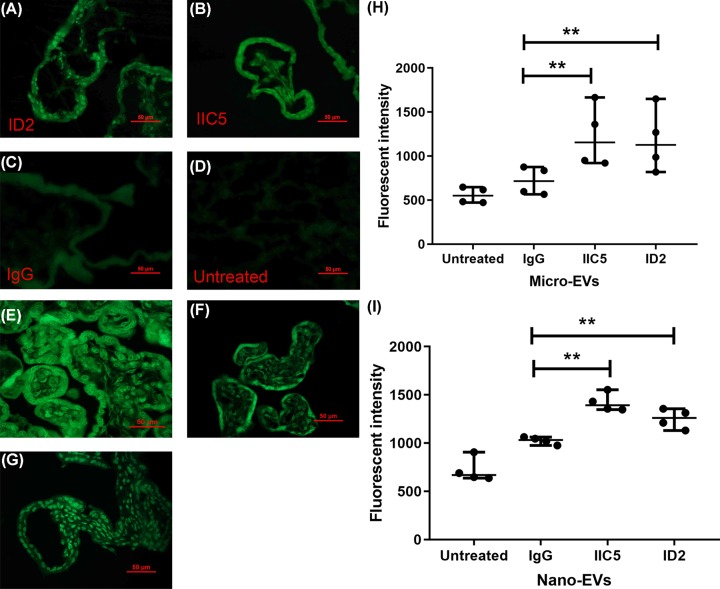
We then measured the misfolded proteins in placental explants that had been treated with ID2 or IIC5 using the fluorescent compound, Thioflavin T (ThT), which has been suggested to use for detection endoplasmic reticulum stress [21]. Compared with control IgG treated or untreated explants, enhanced ThT fluorescent density (green) was detected in placentae that had been treated with either ID2 or IIC5 (Figure 2A–D). We also measured the levels of misfolded proteins in EVs collected from placental explants that had been treated with ID2 or IIC5 or control IgG. The fluorescent intensity was significantly increased in both micro-EVs (Figure 2H, P = 0.028) and nano-EVs (Figure 2I, P = 0.026) from ID2 or IIC5-treated placental explants, compared with control IgG-treated or untreated explants.
Figure 2. The increased levels of misfolded proteins in placental explants and placental EVs following treatment with antiphospholipid antibodies.
Representative ThT staining images of thin sections of placental explants showing that the levels of misfolded proteins (green) were increased in placentae that had been treated with ID2 (A) or IIC5 (B), compared with isotype-matched control IgG (C) or untreated (D) (n = 4). (E) ThT staining images of thin sections of a placenta from a woman with antiphospholipid antibody syndrome or (F) a healthy term placenta. (G) first trimester placental explants was treated with Thapsigargin (1 μM) as positive control of ER stress. Magnification bar = 50 μm. (H and I) ThT fluorescent intensity analysis showing significantly increased fluorescent intensity in EVs collected from placental explants that had been treated with either ID2 or IIC5, compared with an isotype-matched control IgG or untreated explants (n = 4). Data show median and range.
To confirm this effect of antiphospholipid antibodies on increased endoplasmic reticulum stress was not just an in vitro effect, we also demonstrated increased misfolded proteins in the placenta from a woman with obstetric antiphospholipid antibody syndrome (Figure 2E), compared with a healthy term placenta (Figure 2F).
Antiphospholipid antibodies increased the production of TNF-α by placental explants
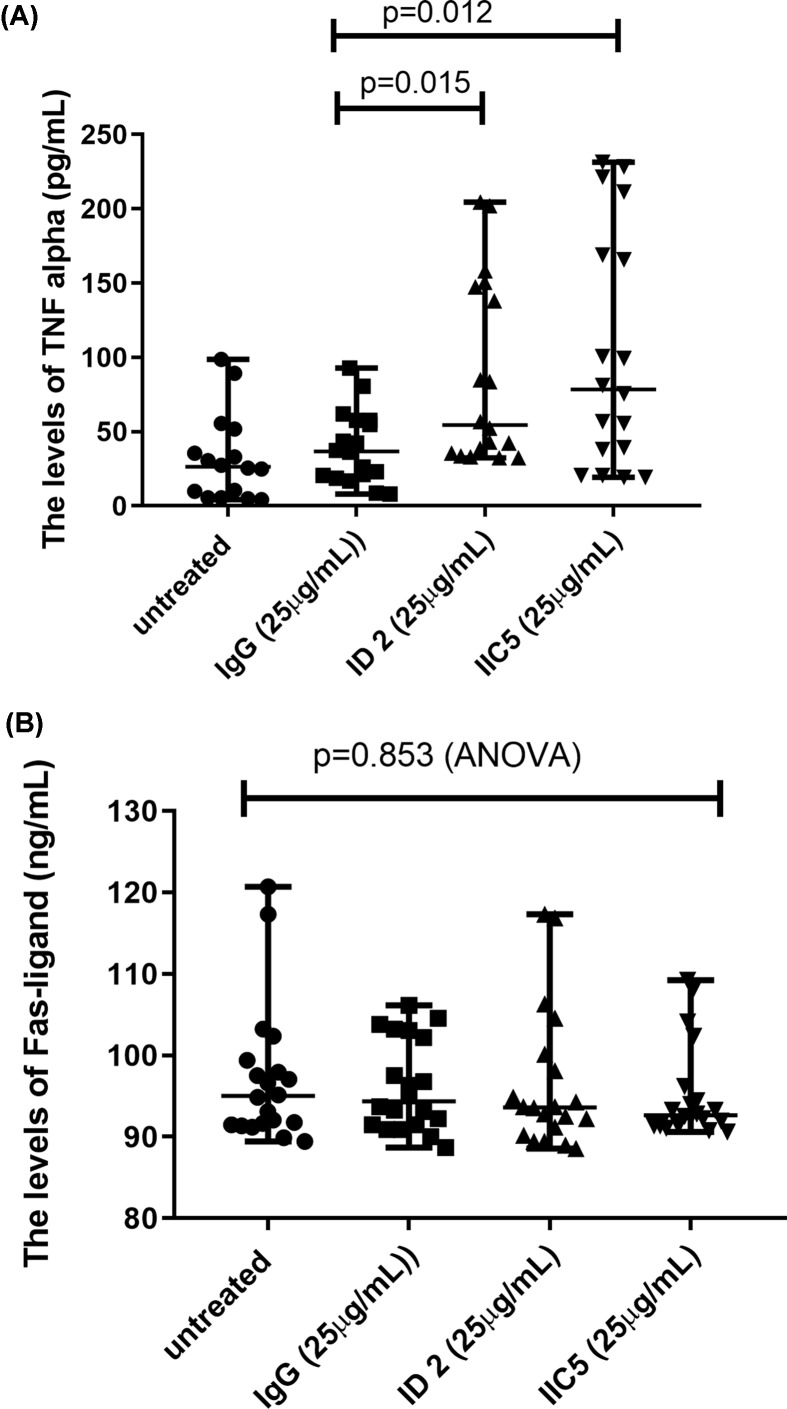
Since previous studies have linked endoplasmic reticulum stress with increased production of TNF-α [14,15], we measured the levels of TNF-α and Fas-ligand (CD95L) in the conditioned media from placental explants that had been treated with antiphospholipid antibodies. The levels of TNF-α were significantly increased in conditioned medium from placental explants that had been treated with either ID 2 or IIC5, compared with control IgG-treated or untreated explants (Figure 3A, P = 0.012 or P = 0.015, respectively). However, the levels of Fas-ligand (CD95) were not changed in the conditioned medium among the groups (Figure 3B, P = 0.853, ANOVA).
Figure 3. Increased levels of TNF-α in placental explant cultures following treatment with antiphospholipid antibodies.
The levels of (A) TNF-α, but not (B) Fas-ligand were significantly increased in conditioned medium from placental explants that had been treated with either ID 2 or IIC5, compared with isotype-matched control IgG-treated or untreated explants. Data show median and range.
The activity of caspase 8 was increased in placentae that had been treated with antiphospholipid antibodies
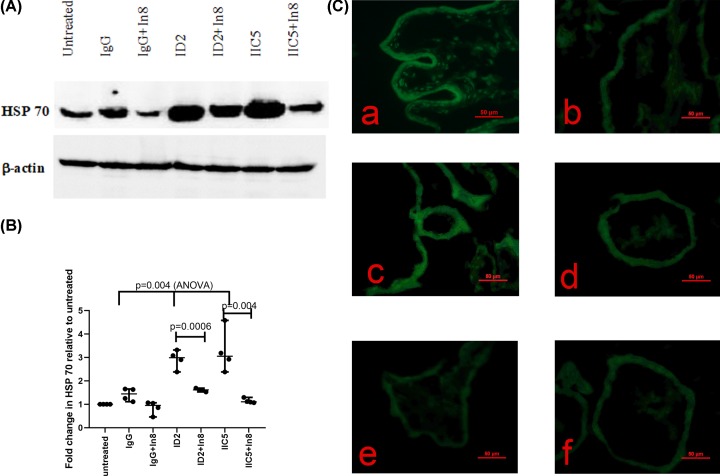
Since TNF-α can activate the extrinsic apoptosis pathway via caspase 8, we then investigated whether there was an increase in caspase 8 activity in the syncytiotrophoblast of placental explants that had been treated with antiphospholipid antibodies. The activity of caspase 8 was increased in placental explants that had been treated with ID2 or IIC5, compared with explants that had been treated with control IgG or that were untreated (Figure 4). Blocking the activity of caspase 8 in antiphospholipid antibody (ID2 or IIC5)-treated placental explants resulted in the reversal of the increase in the levels of HSP 70 (Figure 5A,B) and protein misfolding as measured by ThT fluorescent density (Figure 5C).
Figure 4. Increased activity of caspase 8 in placental explants following treatment with antiphospholipid antibodies.
The activity of caspase 8 in placental explants that had been treated with ID2 (A) or IIC5 (B) was significantly increased, compared with treatment with isotype-matched control IgG (C) (n = 4). Magnification bar = 50 μm.
Figure 5. Caspase 8 activity involving in the increased levels of HSP70 and misfoled protiens in placental explants following treatment with antiphospholipid antibodies.
(A) Representative Western blot demonstrating that the levels of HSP70 were significantly reduced in placental explants that had been treated with either ID2 or IIC5 in the presence of caspase 8 inhibitor (In8). (B) These changes were measured by semi-quantitative analysis after normalising to levels of β-actin. Data show median and range. (C) The increased misfolded proteins in placental explants that had been treated with ID2 (a) or IIC5 (c), compared with treatment with isotype-matched control IgG (e), was reduced in the presence of caspase 8 inhibitor (b or d). Panel (f) is the treatment with isotype-matched control IgG in the presence of caspase 8 inhibitor (n = 4). Magnification bar = 50μm.
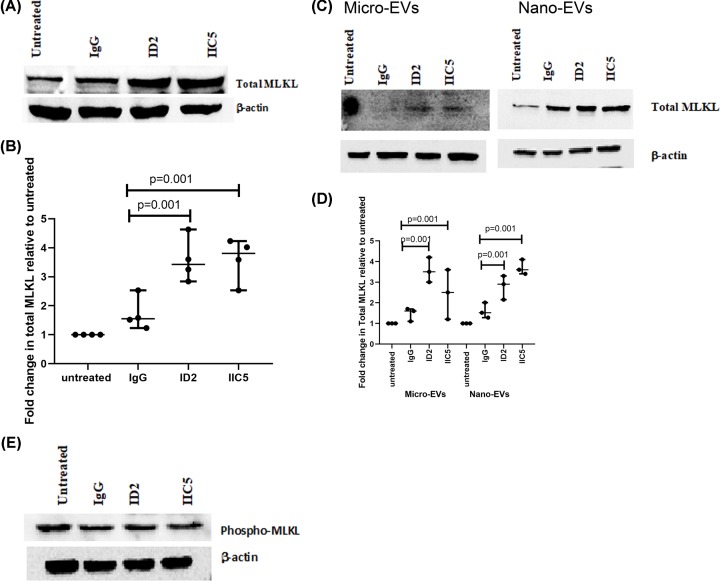
The levels of MLKL, a protein involved in vesicle production and necroptosis, were increased in placental explants and EVs that had been treated with antiphospholipid antibodies
In addition to triggering apoptosis, endoplasmic reticulum stress-induced TNF-α can also trigger the necroptosis programmed cell death pathway via phosphorylation of the mixed lineage kinase domain-like protein (MLKL) [23,24]. Consequently, we investigated the levels of MLKL in placental explants that had been treated with antiphospholipid antibodies and found that compared with either control IgG-treated or untreated explants, the levels of total MLKL in placental explants that had been treated with either ID2 or IIC5 were significantly increased (Figure 6A,B). However, this treatment did not increase the levels of phospho-MLKL that is required for necroptosis (Figure 6E). Paralleling the increase in cellular MLKL, the levels of total MLKL in micro-EVs and nano-EVs extruded from ID2 or IIC5 treated placental explants were also significantly (Figure 6C,D, P < 0.001) increased, compared with levels of MLKL in vesicles from control IgG treated explants (Figure 6C,D).
Figure 6. Increased levels of total MLKL in placental explants and placental EVs following treatment with antiphospholipid antibodies.
Representative Western blots demonstrating that the levels of total MLKL were significantly increased in placental explants (A) or in micro-EVs and nano-EVs (C) from placental explants that had been treated with ID2, IIC5, control isotype-matched IgG or an untreated explant. (B and D) Semi-quantitative analysis of the Western blots in (A) and (B) respectively, after normalization to levels of β-actin (n = 3). Data show median and range. (E) Representative Western blot demonstrating that the levels of phosphor-MLKL were not significantly altered in explants that had been treated with ID2, IIC5, control isotype-matched IgG or an untreated explant (n = 4).
Discussion
The human placenta is entirely covered by a unique single multinucleated cell, the syncytiotrophoblast that has a surface area of some 11–13 m2 in a normal pregnancy at term [25]. The syncytiotrophoblast functions to effect transfer between mother and the fetus and also to protect the placenta/fetus from the maternal immune system such that, having an intact syncytiotrophoblast is essential for human pregnancy. When a mononuclear cell is stressed, there are a number of mechanisms that the cell can call upon to cope with the stress. If these mechanisms fail, the cell can die [26] with little or no effect on the organ and indeed it is estimated that 10 billion cells must die daily to maintain homoeostasis in humans [27]. However, since it is a single cell, death of the syncytiotrophoblast would cause the loss of the pregnancy. It has previously been suggested that rather than dying, the syncytiotrophoblast extrudes damaged/stressed parts of the cell in EVs possibly using components of the machinery that would lead to death in other cells [28,29]. Some mononuclear cells also employ EVs to remove dangerous material including misfolded proteins [30] or promoters of cell death from the cell [31]. The syncytiotrophoblast is bathed in maternal blood and produces vast quantities of EVs in normal pregnancy. These EVs are extruded directly into the maternal blood and target specific maternal organs [5,6]. We have previously shown that EVs produced by the syncytiotrophoblast in normal pregnancies are tolerogenic to both immune and endothelial cells, but EVs from preeclamptic placentae are dangerous/toxic and activate endothelial cells [32–35]. We hypothesize this change in the nature of the EVs may be due to a shift in the pathways associated with EV biogenesis in the syncytiotrophoblast.
Antiphospholipid antibodies enter the syncytiotrophoblast in a receptor-mediated, antigen-dependent fashion and we have previously shown that this process does not involve Fc receptors [9]. Once internalized into the syncytiotrophoblast antiphospholipid antibodies associate with both mitochondria and, as yet, unidentified vesicular structures [9,10]. The antibodies then induce the extrusion of increased numbers of dangerous/toxic EVs that cause the activation of maternal endothelial cells [36]. This suggests that the antibodies are inducing stress in the syncytiotrophoblast and here we show an increase in total misfolded proteins, as well as, an increase in HSP70 (as known as GRP 78 or HSP5A), a marker of endoplasmic reticulum stress, in placental explants that had been treated with antiphospholipid antibodies. Confirming that this is not just an in vitro effect, the placentae from women with Antiphospholipid Antibody Syndrome contained a substantially higher level of misfolded proteins than control placentae. Endoplasmic reticulum stress is characterized by the misfolding of proteins in the endoplasmic reticulum and a physiologic response called the unfolded protein response [37,38]. Thus, it seems that one mechanism by which antiphospholipid antibodies affect placental function is by increasing endoplasmic reticulum stress in the syncytiotrophoblast. Since antiphospholipid antibodies are a major risk factor for a number of pregnancy complications, these findings fit with reports of increased endoplasmic reticulum stress in preeclamptic placentae [39]. In addition, the EVs extruded from placentae after treatment with antiphospholipid antibodies contained increased amounts of both total misfolded proteins and HSP70. These misfolded proteins are potentially toxic to the syncytiotrophoblast and, to avoid that toxicity, it appears they are packaged into EVs for removal. This seems likely to be a mechanism by which cellular homoeostasis is maintained in the stressed syncytiotrophoblast and is consistent with the finding that endoplasmic reticulum stress in BeWo (choriocarcinoma) cells as well as, other non-placental cells resulted in the extrusion of an increased number of EVs that were dangerous/toxic [30,40]. While removal of misfolded proteins via EVs may protect the stressed syncytiotrophoblast, these misfolded proteins are potentially toxic to the maternal cells that are the targets of the placental EVs.
Due to their interaction with mitochondria [10], we anticipated that treating placental explants with antiphospholipid antibodies would increase the activation of caspase 9 and that did occur (unpublished). Unexpectedly, we also saw an increase in the activity of caspase 8 which is usually triggered via the actions of TNF-α or similar molecules. In other cells, endoplasmic reticulum stress has been shown to induce the production of TNF-α which then acts in an autocrine fashion to activate caspase 8 [41]. We demonstrated a similar increase in TNF-α secretion by the syncytiotrophoblast following treatment with antiphospholipid antibodies. However, activation of caspase 8 cannot lead to apoptotic death of the entire syncytiotrophoblast and instead we believe it contributed to the extrusion of toxic EVs and inhibiting the activity of caspase 8 reduced the amount of misfolded proteins and HSP70 in explants treated with antiphospholipid antibodies.
While caspase 8 is typically known for its role in the induction of apoptosis, this caspase also participates in other processes one of which is the balance between apoptosis and necroptosis [42]. Necroptosis is a relatively newly discovered pathway of regulated cell death. Cells dying via necroptosis swell and release danger signals that promote sterile inflammation. While necroptosis can be initiated by multiple ligands binding to death receptors, TNFα-mediated necroptosis is the most studied. TNFα binding to it receptor recruits the adaptor protein, Tumor necrosis factor Receptor type 1-Associated DEATH Domain (TRADD) and the Receptor Interacting Protein Kinase -1 (RIPK1). This death complex may then activate caspase 8-dependent apoptosis [43]. Alternatively, when caspase 8 is unavailable, RIPK1 can recruit and phosphorylate an additional kinase, RIPK3, which in turn phosphorylates the MLKL protein and these three proteins constitute the canonical necroptosome [43]. Phospho-MLKL forms multimers that bind to negatively charged phospholipids (including cardiolipin part of the antigen for antiphospholipid antibodies) and permeabilize the plasma membrane of cells leading to rupture of the cell [44,45]. In a separate process, independent of phosphorylation by RIPK3, MLKL is involved in the trafficking of intracellular vesicles to the cell surface for release as exosomes [31,46]. We found that after treatment of placental explants with antiphospholipid antibodies there was a significant increase in the amount of total, but not phosphorylated, MLKL in the explants, as well as, in both micro-EVs and micro-EVs extruded from the explants hinting at involvement of MLKL in the export of both micro-EVs and micro-EVs from the syncytiotrophoblast. Both RIPK1 and RIPK3 contain caspase 8 cleavage sites and it seems likely that, although total MLKL was increased in response to antiphospholipid antibodies, the concurrent increase in caspase 8 activity induced indirectly by antiphospholipid antibodies resulted in inactivation of RIPK1 and/or RIPK3 such that the necroptosis pathway was not activated. Despite the lack of phosphorylation, an accumulation of MLKL in the syncytiotrophoblast would pose a threat to the cell since, if caspase 8 was overwhelmed or inhibited, activation of a large pool of MLKL by the RIPKs would lead to necroptosis of the syncytiotrophoblast. Our observations suggest that the stress induced by antiphospholipid antibodies in the syncytiotrophoblast resulted in up-regulation of a second pathway related to cell death, in this case necroptosis, but instead of inducing the death of the syncytiotrophoblast there was increased export of EVs containing MLKL to reduce the potential harm from this protein.
The syncytiotrophoblast produces both micro-EVs and nano-EVs (exosomes are a subset of nano-EVs). While the biogenesis of exosomes is well studied, the processes leading to micro-EVs and non-exosomal nano-EV formation are not well known. It is generally assumed that micro-EVs are formed by the outward budding of the plasma membrane [3,47]. It is also assumed that, due to their different origins, exosomes and micro-EVs will have different cargos. Here we show that both micro-EVs and nano-EVs are apparently being used to export harmful misfolded proteins as well as, the potentially harmful, MLKL from the syncytiotrophoblast. Thus, the stressed syncytiotrophoblast appears to be using multiple strategies, involving different EVs and export pathways, to protect itself.
If, following exposure to antiphospholipid antibodies, the syncytiotrophoblast is not functioning adequately because it is responding to endoplasmic reticulum stress, this may cause reduced nutrient/gas transfer across the placenta and lead to fetal growth restriction, one of the complications that occurs in women with Antiphospholipid antibody Syndrome [48]. In addition, the export of both micro-EVs and nano-EVs containing misfolded proteins may harm the maternal cells that take up these vesicles [5,49]. We have previously shown that EVs from placentae treated with antiphospholipid antibodies contain increased amounts of various danger signals including mitochondrial DNA [50] and HMGB1 [51]. The increased load of misfolded proteins delivered to endothelial cells by EVs from antiphospholipid antibody-affected placentae may be an another factor that contributes to the endothelial cell activation that is a hallmark of preeclampsia, or in maternal organ damage.
Conclusion
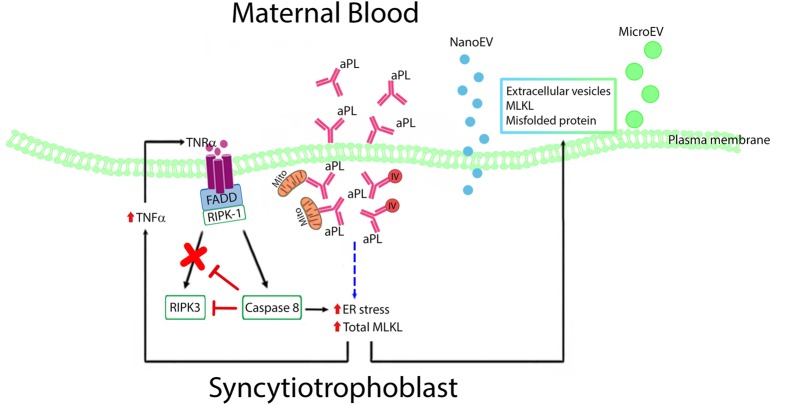
Here, we have shown that antiphospholipid antibodies induce endoplasmic reticulum stress and secretion of TNFα in the syncytiotrophoblast. The antibodies also induced caspase 8 activity and blocking this activity reduced endoplasmic reticulum stress suggesting an autocrine cycle involving caspase 8, TNFα and endoplasmic reticulum stress is induced by antiphospholipid antibodies in the syncytiotrophoblast. The antibodies also caused increased expression of MLKL which could has the potential to induce necroptotic cell death. The syncytiotrophoblast responded to these stresses by increasing the export of dangerous misfolded proteins and MLKL via both micro-EVs and nano-EVs (Figure 7). We suggest that since the single syncytiotrophoblast that covers the entire placenta cannot die without causing pregnancy loss this unique cell uses components of programmed death pathways to increase the export of micro-EVs that contain proteins/molecules that if retained would threaten the survival of this enormous multinucleated cell.
Figure 7. Proposed mechanisms of antiphospholipid antibodies on production of toxic placental EVs.
Cartoon demonstrating that antiphospholipid antibodies are internalized into the syncytiotrophoblast, bind to mitochondria (Mito) and intracellular vesicles (IVs) inducing endoplasmic reticulum stress, TNF-α secretion, and caspase 8 activation. This establishes a feed-forward cycle involving caspase 8, TNF-α and endoplasmic reticulum stress. These actions lead to the syncytiotrophoblast exporting dangerous misfolded proteins and MLKL in both micro-EVs and nano-EVs.
Clinical perspectives
Antiphospholipid antibodies are one of the main maternal factors for developing pregnancy complications.
Although we have previously reported that antiphospholipid antibodies are internalized into the syncytiotrophoblast resulting in the production of toxic EVs, which then induce maternal endothelial cell activation, the underlying mechanism of production of toxic EVs is unclear.
Endoplasmic reticulum stress is associated with placental dysfunction which is seen in preeclampsia.
In the present study, we found that antiphospholipid antibodies induced endoplasmic reticulum stress and secretion of TNF-α in the syncytiotrophoblast and caspase 8 was involved in endoplasmic reticulum stress. Our study suggests a potential mechanism of antiphospholipid antibodies being a maternal risk factor for developing preeclampsia.
Acknowledgements
The authors thank the patients and staff of the Epsom Day Unit, Green Lane Hospital, and the Auckland Medical Aid Centre for the donation and collection of placentae.
Abbreviations
- aPLs
antiphospholipid autoantibodies
- CV%
coefficient of variation
- EV
extracellular vesicle
- HSP70
heat shock protein 70
- MLKL
mixed lineage kinase domain-like
- ThT
Thioflavin-T
Contributor Information
Yunhui Tang, Email: 945808953@qq.com.
Qi Chen, Email: q.chen@auckland.ac.nz.
Competing Interests
The authors declare that there are no competing interests associated with the manuscript.
Funding
This work was supported by grants from the Auckland Medical Research Foundation and the Health Research Council of New Zealand. Y.T. was funded by training grants from The Hospital of Obstetrics and Gynaecology, Fudan University, China.
Open Access
Open access for this article was enabled by the participation of University of Auckland in an all-inclusive Read & Publish pilot with Portland Press and the Biochemical Society under a transformative agreement with CAUL.
Author Contribution
All authors were involved in the manuscript preparation. In addition, Y.T.: performed the experiments, Y.C.: Assisted to perform experiments, Y.N.: designed the summary cartoon, K.G. and A.H.: participated the study design, L.C. and Q.C.: design the study and wrote up the manuscript.
References
- 1.Noris M., Perico N. and Remuzzi G. (2005) Mechanisms of disease: pre-eclampsia. Nat. Clin. Pract. Nephrol. 1, 98–114quiz 20 10.1038/ncpneph0035 [DOI] [PubMed] [Google Scholar]
- 2.Branch D.W., Dudley D.J., Mitchell M.D., Creighton K.A., Abbott T.M., Hammond E.H. et al. (1990) Immunoglobulin G fractions from patients with antiphospholipid antibodies cause fetal death in BALB/c mice: a model for autoimmune fetal loss. Am. J. Obstet. Gynecol. 163, 210–216 10.1016/S0002-9378(11)90700-5 [DOI] [PubMed] [Google Scholar]
- 3.Tong M. and Chamley L.W. (2015) Placental extracellular vesicles and feto-maternal communication. Cold Spring Harb. Perspect. Med. 5, a023028 10.1101/cshperspect.a023028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Covone A.E., Mutton D., Johnson P.M. and Adinolfi M. (1984) Trophoblast cells in peripheral blood from pregnant women. Lancet 2, 841–843 10.1016/S0140-6736(84)90875-4 [DOI] [PubMed] [Google Scholar]
- 5.Tong M., Chen Q., James J.L., Wise M.R., Stone P.R. and Chamley L.W. (2017) In vivo targets of human placental micro-vesicles vary with exposure time and pregnancy. Reproduction 153, 835–845 10.1530/REP-16-0615 [DOI] [PubMed] [Google Scholar]
- 6.Tong M., Stanley J.L., Chen Q., James J.L., Stone P.R. and Chamley L.W. (2017) Placental Nano-vesicles Target to Specific Organs and Modulate Vascular Tone In Vivo. Hum. Reprod. 32, 2188–2198 10.1093/humrep/dex310 [DOI] [PubMed] [Google Scholar]
- 7.Wei J., Blenkiron C., Tsai P., James J.L., Chen Q., Stone P.R. et al. (2017) Placental trophoblast debris mediated feto-maternal signalling via small RNA delivery: implications for preeclampsia. Sci. Rep. 7, 14681 10.1038/s41598-017-14180-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wei J., Lau S.Y., Blenkiron C., Chen Q., James J.L., Kleffmann T. et al. (2016) Trophoblastic debris modifies endothelial cell transcriptome in vitro: a mechanism by which fetal cells might control maternal responses to pregnancy. Sci. Rep. 6, 30632 10.1038/srep30632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Viall C.A., Chen Q., Liu B., Hickey A., Snowise S., Salmon J.E. et al. (2013) Antiphospholipid antibodies internalised by human syncytiotrophoblast cause aberrant cell death and the release of necrotic trophoblast debris. J. Autoimmun. 47, 45–57 10.1016/j.jaut.2013.08.005 [DOI] [PubMed] [Google Scholar]
- 10.Pantham P., Viall C.A., Chen Q., Kleffmann T., Print C.G. and Chamley L.W. (2015) Antiphospholipid antibodies bind syncytiotrophoblast mitochondria and alter the proteome of extruded syncytial nuclear aggregates. Placenta 36, 1463–1473 10.1016/j.placenta.2015.10.006 [DOI] [PubMed] [Google Scholar]
- 11.Padmini E. and Lavanya S. (2011) Over expression of HSP70 and HSF1 in endothelial cells during pre-eclamptic placental stress. Aust. N. Z. J. Obstet. Gynaecol. 51, 47–52 10.1111/j.1479-828X.2010.01246.x [DOI] [PubMed] [Google Scholar]
- 12.Jirecek S., Hohlagschwandtner M., Tempfer C., Knofler M., Husslein P. and Zeisler H. (2002) Serum levels of heat shock protein 70 in patients with preeclampsia: a pilot-study. Wien. Klin. Wochenschr. 114, 730–732 [PubMed] [Google Scholar]
- 13.Tong M., Cheng S.B., Chen Q., DeSousa J., Stone P.R., James J.L. et al. (2017) Aggregated transthyretin is specifically packaged into placental nano-vesicles in preeclampsia. Sci. Rep. 7, 6694 10.1038/s41598-017-07017-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hu P., Han Z., Couvillon A.D., Kaufman R.J. and Exton J.H. (2006) Autocrine tumor necrosis factor alpha links endoplasmic reticulum stress to the membrane death receptor pathway through IRE1alpha-mediated NF-kappaB activation and down-regulation of TRAF2 expression. Mol. Cell. Biol. 26, 3071–3084 10.1128/MCB.26.8.3071-3084.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Denis R.G., Arruda A.P., Romanatto T., Milanski M., Coope A., Solon C. et al. (2010) TNF-alpha transiently induces endoplasmic reticulum stress and an incomplete unfolded protein response in the hypothalamus. Neuroscience 170, 1035–1044 10.1016/j.neuroscience.2010.08.013 [DOI] [PubMed] [Google Scholar]
- 16.Chamley L.W., Konarkowska B., Duncalf A.M., Mitchell M.D. and Johnson P.M. (2001) Is interleukin-3 important in antiphospholipid antibody-mediated pregnancy failure? Fertil. Steril. 76, 700–706 10.1016/S0015-0282(01)01984-7 [DOI] [PubMed] [Google Scholar]
- 17.Bertolaccini M.L., Amengual O., Andreoli L., Atsumi T., Chighizola C.B., Forastiero R. et al. (2014) 14th International Congress on Antiphospholipid Antibodies Task Force. Report on antiphospholipid syndrome laboratory diagnostics and trends. Autoimmunity Rev. 13, 917–930 10.1016/j.autrev.2014.05.001 [DOI] [PubMed] [Google Scholar]
- 18.Chen Q., Stone P.R., McCowan L.M. and Chamley L.W. (2006) Phagocytosis of necrotic but not apoptotic trophoblasts induces endothelial cell activation. Hypertension 47, 116–121 10.1161/01.HYP.0000196731.56062.7c [DOI] [PubMed] [Google Scholar]
- 19.Abumaree M., Stone P. and Chamley L. (2006) An in vitro model of human placental trophoblast deportation/shedding. Mol. Hum. Reprod. 12, 687–692 10.1093/molehr/gal073 [DOI] [PubMed] [Google Scholar]
- 20.Tong M., Kleffmann T., Pradhan S., Johansson C.L., DeSousa J., Stone P.R. et al. (2016) Proteomic characterization of macro-, micro- and nano-extracellular vesicles derived from the same first trimester placenta: relevance for feto-maternal communication. Hum. Reprod. 31, 687–699 10.1093/humrep/dew004 [DOI] [PubMed] [Google Scholar]
- 21.Beriault D.R. and Werstuck G.H. (2013) Detection and quantification of endoplasmic reticulum stress in living cells using the fluorescent compound, Thioflavin T. Biochim. Biophys. Acta 1833, 2293–2301 10.1016/j.bbamcr.2013.05.020 [DOI] [PubMed] [Google Scholar]
- 22.Thastrup O., Cullen P.J., Drobak B.K., Hanley M.R. and Dawson A.P. (1990) Thapsigargin, a tumor promoter, discharges intracellular Ca2+ stores by specific inhibition of the endoplasmic reticulum Ca2(+)-ATPase. Proc. Natl. Acad. Sci. U.S.A. 87, 2466–2470 10.1073/pnas.87.7.2466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Saveljeva S., Mc Laughlin S.L., Vandenabeele P., Samali A. and Bertrand M.J. (2015) Endoplasmic reticulum stress induces ligand-independent TNFR1-mediated necroptosis in L929 cells. Cell Death. Dis. 6, e1587 10.1038/cddis.2014.548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fan H., Tang H.B., Kang J., Shan L., Song H., Zhu K. et al. (2015) Involvement of endoplasmic reticulum stress in the necroptosis of microglia/macrophages after spinal cord injury. Neuroscience 311, 362–373 10.1016/j.neuroscience.2015.10.049 [DOI] [PubMed] [Google Scholar]
- 25.Mayhew T.M. (2014) Turnover of human villous trophoblast in normal pregnancy: What do we know and what do we need to know? Placenta 35, 229–240 10.1016/j.placenta.2014.01.011 [DOI] [PubMed] [Google Scholar]
- 26.Zhang K. and Kaufman R.J. (2008) From endoplasmic-reticulum stress to the inflammatory response. Nature 454, 455–462 10.1038/nature07203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Renehan A.G., Booth C. and Potten C.S. (2001) What is apoptosis, and why is it important? Brit. Med. J. 322, 1536–1538 10.1136/bmj.322.7301.1536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chamley L.W., Holland O.J., Chen Q., Viall C.A., Stone P.R. and Abumaree M. (2014) Review: where is the maternofetal interface? Placenta 35, S74–S80 10.1016/j.placenta.2013.10.014 [DOI] [PubMed] [Google Scholar]
- 29.Huppertz B., Kingdom J., Caniggia I., Desoye G., Black S., Korr H. et al. (2003) Hypoxia favours necrotic versus apoptotic shedding of placental syncytiotrophoblast into the maternal circulation. Placenta 24, 181–190 10.1053/plac.2002.0903 [DOI] [PubMed] [Google Scholar]
- 30.Kanemoto S., Nitani R., Murakami T., Kaneko M., Asada R., Matsuhisa K. et al. (2016) Multivesicular body formation enhancement and exosome release during endoplasmic reticulum stress. Biochem. Biophys. Res. Commun. 480, 166–172 10.1016/j.bbrc.2016.10.019 [DOI] [PubMed] [Google Scholar]
- 31.Yoon S., Kovalenko A., Bogdanov K. and Wallach D. (2017) MLKL, the Protein that Mediates Necroptosis, Also Regulates Endosomal Trafficking and Extracellular Vesicle Generation. Immunity 47, 51e7–65e7 10.1016/j.immuni.2017.06.001 [DOI] [PubMed] [Google Scholar]
- 32.Shen F., Wei J., Snowise S., DeSousa J., Stone P., Viall C. et al. (2014) Trophoblast debris extruded from preeclamptic placentae activates endothelial cells: a mechanism by which the placenta communicates with the maternal endothelium. Placenta 35, 839–847 10.1016/j.placenta.2014.07.009 [DOI] [PubMed] [Google Scholar]
- 33.Chen Q., Guo F., Jin H.Y., Lau S., Stone P. and Chamley L. (2012) Phagocytosis of apoptotic trophoblastic debris protects endothelial cells against activation. Placenta 33, 548–553 10.1016/j.placenta.2012.03.007 [DOI] [PubMed] [Google Scholar]
- 34.Abumaree M.H., Chamley L.W., Badri M. and El-Muzaini M.F. (2012) Trophoblast debris modulates the expression of immune proteins in macrophages: a key to maternal tolerance of the fetal allograft? J. Reprod. Immunol. 94, 131–141 10.1016/j.jri.2012.03.488 [DOI] [PubMed] [Google Scholar]
- 35.Abumaree M.H., Stone P.R. and Chamley L.W. (2006) The effects of apoptotic, deported human placental trophoblast on macrophages: possible consequences for pregnancy. J. Reprod. Immunol. 72, 33–45 10.1016/j.jri.2006.03.001 [DOI] [PubMed] [Google Scholar]
- 36.Chen Q., Guo F., Hensby-Bennett S., Stone P. and Chamley L. (2012) Antiphospholipid antibodies prolong the activation of endothelial cells induced by necrotic trophoblastic debris: implications for the pathogenesis of preeclampsia. Placenta 33, 810–815 10.1016/j.placenta.2012.07.019 [DOI] [PubMed] [Google Scholar]
- 37.Matsushita H., Matsumura Y., Moriya Y., Akasu T., Fujita S., Yamamoto S. et al. (2005) A new method for isolating colonocytes from naturally evacuated feces and its clinical application to colorectal cancer diagnosis. Gastroenterology 129, 1918–1927 10.1053/j.gastro.2005.10.007 [DOI] [PubMed] [Google Scholar]
- 38.Bastida-Ruiz D., Aguilar E., Ditisheim A., Yart L. and Cohen M. (2017) Endoplasmic reticulum stress responses in placentation - A true balancing act. Placenta 57, 163–169 10.1016/j.placenta.2017.07.004 [DOI] [PubMed] [Google Scholar]
- 39.Yung H.W., Atkinson D., Campion-Smith T., Olovsson M., Charnock-Jones D.S. and Burton G.J. (2014) Differential activation of placental unfolded protein response pathways implies heterogeneity in causation of early- and late-onset pre-eclampsia. J. Pathol. 234, 262–276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Collett G.P., Redman C.W., Sargent I.L. and Vatish M. (2018) Endoplasmic reticulum stress stimulates the release of extracellular vesicles carrying danger-associated molecular pattern (DAMP) molecules. Oncotarget 9, 6707–6717 10.18632/oncotarget.24158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hu P., Han Z., Couvillon A.D., Kaufman R.J. and Exton J.H. (2006) Autocrine tumor necrosis factor alpha links endoplasmic reticulum stress to the membrane death receptor pathway through IRE1alpha-mediated NF-kappaB activation and down-regulation of TRAF2 expression. Mol. Cell. Biol. 26, 3071–3084 10.1128/MCB.26.8.3071-3084.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tummers B. and Green D.R. (2017) Caspase-8: regulating life and death. Immunol. Rev. 277, 76–89 10.1111/imr.12541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Grootjans S., Vanden Berghe T. and Vandenabeele P. (2017) Initiation and execution mechanisms of necroptosis: an overview. Cell Death Differ. 24, 1184–1195 10.1038/cdd.2017.65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chen X., Li W., Ren J., Huang D., He W.T., Song Y. et al. (2014) Translocation of mixed lineage kinase domain-like protein to plasma membrane leads to necrotic cell death. Cell Res. 24, 105–121 10.1038/cr.2013.171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wang H., Sun L., Su L., Rizo J., Liu L., Wang L.F. et al. (2014) Mixed lineage kinase domain-like protein MLKL causes necrotic membrane disruption upon phosphorylation by RIP3. Mol. Cell 54, 133–146 10.1016/j.molcel.2014.03.003 [DOI] [PubMed] [Google Scholar]
- 46.Johnstone R.M., Adam M., Hammond J.R., Orr L. and Turbide C. (1987) Vesicle formation during reticulocyte maturation. Association of plasma membrane activities with released vesicles (exosomes). J. Biol. Chem. 262, 9412–9420 [PubMed] [Google Scholar]
- 47.Raposo G. and Stoorvogel W. (2013) Extracellular vesicles: exosomes, microvesicles, and friends. J. Cell Biol. 200, 373–383 10.1083/jcb.201211138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.de Jesus G.R., Agmon-Levin N., Andrade C.A., Andreoli L., Chighizola C.B., Porter T.F. et al. (2014) 14th International Congress on Antiphospholipid Antibodies Task Force report on obstetric antiphospholipid syndrome. Autoimmun. Rev. 13, 795–813 10.1016/j.autrev.2014.02.003 [DOI] [PubMed] [Google Scholar]
- 49.Tong M., Stanley J., Chen Q., James J., Stone P. and Chamley L.W. (2016) In vivo localisation and vascular effects of nano-vesicles derived from normal first trimester human placentae. Placenta 45, 63 10.1016/j.placenta.2016.06.024 [DOI] [Google Scholar]
- 50.Tong M., Johansson C., Xiao F., Stone P.R., James J.L., Chen Q. et al. (2017) Antiphospholipid antibodies increase the levels of mitochondrial DNA in placental extracellular vesicles: Alarmin-g for preeclampsia. Sci. Rep. 7, 16556 10.1038/s41598-017-16448-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Shao J., Zhao M., Tong M., Wei J., Wise M.R., Stone P. et al. (2016) Increased levels of HMGB1 in trophoblastic debris may contribute to preeclampsia. Reproduction 152, 775–784 10.1530/REP-16-0083 [DOI] [PubMed] [Google Scholar]