Abstract
The nutraceuticals market is vast, encompassing many different products with inconsistent levels of evidence available to support their use. This overview represents a Western perspective of the nutraceuticals market, with a brief comparison with that in China, as an illustration of how individual health supplements increase and decrease in popularity in regional terms. Recent changes in sales patterns, mainly taken from the US market, are summarized and a selection of five newer products, which have not been subject to extensive recent review are profiled: astaxanthin, a carotenoid found in red algae, seafood, salmon and trout, as an antioxidant; cannabidiol, a non‐euphoric marijuana ingredient used as mood enhancer and for painful/inflammatory conditions; modified extracts of ginseng used in new indications including dementia and space travel; monk fruit, a non‐sugar high intensity sweetener and nigella seed, a popular food ingredient and Asian medicine, which has experienced an extraordinary rise in sales recently.
Linked Articles
This article is part of a themed section on The Pharmacology of Nutraceuticals. To view the other articles in this section visit http://onlinelibrary.wiley.com/doi/10.1111/bph.v177.6/issuetoc
Abbreviations
- AX
astaxanthin
- CBD
cannabidiol
- DS
dammarane sapogenins
- PPT
20(S)‐protopanaxatriol
- TCM
traditional Chinese medicine
- THC
Δ9‐tetrahydrocannabinol
- TQ
thymoquinone
1. INTRODUCTION
In Western countries, demand for nutraceuticals continues to grow, despite mounting criticism of the general lack of clinical evidence for many products, false claims of efficacy, concerns about quality, and issues surrounding self‐medication in cases of serious illness (Colalto, 2018; Grollman & Marcus, 2016; Izzo, 2018; Minuz, Velo, Violi, & Ferro, 2017).
There is controversy about the term “nutraceutical”—these products have been described as “neither nutritious nor pharmaceutical”—and there is wholesale uncertainty about what they are (Aronson, 2017). The terms “nutraceutical” and “functional food” are often used interchangeably and have no legal definition in most countries. These products are intended to have health benefits in addition to their basic nutritional value and usually contain ingredients which are referred to as generally recognized as safe (GRAS) in the United States and elsewhere and as qualified presumption of safety (QPS) in the European Union (EU). They may be presented as a pharmaceutical dosage form (such as a capsule or tablet) or a food form as part of a recipe. The regulation of these products varies widely according to region and tradition, and many are covered by general legislation for herbal products using different terminology: For example, “botanical drug” is used in the United States, and “phytomedicine” in the EU. In Canada, they are classified as “Natural and Non‐prescription Health Products” and in Australia, they are covered by the Therapeutic Goods Act. In China, many terms are used, such as “Chinese materia medica” and “functional food,” furthermore, these products may contain animal or mineral derivatives. In India, regulation of traditional supplements is covered by the Ministry of AYUSH and in Japan, regulatory categories used include “Kampo products,” “non‐Kampo crude drug products” and “foods with function claims.” The term nutraceutical is a wide umbrella where different naturally occurring products, such as functional foods, fortified foods and dietary supplements (e.g. plant extracts, vitamins, amino acids, minerals, fibre and prebiotics), have found their place, as shown in Figure 1.
Figure 1.
Different types of products described as “nutraceuticals”
For the purposes of this review, we use a working description of dietary health supplements which are usually derived from, and/or frequently added to, food items and are not primarily herbal medicines.
Nutraceutical use is widespread but more prevalent in certain patient groups—the elderly, cancer sufferers, athletes and those trying to lose weight (Alsanad, Howard, & Williamson, 2016; Agbabiaka, Wider, Watson, & Goodman, 2017; Gurley et al., 2018). Many are taking conventional medicines at the same time and the possibility of drug interactions must be considered (Izzo, Hoon‐Kim, Radhakrishnan, & Williamson, 2016; Awortwe, 2017). In the United Kingdom, Alsanad et al. (2016) found a high use (34%) of health supplements in cancer patients. The most popular products taken showed a low potential for drug interaction and few patients reported any adverse events. Agbabiaka, Spencer, Khanom, and Goodman (2018) found a remarkably similar incidence (33.6%) in the elderly but identified some concerns about potential interactions. This is comparable with other Western countries.
Many types of traditional medicine consider food and herbal ingredients as an integral part of treatment, for example, in traditional Chinese medicine (TCM), Ayurveda, Unani and many other systems. These markets cover a wide range of nutraceutical products and some have entered the mainstream global market, for example, ginseng, turmeric, moringa, nigella and fenugreek, as shown in Tables 1 and 2.
Table 1.
Sales of herbal nutraceuticals in 2017 by U.S. mainstream multi‐outlet channels (adapted from Smith et al., 2018)
| Rank (No.) | Common and Latin binomial names | Total US sales ($) | % Change 2016–2017 | Common uses (not exhaustive) |
|---|---|---|---|---|
| 1 (3) | Cranberry, Vaccinium macrocarpon | 68,121,373 | −7.9 | Urinary tract infection |
| 2 (5) | Turmeric, Curcuma longa | 32,465,933 | 46.7 | General health |
| 3 (7) | Garcinia, Garcinia cambogia | 32,298,038 | −17.2 | Weight loss |
| 4 (8) | Green tea, Camelia sinensis | 30,151,628 | −30.4 | General health |
| 5 (9) | Ginger, Zingiber officinalis | 29,532,702 | 5.2 | Gastrointestinal, inflammation |
| 6 (10) | Fenugreek, Trigonella foenum‐graecum | 28,823,510 | 35.5 | Hypercholesterolaemia |
| 7 (11) | Flaxseed, seed oil, Linum usitatissimum | 28,361,026 | −11.1 | General health, menopause |
| 8 (12) | Aloe, Aloe vera | 21,139,780 | 6.3 | General health, skin |
| 9 (18) | Garlic, Allium sativum | 15,918,006 | −1.0 | Cardiovascular conditions |
| 10 (24) | Coconut oil, Cocos nucifera | 9,609,631 | −34.9 | General health, skin |
| 11 (25) | Ginseng, Panax ginseng | 9,450,098 | −2.9 | General health, fatigue, debility |
| 12 (27) | Red yeast rice, Oryza sativa/Monascus purpureus | 9,419,507 | −4.7 | Hypercholesterolaemia |
| 13 (29) | Guarana, Paullinia cupana | 9,099,900 | −13.8 | Stimulant, in “energy” foods |
| 14 (30) | Plant sterols | 8,890,234 | −27.8 | Hypercholesterolaemia |
| 15 (31) | Açai berry, Euterpe oleracea | 8,523,191 | −19.6 | General health |
| 16 (32) | Green coffee, Coffea arabica | 8,358,636 | −38.2 | General health |
| 17 (33) | Wheat/barley grass, Triticum aestivum/Hordeum vulgare | 8,337,413 | 44.2 | General health |
| 18 (34) | Biflavonoid complex | 8,264,590 | −17.9 | General health |
| 19 (36) | Maca, Lepidium meyenii | 6,250,846 | 0.0 | General health |
| 20 (39) | Chia, Salvia hispanica | 3,857,463 | −26.6 | General health |
Table 2.
Sales of herbal nutraceuticals in 2017 by the U.S. natural channel
| Rank | Common and Latin binomial names | Total US sales ($) | % change 2016–2017 | Common uses |
|---|---|---|---|---|
| 1 | Cannabidiol (CBD), from Cannabis sativa | 7,583,438 | 303.0 | General “wellness,” mood support, inflammation, pain |
| 2 | Mushrooms | 5,611,642 | 29 | Stimulation of the immune system, cancer support |
| 3 | Chlorophyll, Chlorella vulgaris | 5,444,533 | −0.2 | General health (“antioxidant”) |
| 4 | Nigella seed, Nigella sativa | 4,675,515 | 202.5 | Many, including gastrointestinal, skin disease, asthma |
| 5 | Cherry fruit, Prunus spp. | 3,626,819 | 8.4 | General health |
| 6 | Stevia, Stevia rebaudiana | 3,470,576 | −0.3 | Sweetener, sugar substitute |
| 7 | Kelp, Laminaria digitata | 2,772,633 | 1.4 | Weight loss, source of iodine |
| 8 | Moringa, Moringa oleifera | 2,738,118 | 32.9 | Multiple, depending on plant part used |
Note: Items NOT included in mainstream sales figures and Table 1.
2. THE CURRENT MARKET AND RECENT TRENDS
Sales and/or usage figures are not available for many regions, and those that are inherently unreliable due to a lack of an adequate definition and differences in the classifications of nutraceuticals, functional foods and dietary supplements in different regions and for different markets. There is a general lack of regulation of the global market overall and many outlets through which consumers can buy these products. Andrew and Izzo (2017) identify polyphenols, carotenoids, phytosterols, amino acids and peptides as important classes of nutraceuticals, explaining their chemistry and assessing the evidence for their efficacy and safety. Products containing polyphenols have especially very high sales, as shown in Table 1, and evidence for efficacy, particularly in cardiovascular conditions, is mounting (Durazzo et al., 2019). Carotenoid and curcuminoid‐containing nutraceuticals (astaxanthin [AX], turmeric) (Brendler and Williamson 2019; Pagano et al., 2018) are also well‐represented in the top selling product, and herbs from TCM and Ayurveda are entering the mainstream global market.
Sales figures for the United States are the most detailed regarding the botanical source of each supplement and will be used here as examples of how trends change. Patterns of herbal nutraceutical use in Western countries are fairly similar to those in the United States, as shown in Tables 1 and 2, but different to those in China, India and other regions. In the United States, in 2017, retail sales of herbal dietary supplements surpassed $8 billion (Smith, Kawa, et al., 2018), whereas in China, by the end of 2014, the overall sales value of Chinese materia medica (CMM) exceeded $US120bn (Dang, Wang, Wang, Yan, & Liu, 2016). In China, the top‐selling herbal health products (nutraceutical or functional food products in terms of raw materials and extracts in 2014 were goji (wolfberry, Lycium chinense) linzhi (reishi) mushroom (Ganoderma lucidum), Asian/Chinese/Korean ginseng (Panax ginseng) and, rather unexpectedly, American ginseng (Panax quinquefolium) (Zhang, Wu, et al., 2017).
Table 1 is extracted from Smith et al. (2018, table 4), the original of which covers the whole “herbal dietary supplements” market sales by U.S. mainstream multi‐outlet channels. We have removed “herbal medicines”—those which are not suitable for general supplement use and are not regular items of diet—although they are among the top‐selling U.S. products. For example, horehound, Marrubium vulgare, is the top‐selling “herbal dietary supplement” and echinacea (various species), ivy leaf (Hedera helix), black cohosh (Actaea racemosa), yohimbe (Pausinystalia johimbe) and saw palmetto (Serenoa repens) are the second, fourth, sixth, 13th and 14th U.S. top‐selling herbal dietary supplements (Smith et al., 2018). Two provisos are worthy of note: firstly, medicinal herbs have been included, likely arbitrarily, in the category “dietary supplements” by the U.S. Dietary Supplement Health and Education Act (Marcus, 2016) and secondly, there is a great deal of overlap between “medicinal” and “food” plants.
The sales ranking assigned in Table 1 is as a nutraceutical and in parentheses is the ranking as a “herbal supplement” by Smith et al. (2018). These statistics have major limitations but can provide a snapshot of how quickly trends vary as products come in and out of favour. Sales figures in bold are supplements which have shown a steep increase in sales (>25%).
Table 2 is also extracted from Smith et al. (2018; table 5) and shows sales figures of the 40 top‐selling “herbal dietary supplements” by the U.S. Natural Channel. This is smaller and more specialized group of outlets. In Table 2, we summarize those nutraceuticals which do not also appear in sales from mainstream outlets. As with Table 1, we have omitted herbal medicines such as ashwagandha and kava. Some of these newer or more specialized products have shown a huge increase in sales: in the case of cannabidiol (CBD) >300% and for nigella seed >200%. None have shown a significant decrease in sales (<1%).
Table 3 shows a selection of other important herbal nutraceuticals which have significant reported sales, much higher than those in the analysis by Smith et al. (2018) and which are predicted to show an increase in sales. Their omission may be due to differences in definitions and the markets surveyed, as Tables 1 and 2 describe the U.S. market only. The higher estimates in Table 3 are likely to be because those figures are for global sales. Some products, such as β‐glucans, are used in a variety of ways, of which pharmaceuticals are only a part (Grandview research, 2019) and it is difficult to assess their market value.
Table 3.
Refined, newer products, with significant global sales, not included above, 2016–2018
| Common and Latin binomial names | Total sales ($) for year | Common and new uses |
|---|---|---|
| Astaxanthin, from Haematococcus pluvialis and other marine sources | 512,800,000 (2016) | Eye health: macular degeneration |
| Monk fruit, Luo Han Guo, Siraitia grosvenorii | 379,400,000 (2018) | Sweetener, sugar substitute, diabetic foods |
| β‐glucans extracted from mushrooms | 410,600,000 (2016). | Immune support, hypercholesterolaemia |
| Modified extracts of Panax ginseng, e.g. dammarane saponins | N/A. Global market huge. | Cognitive impairment/space travel; radiotherapy |
3. MODERN APPLICATIONS AND RESEARCH
Food as medicine is a long‐held tenet of traditional medicine. Newer applications for existing medicines are being investigated, such as for their ability to treat memory impairment in Alzheimer's disease and for depression, where an increasing global population of elderly people makes these conditions a significant social and economic burden.
Modern medical treatments such as radiotherapy and chemotherapy produce many undesirable side effects and the role of herbal supplements in alleviating these is the subject of a great deal of research. Future applications even include attenuating the negative physiological and psychological effects experienced by astronauts during space travel (Dang, Sun, et al., 2014), where research has shown that some Chinese herbs may be useful in counteracting stresses induced in a simulated space environment in animal models, acting through multiple pathways on multiple targets.
4. NEW PRODUCT DEVELOPMENT
A wealth of evidence shows that different phytochemicals in a single herbal extract can produce opposing effects, and consequently, the composition of an extract is crucial. This suggests that if extracts can be manipulated, by chemical modification, selective removal or enhancement of a constituent, the same plant may yield medicines tailored for different indications. For these products, quality assurance is critical.
Five products, associated with rapidly increasing sales and/or research efforts, were selected for a more detailed look at the evidence. We have not included those with no clinical evidence based on well‐defined extracts (wheatgrass/barley grass), nor where the food or medical usage is documented but depends very much on the part of the plant (Moringa; see Anwar, Latif, Ashraf, & Gilani, 2007). The market for mushrooms and β‐glucans is huge but sources of products very wide (Grandview research, 2019), and turmeric has been so widely reviewed recently that there is little more to add. For the following nutraceuticals, a brief overview of (speculative) reasons for their increased popularity, and current evidence for their use provided, with an emphasis on more recent research. For AX and ginseng‐targeted extracts, where specific chemical structural changes are integral to the discussion of the pharmacology, the relevant formula is shown for convenience.
AX: a newer product with good safety data and some clinical evidence in support.
CBD/cannabis oils and edibles: a worrying—and rapidly growing—“wellness” trend.
Ginseng‐targeted extracts: a TCM/global herb with new indications and in new formulations.
Monk fruit: a sugar substitute, a new food use for a TCM herb, with considerable safety data.
Nigella seed: food item and traditional Asian medicine, and an increasingly popular nutraceutical.
5. ASTAXANTHIN
AX is a xanthophyll carotenoid found naturally in micro‐algae, mainly Haematococcus pluvialis, and the aquatic animals that feed on them, the yeast Pfaffia rhodozyma and the bacterium Paracoccus carotinifaciens. It is responsible for the pink or red colour of seafood such as salmon, lobster, crab, fish eggs, trout and shrimp, and as such, it forms part of their normal diet. It is also added to the feed of farmed fish to provide the characteristic red colour (Ambati, Phang, Ravi, & Aswathanarayana, 2014).
5.1. Main active constituents
Natural AX is a mixture of cis‐ and trans‐isomers of 3,3′‐dihydroxy‐β,β‐carotene‐4,4′‐dione. Synthetically produced AX consists only of trans isomers (Figure 2).
Figure 2.
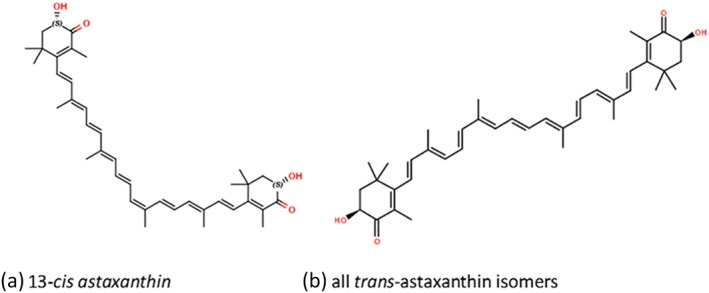
Structure of cis‐ and trans‐isomers of astaxanthin
5.2. Mechanisms of action
The underlying mechanisms for the effects of AX include its high antioxidant capacity, its role as a modulator of the https://www.guidetopharmacology.org/GRAC/FamilyDisplayForward?familyId=86 (PPAR; Choi, 2019) and its complex effects on signalling pathways associated with inflammation and apoptosis (see review by Fakhri et al., 2018). AX enhances the effect of PPARα and suppresses that of PPARβ/δ and PPARγ. However, it has opposing effects on some PPARs, depending on the cell context. Regulation of PPARα and PPARγ activity by AX prevented hepatic injury by maintaining lipid homoeostasis, the cardio‐, neuro‐ and retino‐protective effects may be related to PPAR modulation (Choi, 2019).
AX blocks the NF‐κB‐dependent signalling pathway and prevents expression of inflammatory mediators such as https://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=4974 https://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=4998 and https://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=5074. It can exhibit either anti‐apoptotic or pro‐apoptotic effects by modifying apoptotic proteins. AX enhanced https://www.guidetopharmacology.org/GRAC/FamilyDisplayForward?familyId=910 (Bcl‐2 antagonist of cell death) phosphorylation and down‐regulated the activation of cytochrome c and caspases 3 and 9 through the regulation of MAPK/p38 (https://www.guidetopharmacology.org/GRAC/FamilyDisplayForward?familyId=519). It also activates the PI3K/AKT survival pathway, leading to the amelioration of mitochondrial‐related apoptosis (Fakhri, Abbaszadeh, Dargahi, & Jorjani, 2018). These multiple effects help to explain the wide range of health benefits claimed for AX.
6. CLINICAL EFFICACY
AX does not have a history of traditional medical use but is becoming very popular as a concentrated extract for various health conditions, for which clinical trials have provided supporting evidence (subject to the usual reservations about quality and independence of many nutraceutical studies, Andrew & Izzo, 2017). These indications include prevention and treatment of atherosclerosis (Kishimoto, Yoshida, & Kondo, 2016), for cancer chemoprevention, maintenance and treatment of eye and vision disorders such as macular degeneration (Piermarocchi et al., 2012), for many dermatological conditions (Davinelli, Nielsen, & Scapagnini, 2018) and as a sports supplement to enhance performance and recovery (Brown, Gough, Deb, Sparks, & McNaughton, 2018). Some regulatory authorities allow limited health claims for these indications.
Systematic reviews and meta‐analyses for its possible lipid‐lowering properties and antioxidant effects in humans have been recently published. The authors of a systematic review of randomized clinical trials aiming at evaluating the efficacy of AX supplementation on plasma lipid and glucose concentrations retrieved seven studies for a total of 280 participants. A meta‐analysis of data did not suggest a significant effect of AX on plasma lipid profiles, although a slight glucose‐lowering effect was observed (Ursoniu, Sahebkar, Serban, & Banach, 2015). A further systematic review assessed the possible antioxidant effects in humans. A meta‐analysis of nine randomized controlled trials revealed a borderline significant antioxidant effect of AX (Wu, Xu, Chen, & Zhang, 2019).
6.1. Safety of AX
The effects of natural AX in humans have been investigated in at least 84 human studies, of which 33 used doses of 4 mg or above, and no safety concerns were identified with natural AX supplementation of at least 12 mg·day−1. Animal studies have also shown no changes in liver or other pathologies in rats treated with AX‐rich extracts of Haematococcus pluvialis, Phaffia rhodozyma or Paracoccus carotinifaciens at any dose (Brendler & Williamson, 2019; Edwards et al., 2018).
However, the evidence for synthetic AX is more complex, although studies for non‐genotoxic and genotoxic effects in mice found no tumorigenic effects, even at high doses (≤1,400 mg·kg−1·bw−1·day), two rat carcinogenicity studies showed increased hepatocellular vacuolation, hypertrophy and incidence of multinuclear hepatocytes in female rats at doses of 200 and 1,000 mg·kg−1·bw−1·day. An increase in hepatocellular adenoma, a non‐malignant tumour, occurred in female rats only, at very high doses (Buser, Schierle, Schüep, et al., 2003). Other studies have found no association between liver or any other organ injury and synthetic AX intake (e.g., Buesen et al., 2015; Vega, Edwards, & Belstein, 2015). Edwards et al. (2016) conclude that the effects of AX appear to be “species specific” and of “doubtful human relevance.”
Synthetically produced AX consists only of the trans isomer and has been reported to contain trace amounts of residual solvents and chemical reagents (Edwards et al. 2016). It should not be considered interchangeable for human use until safety parameters are established and clinical trials conducted.
7. CANNABIDIOL
https://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=4150 is the most well‐known of the non‐euphoric phytocannabinoids contained in the marijuana or hemp plant, Cannabis sativa. It was first isolated in 1940 by Adams and co‐workers, but its structure and stereochemistry were determined in 1963 by Mechoulam and Shvo (Crippa, Guimarães, Campos, & Zuardi, 2018; Izzo, Borrelli, Capasso, di Marzo, & Mechoulam, 2009). In contrast to Δ9‐tetrahydrocannabinol (THC), which has euphoric properties due to activation of brain cannabinoid https://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=56 receptors, CBD has very low affinity for both CB1 and https://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=57 receptors, has no known abuse potential and exerts multiple pharmacological actions (e.g. analgesic, anti‐inflammatory and behavioural effects) via a number of mechanisms. These include modulation of the endocannabinoid system and transient receptor potential (https://www.guidetopharmacology.org/GRAC/FamilyDisplayForward?familyId=78) channels, activation of PPARγ and https://www.guidetopharmacology.org/GRAC/FamilyDisplayForward?familyId=1 1A receptors, https://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=109 antagonism and inhibition of https://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=2844 reuptake (Premoli et al., 2019; Rong et al., 2017).
Clinically, CBD is likely most known for its effects on drug‐resistant seizures in patients with severe early‐onset epilepsy. Two randomized double‐blind trials, published in the New England of Medicine, have shown that the addition of CBD (10–20 mg·kg−1) to the conventional antiepileptic regimen resulted in a greater reduction in convulsive‐seizure frequency than placebo among patients with the Dravet syndrome (Devinski et al., 2017) or with Lennox–Gastaut syndrome (Devinski et al., 2018), that are two rare and severe form of epilepsy. This and other robust clinical evidence (Lattanzi et al., 2018) has led to the Food and Drug Administration (FDA) approval of CBD (Epidiolex®, i.e. a 99% pure oral CBD extract) for the treatment of these rare form of epilepsy in patients of 2 years and older.
In addition to the FDA‐approved medicine, CBD products are sold in “smoke” shops, convenience stores and over the Internet (White, 2019). CBD was the 12th highest selling herbal supplement in the U.S. natural channel with total sales of $7,583,438 in 2017, displaying an extraordinary increase of 303% from the previous year (Table 2). Most CBD sales in 2017 were related to products with non‐specific health indications, but sales of condition‐specific CBD formulations, such as those for mood disorders and pain and inflammation, are now emerging (Smith et al., 2018). A concise overview on the experimental and clinical pharmacology of CBD in such conditions is reported below.
7.1. Experimental pharmacology
CBD was initially shown to be non‐euphoric, with the suggestion that it was an inactive compound (Crippa et al., 2018). However, later studies denied this assumption, and several investigations demonstrated that CBD causes multiple effects in the CNS (Silote et al., 2019). CBD exerts behavioural effects in animals predictive of anxiolytic, antipsychotic and antidepressant effects in humans (Rong et al., 2017). CBD has anxiolytic effects—possibly via 5‐HT1A receptor activation—and facilitates the extinction of contextual fear memory—perhaps via indirect activation of CB1 receptors—in rodents (Lee, Bertoglio, Guimarães, & Stevenson, 2017). Also, CBD modulates several targets involved in the neurobiology of depression, although the involvement of the serotonergic neurotransmission in the antidepressant‐like effects of CBD represents the best investigated neurochemical mechanism (Silote et al., 2019). Finally, CBD strongly decreases mesolimbic dopaminergic activity and exerts effects on schizophrenia‐related molecular signalling pathways that are distinct from those associated with more traditional anti‐psychotic drugs (Renard, Norris, Rushlow, & Laviolette, 2017).
Animal studies have shown that CBD promotes analgesia by activating serotonin 5‐HT1A and TRPV1 receptors (Lowin, Schneider, & Pongratz, 2019) and exerts analgesic effects in different models of inflammatory and neuropathic pain. For instance, CBD is effective in models of neuropathic pain induced by chemotherapy (Harris, Sufka, Gul, & ElSohly, 2016; Ward et al., 2014), diabetes (Jesus et al., 2019) nerve chronic constriction (Belardo et al., 2019) and injury (De Gregorio, McLaughlin, et al., 2019). Similarly, CBD may exert analgesic and/or anti‐inflammatory effects in animal models of arthritis (Hammell et al., 2016), airways inflammation (Vuolo et al., 2019), allergic contact dermatitis (Petrosino et al., 2018), oral wounds (Klein et al., 2018) and colitis (Pagano et al., 2016). In arthritis animal models, CBD provided pain relief and reduced inflammatory via a combination of immunosuppressive and anti‐inflammatory effects (Lowin et al., 2019). CBD actions include suppression of both cell‐mediated and humoral immunity and involve inhibition of proliferation, maturation and migration of immune cells, antigen presentation and humoral response (Booz, 2011).
7.2. Clinical efficacy
A very recent systematic review aiming at investigating the possible benefits of CBD in the treatment of mood disorders, specifically schizophrenia, psychotic disorders, anxiety disorders, depression, bipolar disorder and substance‐use disorders identified six case reports and seven trials, for a total of 201 subjects (Khoury et al., 2019). Most of the published studies presented several drawbacks. Although some preliminary promising results were published, the authors conclude that “the evidence regarding efficacy and safety of CBD in psychiatry is still scarce” (Khoury et al., 2019). A further analysis of clinical data concluded that the efficacy of CBD in treating anxiety‐provoking events like public speaking and in schizophrenia are promising but not fully proven (White, 2019).
Cannabinoids have been clinically evaluated to treat neuropathic pain in multiple sclerosis (Nielsen et al., 2018). Sativex® (i.e. a buccal spray containing CBD and THC) has been approved for use in neuropathic pain due to multiple sclerosis in Canada. However, the clinical studies assessing CBD (alone) for pain relief are limited. A recent overview of the clinical data (White, 2019) examined three clinical studies. Two open‐label, single‐arm trials investigated the effect of a CBD‐enriched hemp oil or oral CBD (50 to 150 mg twice a day for 3 weeks) in patients with somatoform psychological and chronic pain (n = 12) and kidney transplant patients (n = 7) respectively. Due to the very poor methodology and sample size, conclusions from these trials cannot be drawn. A third study, a double‐blind crossover randomized trial in patients with multiple sclerosis, spinal cord injury, brachial plexus damage and limb amputation (n = 24), found a significant improvement of pain control in the CBD group (White, 2019).
CBD has been preliminarily evaluated also in inflammatory bowel disease (IBD) patients. In Crohn's disease patients (n = 20) who did not respond to standard treatment with steroids, a low dose of CBD displayed no beneficial effects (Naftali et al., 2017). On the other hand, although a CBD‐rich botanical extract (50 mg for 12 weeks) did not significantly affect the primary endpoint (i.e. the percentage of patients in remission after treatment) in patients with mild‐to‐moderate ulcerative colitis (n = 62), a number of indications suggested that this treatment may be beneficial for symptomatic treatment of ulcerative colitis (Irving et al., 2018).
In summary, although some preliminary result of efficacy has been reported, the evidence of CBD in mood disorders and inflammatory/painful conditions is still limited. Larger and higher quality studies are required before firm conclusions can be drawn.
7.3. Safety of CBD
Historically, CBD has been believed to be extremely safe, with doses up to 600 mg not resulting in psychotic symptoms and chronic use and high doses up to 1,500 mg·day−1 reportedly well tolerated in humans (Bergamaschi, Queiroz, Zuardi, & Crippa, 2011; Welty, Luebke, & Gidal, 2014). In more recent years, in randomized trials in epileptic patients, adverse events have been shown to occur in 87.9% and 72.2% of patients treated with CBD and placebo respectively (Lattanzi et al., 2018). CBD adverse events were somnolence, decreased appetite, diarrhoea and increased serum aminotransferases.
CBD can inhibit the CYP2C and CYP3A4 families of isoenzymes and has been shown to lead to a pharmacokinetic interaction with the antiepileptic drug https://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=7149, resulting in elevation of a clobazam long‐active metabolite (Iffland & Grotenhermen, 2017; Sekar & Pack, 2019). The interaction accentuates sedation, which can be resolved with a reduction of the clobazam dose (Sekar & Pack, 2019).
Because safety data derive mainly from clinical trials, long‐term safety data are needed to fully assess the balance between CBD benefit and harm. The possibility that CBD, like other anticonvulsant drugs, may cause suicidal ideation needs to be monitored (White, 2019).
Finally, it is worthy of note that almost all the safety data derive from the use of Epidiolex®, which is a controlled FDA drug. The possibility that non‐FDA‐approved CBD products freely available in the market may cause adverse events due the variable quality (e.g. contamination, adulteration and THC content in CBD extracts higher than 0.3%) cannot be excluded (White, 2019).
8. MONK FRUIT
Monk fruit, Siraitia grosvenorii (previously classified as Momordica grosvenorii and Thladiantha grosvernorii), is also known as luo han guo, momordica fruit and longevity fruit. It has a long tradition of use in Chinese medicine and is popular in beverages in its own right; however, it is becoming even more widely used globally as a low‐calorie sweetening agent in other beverages and foods. As with Stevia, the market for products which can be used in diabetes and obesity is of increasing importance.
8.1. Main active constituents
Monk fruit contains cucurbitane https://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=8843 glycosides known as mogrosides. These are responsible for the sweet taste and some of the medical applications of monk fruit. Other constituents include flavonoids, polysaccharides and essential oils, some of which show anti‐asthmatic, antitussive, glucose‐lowering, and immunoregulating actions in vitro (Engels & Brinkmann, 2017).
8.2. Traditional use
Monk fruit is used very widely for respiratory conditions in TCM, for bronchitis, tonsillitis, cough, and sore throat. The leaf (luo han ye) is also used to make a tea for these conditions. In the summer, monk fruit is used in beverages to quench thirst and reduce body heat (Engels & Brinkmann, 2017).
8.3. Mechanisms of action
Although there are few published mechanistic studies available to support the medical use, a study in mice has demonstrated that mogroside IIIE attenuates acute lung injury partly via regulation of the https://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=1754/MAPK/NF‐κB axis via https://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=1540 activation (Tao et al., 2017). Preclinical studies have also confirmed antioxidant, antimicrobial, and other properties of the plant (see Engels & Brinkmann, 2017). The glucose‐lowering effects have been confirmed in several animal studies (see Li, Lin, et al., 2014) and more recently, mogroside IIIE has been investigated for its effects in gestational diabetes in mice, which was attenuated through activating of the AMPK signalling pathway (Zou, Zhang, & Zhang, 2018).
8.4. Food use and clinical efficacy
In contrast, a great deal of work has been carried out on the chemical analysis, methods of extraction, metabolism and biotransformation of extracts for use as a non‐calorific sweetener. Toxicity testing has also been extensively performed in animal studies, as is required in the EU for registration as a “novel food.” The mogrosides are anywhere from 200 to 563 times as sweet as sucrose (Engels & Brinkmann, 2017), with mogroside V being the most abundant. Studies have confirmed the structural requirements for a sweet taste (Pawar, Krynitsky, & Rader, 2013), but little information is available as to specific interactions with the sweet taste receptor.
Zhou, Peng, Zhao, Wang, and Li (2017) have reported metabolic differences between type 2 diabetes and normal human microbiota for the transformation of mogroside V, and in healthy and type 2 diabetes model rats (Zhou, Zhang, Li, Wang, & Li, 2018). There are differences in the metabolism and distribution of metabolites, but they are similar in nature to compounds in the fruit and have not given rise to any concerns. In summary, human data of this nutraceutical are very scant and no statement on efficacy can be made.
8.5. Safety of monk fruit
Many animal studies have shown a lack of toxicity of monk fruit extracts (e.g. Jin et al., 2007; Marone, Borzelleca, Merkel, Heimbach, & Kennepohl, 2008; Matsumoto et al., 2009), and in recent human studies, Tey, Salleh, and Henry (2017a, 2017b) found that a beverage containing monk fruit had no effect on total daily energy intake, blood glucose, and insulin responses in healthy human males. Other human studies (not peer reviewed, submitted as part of regulatory applications and not cited) have not given rise to safety concerns. In the United States, monk fruit is considered GRAS (generally regarded as safe) and in Canada it is classified as a medicinal ingredient and is permitted for use as a flavour enhancer and sweetening agent. In the EU, monk fruit is classified as a “novel food” because it was not used as a food or food ingredient before May 15, 1997. Before it can be placed on the EU market, a safety assessment is required and is underway.
9. GINSENG‐TARGETED EXTRACTS
Ginseng is the probably the most widely consumed herbal nutritional product in the world. The root of P. ginseng is used in medicinal food recipes, particularly soups and stews. It is added to confectionary, food products and drinks, pharmaceutical formulations of the extract are taken for their medicinal properties (Liu et al., 2014). Ginseng on the market is mainly cultivated, but wild harvested ginseng is more highly prized. Ancient, prime specimens of wild‐harvested ginseng root have sold at auction for record prices: a 325‐year‐old plant was sold in 2012 for US$1.57 million (See Smith, Williamson, Putnam, Farrimond, & Whalley, 2014, for review). Ginseng is used to promote rejuvenation and longevity, for frailty and stress, weakness and fatigue, both mental and physical (Ogawa‐Ochiai & Kawasaki, 2019), and as supportive treatment for diabetes, cardiovascular disease and many others (Mancuso & Santangelo, 2017). Recently, a number of systematic reviews have critically evaluated the clinical evidence for and against the effectiveness of ginseng in a number of conditions, such as erectile dysfunction (Borrelli, Colalto, Delfino, Iriti, & Izzo, 2018), chronic fatigue (Arring, Millstine, Marks, & Nail, 2018), hypertension (Lee, Lim, Jun, Choi, & Lee, 2017), diabetes (Gui, Xu, Xu, & Yang, 2016), Alzheimer's disease (Wang et al., 2016) and as an ergogenic aid (Lee, Jung, & Lee, 2016).
Pharmacological and clinical evidence are reconciling traditional properties with modern medical theory, and there is a now wealth of global published research on ginseng and the ginsenosides (Xu, Choi, & Huang, 2017), so much so that it is surprising that there is anything new to find. However, newer indications, particularly in the realm of cognition enhancement are being investigated. Ginseng has a traditional use as an adaptogen and research on modified and standardized extracts of ginseng is being carried out under the auspices of the China Astronaut Research and Training Centre, Beijing, as part of a collaborative programme to support health during space travel (Dang et al., 2014). Although a most exciting topic, these studies are also relevant to more prevalent and serious types of cognitive impairment of varying aetiology and for stressors which require adaptation to ever‐changing environments.
Human cognitive studies usually use healthy volunteers, but there is a limited number of astronauts available at any time to take part in clinical studies. In addition, it would be unfeasible and almost certainly unethical to use humans to investigate individual compounds and formulations with improved bioavailability, so animal models are almost universally employed.
9.1. Main active constituents of ginseng
Ginseng contains very many active constituents, from different chemical classes. The most important in cognition are the ginsenosides, of which over 150 are known. They are dammarane‐type saponins classified in series Ra to Rs and fall into two major subtypes: those derived from protopanaxadiol (PPD) and those derived from protopanaxatriol (PPT). Similar compounds are found in other species of Panax, in varying amounts. Ginseng is usually steamed, otherwise processed, fermented or aged, affecting the ginsenoside pattern of the final botanical product (Mancuso & Santangelo, 2017).
9.2. Evidence for effects of ginsenosides in cognitive impairment
A Cochrane review in 2010 concluded that at the time, there was a lack of convincing evidence to show a cognitive enhancing effect of P. ginseng in healthy participants, and no high‐quality evidence about its efficacy in patients with dementia (Geng, Dong, et al., 2010). A systematic review of the evidence for ginseng in Alzheimer's disease found only two RCTs which met all inclusion criteria. Although the results suggested an effect in favour of ginseng, both studies were burdened with serious methodological limitations (Lee, Yang, Kim, & Ernst, 2009).
A more general systematic review of all types of clinical studies (Shergis, Zhang, Zhou, & Xue, 2013) retrieved 16 citations evaluating the effects of ginseng on psychomotor performance. Although this was seen to improve in subjects taking P. ginseng, the authors concluded that the wide range of methods and products involved made the clinical significance difficult to interpret.
To overcome these obstacles, research is now focussing on the effects of individual ginsenosides, refined, standardized and/or chemically modified extracts. Research on the effects of individual ginsenosides in cognitive disorders has been extensively reviewed (Smith et al., 2014), with some more recent studies outlined below.
10. GINSENOSIDE RG1
Rg1 is linked to many different targets and pathways, including PPAR, https://www.guidetopharmacology.org/GRAC/FamilyDisplayForward?familyId=288, toll‐like receptors, arachidonic acid metabolism and apoptosis (Mancuso & Santangelo, 2017). In the area of cognition, Rg1 has shown memory‐enhancing and neuroprotective effects in models of Alzheimer's disease and amyloidogenesis (Smith et al., 2014). Previous studies mainly used avoidance tasks based on punitive stimuli, such as the water maze, shuttle‐box, step‐down, and novel object recognition tests, but more recent evidence from reward‐directed instrumental conditioning tests (a form of associative learning through which the animal learns from the consequences of its behaviours) suggests that Rg1 can reduce memory impairment due to chronic stress, possibly via regulation of the BDNF/TrκB/Erk pathway in the prefrontal cortex (Wang, Xu, et al., 2017). Rg1 also improved memory impairment due to sleep deprivation in mice, with its ability to reduce oxidative stress in the cortex and hippocampus contributing to the mechanism of action (Lu et al., 2017).
11. GINSENOSIDE RB1
Rb1 improves spatial memory and reduced memory impairment in animal models (Smith et al., 2014). More recently, it has been found to attenuate cognitive dysfunction induced by anaesthetics and surgery, by inhibiting neuroinflammation and oxidative stress (Miao et al., 2017).
12. GINSENOSIDES RH1 AND RH2
Rh1 and Rh 2 have anti‐apoptotic, anti‐angiogenic, and effects related to their antioxidant activity. These properties are implicated in cognition, but both have shown direct effects on memory and learning. Rh1 and Rh2 reduced sleep deprivation‐induced memory impairment (Lu et al., 2017; Lu et al., 2018a). Rh1 improved cognition in scopolamine‐treated mice using object location, novel object recognition, Morris water maze, and passive avoidance tests. It significantly enhanced cholinergic activity and suppressed oxidative stress in the hippocampus, and up‐regulated CREB, Egr‐1, c‐Fos, and c‐Jun expression (Lu, Lv, Dong, et al., 2018b). Finally, Rh2 reduces neuroinflammation and oxidative stress, which is of therapeutic interest to counteract neurodegeneration (Hou, Xue, Wang, & Li, 2018).
13. MODIFIED GINSENG EXTRACTS
Hydrolysis of the ginsenosides—as happens naturally in the gut—enhances bioavailability and makes it more predictable. Neuroprotective effects of 20(S)‐protopanaxatriol (PPT, the aglycone of the Rg and Rh series) were shown in scopolamine‐induced cognitive deficits in mice, associated with up‐regulation of hippocampal expression of Egr‐1, c‐Jun, and CREB (Lu et al., 2018c). A hydrolysed, standardized mixture of the total ginsenosides, dammarane sapogenins (DS) has been shown to restore cognitive function in conditions of simulated long‐duration spaceflight (Wu et al., 2017) and to exhibit antidepressant‐like effects in sleep deprivation and chronic restraint and unpredictable mild stress‐induced depressive mice (Jiang et al., 2018).
13.1. Safety of ginseng
The widespread use and modern studies suggest that ginseng—and its various formulations and constituents—are generally safe. These have been well‐reviewed (e.g. Mancuso & Santangelo, 2017). In certain groups of patients, there are contra‐indications to the use of ginseng, such as those with hypertension and some CNS disorders. Insomnia and oestrogenic effects have been observed at high doses. Several cases of suspected drug interactions have been reported with, for example, some anti‐HIV and anti‐cancer drugs. Human studies have found no significant drug interactions with warfarin including in patients who have undergone cardiac valve replacement and those suffering from stroke, and negligible effects on several antihypertensive drugs such as https://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=6981 and https://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=590. For further details, see, for exapmle, Ramanathan and Penzak (2017). The new indications discussed in this review are more specialized than those usually cited and are not suitable for self‐medication by those with memory deficits, including astronauts.
14. NIGELLA SEED
Nigella sativa is known all over the world, cultivated as a popular garden plant and for its seeds, which are known as black cumin, black caraway, black seed along with and many other names. They have been used as both food and medicine since antiquity and are still widely used in Unani medicine, Ayurveda and other Asian traditions. In Islamic medicine, they have been described as “a cure for all diseases except death and ageing”; they are also mentioned in the Bible. Nigella seed has been extensively reviewed regarding its chemistry, biological activity and clinical effects (e.g. Engels & Brinkmann, 2017; Gholamnezhad, Havakhah, et al., 2016). Traditional uses include infertility, fever, bronchitis, asthma, chronic headache, back pain, dysmenorrhea, obesity, diabetes, inflammation and gastrointestinal disorders. The seed oil is used externally for abscesses, nasal ulcers, eczema and swollen joints.
These historical uses do not explain the sudden enormous rise in sales of nigella seeds and there does not seem to be one specific reason for such an increase in popularity, but there has been a rapid rise over the past decade in research publications describing health benefits of nigella seed powder and nigella oil, for improvement in atherogenesis, glucose metabolism, lipid profiles and asthma (Gholamnezhad et al., 2016).
14.1. Main active constituents
The main active component of nigella seeds and oil is thymoquinone (TQ, 2‐methyl‐5‐isopropyl‐1,4‐benzoquinone). Fixed oil constituents include linoleic, oleic, dihomolinoleic and eicosadienoic acids; the essential oil contains TQ (30–48%), thymol, thymohydroquinone, and dithymoquinone. The seed contains traces of alkaloids (nigellidine and nigellicine, nigellicimine along with these compounds and especially TQ, are found in most medicinal preparations).
14.2. Evidence for efficacy and mechanisms of action
Despite the wealth of research on nigella seed and the positive results recorded in most human studies, the clinical evidence is disparate and not especially strong for any indication. Engels and Brinkmann (2017) found at least 38 clinical studies reporting effects of nigella seed powder and oil in respiratory disorders (8), obesity (3) and inflammatory disorders such as rheumatoid arthritis, dysmenorrhea, mastalgia, metabolic conditions and many others. There is an uneven quality in the clinical studies performed and specifically the lack of chemical characterization of the extracts used in them (Gholamnezhad et al., 2016). Systematic reviews and meta‐analyses have assessed the effect of N. sativa preparations on glycaemic control, obesity, hypertension and plasma lipid concentrations (Askari et al., 2019; Maffioli & Parati, 2016; Namazi, Larijani, Ayati, & Abdollahi, 2018; Sahebkar, Beccuti, Simental‐Mendía, Nobili, & Bo, 2016). Collectively, results are moderately promising but not compelling. Very recently, nigella preparations have been evaluated to assess their renal‐stone‐dissolving properties (Ardakani et al., 2019) and in ulcerative colitis patients (Nikkhah‐Bodaghi, Darabi, Agah, & Hekmatdoost, 2019). TQ is generally considered to be the most important active constituent and has been extensively studied preclinically, but surprisingly, only one clinical study on TQ could be found, a pilot study on intractable paediatric seizures, where positive results were reported (Akhondian et al., 2011).
TQ exhibits anti‐inflammatory and immunomodulatory effects by targeting NF‐κB, IL‐1β, and TNF‐α signalling pathways. It shows anticancer activity in animal models due to altering the expression of signal transducer and activator transcription genes and is being investigated for these and many other effects, and for new formulations with improved bioavailability (Goyal et al., 2017).
14.3. Safety of nigella seed
As a widely used food item, and with a long tradition of medicinal use, nigella seed does not give rise to any safety concerns. Studies evaluating the safety of the seed, its fixed oil TQ in rats and mice found no significant acute or chronic toxicity. No changes in hepatic enzyme levels or histopathological modifications have been observed in rats treated with high daily doses for up to 12 weeks. The LD50 of TQ was determined as 870.9 (oral)/104.7 mg·kg−1 (intraperitoneal) mice, and 794.3/57.5 mg·kg−1 in rats (Gholamnezhad et al., 2016). There is little doubt that nigella possesses useful properties, but more rigorous studies need to be carried out before it is possible to assess its general use as a nutraceutical. The use of nigella in serious illness (cancer, asthma and epilepsy) is not appropriate for self‐medication.
15. CONCLUSION
Despite the inconsistencies in market definitions, the variable composition and quality of herbal nutraceutical products available and the lack of good clinical evidence in many cases to support their use, there is an upward trajectory in sales, which for certain products is remarkable. As these trends show, consumers continue to be interested in these herbal supplements, and some are indubitably beneficial, or at least harmless; there is also a worrying tendency for “new” substances, such as cannabis extracts, to be promoted widely and used inappropriately in potentially serious illness.
15.1. Nomenclature of targets and ligands
Key protein targets and ligands in this article are hyperlinked to corresponding entries in http://www.guidetopharmacology.org, the common portal for data from the IUPHAR/BPS Guide to PHARMACOLOGY (Harding et al., 2018), and are permanently archived in the Concise Guide to PHARMACOLOGY 2019/20 (Alexander et al., 2019).
CONFLICT OF INTEREST
The authors declare no conflicts of interest.
Williamson EM, Liu X, Izzo AA. Trends in use, pharmacology, and clinical applications of emerging herbal nutraceuticals. Br J Pharmacol. 2020;177:1227–1240. 10.1111/bph.14943
Contributor Information
Elizabeth M. Williamson, Email: e.m.williamson@reading.ac.uk.
Angelo A. Izzo, Email: aaizzo@unina.it.
REFERENCES
- Agbabiaka, T. B. , Spencer, N. H. , Khanom, S. , & Goodman, C. (2018). Prevalence of drug‐herb and drug‐supplement interactions in older adults: A cross‐sectional survey. The British Journal of General Practice, 68(675), e711–e717. 10.3399/bjgp18X699101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agbabiaka, T. B. , Wider, B. , Watson, L. K. , & Goodman, C. (2017). Concurrent use of prescription drugs and herbal medicinal products in older adults: A systematic review. Drugs & Aging, 34(12), 891–905. 10.1007/s40266-017-0501-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akhondian, J. , Kianifar, H. , Raoofziaee, M. , Moayedpour, A. , Toosi, M. B. , & Khajedaluee, M. (2011). The effect of thymoquinone on intractable pediatric seizures (pilot study). Epilepsy Research, 93(1), 39–43. 10.1016/j.eplepsyres.2010.10.010 [DOI] [PubMed] [Google Scholar]
- Alexander, S. P. H. , Kelly, E. , Mathie, A. , Peters, J. A. , Veale, E. L. Armstrong, J. F. , … Davies, J. A. (2019). CGTP Collaborators. THE CONCISE GUIDE TO PHARMACOLOGY 2019/20: Introduction andOther Protein Targets. Br J Pharmacol, Suppl 1, S1–S20. 10.1111/bph.14747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alsanad, S. M. , Howard, R. L. , & Williamson, E. M. (2016). An assessment of the impact of herb‐drug combinations used by cancer patients. BMC Complementary and Alternative Medicine, 16, 393 10.1186/s12906-016-1372-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ambati, R. R. , Phang, S. M. , Ravi, S. , & Aswathanarayana, R. (2014). Astaxanthin: Sources, extraction, stability, biological activities and its commercial applications—A review. Marine Drugs, 12(1), 128–152. 10.3390/md12010128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrew, R. , & Izzo, A. A. (2017). Principles of pharmacological research of nutraceuticals. Brit. J. Pharmacol., 174, 1177–1194. 10.1111/bph.13779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anwar, F. , Latif, S. , Ashraf, M. , & Gilani, A. H. (2007). Moringa oleifera: A food plant with multiple medicinal uses. Phytotherapy Research, 21, 17–25. 10.1002/ptr.2023 [DOI] [PubMed] [Google Scholar]
- Ardakani, M. R. , Yousefi, M. , Saghebi, S. A. , Sadeghi Vazin, M. , Iraji, A. , & Mosavat, S. H. (2019). Efficacy of black seed (Nigella sativa L.) on kidney stone dissolution: A randomized, double‐blind, placebo‐controlled, clinical trial. Phytotherapy Research, 33, 1404–1412. 10.1002/ptr.6331 [DOI] [PubMed] [Google Scholar]
- Aronson, J. K. (2017). Defining ‘nutraceuticals’: Neither nutritious nor pharmaceutical. Brit. J. Clin Pharmacol, 83, 8–19. 10.1111/bcp.12935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arring, N. M. , Millstine, D. , Marks, L. A. , & Nail, L. M. (2018). Ginseng as a treatment for fatigue: A systematic review. Journal of Alternative and Complementary Medicine, 24, 624–633. 10.1089/acm.2017.0361 [DOI] [PubMed] [Google Scholar]
- Askari, G. , Rouhani, M. H. , Ghaedi, E. , Ghavami, A. , Nouri, M. , & Mohammadi, H. (2019). Effect of Nigella sativa (black seed) supplementation on glycemic control: A systematic review and meta‐analysis of clinical trials. Phytotherapy Research, 33, 1341–1352. 10.1002/ptr.6337 [DOI] [PubMed] [Google Scholar]
- Awortwe, C. , Bruckmueller, H. , & Cascorbi, I. (2019). Interaction of herbal products with prescribed Medications: A systematic review and meta‐analysis. Pharmacological Research, 141, 397–408. 10.1016/j.phrs.2019.01.028 [DOI] [PubMed] [Google Scholar]
- Belardo, C. , Iannotta, M. , Boccella, S. , Rubino, R. C. , Ricciardi, F. , Infantino, R. , … Guida, F. (2019). Oral cannabidiol prevents allodynia and neurological dysfunctions in a mouse model of mild traumatic brain injury. Frontiers in Pharmacology, 10, 352 10.3389/fphar.2019.00352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergamaschi, M. M. , Queiroz, R. H. , Zuardi, A. W. , & Crippa, J. A. (2011). Safety and side effects of cannabidiol, a Cannabis sativa constituent. Current Drug Safety, 6, 237–249. 10.2174/157488611798280924 [DOI] [PubMed] [Google Scholar]
- Booz, G. W. (2011). Cannabidiol as an emergent therapeutic strategy for lessening the impact of Inflammation on oxidative stress. Free Radical Biology & Medicine, 51, 1054–1061. 10.1016/j.freeradbiomed.2011.01.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borrelli, F. , Colalto, C. , Delfino, D. V. , Iriti, M. , & Izzo, A. A. (2018). Herbal dietary supplements for erectile dysfunction: A systematic review and meta‐analysis. Drugs, 78, 643–673. 10.1007/s40265-018-0897-3 [DOI] [PubMed] [Google Scholar]
- Brendler, T. , & Williamson, M. E. (2019). Astaxanthin: How much is too much? A safety review. Phytotherapy Researchin press. 10.1002/ptr.6514 [DOI] [PubMed] [Google Scholar]
- Brown, D. R. , Gough, L. A. , Deb, S. K. , Sparks, S. A. , & McNaughton, L. R. (2018). Astaxanthin in exercise metabolism, performance and recovery: A review. Frontiers in Nutrition, 4, 76 10.3389/fnut.2017.00076 eCollection 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buesen, R. , Schulte, S. , Strauss, V. , Treumann, S. , Becker, M. , Gröters, S. , … van Ravenzwaay, B. (2015). Safety assessment of [3S, 3′S]‐astaxanthin—Subchronic toxicity study in rats. Food and Chemical Toxicology, 81(2015), 129–136. 10.1016/j.fct.2015.04.017 [DOI] [PubMed] [Google Scholar]
- Buser S, Schierle J, Schüep W et al (2003). Ro 11e3741/021 (Astaxanthin): 104‐week oral carcinogenicity study in the Rat. Protocol No. 002V92, 12‐May‐2003, DSM Report 1007905
- Choi, C. I. (2019). Astaxanthin as a peroxisome proliferator‐activated receptor (PPAR) modulator: Its therapeutic implications. Marine Drugs, 17(4), pii: E242 10.3390/md17040242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colalto, C. (2018). What phytotherapy needs: Evidence‐based guidelines for better clinical practice. Phytotherapy Research, 32, 413–425. 10.1002/ptr.5977 [DOI] [PubMed] [Google Scholar]
- Crippa, J. A. , Guimarães, F. S. , Campos, A. C. , & Zuardi, A. W. (2018). Translational investigation of the therapeutic potential of cannabidiol (CBD): Toward a new age. Frontiers in Immunology, 9, 2009 10.3389/fimmu.2018.02009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dang, H. , Sun, X. , et al. (2014). Traditional Chinese medicine—A potential countermeasure to stressors associated with space missions In Human performance in space: Advancing astronautics research in China. Science/AAAS 103 (Vol. 2014) (pp. 21–23). Washington, DC. [Google Scholar]
- Dang, H. , Wang, Q. , Wang, H. , Yan, M. , & Liu, X. (2016). The integration of Chinese material medica into the Chinese health care delivery system, an update. Phytotherapy Research, 30(2), 292–297. 10.1002/ptr.5530 [DOI] [PubMed] [Google Scholar]
- Davinelli, S. , Nielsen, M. E. , & Scapagnini, G. (2018). Astaxanthin in skin health, repair, and disease: A comprehensive review. Nutrients, 2018(10), 522 10.3390/nu10040522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Gregorio, D. , McLaughlin, R. J. , et al. (2019). Cannabidiol modulates serotonergic transmission and reverses both allodynia and anxiety‐like behavior in a model of neuropathic pain. Pain, 160, 136–150. 10.1097/j.pain.0000000000001386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devinsky, O. , Cross, J. H. , Laux, L. , Marsh, E. , Miller, I. , Nabbout, R. , … Cannabidiol in Dravet Syndrome Study Group (2017). Trial of cannabidiol for drug‐resistant seizures in the Dravet Syndrome. The New England Journal of Medicine, 376, 2011–2020. 10.1056/NEJMoa1611618 [DOI] [PubMed] [Google Scholar]
- Devinsky, O. , Patel, A. D. , Cross, J. H. , Villanueva, V. , Wirrell, E. C. , Privitera, M. , … GWPCARE3 Study Group (2018). Effect of cannabidiol on drop seizures in the Lennox‐Gastaut syndrome. The New England Journal of Medicine, 378, 1888–1897. 10.1056/NEJMoa1714631 [DOI] [PubMed] [Google Scholar]
- Durazzo, A. , Lucarini, M. , Souto, E. B. , Cicala, C. , Caiazzo, E. , Izzo, A. A. , … Santini, A. (2019). Polyphenols: A concise overview on the chemistry, occurrence and effects of human health. Phytotherapy Research, 33, 2221–2243. 10.1002/ptr.6419 [DOI] [PubMed] [Google Scholar]
- Edwards, J.A. , Bellion, P. , Beilstein, P. , Rümbeli, R. , & Schierle, J. (2016). Review ofgenotoxicity and rat carcinogenicity investigations with astaxanthin. RegulToxicol Pharmacol, 75, 5–19. 10.1016/j.yrtph.2015.12.009 [DOI] [PubMed] [Google Scholar]
- Engels, G. , & Brinkmann, J. (2017). Nigella. (Nigella sativa, Ranunculaceae). HerbalGram, 114, 8–16. [Google Scholar]
- Fakhri, S. , Abbaszadeh, F. , Dargahi, L. , & Jorjani, M. (2018). Astaxanthin: A mechanistic review on its biological activities and health benefits. Pharmacological Research, 136, 1–20. 10.1016/j.phrs.2018.08.012 [DOI] [PubMed] [Google Scholar]
- Geng, J. , Dong, J. , et al. (2010). Ginseng for cognition. Cochrane Database of Systematic Reviews, (12), CD007769. [DOI] [PubMed] [Google Scholar]
- Gholamnezhad, Z. , Havakhah, S. , et al. (2016). Preclinical and clinical effects of Nigella sativa and its constituent, thymoquinone: A review. Journal of Ethnopharmacology, 90, 372–386. [DOI] [PubMed] [Google Scholar]
- Goyal, S. N. , Prajapati, C. P. , Gore, P. R. , Patil, C. R. , Mahajan, U. B. , Sharma, C. , … Ojha, S. K. (2017, 2017 Sep 21). Therapeutic potential and pharmaceutical development of thymoquinone: A multitargeted molecule of natural origin. Frontiers in Pharmacology., 8, 656 10.3389/fphar.2017.00656 eCollection 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grandview research (2019). β‐glucan market analysis by source (cereal, mushroom, yeasts, seaweed), by application (F&B, Personal Care, Pharmaceuticals, Animal Feed), By Type, By Region, And Segment Forecasts, 2018–2025: https://www.grandviewresearch.com/industry-analysis/beta-glucan-market. Accessed May 2019
- Grollman, A. P. , & Marcus, D. M. (2016). Global hazards of herbal remedies: Lessons from Aristolochia: The lesson from the health hazards of Aristolochia should lead to more research into the safety and efficacy of medicinal plants. EMBO Rep, 17, 619–625. 10.15252/embr.201642375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gui, Q. F. , Xu, Z. R. , Xu, K. Y. , & Yang, Y. M. (2016). The Efficacy of ginseng‐related therapies in type 2 diabetes mellitus: An updated systematic review and meta‐analysis. Medicine (Baltimore), 95, e2584 10.1097/MD.0000000000002584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurley, B. J. , Tonsing‐Carter, A. , Thomas, S. L. , & Fifer, E. K. (2018). Clinically Relevant Herb‐Micronutrient Interactions: When Botanicals, Minerals, and Vitamins Collide. Adv Nutr, 9(4), 524S–532S. 10.1093/advances/nmy029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammell, D. C. , Zhang, L. P. , Ma, F. , Abshire, S. M. , McIlwrath, S. , Stinchcomb, A. L. , & Westlund, K. N. (2016). Transdermal cannabidiol reduces inflammation and pain‐related behaviours in a rat model of arthritis. European Journal of Pain, 20, 936–948. 10.1002/ejp.818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris, H. M. , Sufka, K. J. , Gul, W. , & ElSohly, M. A. (2016). Effects of δ‐9‐tetrahydrocannabinol and cannabidiol on cisplatin‐induced neuropathy in mice. Planta Medica, 82, 1169–1172. 10.1055/s-0042-106303 [DOI] [PubMed] [Google Scholar]
- Harding, S. D. , Sharman, J. L. , Faccenda, E. , Southan, C. , Pawson, A. J. Ireland, S. , … Davies, J. A. (2018). NC‐IUPHAR. The IUPHAR/BPS Guide to PHARMACOLOGY in 2018: updates and expansion to encompass the new guide to IMMUNOPHARMACOLOGY. Nucleic Acids Res, 46(D1), D1091–D1106. 10.1093/nar/gkx1121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou, J. , Xue, J. , Wang, Z. , & Li, W. (2018). Ginsenoside Rg3 and Rh2 protect trimethyltin‐induced neurotoxicity via prevention on neuronal apoptosis and neuroinflammation. Phytotherapy Research, 32, 2531–2540. 10.1002/ptr.6193 [DOI] [PubMed] [Google Scholar]
- Iffland, K. , & Grotenhermen, F. (2017). An update on safety and side effects of cannabidiol: A review of clinical data and relevant animal studies. Cannabis Cannabinoid Res, 2, 139–154. 10.1089/can.2016.0034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irving, P. M. , Iqbal, T. , Nwokolo, C. , Subramanian, S. , Bloom, S. , Prasad, N. , … Wright, S. (2018). A randomized, double‐blind, placebo‐controlled, parallel‐group, pilot study of cannabidiol‐rich botanical extract in the symptomatic treatment of ulcerative colitis. Inflammatory Bowel Diseases, 24, 714–724. 10.1093/ibd/izy002 [DOI] [PubMed] [Google Scholar]
- Izzo, A. A. (2018). The clinical efficacy of herbal dietary supplements: A collection of recent systematic reviews and meta‐analyses. Phytotherapy Research, 32, 1423–1424. 10.1002/ptr.6128 [DOI] [PubMed] [Google Scholar]
- Izzo, A. A. , Borrelli, F. , Capasso, R. , di Marzo, V. , & Mechoulam, R. (2009). Non‐psychotropic plant cannabinoids: New therapeutic opportunities from an ancient herb. Trends in Pharmacological Sciences, 30, 515–527. 10.1016/j.tips.2009.07.006 [DOI] [PubMed] [Google Scholar]
- Izzo, A. A. , Hoon‐Kim, S. , Radhakrishnan, R. , & Williamson, E. M. (2016). A critical approach to evaluating clinical efficacy, adverse events and drug interactions of herbal remedies. Phytotherapy Research, 30, 691–700. 10.1002/ptr.5591 [DOI] [PubMed] [Google Scholar]
- Jesus, C. H. A. , Redivo, D. D. B , Gasparin, A. T. , Sotomaior, B. B. , de Carvalho, M. C. Genaro, K. , … de Cunha, J. M. (2019). Cannabidiolattenuates mechanical allodynia in streptozotocin‐induced diabetic rats viaserotonergic system activation through 5‐HT1A receptors. Brain Res, 1715, 156–164. 10.1016/j.brainres.2019.03.014 [DOI] [PubMed] [Google Scholar]
- Jiang, N. , Zhang, B. Y. , Dong, L. M. , Lv, J. W. , Lu, C. , Wang, Q. , … Liu, X. M. (2018). Antidepressant effects of dammarane sapogenins in chronic unpredictable mild stress‐induced depressive mice. Phytotherapy Research, 32, 1023–1029. 10.1002/ptr.6040 [DOI] [PubMed] [Google Scholar]
- Jin, M. , Muguruma, M. , Moto, M. , Okamura, M. , Kashida, Y. , & Mitsumori, K. (2007). Thirteen‐week repeated dose toxicity of Siraitia grosvenori extract in Wistar Hannover (GALAS) rats. Food and Chemical Toxicology, 45(7), 1231–1237. 10.1016/j.fct.2006.12.030 [DOI] [PubMed] [Google Scholar]
- Khoury, J. M. , Neves, M. C. L. D. , Roque, M. A. V. , Queiroz, D. A. B. , Corrêa de Freitas, A. A. , de Fátima, Â. , … Garcia, F. D. (2019). Is there a role for cannabidiol in psychiatry? The World Journal of Biological Psychiatry, 20, 101–116. 10.1080/15622975.2017.1285049 [DOI] [PubMed] [Google Scholar]
- Kishimoto, Y. , Yoshida, H. , & Kondo, K. (2016). Potential Anti‐Atherosclerotic Properties of Astaxanthin. Marine Drugs, 14, 35 10.3390/md14020035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein, M. , de Quadros De Bortolli, J. , Guimarães, F. S. , Salum, F. G. , Cherubini, K. , & de Figueiredo, M. A. Z. (2018). Effects of cannabidiol, a Cannabis sativa constituent, on oral wound healing process in rats: Clinical and histological evaluation. Phytotherapy Research, 32, 2275–2281. 10.1002/ptr.6165 [DOI] [PubMed] [Google Scholar]
- Lattanzi, S. , Brigo, F. , Trinka, E. , Zaccara, G. , Cagnetti, C. , del Giovane, C. , & Silvestrini, M. (2018). Efficacy and safety of cannabidiol in epilepsy: A systematic review and meta‐analysis. Drugs, 78, 1791–1804. 10.1007/s40265-018-0992-5 [DOI] [PubMed] [Google Scholar]
- Lee, H. W. , Lim, H. J. , Jun, J. H. , Choi, J. , & Lee, M. S. (2017). Ginseng for treating hypertension: A systematic review and meta‐analysis of double blind, randomized, placebo‐controlled trials. Current Vascular Pharmacology, 15, 549–556. 10.2174/1570161115666170713092701 [DOI] [PubMed] [Google Scholar]
- Lee, J. L. C. , Bertoglio, L. J. , Guimarães, F. S. , & Stevenson, C. W. (2017). Cannabidiol regulation of emotion and emotional memory processing: Relevance for treating anxiety‐related and substance abuse disorders. British Journal of Pharmacology, 174, 3242–3256. 10.1111/bph.13724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee, M. S. , Yang, E. J. , Kim, J. I. , & Ernst, E. (2009). Ginseng for cognitive function in Alzheimer's disease: A systematic review. Journal of Alzheimer's Disease, 18(2), 339–344. 10.3233/JAD-2009-1149 [DOI] [PubMed] [Google Scholar]
- Lee, N. H. , Jung, H. C. , & Lee, S. (2016). Red ginseng as an ergogenic aid: A systematic review of clinical trials. Journal of Exercise Nutrition and Biochemistry, 20, 13–19. 10.20463/jenb.2016.0034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, C. , et al. (2014). Chemistry and pharmacology of Siraitia grosvenorii: A review. Chinese Journal of Natural Medicines, 12, 89–1025. 10.1016/S1875-5364(14)60015-7 [DOI] [PubMed] [Google Scholar]
- Liu, X. , Wang, Q. , Song, G. , Zhang, G. , Ye, Z. , & Williamson, E. M. (2014). The classification and application of toxic Chinese Materia Medica. Phytotherapy Research, 28, 334–347. 10.1002/ptr.5006 [DOI] [PubMed] [Google Scholar]
- Lowin, T. , Schneider, M. , & Pongratz, G. (2019). Joints for joints: Cannabinoids in the treatment of rheumatoid arthritis. Current Opinion in Rheumatology, 31, 271–278. 10.1097/BOR.0000000000000590 [DOI] [PubMed] [Google Scholar]
- Lu, C. , Lv, J. , Dong, L. M. , Jiang, N. , Wang, Y. , Wang, Q. , … Liu, X. (2018a). Neuroprotective effects of 20(S)‐protopanaxatriol (PPT) on scopolamine‐induced cognitive deficits in mice. Phytotherapy Research, 32, 1056–1063. 10.1002/ptr.6044 [DOI] [PubMed] [Google Scholar]
- Lu, C. , Lv, J. , Dong, L. M. , et al. (2018b). Neuroprotective effect of ginsenoside Rh1 on scopolamine‐induced cognitive dysfunctions. Neuropsychiatry, 8, 749–760. [Google Scholar]
- Lu, C. , Shi, Z. , Dong, L. , Lv, J. , Xu, P. , Li, Y. , … Liu, X. (2017). Exploring the effect of ginsenoside Rh1 in a sleep deprivation‐induced mouse memory impairment model. Phytotherapy Research, 31, 763–770. 10.1002/ptr.5797 [DOI] [PubMed] [Google Scholar]
- Lu, C. , Wang, Y. , Lv, J. , Jiang, N. , Fan, B. , Qu, L. , … Liu, X. (2018c). Ginsenoside Rh2 reverses sleep deprivation‐induced cognitive deficit in mice. Behavioural Brain Research, 349, 109–115. 10.1016/j.bbr.2018.03.005 [DOI] [PubMed] [Google Scholar]
- Maffioli, P. , & Parati, G. (2016). A systematic review and meta‐analysis of randomized controlled trials investigating the effects of supplementation with Nigella sativa (black seed) on blood pressure. Journal of Hypertension, 34, 2127–2135. [DOI] [PubMed] [Google Scholar]
- Mancuso, C. , & Santangelo, R. (2017). Panax ginseng and Panax quinquefolius: From pharmacology to toxicology. Food and Chemical Toxicology, 107(Pt A), 362–372. 10.1016/j.fct.2017.07.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcus, D. M. (2016). Dietary supplements: What's in a name? What's in the bottle? Drug Testing and Analysis, 8, 410–412. 10.1002/dta.1855 [DOI] [PubMed] [Google Scholar]
- Marone, P. A. , Borzelleca, J. F. , Merkel, D. , Heimbach, J. T. , & Kennepohl, E. (2008). Twenty‐eight‐day dietary toxicity study of Luo Han fruit concentrate in Hsd:SDr rats. Food and Chemical Toxicology, 46(3), 910–919. 10.1016/j.fct.2007.10.013 [DOI] [PubMed] [Google Scholar]
- Matsumoto, S. , Jin, M. , Dewa, Y. , Nishimura, J. , Moto, M. , Murata, Y. , … Mitsumori, K. (2009). Suppressive effect of Siraitia grosvenorii extract on dicyclanil‐promoted hepatocellular proliferative lesions in male mice. The Journal of Toxicological Sciences, 34(1), 109–118. 10.2131/jts.34.109 [DOI] [PubMed] [Google Scholar]
- Miao, H. H. , Zhang, Y. , Ding, G. N. , Hong, F. X. , Dong, P. , & Tian, M. (2017). Ginsenoside Rb1 attenuates isoflurane/surgery‐induced cognitive dysfunction via inhibiting neuroinflammation and oxidative stress. Biomedical and Environmental Sciences, 30(5), 363–372. 10.3967/bes2017.047 [DOI] [PubMed] [Google Scholar]
- Minuz, P. , Velo, G. , Violi, F. , & Ferro, A. (2017). Are nutraceuticals the modern panacea? From myth to science. British Journal of Clinical Pharmacology, 83, 5–7. 10.1111/bcp.13142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naftali, T. , Mechulam, R. , Marii, A. , Gabay, G. , Stein, A. , Bronshtain, M. , … Konikoff, F. M. (2017). Low‐dose cannabidiol is safe but not effective in the treatment for Crohn's disease, a randomized controlled trial. Digestive Diseases and Sciences, 62, 1615–1620. 10.1007/s10620-017-4540-z [DOI] [PubMed] [Google Scholar]
- Namazi, N. , Larijani, B. , Ayati, M. H. , & Abdollahi, M. (2018). The effects of Nigella sativa L. on obesity: A systematic review and meta‐analysis. Journal of Ethnopharmacology, 219, 173–181. 10.1016/j.jep.2018.03.001 [DOI] [PubMed] [Google Scholar]
- Nielsen, S. , Germanos, R. , Weier, M. , Pollard, J. , Degenhardt, L. , Hall, W. , … Farrell, M. (2018). The use of cannabis and cannabinoids in treating symptoms of multiple sclerosis: A systematic review of reviews. Current Neurology and Neuroscience Reports, 18, 8 10.1007/s11910-018-0814-x [DOI] [PubMed] [Google Scholar]
- Nikkhah‐Bodaghi, M. , Darabi, Z. , Agah, S. , & Hekmatdoost, A. (2019). The effects of Nigella sativa on quality of life, disease activity index, and some of inflammatory and oxidative stress factors in patients with ulcerative colitis. Phytotherapy Research, 33, 1027–1032. 10.1002/ptr.6296 [DOI] [PubMed] [Google Scholar]
- Ogawa‐Ochiai, K. , & Kawasaki, K. (2019). Panax ginseng for frailty‐related disorders: A review. Frontiers in Nutrition, 5, 140 10.3389/fnut.2018.00140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pagano, E. , Capasso, R. , et al. (2016). An orally active cannabis extract with high content in cannabidiol attenuates chemically induced intestinal inflammation and hypermotility in the mouse. Frontiers in Pharmacology, 7, 341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pagano, E. , Romano, B. , Izzo, A. A. , & Borrelli, F. (2018). The clinical efficacy of curcumin‐containingnutraceuticals: An overview of systematic reviews. Pharmacol Res, v, 79–91. 10.1016/j.phrs.2018.06.007 [DOI] [PubMed] [Google Scholar]
- Pawar, R. S. , Krynitsky, A. J. , & Rader, J. I. (2013). Sweeteners from plants—with emphasis on Stevia rebaudiana (Bertoni) and Siraitia grosvenorii (Swingle). Analytical and Bioanalytical Chemistry, 405, 4397–4407. https://doi-org.dblibweb.rdg.ac.uk/10.1007/s00216-012-6693-0 [DOI] [PubMed] [Google Scholar]
- Petrosino, S. , Verde, R. , Vaia, M. , Allarà, M. , Iuvone, T. , & di Marzo, V. (2018). Anti‐inflammatory properties of cannabidiol, a nonpsychotropic cannabinoid, in experimental allergic contact dermatitis. The Journal of Pharmacology and Experimental Therapeutics, 365, 652–663. 10.1124/jpet.117.244368 [DOI] [PubMed] [Google Scholar]
- Piermarocchi, S. , Saviano, S. , Parisi, V. , Tedeschi, M. , Panozzo, G. , Scarpa, G. , … Carmis Study Group (2012). Carotenoids in Age‐related Maculopathy Italian Study (CARMIS): Two‐year results of a randomized study. European Journal of Ophthalmology, 22(2), 216–225. 10.5301/ejo.5000069 [DOI] [PubMed] [Google Scholar]
- Premoli, M. , Aria, F. , Bonini, S. A. , Maccarinelli, G. , Gianoncelli, A. , Pina, S. D. , … Mastinu, A. (2019). Cannabidiol: Recent advances and new insights for neuropsychiatric disorders treatment. Life Sciences, 224, 120–127. 10.1016/j.lfs.2019.03.053 [DOI] [PubMed] [Google Scholar]
- Ramanathan, M. R. , & Penzak, S. R. (2017). Pharmacokinetic drug interactions with Panax ginseng . European Journal of Drug Metabolism and Pharmacokinetics, 42, 545–557. 10.1007/s13318-016-0387-5 [DOI] [PubMed] [Google Scholar]
- Renard, J. , Norris, C. , Rushlow, W. , & Laviolette, S. R. (2017). Neuronal and molecular effects of cannabidiol on the mesolimbic dopamine system: Implications for novel schizophrenia treatments. Neuroscience and Biobehavioral Reviews, 75, 157–165. 10.1016/j.neubiorev.2017.02.006 [DOI] [PubMed] [Google Scholar]
- Rong, C. , Lee, Y. , Carmona, N. E. , Cha, D. S. , Ragguett, R. M. , Rosenblat, J. D. , … McIntyre, R. (2017). Cannabidiol in medical marijuana: Research vistas and potential opportunities. Pharmacological Research, 121, 213–218. 10.1016/j.phrs.2017.05.005 [DOI] [PubMed] [Google Scholar]
- Sahebkar, A. , Beccuti, G. , Simental‐Mendía, L. E. , Nobili, V. , & Bo, S. (2016). Nigella sativa (black seed) effects on plasma lipid concentrations in humans: A systematic review and meta‐analysis of randomized placebo‐controlled trials. Pharmacological Research, 106, 37–50. 10.1016/j.phrs.2016.02.008 [DOI] [PubMed] [Google Scholar]
- Sekar K, Pack A (2019). Epidiolex as adjunct therapy for treatment of refractory epilepsy: A comprehensive review with a focus on adverse effects. F1000Res 8.pii: F1000 Faculty Rev‐234. [DOI] [PMC free article] [PubMed]
- Shergis, J. L. , Zhang, A. L. , Zhou, W. , & Xue, C. C. (2013). Panax ginseng in randomised controlled trials: A systematic review. Phytotherapy Research, 27, 949–965. 10.1002/ptr.4832 [DOI] [PubMed] [Google Scholar]
- Silote, G. P. , Sartim, A. , Sales, A. , Eskelund, A. , Guimarães, F. S. , Wegener, G. , & Joca, S. (2019). Emerging evidence for the antidepressant effect of cannabidiol and the underlying molecular mechanisms. Journal of Chemical Neuroanatomy, 98, 104–116. 10.1016/j.jchemneu.2019.04.006 [DOI] [PubMed] [Google Scholar]
- Smith, I. , Williamson, E. M. , Putnam, S. , Farrimond, J. , & Whalley, B. J. (2014). Effects and mechanisms of ginseng and ginsenosides on cognition. Nutrition Reviews, 72, 319–333. 10.1111/nure.12099 [DOI] [PubMed] [Google Scholar]
- Smith, T. , Kawa, K. , et al. (2018). Herbal supplement sales in US increased 8.5% in 2017, topping $8 billion. HerbalGram, 119, 62–71. [Google Scholar]
- Tao, L. , Cao, F. , Xu, G. , Xie, H. , Zhang, M. , & Zhang, C. (2017). Mogroside IIIE attenuates LPS‐induced acute lung injury in mice partly through regulation of the TLR4/MAPK/NF‐κB Axis via AMPK activation. Phytotherapy Research, 31, 1097–1106. 10.1002/ptr.5833 [DOI] [PubMed] [Google Scholar]
- Tey, S. L. , Salleh, N. B. , & Henry, C. (2017b). Effects of aspartame‐, monk fruit‐, stevia‐ and sucrose‐ sweetened beverages on post‐prandial glucose, insulin and energy intake. International Journal of Obesity, 41(3), 450–457. 10.1038/ijo.2016.225 [DOI] [PubMed] [Google Scholar]
- Tey, S. L. , Salleh, N. B. , & Henry, C. J. (2017a). Effects of a non‐nutrient (artificial v. natural) sweeteners on 24‐h glucose profiles. [DOI] [PubMed]
- Ursoniu, S. , Sahebkar, A. , Serban, M. C. , & Banach, M. (2015). Lipid profile and glucose changes after supplementation with astaxanthin: A systematic review and meta‐analysis of randomized controlled trials. Archives of Medical Science, 11, 253–266. 10.5114/aoms.2015.50960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vega, K. , Edwards, J. , & Belstein, P. (2015). Subacute (13‐week) and pre‐natal development toxicity studies of dietary astaxanthin in rats. Regulatory Toxicology and Pharmacology, 73, 819–828. 10.1016/j.yrtph.2015.10.013 [DOI] [PubMed] [Google Scholar]
- Vuolo, F. , Abreu, S. C. , Michels, M. , Xisto, D. G. , Blanco, N. G. , Hallak, J. E. C. , … Dal‐Pizzol, F. (2019). Cannabidiol reduces airway inflammation and fibrosis in experimental allergic asthma. European Journal of Pharmacology, 843, 251–259. 10.1016/j.ejphar.2018.11.029 [DOI] [PubMed] [Google Scholar]
- Wang, K. , Xu, P. , et al. (2017). Effects of ginsenoside Rg1 on learning and memory in a reward‐directed instrumental conditioning task in chronic restraint stressed rats. Phytotherapy Research, 31, 81–89. [DOI] [PubMed] [Google Scholar]
- Wang, Y. , Yang, G. , Gong, J. , Lu, F. , Diao, Q. , Sun, J. , … Liu, J. (2016). Ginseng for Alzheimer's disease: A systematic review and meta‐analysis of randomized controlled trials. Current Topics in Medicinal Chemistry, 16, 529–536. 10.2174/1568026615666150813143753 [DOI] [PubMed] [Google Scholar]
- Ward, S. J. , McAllister, S. D. , Kawamura, R. , Murase, R. , Neelakantan, H. , & Walker, E. A. (2014). Cannabidiol inhibits paclitaxel‐induced neuropathic pain through 5‐HT(1A) receptors without diminishing nervous system function or chemotherapy efficacy. British Journal of Pharmacology, 171, 636–645. 10.1111/bph.12439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welty, T. E. , Luebke, A. , & Gidal, B. E. (2014). Cannabidiol: Promise and pitfalls. Epilepsy Curr, 14, 250–252. 10.5698/1535-7597-14.5.250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- White, C. M. (2019). A review of human studies assessing cannabidiol's (CBD) therapeutic actions and potential. Journal of Clinical Pharmacology, 9, 923–934. [DOI] [PubMed] [Google Scholar]
- Wu, D. , Xu, H. , Chen, J. , & Zhang, L. (2019). Effects of astaxanthin supplementation on oxidative stress. International Journal for Vitamin and Nutrition Research, 15, 1–16. 10.1024/0300-9831/a000497 [DOI] [PubMed] [Google Scholar]
- Wu, X. , Li, D. , Liu, J. , Diao, L. , Ling, S. , Li, Y. , … Li, Y. (2017. May 29). Dammarane sapogenins ameliorates neurocognitive functional impairment induced by simulated long‐duration spaceflight. Frontiers in Pharmacology., 8, 315 10.3389/fphar.2017.00315 eCollection 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu, W. , Choi, H.‐K. , & Huang, L. (2017). State of Panax ginseng research: A global analysis. Molecules, 22(9), 1518 10.3390/molecules22091518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Wu H et al (2017) Analysis of anti‐fatigue herbal products originated from food‐drug herbs. http://app1.sfda.gov.cn/.
- Zhou, G. , Peng, Y. , Zhao, L. , Wang, M. , & Li, X. (2017). Biotransformation of total saponins in Siraitia Fructus by human intestinal microbiota of normal and type 2 diabetic patients. Journal of Agricultural and Food Chemistry, 65, 1518–1524. 10.1021/acs.jafc.6b04498 [DOI] [PubMed] [Google Scholar]
- Zhou, G. , Zhang, Y. , Li, Y. , Wang, M. , & Li, X. (2018). The metabolism of a natural product mogroside V, in healthy and type 2 diabetic rats. Journal of Chromatography. B, Analytical Technologies in the Biomedical and Life Sciences, 1079, 25–33. 10.1016/j.jchromb.2018.02.002 [DOI] [PubMed] [Google Scholar]
- Zou, C. , Zhang, Q. , & Zhang, S. (2018). Mogroside IIIE attenuates gestational diabetes mellitus through activating of AMPK signaling pathway in mice. Journal of Pharmacological Sciences, 138(3), 161–166. 10.1016/j.jphs.2018.09.008 [DOI] [PubMed] [Google Scholar]


