Abstract
Traditional chemotherapy is being considered due to hindrances caused by systemic toxicity. Currently, the administration of multiple chemotherapeutic drugs with different biochemical/molecular targets, known as combination chemotherapy, has attained numerous benefits like efficacy enhancement and amelioration of adverse effects that has been broadly applied to various cancer types. Additionally, seeking natural‐based alternatives with less toxicity has become more important. Experimental evidence suggests that herbal extracts such as Solanum nigrum and Claviceps purpurea and isolated herbal compounds (e.g., curcumin, resveratrol, and matairesinol) combined with antitumoral drugs have the potential to attenuate resistance against cancer therapy and to exert chemoprotective actions. Plant products are not free of risks: Herb adverse effects, including herb–drug interactions, should be carefully considered.
Linked Articles
This article is part of a themed section on The Pharmacology of Nutraceuticals. To view the other articles in this section visit http://onlinelibrary.wiley.com/doi/10.1111/bph.v177.6/issuetoc
Abbreviations
- EGCG
http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=7002
- EMT
epithelial‐mesenchymal transition
- MRP
http://www.guidetopharmacology.org/GRAC/FamilyDisplayForward?familyId=153
- TCM
traditional Chinese medicine
- NSCLC
non‐small‐cell lung cancer
- OSCC
oral squamous cell carcinoma
1. INTRODUCTION
In clinics, chemotherapy for cancer patients is commonly based on the drug indications, recommended dosages, treatment duration, and adverse effects (e.g., nephrotoxicity and hepatotoxicity; Grossi et al., 2010; Sharbaf, Farhangi, & Assadi, 2017; Sulthana et al., 2017). Occasionally, it is difficult to prevent occurrences of adverse effects from chemotherapeutic drugs during therapy. For instance, http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=7069, a widely used chemotherapy agent, frequently induces cardiomyopathy and chronic heart failure with a prevalence between 4% and 36% (cardiomyopathy) and 0.2–8.7% (chronic heart failure) according to cumulative doses (Chatterjee, Zhang, Honbo, & Karliner, 2010; Volkova & Russell, 2011; J. Yu et al., 2018). Although there are reports of nephrotoxicity and immunosuppression from http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=5343 during treatment, it is, nevertheless, a first choice for the treatment of advanced non‐small‐cell lung cancer cells (NSCLC), breast cancer and ovarian cancer (Brown et al., 2013; Browning et al., 2017; Lorusso, Petrelli, Coinu, Raspagliesi, & Barni, 2014; Manohar & Leung, 2018). Moreover, cancer cells may develop drug resistance during treatment with chemotherapy. Accordingly, higher doses need to be applied to achieve a similar tumoricidal effect as the initial dosage. Frequently, higher dosages have a higher possibility of severe side effects (Zheng, 2017). Consequently, taking a combination of drugs with different mechanisms could synergistically potentiate therapeutic efficacy (Glasgow & Chougule, 2015).
Currently, combinations of chemotherapeutic drugs are widely used for various cancer types (Liu et al., 2017; Zhang et al., 2011). Importantly, the advantage of using multiple drugs are seen as the lowering of doses which could lead to lower resistance and the retention of the same efficacy or sometimes a higher efficacy, a synergistic effect (Glasgow & Chougule, 2015; He et al., 2015; Liboiron & Mayer, 2014). The effects of lower toxicity are ignored as they are considered to be harmless. In fact, the accumulation of toxicity from each drug can still cause deleterious systemic responses (F. Li & Zhang, 2015). Therefore, optimizing drug ratios and schedules can provide an opportunity to improve drug combination activity and reduce dosages to attenuate toxicity (L. Wu, Leng, Cun, Foged, & Yang, 2017). Additionally, drug combinations which include dietary supplements and natural products have been postulated to obtain similar effects to conventional chemotherapeutic drugs but with less adverse effects (Lin, Fu, Tsai, Cheng, & Weng, 2017). Three meta‐analyses reviewing traditional herbal medicine have found such products used as chemotherapeutic adjuvants in nasopharyngeal, breast, and pancreatic cancer treatments. The effective outcome has exemplified traditional herbal medicine as a chemotherapeutic adjuvant (W. Kim, Lee, Lee, Min, Baek, et al., 2015a; W. Kim, Lee, Lee, Min, Lee, & Cho, 2015b; Kuo et al., 2018). Since 2006, several clinical trials were conducted to assess the enhancing effect of natural compounds such as http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=7000 or traditional Chinese medicine (TCM) in promoting conventional chemotherapy against various cancers, including lung cancer (especially non‐small‐cell lung cancer), breast cancer, and colon cancer (Table 1). The conventional chemotherapeutic drugs used in these clinical trials include the platinum‐based chemotherapeutic drugs (e.g., cisplatin and http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=7433), http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=4793, and http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=6809. These drugs can cause severe side effects during therapy such as nephrotoxicity caused by cisplatin and a high prevalence of haematopoiesis suppression by gemcitabine (Manohar & Leung, 2018; Takei et al., 2017). Although some of the clinical trials were complete, none of the results was reported in detail (Table 1). The aim of this article is to highlight recent preclinical evidence on the potential of natural products as adjuvants in cancer therapy.
Table 1.
Clinical trials for natural compounds or herbal medicines combining with chemotherapy
| Recruitment status | Natural compounds | Drugs | Phase | Disease | Trial ID |
|---|---|---|---|---|---|
| Clinical trial for natural compound combinations | |||||
| Unknown | Curcumin |
Gemcitabine http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=2892 |
III | Pancreatic cancer | NCT00486460 |
| + | + | + | + | Colon cancer | NCT00295035 |
| Clinical trial for herbal products combinations | |||||
| Completed | Teng‐Long‐Bu‐Zhong‐Tang |
Oxaliplatin http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=6799 |
II | Colon cancer | NCT01975454 |
| TCM |
http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=7154 Doxorubicin HCl |
+ | Breast cancer | NCT00028964 | |
| + |
Docetaxel http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=7105 Gemcitabine |
+ | NSCLC | NCT01780181 | |
| + |
Vinorelbine Platinum‐based chemotherapy |
III | + | NCT01441752 | |
| + |
CDDP 5‐FU |
II | Peritoneal carcinomatosis | NCT02638051 | |
| Jin Fu Kang | Docetaxel | + | NSCLC | NCT00260026 | |
| Recruiting | TCM | Adjuvant chemotherapy | + | Breast cancer | NCT03797248 |
| + | Standard chemotherapy protocols | I | NSCLC | NCT02737735 | |
| Enrolling by invitation | Yiqi‐yangyin‐jiedu decoction | Gefitinib | III | Lung cancer | NCT02929693 |
| Active, not recruiting | PHY906 | http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=5711 | I | Liver cancer | NCT01666756 |
| Unknown | + |
Gefitinib http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=4920 http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=7641 |
NA | Pulmonary adenocarcinoma | NCT01745302 |
| + | Lotrozole | NA | Polycystic ovary syndrome | NCT01431352 | |
Note. For detail information about each clinical trial, see following website: https://clinicaltrials.gov/.
Abbreviations: +, The same with above cell; 5‐FU, 5‐Fluorouracil; CDDP, cisplatin; NA, not applicable; NSCLC, non‐small‐cell lung cancer; TCM, traditional Chinese medicine.
2. HERBAL COMPOUNDS WITH THE POTENTIAL TO SYNERGIZE WITH ANTITUMOR DRUGS
A synergistic effect is described as an increase in efficacy for a combination of components when compared with a single one (Pai, Cottrell, Kashuba, & Bertino, 2015). Data focusing on the toxic episodes of chemotherapy has led to the characterization of novel strategies, including the exploitation of natural compounds in combination therapies. The goals of including natural compounds in cancer chemotherapies are as follows: (a) to widen the therapeutic window of the chemotherapeutic drugs and (b) to decrease the occurrence of chemotherapy resistance (Ouyang et al., 2014). The next section will summarise herbal or folk medicines and natural compounds that act as chemosensitizers, chemoresistance reducers, or chemotherapeutic protectors, in clinical use.
2.1. Natural compounds acting as chemotherapeutic drug sensitizers
Chemosensitization refers to the potentiation of the tumoricidal effect of chemotherapeutic drugs by other low MW compounds, including making cancer cells more predisposed to chemotherapeutic drugs (Oliveira, Mendes, & Torchilin, 2017). The chemosensitizers can be natural products or synthetic compounds. This section will discuss the naturally sourced chemosensitizers that make cancer cells aware of responding therapeutic agents. http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=6785 is a natural alkaloid isolated from Catharanthus roseus that is currently used in acute lymphocytic lymphoma and neuroblastoma (Below & Das, 2019). However, due to its high cytotoxicity and narrow therapeutic window, it is restricted for further use, especially in paediatric malignancy (Parasramka, Talari, Rosenfeld, Guo, & Villano, 2017). Bahmani et al. (2018) found that another plant extract from Centaurea albonitens, could significantly enhance the cytotoxicity of vincristine against leukaemia cell lines without increasing toxicity to normal cells. To reduce the cardiotoxicity and resistance due to doxorubicin, numerous plant extracts have been used with doxorubicin, in screening synergistic effects. So far, an aqueous extract of Solanum nigrum Linn. was shown to potentiate doxorubicin against colorectal cancer and ovarian cancer through autophagy induction (Tai et al., 2013; C. W. Wang et al., 2015). Based on the same idea, polysaccharides isolated from Agrocybe aegerita and aqueous extract of S. nigrum Linn. were found to increase cytotoxicity of http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=4789 (5‐FU) against oesophageal carcinoma, ovarian cancer, and colorectal cancer via regulating pro‐inflammatory cytokine such as http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=5074 and IFN‐γ (Ji, Zheng, Ye, Wu, & Chen, 2013; Tai et al., 2013; C. W. Wang et al., 2015). For increasing the anti‐cancer effects of paclitaxel, three natural phenolic acids (http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=5155, rosmarinic acid, and ursolic acid) were used with paclitaxel in ex vivo breast cancer cells, and found to promote cytotoxicity these cells by modulating the tumour micro‐environment (Carranza‐Torres et al., 2015). Such results show that it is possible to increase the cytotoxic effects of known anti‐cancer agents, with natural compounds. There are numerous studies focusing on synergism between herbal compounds and cancer therapeutic drugs, both in vitro and in vivo. A large percentage of the natural compounds are flavonoids and phenolics, which implies that flavonoids and phenolics have more potential than other subgroups. However, curcumin is the most studied natural compound (Table 2). These studies cover the most prevalent and fatal cancers, for example, lung cancer, breast cancer, and colorectal cancer. Interestingly, these studies focused on curcumin potentiating chemotherapeutic efficacy (by http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=7624, 5‐FU, doxorubicin, and radiation) have shown that curcumin promotes chemotherapy through regulating the expression or activity of the transcription factor NF‐κB. This finding implies that curcumin might target upstream signalling modulators of NF‐κB or NF‐κB itself (Table 2). Most of these herbal enhancers for promoting cytotoxicity of chemotherapeutic agents exert their functions via targeting the stress–stimuli response, that is to oxidative stress and particularly NF‐κB, which seems to be an indicator for determining the potency of chemotherapeutic cytotoxicity. When taken together, natural compounds or herbal products have a high potential to support chemotherapeutic drugs to fight cancer cells.
Table 2.
Herbal compounds act as an enhancer of cancer therapy
| Structure subclass | Natural compound | Chemotherapeutic drug | Cancer | Signal pathway | Referencea |
|---|---|---|---|---|---|
| Cell death via specific signalling pathway | |||||
| Alkaloid | 3,3′‐Diindolylmethane | Cisplatin | Ovary | http://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=2994/Akt | (Zou, Xu, Li, Zhang, & Fan, 2018) |
| Berberine | Radiation | Esophagus | Rad51 | (Liu et al., 2011) | |
| Ethoxysanguinarine | Cisplatin | Lung | CIP2A | (Liu, Ma, Wen, Cheng, & Zhou, 2014) | |
| http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=224 | + | Liver | NF‐κB/AP‐2β | (Hao et al., 2017) | |
| Neferine | Doxorubicin | Lung | http://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=1875/ROS | (Poornima, Kumar, Weng, & Padma, 2014) | |
| http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=10212 | Cisplatin | Ovary | HIF‐1α | (Su et al., 2011) | |
| Piperlongumine | Doxorubicin | Prostate | http://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=1383 | (Piska et al., 2019) | |
| Capsaicinoid | http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=2486 | Radiation | Prostate | NF‐κB | (Venier et al., 2015) |
| Diarylheptanoid | Curcumin | 5‐FU | Gastric | NF‐κB | (Kang et al., 2016) |
| + | Carboplatin | Lung | Akt/NF‐κB | (Kang et al., 2015) | |
| + | + | Breast | FEN1 | (Zou et al., 2018) | |
| + | + | Colorectal | endoG/NF‐κB | (Wang, Liu, & Su, 2014) | |
| + | + | Lymphoma | Rad51, apoptosis‐Caspase | (Zhao et al., 2018) | |
| + | + | Neuroblastoma | Uniquitin | (D'Aguanno et al., 2012) | |
| + | + | Ovary | c‐Myb/STAT3/NF‐κB | (Tian, Tian, Qiao, Li, & Zhang, 2019) | |
| + | Doxorubicin | Gastric | NF‐κB | (Yu, Wu, Dai, Yu, & Si, 2011) | |
| + | Radiation | Prostate | miR‐143 | (Liu, Li, Wang, & Luo, 2017) | |
| + | Rhtrail | Breast | http://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=1880/http://www.guidetopharmacology.org/GRAC/FamilyDisplayForward?familyId=889 | (Park, Cho, Andera, Suh, & Kim, 2013) | |
| Diterpenoid | Adenanthin | http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=2779 | Leukaemia | Prx‐1/C/EBP | (Wei et al., 2016) |
| Cryptotanshinone | Cisplatin | Ovary | http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=4470 and http://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=1633 | (Jiang et al., 2017) | |
| + | http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=2770 | Oral |
http://www.guidetopharmacology.org/GRAC/FamilyIntroductionForward?familyId=581/STAT3 e‐Cadherin/p53/β‐catenin |
(Wang et al., 2017) | |
| Flavonoid | (−)‐Epicatechin | Radiation | Pancreas/Glioma | http://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=1987/p21 | (Elbaz, Lee, Antwih, Liu, Huttemann, & Zielske, 2014) |
| Formononetin | Doxorubicin | Gastric | http://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=2660 | (Liu et al., 2015) | |
| Icariin | 5‐FU | Colorectal | NF‐κB | (Shi et al., 2014) | |
| http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=5215 | Cisplatin | Bile duct | http://www.guidetopharmacology.org/GRAC/FamilyDisplayForward?familyId=781 3K/Akt/http://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=2109/SREP | (Lim, Yang, Bazer, & Song, 2016) | |
| http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=10298 | Paclitaxel | Prostate | PI3K/Akt and http://www.guidetopharmacology.org/GRAC/FamilyDisplayForward?familyId=514 | (Lim, Park, Bazer, & Song, 2017) | |
| WYC02 | Cisplatin | Multi cancer | ATM | (Wang et al., 2012 ) | |
| http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=5346 | Rhtrail | Breast | http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=5188/DR5 | (Manouchehri, Turner, & Kalafatis, 2018) | |
| Silibinin | 5‐FU | Colorectal | PI3K/MAPK/http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=5371/nanog/CD44v6 | (Patel et al., 2018) | |
| Isoprenoid | http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=2771 | + | Liver | NF‐κB | (Zhang et al., 2011) |
| Macrolide | Elaiophylin | Cisplatin | Ovary | http://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=2348 | (Zhao et al., 2015) |
| Monoterpenoid | http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=6413 | + | Oesophagus | PI3K/Akt | (Meng et al., 2018) |
| Organosulfur | http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=6569 | Doxorubicin | Ovary | SFN, http://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=3055 | (Pastorek et al., 2015) |
| Phenolic | http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=6999 | Radiation | Prostate | γ‐H2AX | (Yao et al., 2015) |
| Caffeic acid | http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=4779 | Cervix | http://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=1540/TCA cycle | (Tyszka‐Czochara, Konieczny, & Majka, 2017) | |
| + | + | + | SNAI1/MMP‐9 | (Tyszka‐Czochara, Lasota, & Majka, 2018) | |
| Capsaicin | Docetaxel | Prostate |
http://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=2497/PI3K/Akt/mTOR http://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=2212/AMPK |
(Sanchez, Bort, Mateos‐Gomez, Rodriguez‐Henche, & Diaz‐Laviada, 2019) | |
| Dicoumarol | Doxorubicin | Urinary tract | NADPH quinone oxidoreductase | (Matsui et al., 2010) | |
| Emodin | http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=1016 | Breast |
Ras/ERK PI3K/mTOR |
(Tseng et al., 2017) | |
| Polyyne | Falcarindiol | 5‐FU | Colorectal | ER stress | (Jin et al., 2012) |
| Susquiterpenoid | Heteronemin | Cytarabine | Leukaemia | http://www.guidetopharmacology.org/GRAC/FamilyDisplayForward?familyId=897 farnesylation | (Saikia et al., 2018) |
| β‐Eudesmol |
Doxorubicin 5‐FU |
Bile duct | NADPH quinone oxidoreductase | (Srijiwangsa, Ponnikorn, & Na‐Bangchang, 2018) | |
| Phytosteroid | Polyphyllin D | Cisplatin | Ovary | 18 unique genes | (Al Sawah et al., 2015) |
| Tenacigenin B derivative | Paclitaxel | Ovary | Inhibit Cytochrome P450 | (Xie et al., 2019) | |
| Stilbenoid | Resveratrol | Cisplatin | Lung | Mitochondrial depolarization | (Ma et al., 2015) |
| + | Doxorubicin | Breast | HSP‐27 | (Diaz‐Chavez et al., 2013) | |
| + | + | + | Carbonyl reductase 1 | (Ito et al., 2013) | |
| Tetrahydrofuran | Acetogenin | Doxorubicin | Ovary | Mitochondrial complex I | (Tormo et al., 2003) |
| Tripyrrole | Prodigiosin | Paclitaxel | + | http://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=2795 | (Ho et al., 2009) |
| + | Doxorubicin | Oral | Doxorubicin accumulation | (Lin & Weng, 2018) | |
| Triterpenoid | Brusatol | 5‐FU | Pancreas | e‐cadherin/Twist/vimentin/NF‐κB | (Lu, Lai, Leung, Leung, Li, & Lin, 2017) |
| Triterpenoid | http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=10386 | Cisplatin | Lung | FANCD2 | (Wang, Liu, Cheng, & Zhou, 2015) |
| + | Tanespimycin | Glioblastoma | http://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=618, http://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=2540, Hsp90 | (Boridy, Le, Petrecca, & Maysinger, 2014) | |
| Xanthonoid | Formononetin | Metformin | Breast | ERK1/2/http://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=2844 | (Xin, Wang, Ren, & Guo, 2019) |
| Via apoptosis or autophagy | |||||
| Alkaloid | Berberine | Sorafenib | Liver | Apoptosis‐Intrinsic | (Huang et al., 2018) |
| Indole‐3‐carbinol | Cisplatin | Ovary | + | (Taylor‐Harding et al., 2012) | |
| + | Doxorubicin | Cervix | + | (Adwas, Elkhoely, Kabel, Abdel‐Rahman, & Eissa, 2016) | |
| Carotenoid | Bixin | + | Acute leukaemia | Apoptosis | (Santos, Almeida, Antunes, & Bianchi, 2016) |
| Diarylheptanoid | Curcumin | Cisplatin | Lung | Apoptosis‐Intrinsic | (Baharuddin et al., 2016) |
| + | + | Oral | Apoptosis‐Intrinsic | (Chen et al., 2018) | |
| + | Sorafenib | Liver | Apoptosis‐Intrinsic | (Bahman, Abaza, Khoushiash, & Al‐Attiyah, 2018) | |
| Diterpenoid | Crassin | Doxorubicin | Breast | Apoptosis‐ROS | (Richards, Vellanki, Smith, & Hopkins, 2018) |
| Ent‐kaurane‐type diterpenoids | + | Liver | Apoptosis | (Pham, Iscache, Pham, & Gairin, 2016) | |
| Flavonoid | Eupatorin | + | Colorectal | Apoptosis‐Intrinsic | (Namazi Sarvestani, Sepehri, Delphi, & Moridi Farimani, 2018) |
| http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=4285 | Cisplatin | Lung | Apoptotic/MMPs | (Ma, Wang, Nan, Li, Wang, & Jin, 2016) | |
| Salvigenin | Doxorubicin | Colorectal | Apoptosis‐Intrinsic | (Namazi Sarvestani, Sepehri, Delphi, & Moridi Farimani, 2018) | |
| Lignan | Enterolactone | + | Breast | Apoptosis | (Di, De Silva, Krol, & Alcorn, 2018) |
| Secoisolariciresinol | + | + | + | (Di, De Silva, Krol, & Alcorn, 2018) | |
| Organosulfur | http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=4822 | Temozolomide | Colorectal | Autophagy | (Goder et al., 2015) |
| Alyssin | 5‐FU | Colorectal | Apoptosis‐Extrinsic | (Milczarek et al., 2018) | |
| Phenolic | http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=7062 | Photodynamic therapy | Ehrlich | Apoptosis‐Intrinsic | (Joy, Nishanth Kumar, Soumya, Radhika, Vibin, & Abraham, 2014) |
| Phenolic | Nordihydroguaiaretic acid | Cisplatin | Breast | ROS | (Mundhe, Kumar, Ahmed, Jamdade, Mundhe, & Lahkar, 2015) |
| Phenolic | http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=10302 | + | Lung | Apoptosis‐Intrinsic | (Xu et al., 2013) |
| Sesquiterpenes | Trans‐nerolidol | Doxorubicin | Breast | doxorubicin accumulation | (Hanusova et al., 2017) |
| β‐Caryophyllene oxide | + | + | + | (Hanusova et al., 2017) | |
| β‐Elemene | Cisplatin |
Lung/Brain/Breast/ Cervix/Ovary/Colorectal |
Apoptpsis‐Intrinsic & Extrinsic | (Li et al., 2013) | |
| Stilbenoid | Resveratrol | Sorafenib | Liver | Apoptosis‐Intrinsic | (Bahman, Abaza, Khoushiash, & Al‐Attiyah, 2018) |
| Triterpenoid | Withaferin A | Radiation | Lymphoma | Apoptosis‐ROS, Bcl‐2 | (Yang, Choi, Kim, Choi, & Kwon, 2011) |
| Acetyl‐11‐keto‐β‐boswellic acid | + | Glioblastoma | Apoptosis‐Intrinsic | (Conti et al., 2018) | |
| Xanthonoid | Forbesione | 5‐FU | Bile duct | Apoptosis‐Intrinsic | (Boueroy et al., 2017) |
| Gambogic acid | Doxorubicin | Ovary | Apoptosis‐ROS | (Wang & Yuan, 2013) | |
| Kaempferol | Sorafenib | Liver | Apoptosis‐Intrinsic | (Bahman, Abaza, Khoushiash, & Al‐Attiyah, 2018) | |
| Reducing chemoresistance via specific mechanism | |||||
| Alkaloid | Aaptamine | Cisplatin | Embryonal carcinoma | http://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=598, p53, eIF5A hypusination | (Dyshlovoy et al., 2014) |
| Demethyloxyaaptamine | + | + | TNF | (Dyshlovoy et al., 2014) | |
| Isoaaptamine | + | + | myc, p53, TNF | (Dyshlovoy et al., 2014) | |
| Sinapine | Doxorubicin | Colorectal | http://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=1811‐FRS2α‐ERK1/2 | (Guo, An, Feng, Liu, Wang, & Zhang, 2014) | |
| Diarylheptanoid | Curcumin | Cisplatin | Lung | http://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=2029/BRCA | (Chen, Li, Jiang, Lan, & Chen, 2015) |
| + | + | Ovary | MEG3, miR‐214 | (Zhang, Liu, Xu, & Li, 2017) | |
| + | + | + | miR‐186 | (Tang, Zhang, & Du, 2010) | |
| Flavonoid | Isoliquiritigenin | + | Oral | ALDH1, http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=9981, GRP78 | (Hu, Yu, Hsieh, Liao, Lu, & Chu, 2017) |
| http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=9738 | Paclitaxel | Ovary | Akt/http://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=3008/http://www.guidetopharmacology.org/GRAC/FamilyDisplayForward?familyId=578 | (Yang et al., 2012) | |
| Wogonin | Doxorubicin | Breast | Nrf2 | (Zhong et al., 2013) | |
| Lignan | Silybin | + | Colon | GLUT1 | (Catanzaro et al., 2018) |
| Nucleoside | Clitocine | + | Liver | NF‐κB | (Sun et al., 2012) |
| Organosulfur | Sulforaphane | Cisplatin | Ovary | HIF‐1α | (Pastorek et al., 2015) |
| Phenol | Phenylethyl isothiocyanate | + | In vivo | http://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=2847 glutathionylation | (Li et al., 2016) |
| Emodin | Doxorubicin | Lung | Anthracycline reductases | (Hintzpeter, Seliger, Hofman, Martin, Wsol, & Maser, 2016) | |
| Steroid | Cucurbitacin b | + | Gastric | CIP2A/PP2A/mTORC1 | (Liu et al., 2017) |
| Triterpenoid | Adcx | Paclitaxel | Liver | Akt/autophagy | (Sun et al., 2017) |
| Polyphyllin I | Erlotinib | Lung | http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=4998/STAT3 | (Lou, Chen, Zhu, Deng, Wu, & Wang, 2017) | |
| Via inhibiting drug efflux | |||||
| Alkaloid | Cinchonine | Paclitaxel | Uterine | (Lee et al., 2011) | |
| Hydrocinchonine | Paclitaxel | Uterine | (Lee et al., 2011) | ||
| http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=2342 | + | + | (Lee et al., 2011) | ||
| Diarylheptanoid | Curcuminoid | Doxorubicin | Leukaemia | (Xu, Tian, & Shen, 2013) | |
| Diterpenoid | Tanshinone IIA | Doxorubicin | Gastric | (Xu et al., 2018) | |
| Flavonoid | http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=2829 | Daunorubicin | Breast | (Zhang, Sagawa, Arnold, Tseng, Wang, & Morris, 2010) | |
| Glabridin | Doxorubicin | + | (Qian et al., 2019) | ||
| Lignan | Matairesinol | Doxorubicin | Colon | (Su, Cheng, & Wink, 2015) | |
| + | + | Leukaemia | (Su, Cheng, & Wink, 2015) | ||
| Monoterpene | Borneol‐peg‐np | Paclitaxel | Ovary | (Zou et al., 2017) | |
| Triterpenoid | Maslinic acid | + | Diarthrosis/smooth muscle | (Villar et al., 2014) | |
| http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=3306 | + | + | (Villar et al., 2014) | ||
| Ursolic acid | Doxorubicin | Breast | (Zong, Cheng, Liu, Pi, Liu, & Song, 2019) | ||
| Xanthone | Forbesione | Doxorubicin | Bile duct | NF‐κB & p‐Glycoprotein | (Hahnvajanawong et al., 2014) |
| Isomorellin | + | + | (Hahnvajanawong et al., 2014) | ||
| Xanthonoid | Mangiferin | + | Breast | p‐Glycoprotein, MRP‐1, http://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=792 | (Louisa, Soediro, & Suyatna, 2014) |
| Gambogic acid | Multidrugs | Multi‐cancer | p‐Glycoprotein | (Wang et al., 2013) | |
Note. Intrinsic: Bcl‐2/Bcl‐XL/caspase‐3, 9; Extrinsic: DR/Bid/caspase‐3, 7, 8; +, the same with above cell.
For Reference list, see Data S1.
2.2. Herbal compounds reduce resistance against cancer therapy
Clinically, herbal compounds can reduce resistance against cancer therapies, and this has become a critical concern. Up to now, drug resistance (excluding radiation‐resistance) in cancer cells remains the most challenging aspect of cancer treatment, especially in NSCLC and prostate cancer (Chang, 2011; Wade & Kyprianou, 2018). Such resistance in cancers reveals a transformation of cancer cells from drug susceptible to resistant, which leads to higher toxicity and expenditures in treatments (Housman et al., 2014; Zheng, 2017). About 90% of treatment failures in recurrent cancer therapy and 80–90% of cancer death is strongly correlated to cancer resistance (Mansoori, Mohammadi, Davudian, Shirjang, & Baradaran, 2017; Yuan et al., 2017).
Prevailing mechanisms of chemoresistance are classified into seven phases: drug flux, DNA damage repair, cell death inhibition, epithelial‐mesenchymal transition (EMT), drug target alteration, drug inactivation, and epigenetics (Housman et al., 2014), and notably, drug flux is the most concerned issue in this topic. Cancer cells pump chemotherapeutic agents out of the cells using the http://www.guidetopharmacology.org/GRAC/FamilyDisplayForward?familyId=153 (MRPs, also known as MDR or the ABCC family of transporters) and the Hedgehog receptor Patched 1 (protein patched homolog 1, PTCH1), which reduces drug accumulation within cancer cells and, thereby, lower drug efficacy (Amiri‐Kordestani, Basseville, Kurdziel, Fojo, & Bates, 2012; Bidet et al., 2012). MRPs, particularly http://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=779 (also known as p‐glycoprotein, P‐gp), are found over‐expression in recurrent cancer cells and which over‐expression is associated with poor prognosis (Chen et al., 2016; W. Li et al., 2016). PTCH1 is a newly discovered drug efflux transporter also found to be overexpressed in many metastatic cancers (Hasanovic & Mus‐Veteau, 2018). In addition to drug efflux, PTCH1 also acts as a receptor in Hedgehog/Gli signalling pathway that activates http://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=239 (Smo)/Gli transduction and leads to growth factor expression (Armas‐Lopez, Zuniga, Arrieta, & Avila‐Moreno, 2017; Rimkus, Carpenter, Qasem, Chan, & Lo, 2016). Some chemotherapeutic agents specially target growth factor signalling, for example, http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=4941 (Iressa®, http://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=1797 inhibitor), http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=5082 (Herceptin®, http://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=2019/neu inhibitor), and http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=6771 (Avastin®, http://www.guidetopharmacology.org/GRAC/FamilyDisplayForward?familyId=324 inhibitor; National Cancer Institute, 2002a, 2002b). However, cancer cells turn on EMT, which leads to anoikis resistance and continuous activation of growth factor signalling during cancer invasion (J. Wang et al., 2016). EMT‐induced chemoresistance has been identified in several cancer types, including lung cancer, prostate cancer, and breast cancer (Fischer et al., 2015; J. Huang, Li, & Ren, 2015; Wade & Kyprianou, 2018).
Numerous cancer drugs, including platinum‐based chemotherapeutic drugs, http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=7242, http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=6823, and doxorubicin, belong to the group known as DNA damage agents (Cheung‐Ong, Giaever, & Nislow, 2013). Therefore, DNA repairing capacity would directly affect these cancer drugs' effects (Nagel et al., 2017; Sakthivel & Hariharan, 2017). However, Wang et al. have explored the involvement of Wip1, which is an inhibitor of the http://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=1934 kinase‐mediated DNA repairing system, in cancer resistance of oral squamous cell carcinoma (OSCC). Wip1 activation is thought to potentiate the cytotoxicity of cisplatin against OSCC (L. Wang, Mosel, Oakley, & Peng, 2012). Unexpectedly, a positive correlation between Wip1 expression and cisplatin resistance in OSCC has now emerged (L. Wang et al., 2012). Thus, both positive and negative relationships between DNA repairing mechanisms and chemoresistance have been found and further investigation is needed to clarify the characteristics of DNA repairing mechanisms in chemoresistance. Figure 1 summarizes the above discussion, showing seven mechanisms of chemoresistance proposed, and giving examples of chemotherapeutic drugs affected by particular chemoresistance mechanisms.
Figure 1.
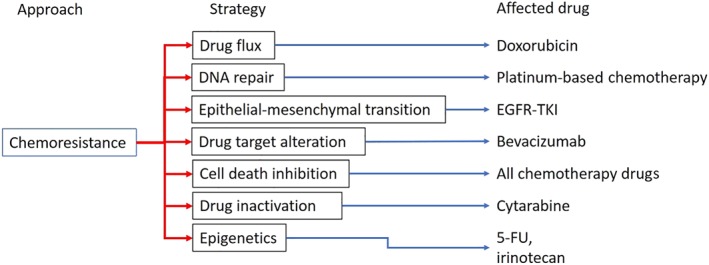
Recent‐known mechanism of chemoresistance
Recent studies on the effects of natural compounds against chemoresistance show that they inhibit MDR protein activity or further reduce MDR protein expressions (Turrini, Ferruzzi, & Fimognari, 2014). Table 2 lists these MDR inhibitors and compounds acting through other mechanisms. This Table shows that MDR inhibition accounts for the highest rate. Interestingly, silybin, a natural lignan isolated from Silybum marianum, allows doxorubicin to overcome drug resistance in colorectal cancer by inhibiting http://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=875 (GLUT1) expression. GLUT1 expression could be regulated by the Wnt/http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=5371 signalling pathway that has been identified as a cisplatin resistance promoter through the ATM‐mediated signalling pathway in laryngeal squamous cell carcinoma cells (L. Wang et al., 2019). Eleven different polyoxypregnanes isolated from Marsdenia tenacissima can combat doxorubicin resistance in multidrug resistance cancer cell lines via inhibition of ABC transporters (To et al., 2017). A series of bisbenzylisoquinoline alkaloids inhibit the transporter P‐gp, which leads to high doxorubicin accumulation in MCF‐7/ADR breast cancer cell to provide much increased cytotoxicity (Sun & Wink, 2014). Investigation of the antitumor activity of six ergot alkaloids from Claviceps purpurea, showed that these ergot alkaloids might bypass chemoresistance mechanisms through unknown signalling pathways in multiple cancers (Mrusek, Seo, Greten, Simon, & Efferth, 2015). Additionally, ellagic acid and http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=8741 prevented induction of resistance in ovarian cancer towards cisplatin (Engelke, Hamacher, Proksch, & Kassack, 2016). (Z)‐3,4,3′,5′‐tetramethoxystilbene, a stilbenoid, increased antitumor efficacy of cisplatin in cisplatin‐resistant osteosarcoma cells in in vitro and in vivo (H. Xu, 2016), and another stilbenoid, resveratrol, increased cisplatin uptake and efficacy (Osman et al., 2015). β‐Phenylethyl isothiocyanate and http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=2428 down‐regulated intracellular http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=6737 level and concurrently reversed resistance to doxorubicin and cisplatin in resistant‐uterine sarcoma cells (Angelini, Conti, Ciofani, Cuccurullo, & Di Ilio, 2013; W. J. Wu et al., 2013). Taken together, the data suggest herbal compounds exert beneficial effects in the treatment of recurrent cancers, when combined with current therapies.
2.3. Chemopreventive effect of herbal compounds
The non‐selective character of most chemotherapeutic drugs usually initiates systemic symptoms as adverse or side effects during therapy (de Oliveira Junior et al., 2018). These adverse effects include cardiotoxicity, nephrotoxicity, hepatotoxicity, and peripheral neuropathy (Duwe et al., 2017; Ma, Kavelaars, Dougherty, & Heijnen, 2018; Santoni et al., 2017; Sharbaf et al., 2017). Sometimes, the adverse effects severely affect the daily quality of life for patients (X. Wu et al., 2016). Many of the adverse effect of chemotherapeutic drugs are caused by the drug itself and its metabolites, usually by inducing ROS formation (Varricchi et al., 2018). Accordingly, a study of doxorubicin metabolism indicated that it was the main cause of doxorubicin‐induced cardiomyopathy, through the generation of toxic intermediates and ROS, leadong to the apoptosis of cardiomyocytes (Renu, Abilash, Tirupathi Pichiah, & Arunachalam, 2018). In addition, many naturally sourced antioxidants are present in plant and herbal sources (D. P. Xu et al., 2017). Therefore, herbal compounds intended to alleviate the adverse effects of chemotherapeutic drugs have been assessed for antioxidation or ROS scavenger effects, in vitro and in vivo. One study in vitro, using cardiomyocytes and doxorubicin indicated that saffron extract could activate http://www.guidetopharmacology.org/GRAC/FamilyDisplayForward?familyId=285/http://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=1525 and http://www.guidetopharmacology.org/GRAC/FamilyDisplayForward?familyId=514 pathways resulting in decreased cardiomyocytic apoptosis (Chahine, Nader, Duca, Martiny, & Chahine, 2016). In another report focused on irinotecan toxicity, the TCM, Gegen Qinlian decoction, ameliorated gut inflammation by activating the http://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=2757/http://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=3057 pathway and might result in up‐regulation of tight junction and down‐regulation of inflammatory cytokines (Y. Wu et al., 2019). Other studies have also shown that anthocyanin from black rice tested in vitro with on cardiomyocytes attenuated cardiotoxicity via http://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=620/http://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=2021 and HSF‐1 signalling pathways, and http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=7002 (EGCG) reduceds NADPH‐cytochrome P‐450 reductase activity (the key enzyme of doxorubicin toxicity; Dudka et al., 2005; P. C. Huang et al., 2016). In a cell model, http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=10047 and 3,3′‐di‐indolylmethane were cardioprotective in mouse models through the Nrf2/ARE pathway as well (Adwas et al., 2016; Hajra, Basu, Singha Roy, Patra, & Bhattacharya, 2017).
Several herbal compounds including nordihydroguaiaretic acid, eriodictyol‐7‐O‐glucoside, and http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=2499 ameliorated cisplatin‐induced renal injury, in vitro and in vivo (Hosseinimehr et al., 2015; Hu, Zhang, Wang, Lou, & Ren, 2012; Mundhe et al., 2015). Resveratrol, http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=2826, and epigallocatechin‐3‐gallate attenuated apoptosis of haematopoietic cells via reducing DNA damage (Alotaibi, Bhatnagar, Najafzadeh, Gupta, & Anderson, 2013; Olas & Wachowicz, 2004; Sonaa, Usha, & Ja In, 2013). Again, in photodynamic therapy research, Pinus halepensis bark extract prevented the photosensitivity in SCID mice model (Petri et al., 2012). Coniferyl aldehyde, found in wine, reduced radiation damage via phosphorylation of HSF‐1 and further increased the activation of ERK1/2 (S. Y. Kim, Lee, Nam, Seo, & Lee, 2015). All these experiments assessed the benefits of the chemoprotective ability of herbal compounds in cancer therapy, derived from a reduction of side effects and a consequent reduction in dose. Nonetheless, drug–herbal interactions, leading to injury may also be important dis‐advantages of combination therapy.
3. HERBAL TOXICITIES AND FUTURE REMARKS
An increasing number of cases require closer attention to the additional toxicity induced by the herbal component or by the herbal–drug combination (Table 3). In these examples of herbal‐induced drug injury, some popular formulations or compounds are included, such as curcumin and chokeberry, which is usually used for increasing patient stamina to overcome the adverse effects of cancer therapy. A meta‐analysis has collected 97 herbal‐induced toxicity cases in Korea and found that both single and multiple herbal preparations could induce hepatocellular toxicity, including Polygonum multiflorum and Dictamnus dasycarpus (W. J. Lee, Kim, Lee, & Son, 2015). A following review collects studies about monoterpene‐ and susquiterpenes‐induced hepatotoxicity and summarize that some common terpenes (e.g. http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=2422 and limonene) might injure liver through generating ROS and imparing antioxidant defenses (Zarybnicky, Bousova, Ambroz, & Skalova, 2018). In addition to ROS generation, another hepatotoxic mechanism is simultaneously observed through the modulation of cytochrome P450 (Brewer & Chen, 2017). Coumarins, furanocoumarins, (−)‐epigallocatechin‐3‐gallate, and http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=2489 have all demonstrated a potent inhibition of cytochrome P450 isoforms, especially http://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=1337 which is a key enzyme for oral drug detoxification, and MDRs, which may be inhibited and thus prolong the t 1/2 of the drug (Brewer & Chen, 2017; Pal & Mitra, 2006; Shamsi, Tran, Tan, Tan, & Lim, 2017). These findings of toxicity from using herbal medicine for cancer therapy suggest that precautions should be taken against the herb‐induced or drug‐induced liver injury.
Table 3.
Natural compounds as potential adjuvants to cancer therapy: Unpredictable adverse events
| Herbal compounds | Chemotherapeutic drugs | Cancer or normal cell type | Adverse effect and relevant mechanism | Referencea |
|---|---|---|---|---|
| Curcumin | Doxorubicin | Cardiac muscle cells | Apoptosis‐ROS | (Hosseinzadeh, Behravan, Mosaffa, Bahrami, Bahrami, & Karimi, 2011) |
| http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=6815 | Cervix/Breast/Colorectal | Offset cancer cell death via http://www.guidetopharmacology.org/GRAC/FamilyDisplayForward?familyId=331/γ‐H2AX | (Saleh, El‐awady, Eissa, & Abdel‐Rahman, 2012) | |
| http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=4520 | Taxane | Breast cancer | Increase peripheral neuropathy | (Hershman et al., 2013) |
| Chokeberry | http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=2774 | Liposarcoma | Induce rhabdomyolysis | (Strippoli, Lorusso, Albano, & Guida, 2013) |
| Bu Zhong Yi Qi Wan | http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=7301/radiation | Glioblastoma | Induce acute liver toxicity | (Melchardt et al., 2014) |
For Reference list, see Data S2.
Accordingly, the best fit between possible adverse effect and anticancer efficacy is urgently needed in terms of clinical application. The balance between adverse effect and anticancer efficacy can be discussed at two levels, acute and chronic toxicity. Acute toxicity, especially hepatotoxicity, nephrotoxicity, and cardiotoxicity, could be determined during administration. Cardiotoxicity could be measured by left ventricular ejection fraction (LVEF), which directly shows the pumping ability of the heart (Florescu, Cinteza, & Vinereanu, 2013). For hepatotoxicity, clinical criteria of chemotherapy‐induced hepatotoxicity is regularly defined by the serum levels of alanine aminotransferase (ALT), aspartate aminotransferase (AST), total bilirubin (TBIL), alkaline phosphatase (ALP), and http://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=1392 (γ‐GT) in which increases to 2 or 3 times higher than the normal upper limit, as acute liver injury is occurring (Y. C. Yu et al., 2017). Nephrotoxicity is defined by serum creatinine level and GFRs (measured by urine volume produced in particular time period) and has five stages from risk to end‐stage renal disease (ESRD; Horie et al., 2018). The criteria of biochemical examinations could guide oncologists and researchers to monitor possible toxicities, which can be used to determine the benefits of anticancer efficacy and, subsequently, to proceed or to cease treatment. In practice, a physician could take a more restricted posture towards advancement of ALT and AST levels based on normal ranges, to securely assure the ongoing therapy. Likewise, the above criteria could be applied to monitor the chronic toxicity in liver, heart, and kidney which potent natural compounds/conventional drugs combination could provide greater anticancer efficacy without exceeding about criteria.
Prospectively, the immunostimulatory effect of natural compounds in chemotherapy is a critical issue, as chemotherapy‐induced immunosuppression could cause severe opportunistic infections (Galluzzi, Buqué, Kepp, Zitvogel, & Kroemer, 2015). Some natural compounds and herbal products have proved as immunomodulators in vivo, such as Ashwagangha (Withania somnifera) and Brahmi (Bacopa monnieri), which improve https://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=4968 and https://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=4985 expression after LPS exposure (Yamada, Hung, Park, Park, & Lim, 2011). Moreover, the immunomodulating activity of astragaloside (the major components of huang‐qi, Astragalus membranaceus) has been linked to http://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=1852 modulation that is essential for T‐cell activation (Qi, Gao, Hou, & Wan, 2017; Rheinlander, Schraven, & Bommhardt, 2018; Wan et al., 2013). Combining the immunostimulatory efficacy of herbal products with immunosuppressive chemotherapeutic drugs like gemcitabine, the side effects of immunosuppression might be relieved. Furthermore, these two studies propose a new approach for appraising the enhancement of the potential of natural compounds in combination with chemotherapeutic drugs. Before applying folk, herb, and natural compounds in combination therapy, the antitumor efficacy of folk, herb, and natural compounds needs to be assessed first.
4. CONCLUSION
Collectively, the mechanisms of natural compounds acting as chemotherapeutic adjuvants could be summarized into three approaches: directly potentiating tumoricidal effect (sensitizing cancer cells to be more responsive to chemotherapeutic drugs), reversing chemoresistance (diminishing drug efflux or overcoming other mechanism to increase the accumulation of chemotherapeutic drugs in cancer cells), and alleviating toxicity induced by chemotherapeutic drugs (promoting the repairing mechanism in normal cells against damage of chemotherapeutic drugs; Figure 2). After demonstrating anticancer activity as monotherapy, natural compounds could further enhance their application by being chemotherapeutic adjuvants or cooperating drugs in combination therapy. Using TCM or traditional herbal medicine as a chemotherapeutic adjuvant in treating NSCLC or gastric cancer could improve the quality of life of patients, ameliorate myelosuppression, and possibly reduce mortality (Hou et al., 2017; Y. K. Lee, Bae, Yoo, & Cho, 2018; X. Wu et al., 2016). Further studies should look at herbal compounds or low MW compounds that can be applied as an alternative potent supplement for cancer therapy to attenuate any adverse effects and chemoresistance. However, the toxicity of herbal–drug interactions for liver or kidney injury needs to be extensively considered as a precaution during new drug discovery and development.
Figure 2.
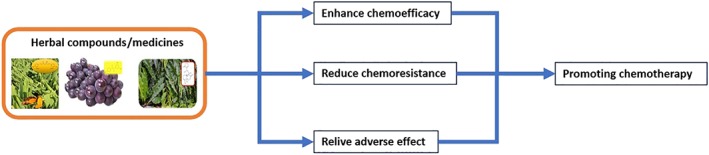
Putative mechanism of natural compounds in chemotherapeutic synergism
4.1. Nomenclature of targets and ligands
Key protein targets and ligands in this article are hyperlinked to corresponding entries in http://www.guidetopharmacology.org, the common portal for data from the UPHAR/BPS Guide to PHARMACOLOGY (Harding et al., 2018), and are permanently archived in the Concise Guide to PHARMACOLOGY 2017/18 (Alexander, Christopoulos et al., 2017; Alexander, Cidlowski et al., 2017; Alexander, Fabbro et al., 2017; Alexander, Kelly et al., 2017a, b).
CONFLICT OF INTEREST
The authors declare no conflicts of interest.
Supporting information
Data S1. Supporting Information
Data S2. Supporting Information
Lin S‐R, Chang C‐H, Hsu C‐F, et al. Natural compounds as potential adjuvants to cancer therapy: Preclinical evidence. Br J Pharmacol. 2020;177:1409–1423. 10.1111/bph.14816
Shian‐Ren Lin and Chia‐Hsiang Chang contribute equally in this study.
REFERENCES
- Adwas, A. A. , Elkhoely, A. A. , Kabel, A. M. , Abdel‐Rahman, M. N. , & Eissa, A. A. (2016). Anti‐cancer and cardioprotective effects of indol‐3‐carbinol in doxorubicin‐treated mice. Journal of Infection and Chemotherapy, 22, 36–43. 10.1016/j.jiac.2015.10.001 [DOI] [PubMed] [Google Scholar]
- Alexander, S. P. H. , Christopoulos, A. , Davenport, A. P. , Kelly, E. , Marrion, N. V. , Peters, J. A. , … CGTP Collaborators (2017). The concise guide to PHARMACOLOGY 2017/18: G protein‐coupled receptors. British Journal of Pharmacology, 174, S17–S129. 10.1111/bph.13878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander, S. P. H. , Cidlowski, J. A. , Kelly, E. , Marrion, N. V. , Peters, J. A. , Faccenda, E. , … CGTP Collaborators (2017). The concise guide to PHARMACOLOGY 2017/18: Nuclear hormone receptors. British Journal of Pharmacology, 174, S208–S224. 10.1111/bph.13880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander, S. P. H. , Fabbro, D. , Kelly, E. , Marrion, N. V. , Peters, J. A. , Faccenda, E. , … CGTP Collaborators (2017). The concise guide to PHARMACOLOGY 2017/18: Enzymes. British Journal of Pharmacology, 174, S272–S359. 10.1111/bph.13877 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander, S. P. , Kelly, E. , Marrion, N. V. , Peters, J. A. , Faccenda, E. , Harding, S. D. , … CGTP Collaborators (2017a). The concise guide to PHARMACOLOGY 2017/18: Other proteins. British Journal of Pharmacology, 174(Suppl 1), S1–S16. 10.1111/bph.13882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander, S. P. H. , Kelly, E. , Marrion, N. V. , Peters, J. A. , Faccenda, E. , Harding, S. D. , … CGTP Collaborators (2017b). The concise guide to PHARMACOLOGY 2017/18: Transporters. British Journal of Pharmacology, 174, S360–S446. 10.1111/bph.13883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alotaibi, A. , Bhatnagar, P. , Najafzadeh, M. , Gupta, K. C. , & Anderson, D. (2013). Tea phenols in bulk and nanoparticle form modify DNA damage in human lymphocytes from colon cancer patients and healthy individuals treated in vitro with platinum‐based chemotherapeutic drugs. Nanomedicine (London, England), 8, 389–401. 10.2217/nnm.12.126 [DOI] [PubMed] [Google Scholar]
- Amiri‐Kordestani, L. , Basseville, A. , Kurdziel, K. , Fojo, A. T. , & Bates, S. E. (2012). Targeting MDR in breast and lung cancer: Discriminating its potential importance from the failure of drug resistance reversal studies. Drug Resistance Updates, 15, 50–61. 10.1016/j.drup.2012.02.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angelini, A. , Conti, P. , Ciofani, G. , Cuccurullo, F. , & Di Ilio, C. (2013). Modulation of multidrug resistance P‐glycoprotein activity by antiemetic compounds in human doxorubicin‐resistant sarcoma cells (MES‐SA/Dx‐5): Implications on cancer therapy. Journal of Biological Regulators and Homeostatic Agents, 27, 1029–1037. [PubMed] [Google Scholar]
- Armas‐Lopez, L. , Zuniga, J. , Arrieta, O. , & Avila‐Moreno, F. (2017). The Hedgehog‐GLI pathway in embryonic development and cancer: Implications for pulmonary oncology therapy. Oncotarget, 8, 60684–60703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bahmani, F. , Esmaeili, S. , Bashash, D. , Dehghan‐Nayeri, N. , Mashati, P. , & Gharehbaghian, A. (2018). Centaurea albonitens extract enhances the therapeutic effects of Vincristine in leukemic cells by inducing apoptosis. Biomedicine & Pharmacotherapy, 99, 598–607. 10.1016/j.biopha.2018.01.101 [DOI] [PubMed] [Google Scholar]
- Below, J. , & Das, J. M. (2019). Vincristine. Treasure Island (FL): StatPearls. [Google Scholar]
- Bidet, M. , Tomico, A. , Martin, P. , Guizouarn, H. , Mollat, P. , & Mus‐Veteau, I. (2012). The Hedgehog receptor patched functions in multidrug transport and chemotherapy resistance. Molecular Cancer Research, 10, 1496–1508. 10.1158/1541-7786.MCR-11-0578 [DOI] [PubMed] [Google Scholar]
- Brewer, C. T. , & Chen, T. (2017). Hepatotoxicity of herbal supplements mediated by modulation of cytochrome P450. International Journal of Molecular Sciences, 18, 2353 10.3390/ijms18112353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown, T. , Pilkington, G. , Bagust, A. , Boland, A. , Oyee, J. , Tudur‐Smith, C. , … Dickson, R. (2013). Clinical effectiveness and cost‐effectiveness of first‐line chemotherapy for adult patients with locally advanced or metastatic non‐small cell lung cancer: A systematic review and economic evaluation. Health Technology Assessment, 17, 1–278. 10.3310/hta17060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Browning, R. J. , Reardon, P. J. T. , Parhizkar, M. , Pedley, R. B. , Edirisinghe, M. , Knowles, J. C. , & Stride, E. (2017). Drug delivery strategies for platinum‐based chemotherapy. ACS Nano, 11, 8560–8578. 10.1021/acsnano.7b04092 [DOI] [PubMed] [Google Scholar]
- Carranza‐Torres, I. E. , Guzman‐Delgado, N. E. , Coronado‐Martinez, C. , Banuelos‐Garcia, J. I. , Viveros‐Valdez, E. , Moran‐Martinez, J. , & Carranza‐Rosales, P. (2015). Organotypic culture of breast tumor explants as a multicellular system for the screening of natural compounds with antineoplastic potential. BioMed Research International, 2015, 618021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chahine, N. , Nader, M. , Duca, L. , Martiny, L. , & Chahine, R. (2016). Saffron extracts alleviate cardiomyocytes injury induced by doxorubicin and ischemia‐reperfusion in vitro. Drug and Chemical Toxicology, 39, 87–96. 10.3109/01480545.2015.1036281 [DOI] [PubMed] [Google Scholar]
- Chang, A. (2011). Chemotherapy, chemoresistance and the changing treatment landscape for NSCLC. Lung Cancer, 71, 3–10. 10.1016/j.lungcan.2010.08.022 [DOI] [PubMed] [Google Scholar]
- Chatterjee, K. , Zhang, J. , Honbo, N. , & Karliner, J. S. (2010). Doxorubicin cardiomyopathy. Cardiology, 115, 155–162. 10.1159/000265166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, Z. , Shi, T. , Zhang, L. , Zhu, P. , Deng, M. , Huang, C. , … Li, J. (2016). Mammalian drug efflux transporters of the ATP binding cassette (ABC) family in multidrug resistance: A review of the past decade. Cancer Letters, 370, 153–164. 10.1016/j.canlet.2015.10.010 [DOI] [PubMed] [Google Scholar]
- Cheung‐Ong, K. , Giaever, G. , & Nislow, C. (2013). DNA‐damaging agents in cancer chemotherapy: Serendipity and chemical biology. Chemistry & Biology, 20, 648–659. 10.1016/j.chembiol.2013.04.007 [DOI] [PubMed] [Google Scholar]
- Dudka, J. , Jodynis‐Liebert, J. , Korobowicz, E. , Burdan, F. , Korobowicz, A. , Szumilo, J. , … Murias, M. (2005). Activity of NADPH‐cytochrome P‐450 reductase of the human heart, liver and lungs in the presence of (−)‐epigallocatechin gallate, quercetin and resveratrol: an in vitro study. Basic & Clinical Pharmacology & Toxicology, 97, 74–79. 10.1111/j.1742-7843.2005.pto_98.x [DOI] [PubMed] [Google Scholar]
- Duwe, G. , Knitter, S. , Pesthy, S. , Beierle, A. S. , Bahra, M. , Schmelzle, M. , … Andreou, A. (2017). Hepatotoxicity following systemic therapy for colorectal liver metastases and the impact of chemotherapy‐associated liver injury on outcomes after curative liver resection. European Journal of Surgical Oncology, 43, 1668–1681. 10.1016/j.ejso.2017.05.008 [DOI] [PubMed] [Google Scholar]
- Engelke, L. H. , Hamacher, A. , Proksch, P. , & Kassack, M. U. (2016). Ellagic acid and resveratrol prevent the development of cisplatin resistance in the epithelial ovarian cancer cell line A2780. Journal of Cancer, 7, 353–363. 10.7150/jca.13754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer, K. R. , Durrans, A. , Lee, S. , Sheng, J. , Li, F. , Wong, S. T. C. , … Gao, D. (2015). Epithelial‐to‐mesenchymal transition is not required for lung metastasis but contributes to chemoresistance. Nature, 527, 472–476. 10.1038/nature15748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Florescu, M. , Cinteza, M. , & Vinereanu, D. (2013). Chemotherapy‐induced cardiotoxicity. Maedica (Buchar), 8, 59–67. [PMC free article] [PubMed] [Google Scholar]
- Galluzzi, L. , Buqué, A. , Kepp, O. , Zitvogel, L. , & Kroemer, G. (2015). Immunological effects of conventional chemotherapy and targeted anticancer agents. Cancer Cell, 28, 690–714. 10.1016/j.ccell.2015.10.012 [DOI] [PubMed] [Google Scholar]
- Glasgow, M. D. , & Chougule, M. B. (2015). Recent developments in active tumor targeted multifunctional nanoparticles for combination chemotherapy in cancer treatment and imaging. Journal of Biomedical Nanotechnology, 11, 1859–1898. 10.1166/jbn.2015.2145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grossi, F. , Kubota, K. , Cappuzzo, F. , de Marinis, F. , Gridelli, C. , Aita, M. , & Douillard, J. Y. (2010). Future scenarios for the treatment of advanced non‐small cell lung cancer: Focus on taxane‐containing regimens. The Oncologist, 15, 1102–1112. 10.1634/theoncologist.2010-0322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajra, S. , Basu, A. , Singha Roy, S. , Patra, A. R. , & Bhattacharya, S. (2017). Attenuation of doxorubicin‐induced cardiotoxicity and genotoxicity by an indole‐based natural compound 3,3′‐diindolylmethane (DIM) through activation of Nrf2/ARE signaling pathways and inhibiting apoptosis. Free Radical Research, 51, 812–827. 10.1080/10715762.2017.1381694 [DOI] [PubMed] [Google Scholar]
- Harding, S. D. , Sharman, J. L. , Faccenda, E. , Southan, C. , Pawson, A. J. , Ireland, S. , … NC‐IUPHAR (2018). The IUPHAR/BPS Guide to PHARMACOLOGY in 2018: Updates and expansion to encompass the new guide to IMMUNOPHARMACOLOGY. Nucleic Acids Research, 46, D1091–D1106. 10.1093/nar/gkx1121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasanovic, A. , & Mus‐Veteau, I. (2018). Targeting the multidrug transporter ptch1 potentiates chemotherapy efficiency. Cell, 7, E107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He, Z. , Huang, J. , Xu, Y. , Zhang, X. , Teng, Y. , Huang, C. , … Sun, W. (2015). Co‐delivery of cisplatin and paclitaxel by folic acid conjugated amphiphilic PEG‐PLGA copolymer nanoparticles for the treatment of non‐small lung cancer. Oncotarget, 6, 42150–42168. 10.18632/oncotarget.6243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horie, S. , Oya, M. , Nangaku, M. , Yasuda, Y. , Komatsu, Y. , Yanagita, M. , … Muto, S. (2018). Guidelines for treatment of renal injury during cancer chemotherapy 2016. Clinical and Experimental Nephrology, 22, 210–244. 10.1007/s10157-017-1448-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosseinimehr, S. J. , Asadian, R. , Naghshvar, F. , Azizi, S. , Jafarinejad, M. , Noaparast, Z. , … Hosseini, S. A. H. (2015). Protective effects of thymol against nephrotoxicity induced by cisplatin with using 99mTc‐DMSA in mice. Renal Failure, 37, 280–284. 10.3109/0886022X.2014.991998 [DOI] [PubMed] [Google Scholar]
- Hou, B. , Liu, R. , Qin, Z. , Luo, D. , Wang, Q. , & Huang, S. (2017). Oral Chinese herbal medicine as an adjuvant treatment for chemotherapy, or radiotherapy, induced myelosuppression: A systematic review and meta‐analysis of randomized controlled trials. Evidence‐Based Complementary and Alternative Medicine, 2017, 3432750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Housman, G. , Byler, S. , Heerboth, S. , Lapinska, K. , Longacre, M. , Snyder, N. , & Sarkar, S. (2014). Drug resistance in cancer: An overview. Cancers (Basel), 6, 1769–1792. 10.3390/cancers6031769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu, Q. , Zhang, D. D. , Wang, L. , Lou, H. , & Ren, D. (2012). Eriodictyol‐7‐O‐glucoside, a novel Nrf2 activator, confers protection against cisplatin‐induced toxicity. Food and Chemical Toxicology, 50, 1927–1932. 10.1016/j.fct.2012.03.059 [DOI] [PubMed] [Google Scholar]
- Huang, J. , Li, H. , & Ren, G. (2015). Epithelial‐mesenchymal transition and drug resistance in breast cancer (Review). International Journal of Oncology, 47, 840–848. 10.3892/ijo.2015.3084 [DOI] [PubMed] [Google Scholar]
- Huang, P. C. , Kuo, W. W. , Shen, C. Y. , Chen, Y. F. , Lin, Y. M. , Ho, T. J. , … Huang, C. Y. (2016). Anthocyanin attenuates doxorubicin‐induced cardiomyotoxicity via estrogen receptor‐α/β and stabilizes HSF1 to inhibit the IGF‐IIR apoptotic pathway. International Journal of Molecular Sciences, 17, 1588 10.3390/ijms17091588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji, Y. , Zheng, M. F. , Ye, S. G. , Wu, X. B. , & Chen, J. Y. (2013). Agrocybe aegerita polysaccharide combined with chemotherapy improves tumor necrosis factor‐α and interferon‐γ levels in rat esophageal carcinoma. Diseases of the Esophagus, 26, 859–863. 10.1111/j.1442-2050.2012.01397.x [DOI] [PubMed] [Google Scholar]
- Kim, S. Y. , Lee, H. J. , Nam, J. W. , Seo, E. K. , & Lee, Y. S. (2015). Coniferyl aldehyde reduces radiation damage through increased protein stability of heat shock transcriptional factor 1 by phosphorylation. International Journal of Radiation Oncology, Biology, Physics, 91, 807–816. 10.1016/j.ijrobp.2014.11.031 [DOI] [PubMed] [Google Scholar]
- Kim, W. , Lee, W. B. , Lee, J. , Min, B. I. , Lee, H. , & Cho, S. H. (2015b). Traditional herbal medicine as adjunctive therapy for nasopharyngeal cancer: A systematic review and meta‐analysis. Integrative Cancer Therapies, 14, 212–220. 10.1177/1534735415572881 [DOI] [PubMed] [Google Scholar]
- Kim, W. , Lee, W. B. , Lee, J. W. , Min, B. I. , Baek, S. K. , Lee, H. S. , & Cho, S. H. (2015a). Traditional herbal medicine as adjunctive therapy for breast cancer: A systematic review. Complementary Therapies in Medicine, 23, 626–632. 10.1016/j.ctim.2015.03.011 [DOI] [PubMed] [Google Scholar]
- Kuo, Y. T. , Liao, H. H. , Chiang, J. H. , Wu, M. Y. , Chen, B. C. , Chang, C. M. , … Yen, H. R. (2018). Complementary Chinese herbal medicine therapy improves survival of patients with pancreatic cancer in Taiwan: A nationwide population‐based cohort study. Integrative Cancer Therapies, 17, 411–422. 10.1177/1534735417722224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee, W. J. , Kim, H. W. , Lee, H. Y. , & Son, C. G. (2015). Systematic review on herb‐induced liver injury in Korea. Food and Chemical Toxicology, 84, 47–54. 10.1016/j.fct.2015.06.004 [DOI] [PubMed] [Google Scholar]
- Lee, Y. K. , Bae, K. , Yoo, H. S. , & Cho, S. H. (2018). Benefit of adjuvant traditional herbal medicine with chemotherapy for resectable gastric cancer. Integrative Cancer Therapies, 17, 619–627. 10.1177/1534735417753542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, F. , & Zhang, N. (2015). Ceramide: Therapeutic potential in combination therapy for cancer treatment. Current Drug Metabolism, 17, 37–51. 10.2174/1389200216666151103120338 [DOI] [PubMed] [Google Scholar]
- Li, W. , Zhang, H. , Assaraf, Y. G. , Zhao, K. , Xu, X. , Xie, J. , … Chen, Z. S. (2016). Overcoming ABC transporter‐mediated multidrug resistance: Molecular mechanisms and novel therapeutic drug strategies. Drug Resistance Updates, 27, 14–29. 10.1016/j.drup.2016.05.001 [DOI] [PubMed] [Google Scholar]
- Liboiron, B. D. , & Mayer, L. D. (2014). Nanoscale particulate systems for multidrug delivery: Towards improved combination chemotherapy. Therapeutic Delivery, 5, 149–171. 10.4155/tde.13.149 [DOI] [PubMed] [Google Scholar]
- Lin, S. R. , Fu, Y. S. , Tsai, M. J. , Cheng, H. , & Weng, C. F. (2017). Natural compounds from herbs that can potentially execute as autophagy inducers for cancer therapy. International Journal of Molecular Sciences, 18, e1412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, B. , Han, L. , Liu, J. , Han, S. , Chen, Z. , & Jiang, L. (2017). Co‐delivery of paclitaxel and TOS‐cisplatin via TAT‐targeted solid lipid nanoparticles with synergistic antitumor activity against cervical cancer. International Journal of Nanomedicine, 12, 955–968. 10.2147/IJN.S115136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorusso, D. , Petrelli, F. , Coinu, A. , Raspagliesi, F. , & Barni, S. (2014). A systematic review comparing cisplatin and carboplatin plus paclitaxel‐based chemotherapy for recurrent or metastatic cervical cancer. Gynecologic Oncology, 133, 117–123. 10.1016/j.ygyno.2014.01.042 [DOI] [PubMed] [Google Scholar]
- Ma, J. , Kavelaars, A. , Dougherty, P. M. , & Heijnen, C. J. (2018). Beyond symptomatic relief for chemotherapy‐induced peripheral neuropathy: Targeting the source. Cancer, 124, 2289–2298. 10.1002/cncr.31248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manohar, S. , & Leung, N. (2018). Cisplatin nephrotoxicity: A review of the literature. Journal of Nephrology, 31, 15–25. 10.1007/s40620-017-0392-z [DOI] [PubMed] [Google Scholar]
- Mansoori, B. , Mohammadi, A. , Davudian, S. , Shirjang, S. , & Baradaran, B. (2017). The different mechanisms of cancer drug resistance: A brief review. Adv Pharm Bull, 7, 339–348. 10.15171/apb.2017.041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mrusek, M. , Seo, E. J. , Greten, H. J. , Simon, M. , & Efferth, T. (2015). Identification of cellular and molecular factors determining the response of cancer cells to six ergot alkaloids. Investigational New Drugs, 33, 32–44. 10.1007/s10637-014-0168-4 [DOI] [PubMed] [Google Scholar]
- Mundhe, N. A. , Kumar, P. , Ahmed, S. , Jamdade, V. , Mundhe, S. , & Lahkar, M. (2015). Nordihydroguaiaretic acid ameliorates cisplatin induced nephrotoxicity and potentiates its anti‐tumor activity in DMBA induced breast cancer in female Sprague‐Dawley rats. International Immunopharmacology, 28, 634–642. 10.1016/j.intimp.2015.07.016 [DOI] [PubMed] [Google Scholar]
- Nagel, Z. D. , Kitange, G. J. , Gupta, S. K. , Joughin, B. A. , Chaim, I. A. , Mazzucato, P. , … Samson, L. D. (2017). DNA repair capacity in multiple pathways predicts chemoresistance in glioblastoma multiforme. Cancer Research, 77, 198–206. 10.1158/0008-5472.CAN-16-1151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Cancer Institute (2002a). Breast cancer treatment (PDQ(R)): Health professional version. Bethesda (MD): PDQ Cancer Information Summaries. [Google Scholar]
- National Cancer Institute (2002b). Non‐small cell lung cancer treatment (PDQ(R)): Health professional version. Bethesda (MD): PDQ Cancer Information Summaries. [Google Scholar]
- Olas, B. , & Wachowicz, B. (2004). Resveratrol reduces oxidative stress induced by platinum compounds in blood platelets. General Physiology and Biophysics, 23, 315–326. [PubMed] [Google Scholar]
- de Oliveira Junior, R. G. , Christiane Adrielly, A. F. , da Silva Almeida, J. R. G. , Grougnet, R. , Thiery, V. , & Picot, L. (2018). Sensitization of tumor cells to chemotherapy by natural products: A systematic review of preclinical data and molecular mechanisms. Fitoterapia, 129, 383–400. 10.1016/j.fitote.2018.02.025 [DOI] [PubMed] [Google Scholar]
- Oliveira, M. S. , Mendes, L. P. , & Torchilin, V. P. (2017). Targeted delivery of anticancer drugs: New trends in lipid nanocarriers In Ficai, A., & Grumezescu, A. M. (Eds.), Nanostructures for cancer therapy (pp. 455–484). Philadelphia, United States: Elsevier. [Google Scholar]
- Osman, A. M. , Al‐Malki, H. S. , Al‐Harthi, S. E. , El‐Hanafy, A. A. , Elashmaoui, H. M. , & Elshal, M. F. (2015). Modulatory role of resveratrol on cytotoxic activity of cisplatin, sensitization and modification of cisplatin resistance in colorectal cancer cells. Molecular Medicine Reports, 12, 1368–1374. 10.3892/mmr.2015.3513 [DOI] [PubMed] [Google Scholar]
- Ouyang, L. , Luo, Y. , Tian, M. , Zhang, S. Y. , Lu, R. , Wang, J. H. , … Li, X. (2014). Plant natural products: From traditional compounds to new emerging drugs in cancer therapy. Cell Proliferation, 47, 506–515. 10.1111/cpr.12143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pai, M. P. , Cottrell, M. L. , Kashuba, A. D. M. , & Bertino, J. S. (2015). 19—Pharmacokinetics and pharmacodynamics of anti‐infective agents In Bennett J. E., Dolin R., & Blaser M. J. (Eds.), Mandell, Douglas, and Bennett's principles and practice of infectious diseases (Eighth ed.) (pp. 252–262.e252). Philadelphia: Content Repository Only! [Google Scholar]
- Pal, D. , & Mitra, A. K. (2006). MDR‐ and CYP3A4‐mediated drug‐herbal interactions. Life Sciences, 78, 2131–2145. 10.1016/j.lfs.2005.12.010 [DOI] [PubMed] [Google Scholar]
- Parasramka, S. , Talari, G. , Rosenfeld, M. , Guo, J. , & Villano, J. L. (2017). Procarbazine, lomustine and vincristine for recurrent high‐grade glioma. Cochrane Database of Systematic Reviews, 7, CD011773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petri, A. , Alexandratou, E. , Kyriazi, M. , Rallis, M. , Roussis, V. , & Yova, D. (2012). Combination of Fospeg‐IPDT and a natural antioxidant compound prevents photosensitivity in a murine prostate cancer tumour model. Photodiagnosis and Photodynamic Therapy, 9, 100–108. 10.1016/j.pdpdt.2011.11.002 [DOI] [PubMed] [Google Scholar]
- Qi, Y. , Gao, F. , Hou, L. , & Wan, C. (2017). Anti‐inflammatory and immunostimulatory activities of astragalosides. The American Journal of Chinese Medicine, 45, 1157–1167. 10.1142/S0192415X1750063X [DOI] [PubMed] [Google Scholar]
- Renu, K. , Abilash, V. G. , Tirupathi Pichiah, P. B. , & Arunachalam, S. (2018). Molecular mechanism of doxorubicin‐induced cardiomyopathy—An update. European Journal of Pharmacology, 818, 241–253. 10.1016/j.ejphar.2017.10.043 [DOI] [PubMed] [Google Scholar]
- Rheinlander, A. , Schraven, B. , & Bommhardt, U. (2018). CD45 in human physiology and clinical medicine. Immunology Letters, 196, 22–32. 10.1016/j.imlet.2018.01.009 [DOI] [PubMed] [Google Scholar]
- Rimkus, T. K. , Carpenter, R. L. , Qasem, S. , Chan, M. , & Lo, H. W. (2016). Targeting the sonic hedgehog signaling pathway: Review of smoothened and GLI inhibitors. Cancers (Basel), 8, 22 10.3390/cancers8020022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakthivel, K. M. , & Hariharan, S. (2017). Regulatory players of DNA damage repair mechanisms: Role in cancer chemoresistance. Biomedicine & Pharmacotherapy, 93, 1238–1245. 10.1016/j.biopha.2017.07.035 [DOI] [PubMed] [Google Scholar]
- Santoni, M. , Guerra, F. , Conti, A. , Lucarelli, A. , Rinaldi, S. , Belvederesi, L. , … Berardi, R. (2017). Incidence and risk of cardiotoxicity in cancer patients treated with targeted therapies. Cancer Treatment Reviews, 59, 123–131. 10.1016/j.ctrv.2017.07.006 [DOI] [PubMed] [Google Scholar]
- Shamsi, S. , Tran, H. , Tan, R. S. , Tan, Z. J. , & Lim, L. Y. (2017). Curcumin, Piperine, and capsaicin: A comparative study of spice‐mediated inhibition of human cytochrome P450 isozyme activities. Drug Metabolism and Disposition, 45, 49–55. 10.1124/dmd.116.073213 [DOI] [PubMed] [Google Scholar]
- Sharbaf, F. G. , Farhangi, H. , & Assadi, F. (2017). Prevention of chemotherapy‐induced nephrotoxicity in children with cancer. International Journal of Preventive Medicine, 8, 76 10.4103/ijpvm.IJPVM_40_17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonaa, E. , Usha, S. , & Ja In, J. (2013). An ex vivo study of selenium, genistein on the morphological and nuclear changes in anticancer drug‐induced apoptosis in human peripheral blood lymphocytes. BioFactors, 39, 279–293. 10.1002/biof.1069 [DOI] [PubMed] [Google Scholar]
- Sulthana, S. , Banerjee, T. , Kallu, J. , Vuppala, S. R. , Heckert, B. , Naz, S. , … Santra, S. (2017). Combination therapy of NSCLC using Hsp90 inhibitor and doxorubicin carrying functional nanoceria. Molecular Pharmaceutics, 14, 875–884. 10.1021/acs.molpharmaceut.6b01076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun, Y. F. , & Wink, M. (2014). Tetrandrine and fangchinoline, bisbenzylisoquinoline alkaloids from Stephania tetrandra can reverse multidrug resistance by inhibiting P‐glycoprotein activity in multidrug resistant human cancer cells. Phytomedicine, 21, 1110–1119. 10.1016/j.phymed.2014.04.029 [DOI] [PubMed] [Google Scholar]
- Tai, C. J. , Wang, C. K. , Tai, C. J. , Lin, Y. F. , Lin, C. S. , Jian, J. Y. , … Chang, C. C. (2013). Aqueous extract of Solanum nigrum leaves induces autophagy and enhances cytotoxicity of cisplatin, doxorubicin, docetaxel, and 5‐fluorouracil in human colorectal carcinoma cells. Evidence‐Based Complementary and Alternative Medicine, 2013, 514719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takei, Y. , Takahashi, Y. , Machida, S. , Taneichi, A. , Takahashi, S. , Nagashima, T. , … Fujiwara, H. (2017). Response to and toxicity of gemcitabine for recurrent ovarian cancer according to number of previous chemotherapy regimens. The Journal of Obstetrics and Gynaecology Research, 43, 358–364. 10.1111/jog.13203 [DOI] [PubMed] [Google Scholar]
- To, K. K. W. , Wu, X. , Yin, C. , Chai, S. , Yao, S. , Kadioglu, O. , … Lin, G. (2017). Reversal of multidrug resistance by Marsdenia tenacissima and its main active ingredients polyoxypregnanes. Journal of Ethnopharmacology, 203, 110–119. [DOI] [PubMed] [Google Scholar]
- Turrini, E. , Ferruzzi, L. , & Fimognari, C. (2014). Natural compounds to overcome cancer chemoresistance: Toxicological and clinical issues. Expert Opinion on Drug Metabolism & Toxicology, 10, 1677–1690. 10.1517/17425255.2014.972933 [DOI] [PubMed] [Google Scholar]
- Varricchi, G. , Ameri, P. , Cadeddu, C. , Ghigo, A. , Madonna, R. , Marone, G. , … Tocchetti, C. G. (2018). Antineoplastic drug‐induced cardiotoxicity: A redox perspective. Frontiers in Physiology, 9, 167 10.3389/fphys.2018.00167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkova, M. , & Russell, R. 3rd (2011). Anthracycline cardiotoxicity: Prevalence, pathogenesis and treatment. Current Cardiology Reviews, 7, 214–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wade, C. A. , & Kyprianou, N. (2018). Profiling prostate cancer therapeutic resistance. International Journal of Molecular Sciences, 19, 904 10.3390/ijms19030904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan, C. P. , Gao, L. X. , Hou, L. F. , Yang, X. Q. , He, P. L. , Yang, Y. F. , … Zuo, J. P. (2013). Astragaloside II triggers T cell activation through regulation of CD45 protein tyrosine phosphatase activity. Acta Pharmacologica Sinica, 34, 522–530. 10.1038/aps.2012.208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, C. W. , Chen, C. L. , Wang, C. K. , Chang, Y. J. , Jian, J. Y. , Lin, C. S. , … Tai, C. J. (2015). Cisplatin‐, doxorubicin‐, and docetaxel‐induced cell death promoted by the aqueous extract of solanum nigrum in human ovarian carcinoma cells. Integrative Cancer Therapies, 14, 546–555. 10.1177/1534735415588826 [DOI] [PubMed] [Google Scholar]
- Wang, J. , Wei, Q. , Wang, X. , Tang, S. , Liu, H. , Zhang, F. , … Luu, H. H. (2016). Transition to resistance: An unexpected role of the EMT in cancer chemoresistance. Genes & Diseases, 3, 3–6. 10.1016/j.gendis.2016.01.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, L. , Mosel, A. J. , Oakley, G. G. , & Peng, A. (2012). Deficient DNA damage signaling leads to chemoresistance to cisplatin in oral cancer. Molecular Cancer Therapeutics, 11, 2401–2409. 10.1158/1535-7163.MCT-12-0448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, L. , Sun, J. , Gao, P. , Su, K. , Wu, H. , Li, J. , … Lou, W. (2019). Wnt1‐inducible signaling protein 1 regulates laryngeal squamous cell carcinoma glycolysis and chemoresistance via the YAP1/TEAD1/GLUT1 pathway. Journal of Cellular Physiology, 234, 15941–15950. [DOI] [PubMed] [Google Scholar]
- Wu, L. , Leng, D. , Cun, D. , Foged, C. , & Yang, M. (2017). Advances in combination therapy of lung cancer: Rationales, delivery technologies and dosage regimens. Journal of Controlled Release, 260, 78–91. 10.1016/j.jconrel.2017.05.023 [DOI] [PubMed] [Google Scholar]
- Wu, W. J. , Zhang, Y. , Zeng, Z. L. , Li, X. B. , Hu, K. S. , Luo, H. Y. , … Xu, R. H. (2013). β‐phenylethyl isothiocyanate reverses platinum resistance by a GSH‐dependent mechanism in cancer cells with epithelial‐mesenchymal transition phenotype. Biochemical Pharmacology, 85, 486–496. 10.1016/j.bcp.2012.11.017 [DOI] [PubMed] [Google Scholar]
- Wu, X. , Chung, V. C. , Lu, P. , Poon, S. K. , Hui, E. P. , Lau, A. Y. , … Wu, J. C. (2016). Chinese herbal medicine for improving quality of life among nonsmall cell lung cancer patients: Overview of systematic reviews and network meta‐analysis. Medicine (Baltimore), 95, e2410 10.1097/MD.0000000000002410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu, Y. , Wang, D. , Yang, X. , Fu, C. , Zou, L. , & Zhang, J. (2019). Traditional Chinese medicine Gegen Qinlian decoction ameliorates irinotecan chemotherapy‐induced gut toxicity in mice. Biomedicine & Pharmacotherapy, 109, 2252–2261. 10.1016/j.biopha.2018.11.095 [DOI] [PubMed] [Google Scholar]
- Xu, D. P. , Li, Y. , Meng, X. , Zhou, T. , Zhou, Y. , Zheng, J. , … Li, H. B. (2017). Natural antioxidants in foods and medicinal plants: Extraction, assessment and resources. International Journal of Molecular Sciences, 18, 96 10.3390/ijms18010096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu, H. (2016). (Z)‐3,4,3′,5′‐tetramethoxystilbene, a natural product, induces apoptosis and reduces viability of paclitaxel‐and cisplatin‐resistant osteosarcoma cells. Journal of Cancer Research and Therapeutics, 12, 1261–1265. 10.4103/0973-1482.158035 [DOI] [PubMed] [Google Scholar]
- Yamada, K. , Hung, P. , Park, T. K. , Park, P. J. , & Lim, B. O. (2011). A comparison of the immunostimulatory effects of the medicinal herbs Echinacea, Ashwagandha and Brahmi. Journal of Ethnopharmacology, 137, 231–235. 10.1016/j.jep.2011.05.017 [DOI] [PubMed] [Google Scholar]
- Yu, J. , Wang, C. , Kong, Q. , Wu, X. , Lu, J. J. , & Chen, X. (2018). Recent progress in doxorubicin‐induced cardiotoxicity and protective potential of natural products. Phytomedicine, 40, 125–139. 10.1016/j.phymed.2018.01.009 [DOI] [PubMed] [Google Scholar]
- Yu, Y. C. , Mao, Y. M. , Chen, C. W. , Chen, J. J. , Chen, J. , Cong, W. M. , … Chinese Medical Association (CMA) (2017). CSH guidelines for the diagnosis and treatment of drug‐induced liver injury. Hepatology International, 11, 221–241. 10.1007/s12072-017-9793-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan, R. , Hou, Y. , Sun, W. , Yu, J. , Liu, X. , Niu, Y. , … Chen, X. (2017). Natural products to prevent drug resistance in cancer chemotherapy: A review. Annals of the new York Academy of Sciences, 1401, 19–27. 10.1111/nyas.13387 [DOI] [PubMed] [Google Scholar]
- Zarybnicky, T. , Bousova, I. , Ambroz, M. , & Skalova, L. (2018). Hepatotoxicity of monoterpenes and sesquiterpenes. Archives of Toxicology, 92, 1–13. 10.1007/s00204-017-2062-2 [DOI] [PubMed] [Google Scholar]
- Zhang, Y. , Yang, M. , Ji, Q. , Fan, D. , Peng, H. , Yang, C. , … Zhou, Y. (2011). Anoikis induction and metastasis suppression by a new integrin αvβ3 inhibitor in human melanoma cell line M21. Investigational New Drugs, 29, 666–673. 10.1007/s10637-010-9616-y [DOI] [PubMed] [Google Scholar]
- Zheng, H. C. (2017). The molecular mechanisms of chemoresistance in cancers. Oncotarget, 8, 59950–59964. 10.18632/oncotarget.19048 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1. Supporting Information
Data S2. Supporting Information


