Abstract
Dietary fibre, such as indigestible oligosaccharides and polysaccharides, occurs in many foods and has gained considerable importance related to its beneficial effects on host health and specific diseases. Dietary fibre is neither digested nor absorbed in the small intestine and modulates the composition of the gut microbiota. New evidence indicates that dietary fibre also interacts directly with the epithelium and immune cells throughout the gastrointestinal tract by microbiota‐independent effects. This review focuses on how dietary fibre improves human health and the reported health benefits that are connected to molecular pathways, in (a) a microbiota‐independent manner, via interaction with specific surface receptors on epithelial and immune cells regulating intestinal barrier and immune function, and (b) a microbiota‐dependent manner via maintaining intestinal homeostasis by promoting beneficial microbes, including Bifidobacteria and Lactobacilli, limiting the growth, adhesion, and cytotoxicity of pathogenic microbes, as well as stimulating fibre‐derived microbial short‐chain fatty acid production.
Linked Articles
This article is part of a themed section on The Pharmacology of Nutraceuticals. To view the other articles in this section visit http://onlinelibrary.wiley.com/doi/10.1111/bph.v177.6/issuetoc
Abbreviations
- 2′FL
2′fucosyllactose
- 6′SL
6′sialyllactose
- AhR
aryl hydrocarbon receptor
- AMPK
5′AMP‐activated protein kinase
- AP
Angelica polysaccharides
- AP‐1
activator protein‐1
- CaSR
calcium‐sensing receptor
- COS
chitosan oligosaccharides
- DCs
dendritic cells
- FOS
fructo‐oligosaccharides
- Gal/GalNAc
galactose/N‐acetylgalactosamine
- GI
gastrointestinal
- GOS
galacto‐oligosaccharides
- HDAC
histone deacetylase
- HMOs
human milk oligosaccharides
- IBD
inflammatory bowel disease
- IECs
intestinal epithelial cells
- ITF
inulin‐type fructans
- MCP‐1
monocyte chemoattractant protein‐1
- MCs
mast cells
- MIP
macrophage inflammatory protein
- NODs
nucleotide‐binding oligomerization domain‐containing proteins
- PBMCs
peripheral blood mononuclear cells
- PGlyRP3
peptidoglycan recognition protein 3
- POS
pectic‐oligosaccharides
- RS
resistant starches
- SCFAs
short‐chain fatty acids
- TLRs
toll‐like receptors
- Tregs
regulatory T cells
1. INTRODUCTION
The CODEX Alimentarius Commission defined dietary fibre as a group of carbohydrate polymer compounds with 10 or more monomeric units (a footnote also allows the inclusion of polymers with a degree of polymerization (DP) 3–9), which are neither digested nor absorbed in the human small intestine (Jones, 2014). Food products that naturally contain dietary fibre, such as cereals, fruits, vegetables, nuts, beans and seafood, but also breast milk and application of prebiotics in functional food are main sources of dietary fibre intake. Some dietary fibre affects the digestion rate by reducing gastric emptying, limiting digestive enzyme activity, and restricting the rate and extent of nutrient absorption in the gut. After ingestion, dietary fibre passes through the oesophagus and stomach and reaches the small intestine followed by fermentation by the microbiota in the caecum and colon, and finally, absorption by the host (Qi, Al‐Ghazzewi, & Tester, 2018).
The human gut microbiome, that is, the microbiota that populate the human gastrointestinal (GI) tract, plays a significant role in maintaining gut homeostasis, including the digestion of dietary fibre, production of nutrients, vitamins and hormones, as well as protection against pathogens and maintaining immune homeostasis (Koh, De Vadder, Kovatcheva‐Datchary, & Backhed, 2016). Dietary habits are crucial for modulating the composition and function of the gut microbiome. Various types of dietary fibre have received considerable interest related to their microbiota‐dependent effects on host health and certain diseases (Makki, Deehan, Walter, & Backhed, 2018). The composition of the gut microbiome, including the presence of Bifidobacterium and Lactobacillus species, is important for health‐promoting effects, such as antiallergic and anti‐inflammatory properties (Maslowski & Mackay, 2011). The main end products of intestinal bacterial fermentation of dietary fibre, the short‐chain fatty acids (SCFAs), particularly acetate, propionate and butyrate, are regulators of gut homeostasis. These fibre‐induced microbial changes and microbial fermentation products have a considerable impact on the immune system and hence on the prevention and treatment of diseases (Bolognini, Tobin, Milligan, & Moss, 2016).
In addition to its microbiota‐dependent effects, some dietary fibre interacts with several cell types and protects intestinal epithelial barrier function, has immunomodulatory properties and can inhibit inflammation independent of the microbiota.
Here, we provide a detailed review of direct, that is microbiota‐independent, effects of dietary fibre (Figure 1) on intestinal barrier function, immunity as well as other relevant actions. We also discuss the microbiota‐dependent effects of dietary fibre on intestinal commensal and pathogenic microbiota, as well as fibre‐induced SCFA production and how these microbiota‐dependent and ‐independent effects relate to the modulation of gut homeostasis in health and disease.
Figure 1.
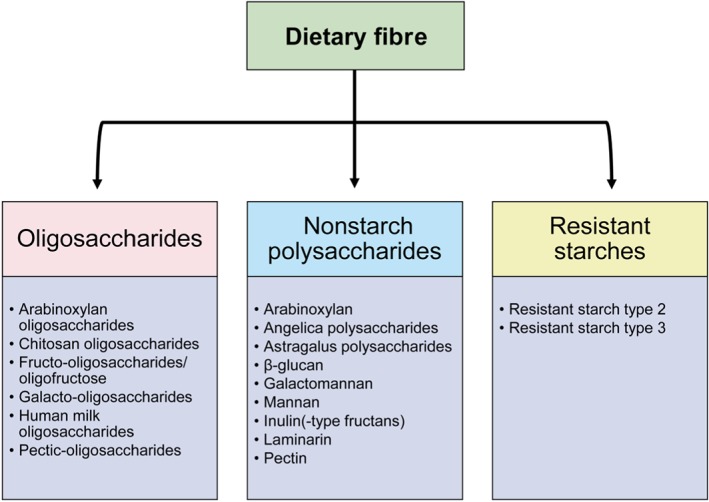
Groups of dietary fibre discussed in this review. Dietary fibre is usually categorized based on their chemical structure
2. MICROBIOTA‐INDEPENDENT EFFECTS OF DIETARY FIBRE
The intestinal mucosa is continuously exposed to food components as well as pathogenic and commensal microbes. This requires a homeostatic balance that allows for tolerating food antigens as well as beneficial nonpathogenic commensal microbes, while defending against pathogenic microbes. The disruption of this homeostatic balance has been associated with the development of multiple (inflammatory) diseases, such as inflammatory bowel disease (IBD). To maintain intestinal homeostasis a close interaction between the luminal microbiota, intestinal epithelium and immune cells is required (Rescigno, 2011).
Intestinal epithelial cells (IECs), which are responsible for the largest mucosal surface of the human body, are in direct contact with the external environment. Their main functions are the digestion of food and the absorption of nutrients, but they also act as a physical and biochemical barrier, preventing potentially harmful antigens from invading the body. The luminal secretion of mucins by goblet cells provides the first protection against luminal antigens. Moreover, IECs are sealed by junctional complexes, such as tight and adherens junctions, which are important regulators of epithelial barrier function. Disruption of these junctional complexes leads to increased epithelial permeability (also known as a “leaky gut”) and predisposes to or enhances acute and chronic https://www.sciencedirect.com/topics/pharmacology-toxicology-and-pharmaceutical-science/disease-exacerbation (Peterson & Artis, 2014). Related to the continuous exposure to pathogens, antigens and toxins, the intestinal epithelium shows rapid self‐renewal in the event of tissue damage (Sancho, Batlle, & Clevers, 2003).
Innate as well as adaptive immune cells including dendritic cells (DCs), macrophages, and mast cells (MCs) are influenced by IEC‐derived signals (e.g. cytokines and chemokines, direct cell–cell contact; Rescigno, 2011). Dendritic cells efficiently acquire antigens from the intestinal lumen and can induce tolerance and immunity (Coombes & Powrie, 2008). Macrophages maintain the intestinal homeostasis and are important components of protective immunity with high https://www.sciencedirect.com/topics/immunology-and-microbiology/phagocytosis (Bain & Schridde, 2018). Mast cells contribute to host defence against invading pathogens by regulating epithelial function and integrity, and by raising innate and adaptive mucosal immune responses (Bischoff, 2009).
Dietary fibre passes the upper GI tract before reaching the large intestine to be fermented and interacts with IECs and immune cells along its intestinal journey. The direct interaction of fibre with different intestinal cell types promotes gut homeostasis and intestinal epithelial barrier function, and it supports intestinal immune responses via effects on dendritic cells, macrophage and mast cells.
2.1. The effect of dietary fibre on intestinal epithelial barrier function
Several studies have highlighted the importance of dietary fibre in protecting intestinal barrier integrity. The human milk oligosaccharide (HMO) and lacto‐N‐neotetraose induced an increase in transepithelial electrical resistance in Caco‐2Bbe cells (Holscher, Davis, & Tappenden, 2014). Galacto‐oligosaccharides (GOS) mitigates the harmful effects of the mycotoxin deoxynivalenol on the intestinal barrier of Caco‐2 cells, accelerating tight junction reassembly and stabilizing the expression and cellular distribution of the tight junction protein, claudin‐3 (Akbari et al., 2015). In addition, GOS enhanced the mucosal barrier function via direct modulation of goblet cell function by up‐regulating gene and protein expression levels of secretory products and Golgi‐sulfotransferases (Bhatia et al., 2015). Astragalus polysaccharide (traditional Chinese medicinal herb) prevented the decreased occludin mRNA expression in lipopolysaccharides (LPS‐challenged Caco‐2 cells (Wang, Li, Yang, & Yao, 2013) and a product rich in β‐galactomannan protected the S. Enteritidis‐infected Caco‐2 intestinal epithelial barrier and recovered the zonula occludens‐1 and occludin localization (Brufau et al., 2016).
The epithelial glycocalyx is important for bacterial–epithelial cell interactions and can support microbial colonization and gut barrier function. 2′‐Fucosyllactose (2′FL), 3′FL, and inulins were able to stimulate intestinal epithelial glycocalyx development, while pectins were less effective (Kong et al., 2019).
Dietary fibre also promotes intestinal barrier function by direct effects on the proliferation, migration, differentiation and apoptosis of IECs. For example, HMOs induced apoptosis and inhibited proliferation in IECs by promoting arrest in the G2/M phase (Kuntz, Kunz, & Rudloff, 2009) and Holscher et al. (2014; 2017) indicated that there is an association between the inhibition in cell proliferation and an increase in cellular differentiation (Holscher et al., 2014; Holscher, Bode, & Tappenden, 2017). High temperature‐modified citrus pectins repressed the proliferation of G2/M cell‐cycle arrest and induced apoptosis in a https://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=1619‐dependent way in different cancer cell lines (Hao et al., 2013). In contrast, arabinoxylan and mixed‐linked β‐glucans had no effect on the proliferation of Caco‐2 and HT‐29 cells (Samuelsen, Rieder, Grimmer, Michaelsen, & Knutsen, 2011).
In summary, different dietary fibre can protect intestinal barrier function via modulation of epithelial tight junction proteins, goblet cell function and, possibly, via regulation of epithelial cell and glycocalyx maturation.
2.2. Potential molecular mechanisms of the regulation of intestinal epithelial barrier function by dietary fibre
2.2.1. Activation of AMP‐activated protein kinase by dietary fibre
AMP‐activated protein kinase (https://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=1540) is an important regulator of IECs including their protective barrier function and tight junction assembly (Sun, Yang, Rogers, Du, & Zhu, 2017). Chitosan oligosaccharides (COS) promoted the assembly of tight junction proteins in an IEC line by activation of AMPK via calcium‐sensing receptor (https://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=54)‐phospholipase C (https://www.guidetopharmacology.org/GRAC/FamilyDisplayForward?familyId=274)‐https://www.guidetopharmacology.org/GRAC/FamilyDisplayForward?familyId=123 receptor channel‐dependent pathway mediated calcium release from endoplasmic reticulum (Muanprasat et al., 2015). There is also a potential role for toll‐like receptors 2 (https://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=1752) in regulating intestinal epithelial barrier function, since activation of TLR2 by inulin‐type fructans (ITF) and lemon pectins promoted the intestinal epithelial integrity, probably via a TLR2/MyD88/NF‐κB signalling pathway (Vogt et al., 2014; Vogt et al., 2016). In addition, TLR2 signalling is involved in epithelial proliferation and apoptosis of terminally differentiated epithelial cells (Hormann et al., 2014). There are even indications that TLR2 signalling promotes mucin production (Jiang et al., 2017; Wu et al., 2007).
2.2.2. Dietary fibre interaction with the epidermal growth factor receptor
The epidermal growth factor receptor (https://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=1797) can induce IEC proliferation and differentiation (Yarden, 2001). Neutral and acidic HMOs caused IEC cycle arrest by interacting with the EGFR as measured by EGFR phosphorylation. These oligosaccharides activated EGFR downstream signalling pathways, such as https://www.guidetopharmacology.org/GRAC/FamilyDisplayForward?familyId=519 MAPK and https://www.guidetopharmacology.org/GRAC/FamilyDisplayForward?familyId=5141/2 (Kuntz et al., 2009).
Taken together, different dietary fibre contains intestinal barrier protective properties by recovering the junctional network, modulating goblet cell function and possibly by increasing epithelial cell differentiation and glycocalyx maturation which is possibly related to direct interactions with AMPK, TLRs, and EGFR. In addition, these types of dietary fibre modulate cytokine production and release by IECs and other cell types, thereby indirectly modifying intestinal barrier function.
2.3. The effect of dietary fibre on intestinal epithelial immunity
Dietary fibre can promote intestinal immunity by direct effects on IECs and on cells of the intestinal immune system including dendritic cells, macrophages and mast cells.
2.3.1. Immunological effects of dietary fibre that are mediated by IECs
Several in vitro studies have described the effects of dietary fibre on intestinal epithelial‐derived cytokine and chemokine production and release using different IEC lines. Inulin, GOS and fructo‐oligosaccharides (FOS) increased human https://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=819, monocyte chemoattractant protein https://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=771, macrophage inflammatory protein https://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=827 and https://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=821 secretion in some types of IECs (Ortega‐Gonzalez et al., 2014), whereas α3‐sialyllactose and chicory FOS inhibited baseline https://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=4977 release in Caco‐2 cells (Zenhom et al., 2011). In contrast, arabinoxylan and mixed‐linked β‐glucans did not affect the basal production of IL‐8 by Caco‐2 and HT‐29 cells (Samuelsen et al., 2011), which is in agreement with the unaltered basal expression of proinflammatory mediators in human colonic epithelial cells incubated with pooled HMOs (Newburg, Ko, Leone, & Nanthakumar, 2016).
The inhibition of cytokine/chemokine expression and release by several types of dietary fibre, including HMOs (He, Liu, et al., 2016; Newburg et al., 2016; Zehra et al., 2018), GOS (Akbari et al., 2015), arabinoxylan hydrolysates (Mendis, Leclerc, & Simsek, 2016), arabinoxylans, mannose and galactomannans (Hermes, Manzanilla, Martin‐Orue, Perez, & Klasing, 2011), was described in in vitro studies using different IECs exposed to inflammatory triggers. For example, HMOs and galactosyllactoses inhibit https://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=5074, Salmonella‐ and Listeria‐induced IL‐8 and https://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=808 expression and release in T84, NCM‐460 and H4 cells. The HMOs, 6′‐sialyllactose (6′SL), can decrease IL‐8 and MIP‐3α release in T84 and HT‐29 cells stimulated with antigen–antibody complex (Zehra et al., 2018), whereas 2′FL is effective in inhibiting the LPS‐dependent induction of IL‐8 in T84 cells (He, Liu, et al., 2016). HMOs from colostrum are able to attenuate PAMP‐induced inflammatory cytokine protein levels, including IL‐8, https://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=4998, MCP‐1/2, and https://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=4974 (He, Liu, Leone, & Newburg, 2014). In addition, GOS suppress the secretion of IL‐8 in Caco‐2 cells stimulated with the mycotoxin deoxynivalenol, while FOS and inulin are not effective in this study (Akbari et al., 2015).
Different arabinoxylan hydrolysates exert differential effects on LPS‐induced IL‐8 and TNF‐α release in colon cancer cell lines (Caco‐2 and HT‐29 cells; Mendis, Leclerc, & Simsek, 2017). Feedstuffs containing arabinoxylans, mannose, or galactomannans are able to decrease the Escherichia coli‐induced IL‐1β, IL‐8 and TNF‐α expression in a porcine IEC line (IPEC‐J2; Hermes et al., 2011).
2.3.2. The effect of dietary fibre on intestinal dendritic cells
Several in vitro studies have shown that dietary fibre promotes immunological responses by acting on intestinal dendritic cells. For example, a GOS/FOS mixture stimulated https://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=4975 release by human monocyte‐derived dendritic cells (Lehmann et al., 2015), whereas pectin reduced their synthetic lipopeptide P3CSK4‐induced release of IL‐6 and IL‐10 (Sahasrabudhe et al., 2018). Different dietary fibre (GOS, inulin, pectin, arabinoxylan and β‐glucan) caused a more regulatory status of dendritic cells as indicated by an elevated IL‐10/IL‐12 ratio (Bermudez‐Brito et al., 2015; Mikkelsen, Jespersen, Mehlsen, Engelsen & Frøkiær, 2014). The immunomodulating effects of dietary fibre on dendritic cells might be dependent on its interaction with (released factors from) IECs as described by Bermudez‐Brito et al. (2015) using coculture systems. It has been shown that production of different cytokines and chemokines, including https://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=4968, IL‐12, IL‐1, IL‐6, IL‐8, MCP‐1, https://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=756, https://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=758 and TNF‐α, released by dendritic cells is decreased after incubation with dietary fibre‐stimulated Caco‐2 cell medium (GOS, inulin, pectin, arabinoxylan, β‐glucan and resistant starches (RS); Bermudez‐Brito, Rosch, Schols, Faas, & de Vos, 2015; Bermudez‐Brito, Sahasrabudhe, et al., 2015).
2.3.3. The effect of dietary fibre on intestinal macrophages
Dietary fibre has been shown to stimulate basal proinflammatory cytokine release by macrophages and monocytes. FOS and inulin reportedly induced TNF‐α and IL‐10 release by rat and human monocytes (Capitan‐Canadas et al., 2014). Stimulation of murine peritoneal macrophages with oat β‐glucan resulted in the production of IL‐1 (Estrada et al., 1997) and an increase in phagocytic activity (Yun, Estrada, Van Kessel, Park, & Laarveld, 2003). In RAW264.7 cells, a murine macrophage cell line, pectin decreased the P3CSK4‐induced IL‐6 production (Sahasrabudhe et al., 2018), and arabinoxylan hydrolysates modulated the LPS‐induced NO production (Mendis et al., 2016).
2.3.4. The effect of dietary fibre on intestinal mast cells
Dietary fibre can inhibit mast cell activation. Angelica polysaccharides (AP) inhibited the release of proinflammatory cytokines, such as IL‐1, TNF‐α, IL‐6, https://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=4996 and MCP‐1, in addition also possessed inhibitory effects on allergic mediator release, including β‐hexosaminidase, leukotrienes C4 and histamine by RBL‐2H3 MCs (Mao et al., 2016). These effects might be related to an AP‐induced down‐regulation of essential proteins in the Gab2/PI3K/https://www.guidetopharmacology.org/GRAC/FamilyDisplayForward?familyId=285 and https://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=2026/https://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=2230 pathways and/or the AP‐induced decrease in Ca2+ influx (Mao et al., 2016). Two different sulfated polysaccharides repressed the IgE‐mediated activation in the same cell line (Ngatu et al., 2017). The HMO, 6′SL, inhibited the degranulation of murine bone marrow‐derived mast cells, whereas 2′FL had no effect (Castillo‐Courtade et al., 2015).
2.3.5. The effect of dietary fibre on blood mononuclear cells
In addition, dietary fibre may promote the development of the intestinal immune system as suggested by studies done on cord blood mononuclear cells from healthy newborns. Low‐molecular‐weight fucoidan stimulated IL‐4 and https://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=4980 production in these cells, whereas the acidic oligosaccharides increased IL‐13 production and the percentage of IFN‐γ producing T cells. Both types of dietary fibre significantly increased https://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=1695 expression, indicative of their effects on lymphocyte function and maturation (Eiwegger et al., 2004). Acidic oligosaccharides skewed the Th‐2 type T‐cell phenotype towards a more balanced Th‐1/Th‐2 profile in cord blood cells as well as in peripheral blood mononuclear cells (PBMCs) from adults (Eiwegger et al., 2010). In addition shorter chain fructans induced a more regulatory cytokine balance compared to longer chain fructans, as measured by IL‐10/IL‐12 ratios in human PBMCs (Vogt et al., 2013).
2.4. Potential molecular mechanisms of the regulation of intestinal immunity by dietary fibre
2.4.1. Dietary fibre binds to carbohydrate‐binding domains
The immune‐modulatory effects of dietary fibre on macrophages and dendritic cells are mediated, at least in part, by binding to carbohydrate receptors, including Ca+‐dependent https://www.guidetopharmacology.org/GRAC/FamilyDisplayForward?familyId=945, such as mannose receptor, https://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=2930, macrophage galactose‐specific lectin and langerin as well as Ca+‐independent C‐type lectin receptors, like dectin‐1, dectin‐2, and complement receptor 3. These receptors can recognize mannose, β‐glucans, fucose‐containing glycans, glucose, galactose and/or chitin (Wismar, Brix, Frokiaer, & Laerke, 2010). After binding, intracellular signalling pathways are activated, ultimately leading to the modulation of immune responses (Drickamer & Taylor, 2015; Goodridge, Wolf, & Underhill, 2009).
HMOs possess binding epitopes for selectin ligands and therefore possibly binds selectins, known as carbohydrate‐binding proteins, which support leukocyte adhesion to the blood vessel wall (Schumacher, Bendas, Stahl, & Beermann, 2006). HMOs are complex glycans that interact with https://www.guidetopharmacology.org/GRAC/FamilyDisplayForward?familyId=992 (Noll et al., 2016). Galectins also contain carbohydrate‐recognition domains and serve as pattern recognition receptors for dietary fibre. For example, intestinal epithelium‐derived galectin‐9 was involved in the immunomodulating effects of non‐digestible oligosaccharides as demonstrated in a coculture model with IECs and PBMCs (de Kivit et al., 2013).
2.4.2. Dietary fibre binds to TLRs
Various types of dietary fibre act as TLR ligands, with downstream phosphorylation of IκB and effects on cytokine production. Two different types of resistant starches (RS), including RS type 2 and type 3, predominantly bind to TLR2 and https://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=1755, respectively (Bermudez‐Brito, Rosch, et al., 2015). ITF can bind to TLR2 located on IECs (Vogt et al., 2014) and PBMCs and to a lesser extent https://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=1754, 5, 7, 8 and nucleotide‐binding oligomerization domain‐containing proteins 2 (https://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=1763) on PBMCs, which resulted in NF‐κB/activator protein‐1 (AP‐1) activation (Vogt et al., 2013). The chain length of ITF is important for TLR binding and the induced activation pattern, including cytokine release (Vogt et al., 2013; Vogt et al., 2017). Interestingly, pectin inhibited TLR2‐https://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=1751 activation (Sahasrabudhe et al., 2018).
GOS, FOS and inulin are TLR4 ligands in IECs and induced cytokine production dependent on TLR4/MyD88/NF‐κB and secondarily on MAPK (Ortega‐Gonzalez et al., 2014). Monocytes and dendritic cells can also bind GOS, FOS, and inulin via TLR4 (Capitan‐Canadas et al., 2014; Lehmann et al., 2015). Different HMOs controlled immune responses via https://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=1753 and TLR4 signalling as reviewed by He, Lawlor, and Newburg (2016).
CD14 expression in enterocytes mediated LPS‐TLR4 stimulation and corresponding IL‐8 response and 2′FL suppressed CD14 expression and repressed NF‐κB and p‐ERK levels, whereas https://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=2994 phosphorylation and expression of IκB and suppressor of cytokine signalling 2 were increased (He, Liu, et al., 2016).
In summary, dietary fibre binds and activates different TLRs, mainly TLR2, 3, and 4, in a cell type‐independent manner.
2.4.3. Activation of PPARγ by dietary fibre
Previous research has highlighted the role of https://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=595 in regulating inflammation and immunity (Daynes & Jones, 2002). Oligosaccharides (α3‐sialyllactose or FOS) exerted an anti‐inflammatory effect by activation of PPARγ, which was at least partly dependent on one of the members of the novel family of pattern recognition molecules in innate immunity, the peptidoglycan recognition protein 3 (PGlyRP3; Zenhom, Hyder, de Vrese, et al., 2011). PGlyRPs are expressed in enterocytes and can recognize microbes. In contrast to TLRs and NODs, PGlyRP3 seems to have an anti‐inflammatory role in IECs. This anti‐inflammatory effect is mediated via NF‐κB pathway suppression, as also observed in reduced gene expression and nuclear translocation of NF‐κB induced by α3‐sialyllactose and FOS (Zenhom et al., 2011; Zenhom, Hyder, de Vrese, et al., 2011). The anti‐inflammatory effect of 6′SL was PPARγ dependent and associated with a decreased activity of the transcription factors AP‐1 and NF‐κB (Zehra et al., 2018).
Taken together, dietary fibre can alter intracellular signalling via binding to specific cell surface receptors on different cell types that regulate epithelial cell maturation, barrier function and mucosal immunity (summarized in Table 1). The mechanisms and scope of these effects are determined by the chemical structure, the purity the origin of dietary fibre and its target cell type.
Table 1.
Microbiota‐independent effects of dietary fibre on intestinal barrier function and immunity
| Dietary fibre | Cell type | Effect | Mechanism | Reference |
|---|---|---|---|---|
| Acidic and neutral human milk oligosaccharides | HT‐29, HIEC, and Caco‐2 cells | Proliferation |
Interaction with EGFR Modulation of downstream EGFR/Ras/Raf/ERK |
(Kuntz et al., 2009) |
| Galacto‐oligosaccharides | LS174T cells | Barrier | Stimulation of Goblet cells | (Bhatia et al., 2015) |
| Chitosan oligosaccharides | T84 cells |
Barrier
Immune |
AMPK activation via CaSR‐PLC‐IP3 receptor channel‐mediated calcium release AMPK‐independent inhibition of NF‐κB signalling |
(Muanprasat et al., 2015) |
| Human milk oligosaccharides, galacto‐oligosaccharides, inulin, inulin‐type fructans, fructo‐oligosaccharides, α3‐sialyllactose |
T84, NCM‐460, H4, IEC18, Caco‐2 cells Reporter cells |
Immune
Barrier |
Modulation NF‐κB signalling | (Newburg et al., 2016; Ortega‐Gonzalez et al., 2014; Vogt et al., 2013; Vogt et al., 2014; Vogt et al., 2017;Zehra et al., 2018 ; Zenhom, Hyder, de Vrese, et al., 2011) |
| Resistant starches (α‐glucan‐based) |
Human DCs Reporter cells Caco‐2 cells |
Immune |
TLR2 and TLR5 binding |
(Bermudez‐Brito, Rosch, et al., 2015) |
|
Inulin‐type fructans Lemon pectins |
Reporter cells T84 cells |
Immune
Barrier |
Binding to TLR2 (and to a lesser extent to TLR4, 5, 7, 8, and NOD2) | (Vogt et al., 2013; Vogt et al., 2014; Vogt et al., 2016) |
| Pectin |
Human DCs Mouse macrophages |
Immune |
TLR2 binding Inhibition of TLR2–TLR1 pathway |
(Sahasrabudhe et al., 2018) |
| Fructo‐oligosaccharides, inulin, galacto‐oligosaccharides |
Rat monocytes Human monocyte‐derived DCs IEC18, HT‐29, and Caco‐2 cells |
Immune | TLR4 binding | (Capitan‐Canadas et al., 2014; Lehmann et al., 2015; Ortega‐Gonzalez et al., 2014) |
| Human milk oligosaccharides, 2′fucosyllactose | T84 and H4 cells | Immune |
Suppressing CD14 expression Modulation of downstream TLR4‐LPS‐CD14 |
(He, Liu, et al., 2016) |
| Galacto‐oligosaccharides, (chicory) inulin, inulin‐type fructans, sugar beet pectin, wheat arabinoxylan, barley β‐glucan |
Human DCs Caco‐2 and IEC18 cells Reporter cells |
Immune | MyD88 dependent | (Bermudez‐Brito, Sahasrabudhe, et al., 2015; Ortega‐Gonzalez et al., 2014; Vogt et al., 2013) |
| Human milk oligosaccharides, α3‐sialyllactose, fructo‐oligosaccharides | T84 and Caco‐2 cells | Immune | PPARγ dependent | (Zehra et al., 2018; Zenhom, Hyder, de Vrese, et al., 2011) |
| Mannose, β‐glucans, fucose‐containing glycans, glucose, galactose, and/or chitin | DCs, macrophages | Immune | Binding to C‐type lectin receptors | (reviewed in Wismar et al., 2010) |
| Galacto‐oligosaccharides, fructo‐oligosaccharides |
HT‐29 and T84 cells Human PBMCs |
Immune | Galectin‐9 | (de Kivit et al., 2013) |
| Angelica polysaccharides | Mast cells | Immune | Modulation of Gab2/PI3‐K/Akt and Fyn/Syk pathways | (Mao et al., 2016) |
2.5. In vivo relevance
Although IEC lines, including Caco‐2 and HT‐29, in general provide a powerful tool to investigate properties and molecular mechanism of the intestinal epithelium, results gained from these in vitro models can be difficult to extrapolate to the (human) in vivo situation. Despite the abundant literature concerning microbiota‐independent effects is related to in vitro studies, an in vivo study with germ‐free mice by Lleywellyn et al. (2018) showed that psyllium fibre has both microbiota‐dependent and ‐independent effects and reduced the severity of colitis related to an improved intestinal barrier function and stimulation of the innate and antimicrobial immunity, confirming the biological relevance of microbiota‐independent effects of dietary fibres (Llewellyn et al., 2018). However, the effect on colitis of psyllium fibre was significantly greater in SPF animals. Other in vivo studies with dietary fibres using germ‐free mice highlighted the importance of the microbiota to maintain gut homeostasis and to prevent diseases (Charbonneau et al., 2016; Turnbaugh et al., 2009).
3. MICROBIOTA‐DEPENDENT EFFECTS OF DIETARY FIBRE ON GUT HOMEOSTASIS
The human gut microbiome is constituted by numerous microbial communities comprising hundreds of individual bacterial species, including beneficial (non‐invasive) microbes (e.g. Bifidobacterium and Lactobacillus spp.) and potential pathogenic (invasive) microbes (e.g. E. coli; Bik et al., 2018). Bifidobacterium and Lactobacillus spp. have anti‐inflammatory effects (Gill, Rutherfurd, & Cross, 2001), stimulate brain functions (Mayer, Tillisch, & Gupta, 2015), modulate metabolism (Brusaferro et al., 2018) and antagonize intestinal pathogens (Servin, 2004). Pathogenic microbes, such as enteropathogenic and enterohemorrhagic E. coli and Clostridium difficile, can cause gut pathology by producing toxins and causing infections. Dietary fibre can help to maintain intestinal homeostasis and to prevent diseases by promoting and limiting the growth and effects of beneficial and pathogenic microbes, respectively (summarized in Table 2). The majority of data supporting the microbiota‐dependent effects of dietary fibre are based on in vitro and in vivo animal studies, while well‐designed clinical trials are scarce (Table 2).
Table 2.
Microbiota‐dependent effects of dietary fibre on gut homeostasis
| Dietary fibre | Type of study | Microbiome change | Mechanism | Disease | Reference |
|---|---|---|---|---|---|
| Arabinoxylan‐oligosaccharides |
In vivo Clinical study |
Bifidobacterium ↑ Lactobacillus ↑ |
Bifidobacterium and/or Lactobacillus stimulation: (a) improving intestinal barrier function, (b) immunomodulation, (c) influencing brain function through the production of neuropeptides, and (d) antimicrobial activities |
Metabolic syndrome Metabolic endotoxemia IBD Diabetes Obesity Food poisoning Irritable bowel syndrome Anxiety |
(Cani et al., 2009; Cherbut, Michel, & Lecannu, 2003; Francois et al., 2012; Frost et al., 2014; Goehring et al., 2016; Gopalakrishnan et al., 2012; Lindsay et al., 2006; Mandadzhieva, Ignatova‐Ivanova, Kambarev, Iliev, & Ivanova, 2011; Silk, Davis, Vulevic, Tzortzis, & Gibson, 2009; Tang et al., 2015; Tarr et al., 2015; Videla et al., 2001) |
| Galacto‐oligosaccharides |
In vivo In vitro Clinical study |
||||
| Fructo‐oligosaccharides |
In vivo In vitro Clinical study |
||||
| Inulin‐type fructans | In vivo | ||||
| Human milk oligosaccharides |
In vivo Clinical study |
||||
| Laminarin | In vivo | ||||
| Resistant starches | In vivo | ||||
| Galacto‐oligosaccharides | In vitro |
Vibrio cholerae ↓ Entamoeba histolytica ↓ |
Inhibition of pathogen‐induced cytotoxicity: (a) direct binding to toxins or lectins, (b) inhibiting the expression of toxins or toxin‐related genes, and (c) neutralizing or interfering with toxins |
Diarrhoea GI disease E. histolytica infection Urinary tract infections Childhood shigellosis Colitis |
(Di et al., 2017; Gonia et al., 2015; Jantscher‐Krenn et al., 2012; Koleva, Valcheva, Sun, Ganzle, & Dieleman, 2012; Nguyen et al., 2016; Olano‐Martin, Williams, Gibson, & Rastall, 2003; Rabbani et al., 2009; Sinclair, de Slegte, Gibson, & Rastall, 2009) |
| Human milk oligosaccharides | In vitro |
Uropathogenic Escherichia coli ↓ Clostridium difficile ↓ Candida albicans ↓ |
|||
| Fructo‐oligosaccharides | In vivo | C. difficile ↓ | |||
| Inulin‐type fructans | In vivo | C. difficile ↓ | |||
| Pectic‐oligosaccharides | In vitro | E. coli ↓ | |||
| Pectin | In vitro | E. coli ↓ | |||
| Resistant starches | Clinical study | Shigella ↓ | |||
| Galacto‐oligosaccharides |
In vivo In vitro |
E. coli ↓ E. histolytica ↓ Cronobacter sakazakii ↓ Citrobacter rodentium ↓ |
Antiadhesion: (a) antiadhesive and (b) decoy glycan effects |
Enteric infection GI disease Diarrhoea E. histolytica infection Food poisoning |
(Di et al., 2017; Ganan et al., 2010; Jantscher‐Krenn et al., 2012; Kittana et al., 2018; Quintero et al., 2011; Shoaf, Mulvey, Armstrong, & Hutkins, 2006; Sinclair et al., 2009) |
| Human milk oligosaccharides | In vitro |
E. histolytica ↓ Campylobacter jejuni ↓ |
|||
| Pectic‐oligosaccharides | In vitro | E. coli ↓ | |||
| Pectin | In vitro | E. coli ↓ | |||
| Human milk oligosaccharides | In vitro | Streptococcus agalactiae ↓ | Growth inhibition: (a) glycosyltransferase catalysis, (b) antimicrobial activities, (c) pore formation and permeabilization of cell walls, and (d) blockage of nutrient flow |
Invasive bacterial infections in newborns GI disease |
(Li, Xia, Nie, & Shan, 2016; Lin et al., 2017; Vishu Kumar, Varadaraj, Gowda, & Tharanathan, 2005) |
| Pectic‐oligosaccharides | In vitro |
Clostridium perfringens ↓ Bacteroides fragilis ↓ |
|||
| Chitosan oligosaccharides | In vitro |
Bacillus cereus ↓ E. coli ↓ |
3.1. Dietary fibre increases Bifidobacterium and/or Lactobacillus spp. growth
Dietary fibre intervention, especially with ITF, FOS and GOS, has been widely used to stimulate the growth of Bifidobacterium and/or Lactobacillus species and the production of SCFAs (Turnbaugh et al., 2009). There are different mechanisms of action related to the beneficial effects of dietary fibre‐stimulated Bifidobacterium and Lactobacillus spp. growth on human health: (a) improving intestinal barrier function, (b) immunomodulation, (c) influencing brain function and (d) antimicrobial activities.
Bifidobacterium and Lactobacillus spp. have been shown to improve intestinal epithelial barrier function (Madsen et al., 2001). An increase in the number of Lactobacillus and Bifidobacterium spp. in the caecum induced by oligofructose supplementation controls endogenous production of the intestinotrophic hormone, https://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=1140, and consequently improved gut barrier function in obese mice (Cani et al., 2009).
Studies in healthy humans indicated that wheat bran extract containing arabinoxylan oligosaccharides can increase fecal bifidobacteria levels and exert beneficial effects on gut health parameters (Francois et al., 2012). The growth of Bifidobacteria induced by fructans in the diet was associated with an increase in villus height and crypt depth, resulting in thicker mucosal layers in the rat jejunum and colon (Kleessen, Hartmann, & Blaut, 2003).
Gut commensal microbes can also affect the host immune system. Lactobacillus acidophilus and Lactobacillus rhamnosus increased the number of regulatory T cells (Tregs) and inhibited the development of dermatitis and asthma, respectively (Jang et al., 2012; Shah et al., 2012). Food‐derived laminarin (a kind of glucan polysaccharides), an antagonist of the https://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=2927 receptor (a C‐type lectin receptor expressed on dendritic cells and macrophages), inhibited the development of dextran sulphate sodium (DSS) induced colitis via promoting the growth and colonization of Lactobacillus murinus leading to an increase in Tregs (Tang et al., 2015). In addition, the administration of inulin and FOS decreased intestinal inflammation and reduced the severity of colitis, which was associated with increased butyrate, lactate and lactobacilli spp. in the colon (Cherbut et al., 2003; Videla et al., 2001).
The oral administration of L. rhamnosus and Bifidobacterium lactis enhanced NK cell‐mediated tumoricidal activity in mouse spleens (Gill, Rutherfurd, Prasad, & Gopal, 2000) and improved the ability of the elderly to resist immune aging (Gill et al., 2001). Gopalakrishnan et al. (2012) reported that GOS can reduce the severity of colitis manifested by less infiltration of inflammatory cells in the caecum and colon along with a reduction in goblet cell depletion, which was mainly because of a GOS‐induced increase in Bifidobacterium spp. leading to enhanced NK cell function and https://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=4981 production.
Bifidobacterium strains exhibited anti‐inflammatory and immunoregulatory effects and increased IL‐10 release by dendritic cells and decreased IFN‐γ production by T cells (Hart et al., 2004). A clinical study in patients with Crohn's disease showed that FOS stimulated the growth of bifidobacteria in both faeces and mucosa, and these changes were related to immunoregulatory effects on mucosal dendritic cell functions and IL‐10 release (Lindsay et al., 2006).
In a large prospective study, it has been observed that long‐term intake of dietary fibre is associated with a lower risk of Crohn's disease (Ananthakrishnan et al., 2013). Arslanoglu et al. (2008) suggested that early nutritional intervention with GOS/FOS seems to modulate the infant's immune system, leading to a protective effect against allergy and infection. In addition, 2′FL stimulated immune development in infants as observed by lower plasma cytokine profiles, such as IL‐1, IL‐6, and TNF‐α (Goehring et al., 2016).
Furthermore, intestinal bacteria, including Lactobacillus and Bifidobacterium spp., importantly modulate brain functions and may be involved neuropsychiatric diseases (Mayer et al., 2015). Dietary fibre can modulate brain signalling via the microbial gut–brain axis, control emotional behaviour and ameliorate cognitive and behavioural disorders in animal models and humans, in part, by increasing Lactobacillus and Bifidobacterium spp. gut populations (Clark & Mach, 2016; Luna & Foster, 2015). The administration of a probiotic formulation consisting of Lactobacillus helveticus and Bifidobacterium longum exhibited anxiolytic‐like activity in rats and beneficial remission of psychological distress in healthy women (Messaoudi et al., 2011). SCFAs, primarily acetate, have been shown to cross the blood–brain barrier and affect brain function. Acetate derived from the fermentable carbohydrate inulin directly suppressed appetite by activating the enzyme acetyl‐CoA carboxylase in the brain and by producing regulatory neuropeptides (Frost et al., 2014). In patients with irritable bowel syndrome, GOS stimulated gut bifidobacteria alleviated bloating symptoms and reduced anxiety scores (Silk et al., 2009). HMOs (3′SL or 6′SL) that promote bifidobacterial growth and modulate inflammatory responses prevented stress‐induced changes in gut microbiota and diminished anxiety‐like behaviour, potentially via modulating the microbial gut–brain axis (Tarr et al., 2015).
Lactobacilli and Bifidobacteria exhibit adhesion properties that interfere with the adhesion of bacterial pathogens to IECs and participate in the antimicrobial activity of host GI defence (Servin, 2004). Two different Bifidobacterium strains isolated from infant faeces inhibited the entry of Salmonella enterica serotype Salmonella typhimurium into Caco‐2 cells (Lievin et al., 2000). Different Lactobacillus spp. (e.g., Lactobacillus plantarum) exhibited an anti‐Salmonella effect via antimicrobial activity and competitive adhesion to mucin and HT‐29 cell lines. Brink et al. (2006) showed that the prebiotic saba starch functioned better than L. plantarum against Salmonella in vitro (Brink, Todorov, Martin, Senekal, & Dicks, 2006; Uraipan, Brigidi, & Hongpattarakere, 2014). Moreover, Lactobacillus spp. cultured and fermented with FOS showed antimicrobial activity against Listeria innocua and Enterobacter aerogenes (Mandadzhieva et al., 2011).
There is abundant literature concerning the effects of dietary fibre on the growth of Bifidobacterium and Lactobacillus spp. (So et al., 2018), however dietary fibre can also stimulate other microbes. For example, COS inhibited gut dysbiosis by promoting Akkermansia muciniphila (Zheng et al., 2018), whereas inulin has been found to increase faecal Faecalibacterium prausnitzii in healthy humans (Ramirez‐Farias et al., 2009).
In summary, dietary fibre regulates the abundance of important beneficial commensal bacteria in the GI tract, such as Bifidobacterium and Lactobacillus spp., thereby improving intestinal barrier function, immune regulation, antimicrobial activity and neuropsychiatric disorders.
3.2. Dietary fibre inhibits pathogen‐induced cytotoxicity
Dietary fibre can also interfere with pathogenic microbes, for example, toxin‐producing microbes. Dietary fibre protects from the effects of pathogenic microbes by (a) directly binding to toxins or lectins, (b) inhibiting the expression of toxins or toxin‐related genes and (c) neutralizing or interfering with toxins.
C. difficile toxin A and toxin B have carbohydrate‐binding sites that bind to HMOs (El‐Hawiet, Kitova, & Klassen, 2015). This suggests that HMOs can specifically abolish cytotoxicity by modifying the diethylpyrocarbonate of histidine residues in toxins (Nguyen et al., 2016). Furthermore, HMOs also have the ability to bind to Shiga toxin type 2 holotoxin (Stx2) and the B subunit homopentamers of cholera toxin, heat‐labile toxin and Shiga toxin type 1 (CTB5, HLTB5 and Stx1B5; El‐Hawiet et al., 2015). GOS are usually added to infant formula to simulate the beneficial effects of HMOs. GOS and HMOs both contain terminal galactose (Gal), which can bind to specific lectin of protozoan parasite Entamoeba histolytica. HMOs and/or GOS rescued the destruction of IECs by binding to the galactose/N‐acetylgalactosamine (Gal/GalNAc) lectin on the surface of E. histolytica trophozoites to reduce the cytotoxicity and adhesion of E. histolytica to intestine (Jantscher‐Krenn et al., 2012).
HMOs decreased invasion and toxicity of Candida albicans, a fungal colonizer of the neonatal gut, by shortening hyphal length and by reducing the expression of hyphal‐specific genes (mycelial cell wall protein‐encoding genes), possibly protecting premature infants from C. albicans‐induced intestinal disorders (Gonia et al., 2015). ITF and FOS decreased gene expression of Clostridium cluster XI and C. difficile toxin B (TcdB) in the faeces of rats in an IBD model, thereby reducing chronic inflammation (Koleva et al., 2012).
Pectin and pectic‐oligosaccharides (POS) were shown to neutralize and interfere with toxins and reduce cytotoxicity of Shiga‐like toxins from E. coli O157:H7 in vitro, as well as protect cells from Shiga‐like toxin‐induced damage (Di et al., 2017; Olano‐Martin et al., 2003). A clinical study in patients with severe bloody dysentery caused by Shigella infection showed that green banana (rich in RS) improved childhood shigellosis, reduced myeloperoxidase activity in mucus and blood, and increased SCFA concentrations in faeces (Rabbani et al., 2009).
3.3. Dietary fibre acts as anti‐adhesives and decoy glycan receptors
Soluble decoy carbohydrates can bind to pathogenic microbes, thereby preventing their binding and damage to the gut epithelium. The carbohydrate moiety on epithelial cells serves as a binding site for adhesins expressed by many intestinal pathogens such as Salmonella, enteropathogenic E. coli and enterohemorrhagic E. coli. GOS act as anti‐adhesives that competitively inhibit the adhesion of pathogens to GI epithelium. in vitro studies showed that the adherence of enteropathogenic E. coli and Cronobacter sakazakii to host epithelial cells can be inhibited by GOS (Quintero et al., 2011; Shoaf et al., 2006). In addition, GOS competitively inhibited Vibrio cholerae toxin binding to its cell surface toxin receptor (GM‐1) on the host cell surface (Sinclair et al., 2009). In addition, GOS significantly reduced the adhesion of the murine bacterial pathogen Citrobacter rodentium to the epithelial cell surface in a dose‐dependent manner in vitro, but not in vivo, which might be related to the expression of adhesins that are insensitive to GOS when C. rodentium grows in vivo (Kittana et al., 2018).
Similar to GOS, HMOs also act as anti‐adhesives and decoy glycan receptors, preventing adherence of pathogens to host intestinal epithelium, thereby decreasing the risk of infections (Jantscher‐Krenn et al., 2012; Ruiz‐Palacios, Cervantes, Ramos, Chavez‐Munguia, & Newburg, 2003). Breastfeeding results in a lower incidence of diarrhoea, which could be related to the reduced binding and colonization of the intestinal pathogen Campylobacter jejuni induced by HMOs (Ruiz‐Palacios et al., 2003). In addition, the anti‐adhesive activity of HMOs (as well as GOS) also prevented the attachment, invasion and infection of E. histolytica to human IECs (Jantscher‐Krenn et al., 2012). HMOs and GOS bind to Gal/GalNAc lectin on the surface of E. histolytica to interfere with its specific binding site, and this antiadhesive and cytoprotective effect depends on the free terminal Gal.
Di et al. (2017) reported that pectin and POS blocked the binding of E. coli O157:H7 to human HT‐29 cells. POS have the ability to compete with Shiga toxin 2 binding to the neutral glycolipid globotriaosylceramide Gb3 receptor on the human IEC surface. Low MW and de‐esterification of POS facilitates the anti‐adhesive activity of Shiga toxin‐producing E. coli to human IECs (Di et al., 2017). Moreover, POS concentration dependently inhibited the adherence and invasion of C. jejuni to Caco‐2 cells (Ganan et al., 2010).
3.4. Dietary fibre inhibits the growth of pathogenic microbes
Notably, some types of dietary fibre exhibit antibacterial effects and directly inhibit the growth of pathogenic microbes independent of host immunity. For example, non‐sialylated HMOs directly inhibited the growth of Streptococcus agalactiae. This inhibition was specific for S. agalactiae and not observed for uropathogenic E. coli, Pseudomonas aeruginosa, or Staphylococcus aureus (Lin et al., 2017). Moreover, this growth inhibition by HMOs was dependent on the expression of bacterial glycosyltransferase encoded by the gbs0738 gene in S. agalactiae. This glycosyltransferase might catalyse the incorporation of HMOs components into the cell wall of bacteria to inhibit the growth of S. agalactiae (Lin et al., 2017). POS completely inhibited the growth of Clostridium perfringens and Bacteroides fragilis strains, whereas FOS enhanced the growth of C. perfringens and B. fragilis strains. This indicates that POS and HMOs not only display prebiotic properties but also exhibit antimicrobial activities (Li et al., 2016). Another study found that COS showed better growth inhibitory activity against both Gram‐positive and ‐negative bacteria, such as Bacillus cereus, E. coli, Yersinia enterocolitica, and Bacillus licheniformis, compared to natural chitosan. Higher DP COS (monomers) with a low degree of acetylation exhibited optimal growth inhibition and lead to pore formation and permeabilization of B. cereus cell walls. In addition, the blockage of nutrient flow caused by aggregation of COS was the main cause of growth inhibition and lysis of E. coli (Vishu Kumar et al., 2005).
Although only a few in vitro studies have shown that dietary fibre directly inhibits the growth of pathogenic microbes, it may open new therapeutic avenues for the treatment of pathogen infections and may contribute to reducing the use of antibiotics.
4. DIETARY FIBRE‐DERIVED MICROBIAL METABOLITE PRODUCTION
A substantial part of the potential health benefits of dietary fibre is related to its metabolites produced by the gut microbiota. The most prominent dietary fibre‐derived metabolites are SCFAs, including acetate, propionate, and butyrate, which are found in high concentrations in the intestine, reaching around 13 mmol·kg−1 of intestinal content in the terminal ileum, increasing to 131 mmol·kg−1 in the caecum, with lower concentrations in the distal colon (80 mmol·kg−1; Cummings, Pomare, Branch, Naylor, & Macfarlane, 1987). The amount and types of SCFAs that are produced largely depend on substrate availability, bacterial species composition of the microbiome and intestinal transit time (Macfarlane & Macfarlane, 2003). Acetate, propionate, and butyrate are the most abundant SCFAs (≥95%) in the GI tract (den Besten et al., 2013). Generally, Bacteroidetes are potent producers of acetate and propionate, whereas butyrate is mainly produced by Firmicutes (Louis & Flint, 2017; Macfarlane & Macfarlane, 2003).
Potent substrates for SCFAs production are polysaccharides, oligosaccharides, proteins, peptides and glycoprotein precursors by anaerobic microorganisms (Blachier, Mariotti, Huneau, & Tomé, 2007), although in quantitative terms, the carbohydrates (polysaccharides and oligosaccharides) are most important for SCFAs production.
4.1. The effect of short‐chain fatty acids (SCFAs) on gut homeostasis
Similar to dietary fibre, SCFAs may contribute to gut homeostasis by regulating mucus (over)production and secretion (Schroeder et al., 2018). Indeed, in vitro studies indicated butyrate and propionate as inducers of mucin (MUC2) synthesis and secretion by intestinal cells (Burger‐van Paassen et al., 2009). Furthermore, SCFAs preserved intestinal barrier function by decreasing tight junction permeability (Suzuki, Yoshida, & Hara, 2008), potentially through activation of AMPK (Peng, Li, Green, Holzman, & Lin, 2009). Although the authors compared the effects of butyrate to similar effects of (low concentrations of) histone deacetylase (https://www.guidetopharmacology.org/GRAC/FamilyDisplayForward?familyId=848) inhibitor https://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=7005, a recent in vivo study suggested butyrate promoted tight junction protein expression in a https://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=312‐dependent manner (Feng et al., 2018). Finally, SCFAs promote gut health by decreasing local pH, thereby inhibiting or promoting the colonization of different taxa (Tramontano et al., 2018).
4.2. Regulation of gut immunity and systemic pathology by SCFAs
SCFAs regulate local immunity and systemic pathology. Interestingly, these microbial metabolites are connected to (novel) molecular pathways (Bolognini et al., 2016).
4.2.1. SCFAs as histone deacetylase (HDAC) inhibitors
Butyrate, and to a lesser extent propionate, is a potent HDAC inhibitor in intestinal cells and associated with the regulation of intestinal immunity and gut homeostasis (Schilderink, Verseijden, & de Jonge, 2013). Via HDAC inhibition, butyrate and other SCFAs display anticancer activities by inducing apoptosis and differentiation (Hinnebusch, Meng, Wu, Archer, & Hodin, 2002). Interestingly, cancerous colonocytes shifted their primary energy source from butyrate to glucose, resulting in a threefold accumulation of butyrate within the cell (known as the Warburg effect) inhibiting the proliferation of the cancerous colonocytes (Donohoe et al., 2012). Butyrate modulated the function of intestinal macrophages via HDAC‐mediated down‐regulation of proinflammatory mediators, including NO, IL‐6 and IL‐12 (Chang, Hao, Offermanns, & Medzhitov, 2014). In 2013, two reports suggested that intestinal immunity is promoted by butyrate via histone H3 acetylation, as well as via the generation of colonic Tregs in a https://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=226‐dependent manner (Furusawa et al., 2013; Smith et al., 2013). Remarkably, the co‐occurring HDAC inhibition and stimulation of GPCR receptors by SCFAs both diminished endothelial cell activation, in a similar fashion (Li, van Esch, Henricks, Folkerts, & Garssen, 2018). More distant from the gut, elevated acetate levels were shown to reduce allergic airway disease in mice by enhancing Tregs in the lung through https://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=2620 inhibition (Thorburn et al., 2015). Taken together, butyrate and other SCFAs can modulate immune cell function and disease, holding potential clinical implications.
4.2.2. SCFAs as GPCR ligands
SCFAs are ligands for cell membrane receptors such as GPCR43 (also called FFA2), https://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=227 (also called FFA3) and GPCR109a (also called https://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=312). GPCR43 can potently be stimulated by acetate, propionate and butyrate, while GPCR41 has a higher affinity for butyrate and propionate (Brown et al., 2003). In vivo studies in conventional and germ‐free mice showed that butyrate suppresses colonic inflammation and carcinogenesis via GPCR109a (Singh et al., 2014). Singh et al. (2014) showed that butyrate‐stimulated signalling of GPCR109a increases the generation of mouse Tregs, elevates https://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=4983 secretion by IECs and increases IL‐10 production by T cells. An interesting study by Maslowski et al. (2009) showed that administration of acetate to the drinking water of mice could diminish inflammatory responses in models of colitis, arthritis and allergic airway disease in a GPCR43‐dependent manner. Similarly, administration of propionate reduced allergic airway disease in mice via haematopoiesis of dendritic cells and reduced Th2 effector function in a GPCR41‐dependent manner (Trompette et al., 2014). Due to the ubiquitous expression of the receptors on different cell types and the reported anticancer plus anti‐inflammatory effects of SCFA‐mediated GPCR stimulation, the pharmacological development of GPCR‐selective compounds might be of therapeutic interest (Bolognini et al., 2016).
4.2.3. SCFAs as regulators of transcriptional activity
Nuclear PPARs can be stimulated by SCFAs with a potency order of butyrate > propionate > acetate (Alex et al., 2013). Byndloss et al. (2017) showed that https://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=595 inhibits dysbiotic Enterobacteriaceae expansion when stimulated by butyrate. Butyrate can also regulate transcription factor activity directly: https://www.guidetopharmacology.org/GRAC/FamilyDisplayForward?familyId=996 activity is strongly inhibited by butyrate demonstrating therapeutic potential in experimental models of colitis and Crohn's disease (Segain et al., 2000). Furthermore, butyrate‐regulated genes often contain several binding sites for specificity protein 1 and AP‐1, suggesting that butyrate (and to a lesser extent propionate) can exert its effects via activation of these transcription factors and associated pathways (Nepelska et al., 2012; Yu, Waby, Chirakkal, Staton, & Corfe, 2010). Finally, the aryl hydrocarbon receptor (https://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=2951) emerged as a critical regulator of immune and metabolic processes in the GI tract. Recently, butyrate was found to induce AhR activity and transcription of AhR‐dependent genes in human IECs (Marinelli et al., 2019).
Taken together, dietary fibre‐derived SCFAs are important promotors of colonic health and positive regulators of immune responses.
5. CONCLUDING REMARKS AND FUTURE PERSPECTIVES
This review describes the impact of different dietary fibre on the microbiome composition but also emphasizes the microbiota‐independent effects (Figure 2). It has been well demonstrated that dietary fibre promotes commensal bacteria in the GI tract, such as Bifidobacteria and Lactobacilli. Bifidobacteria and Lactobacilli have been shown to directly compete with pathogenic bacteria but can also stimulate the intestinal barrier, immune and brain function. These dietary fibres do not only stimulate commensal bacteria but also directly influence pathogens via inhibition of pathogen growth, competitive inhibition of adhesion, abolishment of cytotoxicity and toxin production induced by pathogens, which have been associated with a range of beneficial health effects. A substantial part of these potential health benefits is related to dietary fibre‐derived microbial metabolite production, including SCFAs. SCFAs can promote intestinal immune homeostasis through inhibition of HDAC and via several other receptor‐mediated pathways, such as GPCR41, GPCR43, GPCR109a, and PPARs.
Figure 2.
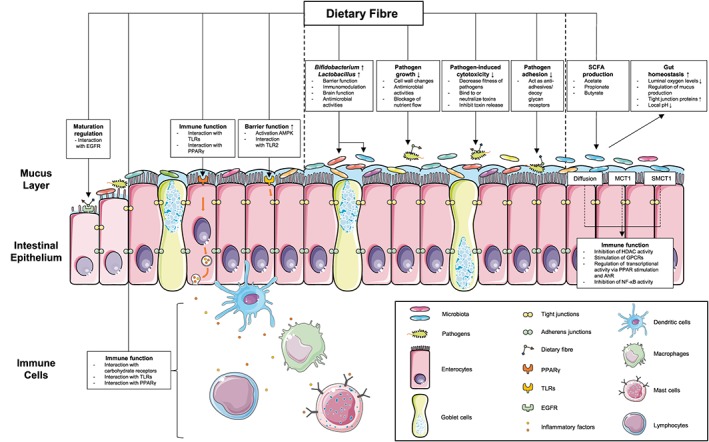
Mechanisms of microbiota‐dependent and ‐independent effects of dietary fibre. Dietary fibre promotes gut homeostasis by directly affecting the intestinal commensal microbiota composition (e.g., increased growth of Bifidobacterium and Lactobacillus), leading to increased barrier function, immunomodulatory effects, stimulating brain function and antimicrobial activities. Dietary fibre also protects gut homeostasis by inhibiting the cytotoxicity, adhesion or growth of pathogenic microorganisms. Dietary fibre is fermented into SCFAs by the microbiota and these SCFAs further maintain intestinal (immune) homeostasis by inhibiting HDAC, stimulating GPCRs or regulating the transcriptional activity. Importantly, dietary fibre exhibits a microbiota‐independent effect on the intestinal epithelium and different immune cells, including dendritic cells, macrophages, mast cells and lymphocytes. These microbiota‐independent effects include regulating epithelial cell maturation, enhancing epithelial barrier function and stimulating the intestinal immune system via specific PPRs on epithelial cells (EGFR, PPARγ and TLRs) and immune cells (TLRs and carbohydrate receptors) or via interaction with regulatory molecules (e.g. AMPK). AMPK, AMP‐activated protein kinase; AhR, aryl hydrocarbon receptor; EGFR, EGF receptor; HDAC, histone deacetylase; MCT1, monocarboxylate transporter 1; SCFAs, short‐chain fatty acids; SMCT1, sodium‐coupled monocarboxylate transporter 1; TLRs, toll‐like receptors
In addition, several microbiota‐independent effects of dietary fibre are important for maintaining intestinal epithelial barrier function and promoting gut immune responses (Figure 2). Various dietary fibre can bind to receptors on epithelial cells (EGFR, PPARγ, and TLRs) and different immune cells (TLRs and carbohydrate‐binding domains) or interact with regulatory molecules (e.g., AMPK) to regulate intestinal barrier function and immune homeostasis. These in vitro studies suggest that multiple dietary fibres exhibit diverse effects on IEC‐derived cytokine and chemokine production and release. This may be associated with the structure–function relationship among dietary fibre structure and their immunomodulatory properties. However, the different types of IECs and triggers used in the in vitro studies can contribute to heterogenicity between the studies. In addition, more in vivo studies with germ‐free mice are needed to confirm the biological relevance of the microbiota‐independent effects. The scope and mechanisms of its effects on gut homeostasis emphasize that dietary fibre does more than modifying the gut microbiota and highlight its multifaceted potential in the treatment or prevention of (chronic) diseases.
The use of dietary fibre to maintain health and prevent diseases is rapidly expanding, and dietary fibre‐based nutraceuticals and functional foods are explored for their effects on several diseases (Gul, Singh, & Jabeen, 2016). Both pharmacologists and nutritionists are increasingly aware of the importance of maintaining and restoring (gut) homeostasis and agree that multifactorial diseases require multitargeting approaches combining pharmacology and nutrition (Georgiou, Garssen, & Witkamp, 2011). The new developments in nutraceutical and functional food research using omics techniques have gained interest in the concept of personalized nutrition (Verma, Hontecillas, Tubau‐Juni, Abedi, & Bassaganya‐Riera, 2018), which seems to be promising for dietary fibre as well. Personalized nutrition is important related to dietary fibre intake, since there is a high interindividual variability of the human microbiome, which will lead to significant variability in response to dietary fibre intake. (Tap et al., 2015). Since most of the studies performed so far focus on the effects of individual dietary fibre, it will be a major challenge to study the interaction of different types of dietary fibre and the interaction of dietary fibre with other nutritional components.
In this respect, well‐conducted in vitro, in vivo and https://www.sciencedirect.com/topics/pharmacology-toxicology-and-pharmaceutical-science/clinical-study are needed to establish the efficacy and mechanisms of action of dietary fibre in treating patients and in utilizing dietary fibre‐based approaches for the prevention of disease.
5.1. Nomenclature of targets and ligands
Key protein targets and ligands in this article are hyperlinked to corresponding entries in http://www.guidetopharmacology.org, the common portal for data from the IUPHAR/BPS Guide to PHARMACOLOGY (Harding et al., 2018), and are permanently archived in the Concise Guide to PHARMACOLOGY 2017/18 (Alexander et al., 2017).
CONFLICT OF INTEREST
The authors declare no conflicts of interest.
ACKNOWLEDGEMENTS
This work was partly funded by the public–private partnership ‘CarboKinetics’ coordinated by the Carbohydrate Competence Center (CCC, http://www.cccresearch.nl). CarboKinetics is financed by participating industrial partners Agrifirm Innovation Center B.V., Cooperatie AVEBE U.A., DSM Food Specialties B.V., FrieslandCampina Nederland B.V., Nutrition Sciences N.V., VanDrie Holding N.V., and Sensus B.V. and allowances of The Netherlands Organisation for Scientific Research (NWO). Research grant funding was received from the China Scholarship Council for Y.C.
Cai Y, Folkerts J, Folkerts G, Maurer M, Braber S. Microbiota‐dependent and ‐independent effects of dietary fibre on human health. Br J Pharmacol. 2020;177:1363–1381. 10.1111/bph.14871
REFERENCES
- Akbari, P. , Braber, S. , Alizadeh, A. , Verheijden, K. A. , Schoterman, M. H. , Kraneveld, A. D. , … Fink‐Gremmels, J. (2015). Galacto‐oligosaccharides protect the intestinal barrier by maintaining the tight junction network and modulating the inflammatory responses after a challenge with the mycotoxin deoxynivalenol in human Caco‐2 cell monolayers and B6C3F1 mice. The Journal of Nutrition, 145, 1604–1613. 10.3945/jn.114.209486 [DOI] [PubMed] [Google Scholar]
- Alex, S. , Lange, K. , Amolo, T. , Grinstead, J. S. , Haakonsson, A. K. , Szalowska, E. , … Kersten, S. (2013). Short‐chain fatty acids stimulate angiopoietin‐like 4 synthesis in human colon adenocarcinoma cells by activating peroxisome proliferator‐activated receptor γ. Molecular and Cellular Biology, 33, 1303–1316. 10.1128/MCB.00858-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander, S. P. H. , Christopoulos, A. , Davenport, A. P. , Kelly, E. , Marrion, N. V. , Peters, J. A. , … CGTP Collaborators (2017). The Concise Guide to PHARMACOLOGY 2017/18: G protein‐coupled receptors. British Journal of Pharmacology, 174, S17–S129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ananthakrishnan, A. N. , Khalili, H. , Konijeti, G. G. , Higuchi, L. M. , de Silva, P. , Korzenik, J. R. , … Chan, A. T. (2013). A prospective study of long‐term intake of dietary fiber and risk of Crohn's disease and ulcerative colitis. Gastroenterology, 145, 970–977. 10.1053/j.gastro.2013.07.050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arslanoglu, S. , Moro, G. E. , Schmitt, J. , Tandoi, L. , Rizzardi, S. , & Boehm, G. (2008). Early dietary intervention with a mixture of prebiotic oligosaccharides reduces the incidence of allergic manifestations and infections during the first two years of life. The Journal of Nutrition, 138, 1091–1095. [DOI] [PubMed] [Google Scholar]
- Bain, C. C. , & Schridde, A. (2018). Origin, differentiation, and function of intestinal macrophages. Frontiers in Immunology, 9, 2733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bermudez‐Brito, M. , Rosch, C. , Schols, H. A. , Faas, M. M. , & de Vos, P. (2015). Resistant starches differentially stimulate Toll‐like receptors and attenuate proinflammatory cytokines in dendritic cells by modulation of intestinal epithelial cells. Molecular Nutrition & Food Research, 59, 1814–1826. [DOI] [PubMed] [Google Scholar]
- Bermudez‐Brito, M. , Sahasrabudhe, N. M. , Rosch, C. , Schols, H. A. , Faas, M. M. , & de Vos, P. (2015). The impact of dietary fibers on dendritic cell responses in vitro is dependent on the differential effects of the fibers on intestinal epithelial cells. Molecular Nutrition & Food Research, 59, 698–710. [DOI] [PubMed] [Google Scholar]
- den Besten, G. , van Eunen, K. , Groen, A. K. , Venema, K. , Reijngoud, D. J. , & Bakker, B. M. (2013). The role of short‐chain fatty acids in the interplay between diet, gut microbiota, and host energy metabolism. Journal of Lipid Research, 54, 2325–2340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhatia, S. , Prabhu, P. N. , Benefiel, A. C. , Miller, M. J. , Chow, J. , Davis, S. R. , & Gaskins, H. R. (2015). Galacto‐oligosaccharides may directly enhance intestinal barrier function through the modulation of goblet cells. Molecular Nutrition & Food Research, 59, 566–573. 10.1002/mnfr.201400639 [DOI] [PubMed] [Google Scholar]
- Bik, E. M. , Ugalde, J. A. , Cousins, J. , Goddard, A. D. , Richman, J. , & Apte, Z. S. (2018). Microbial biotransformations in the human distal gut. British Journal of Pharmacology, 175, 4404–4414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bischoff, S. C. (2009). Physiological and pathophysiological functions of intestinal mast cells. Seminars in Immunopathology, 31, 185–205. 10.1007/s00281-009-0165-4 [DOI] [PubMed] [Google Scholar]
- Blachier, F. , Mariotti, F. , Huneau, J. F. , & Tomé, D. (2007). Effects of amino acid‐derived luminal metabolites on the colonic epithelium and physiopathological consequences. Amino Acids, 33, 547–562. 10.1007/s00726-006-0477-9 [DOI] [PubMed] [Google Scholar]
- Bolognini, D. , Tobin, A. B. , Milligan, G. , & Moss, C. E. (2016). The pharmacology and function of receptors for short‐chain fatty acids. Molecular Pharmacology, 89, 388–398. 10.1124/mol.115.102301 [DOI] [PubMed] [Google Scholar]
- Brink, M. , Todorov, S. D. , Martin, J. H. , Senekal, M. , & Dicks, L. M. (2006). The effect of prebiotics on production of antimicrobial compounds, resistance to growth at low pH and in the presence of bile, and adhesion of probiotic cells to intestinal mucus. Journal of Applied Microbiology, 100, 813–820. [DOI] [PubMed] [Google Scholar]
- Brown, A. J. , Goldsworthy, S. M. , Barnes, A. A. , Eilert, M. M. , Tcheang, L. , Daniels, D. , … Dowell, S. J. (2003). The Orphan G protein‐coupled receptors GPR41 and GPR43 are activated by propionate and other short chain carboxylic acids. The Journal of Biological Chemistry, 278, 11312–11319. 10.1074/jbc.M211609200 [DOI] [PubMed] [Google Scholar]
- Brufau, M. T. , Campo‐Sabariz, J. , Bou, R. , Carne, S. , Brufau, J. , Vila, B. , … Martín‐Venegas, R. (2016). Salmosan, a β‐galactomannan‐rich product, protects epithelial barrier function in Caco‐2 cells infected by Salmonella enterica Serovar Enteritidis. The Journal of Nutrition, 146, 1492–1498. [DOI] [PubMed] [Google Scholar]
- Brusaferro, A. , Cozzali, R. , Orabona, C. , Biscarini, A. , Farinelli, E. , Cavalli, E. , … Esposito, S. (2018). Is it time to use probiotics to prevent or treat obesity? Nutrients, 10, E1613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burger‐van Paassen, N. , Vincent, A. , Puiman, P. J. , van der Sluis, M. , Bouma, J. , Boehm, G. , … Renes, I. B. (2009). The regulation of intestinal mucin MUC2 expression by short‐chain fatty acids: Implications for epithelial protection. Biochemical Journal, 420, 211–219. 10.1042/BJ20082222 [DOI] [PubMed] [Google Scholar]
- Byndloss, M. X. , Olsan, E. E. , Rivera‐Chávez, F. , Tiffany, C. R. , Cevallos, S. A. , Lokken, K. L. , … Bäumler, A. J. (2017). Microbiota‐activated PPAR‐γ signaling inhibits dysbiotic Enterobacteriaceae expansion. Science (New York, N.Y.), 357, 570–575. 10.1126/science.aam9949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cani, P. D. , Possemiers, S. , Van de Wiele, T. , Guiot, Y. , Everard, A. , Rottier, O. , … Delzenne, N. M. (2009). Changes in gut microbiota control inflammation in obese mice through a mechanism involving GLP‐2‐driven improvement of gut permeability. Gut, 58, 1091–1103. 10.1136/gut.2008.165886 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capitan‐Canadas, F. , Ortega‐Gonzalez, M. , Guadix, E. , Zarzuelo, A. , Suarez, M. D. , de Medina, F. S. , & Martínez‐Augustin, O. (2014). Prebiotic oligosaccharides directly modulate proinflammatory cytokine production in monocytes via activation of TLR4. Molecular Nutrition & Food Research, 58, 1098–1110. [DOI] [PubMed] [Google Scholar]
- Castillo‐Courtade, L. , Han, S. , Lee, S. , Mian, F. M. , Buck, R. , & Forsythe, P. (2015). Attenuation of food allergy symptoms following treatment with human milk oligosaccharides in a mouse model. Allergy, 70, 1091–1102. 10.1111/all.12650 [DOI] [PubMed] [Google Scholar]
- Chang, P. V. , Hao, L. , Offermanns, S. , & Medzhitov, R. (2014). The microbial metabolite butyrate regulates intestinal macrophage function via histone deacetylase inhibition. Proceedings of the National Academy of Sciences of the United States of America, 111, 2247–2252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charbonneau, M. R. , O'Donnell, D. , Blanton, L. V. , Totten, S. M. , Davis, J. C. , Barratt, M. J. , … Gordon, J. I. (2016). Sialylated milk oligosaccharides promote microbiota‐dependent growth in models of infant undernutrition. Cell, 164, 859–871. 10.1016/j.cell.2016.01.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherbut, C. , Michel, C. , & Lecannu, G. (2003). The prebiotic characteristics of fructooligosaccharides are necessary for reduction of TNBS‐induced colitis in rats. The Journal of Nutrition, 133, 21–27. 10.1093/jn/133.1.21 [DOI] [PubMed] [Google Scholar]
- Clark, A. , & Mach, N. (2016). Exercise‐induced stress behavior, gut‐microbiota‐brain axis and diet: A systematic review for athletes. Journal of the International Society of Sports Nutrition, 13, 43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coombes, J. L. , & Powrie, F. (2008). Dendritic cells in intestinal immune regulation. Nature Reviews. Immunology, 8, 435–446. 10.1038/nri2335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cummings, J. H. , Pomare, E. W. , Branch, W. J. , Naylor, C. P. E. , & Macfarlane, G. T. (1987). Short chain fatty‐acids in human large‐intestine, portal, hepatic and venous‐blood. Gut, 28, 1221–1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daynes, R. A. , & Jones, D. C. (2002). Emerging roles of PPARs in inflammation and immunity. Nature Reviews. Immunology, 2, 748–759. [DOI] [PubMed] [Google Scholar]
- Di, R. , Vakkalanka, M. S. , Onumpai, C. , Chau, H. K. , White, A. , Rastall, R. A. , … Hotchkiss, A. T. Jr. (2017). Pectic oligosaccharide structure‐function relationships: Prebiotics, inhibitors of Escherichia coli O157:H7 adhesion and reduction of Shiga toxin cytotoxicity in HT29 cells. Food Chemistry, 227, 245–254. 10.1016/j.foodchem.2017.01.100 [DOI] [PubMed] [Google Scholar]
- Donohoe, D. R. , Collins, L. B. , Wali, A. , Bigler, R. , Sun, W. , & Bultman, S. J. (2012). The Warburg effect dictates the mechanism of butyrate‐mediated histone acetylation and cell proliferation. Molecular Cell, 48, 612–626. 10.1016/j.molcel.2012.08.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drickamer, K. , & Taylor, M. E. (2015). Recent insights into structures and functions of C‐type lectins in the immune system. Current Opinion in Structural Biology, 34, 26–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eiwegger, T. , Stahl, B. , Haidl, P. , Schmitt, J. , Boehm, G. , Dehlink, E. , … Szépfalusi, Z. (2010). Prebiotic oligosaccharides: In vitro evidence for gastrointestinal epithelial transfer and immunomodulatory properties. Pediatric Allergy and Immunology, 21, 1179–1188. 10.1111/j.1399-3038.2010.01062.x [DOI] [PubMed] [Google Scholar]
- Eiwegger, T. , Stahl, B. , Schmitt, J. , Boehm, G. , Gerstmayr, M. , Pichler, J. , … Szépfalusi, Z. (2004). Human milk‐derived oligosaccharides and plant‐derived oligosaccharides stimulate cytokine production of cord blood T‐cells in vitro. Pediatric Research, 56, 536–540. 10.1203/01.PDR.0000139411.35619.B4 [DOI] [PubMed] [Google Scholar]
- El‐Hawiet, A. , Kitova, E. N. , & Klassen, J. S. (2015). Recognition of human milk oligosaccharides by bacterial exotoxins. Glycobiology, 25, 845–854. [DOI] [PubMed] [Google Scholar]
- Estrada, A. , Yun, C. H. , Van Kessel, A. , Li, B. , Hauta, S. , & Laarveld, B. (1997). Immunomodulatory activities of oat β‐glucan in vitro and in vivo. Microbiology and Immunology, 41, 991–998. 10.1111/j.1348-0421.1997.tb01959.x [DOI] [PubMed] [Google Scholar]
- Feng, W. , Wu, Y. , Chen, G. , Fu, S. , Li, B. , Huang, B. , … Liu, J. (2018). Sodium butyrate attenuates diarrhea in weaned piglets and promotes tight junction protein expression in colon in a GPR109A‐dependent manner. Cellular Physiology and Biochemistry: International Journal of Experimental Cellular Physiology, Biochemistry, and Pharmacology, 47, 1617–1629. [DOI] [PubMed] [Google Scholar]
- Francois, I. E. , Lescroart, O. , Veraverbeke, W. S. , Marzorati, M. , Possemiers, S. , Evenepoel, P. , … Broekaert, W. F. (2012). Effects of a wheat bran extract containing arabinoxylan oligosaccharides on gastrointestinal health parameters in healthy adult human volunteers: A double‐blind, randomised, placebo‐controlled, cross‐over trial. The British Journal of Nutrition, 108, 2229–2242. 10.1017/S0007114512000372 [DOI] [PubMed] [Google Scholar]
- Frost, G. , Sleeth, M. L. , Sahuri‐Arisoylu, M. , Lizarbe, B. , Cerdan, S. , Brody, L. , … Bell, J. D. (2014). The short‐chain fatty acid acetate reduces appetite via a central homeostatic mechanism. Nature Communications, 5, 3611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furusawa, Y. , Obata, Y. , Fukuda, S. , Endo, T. A. , Nakato, G. , Takahashi, D. , … Ohno, H. (2013). Commensal microbe‐derived butyrate induces the differentiation of colonic regulatory T cells. Nature, 504, 446–450. 10.1038/nature12721 [DOI] [PubMed] [Google Scholar]
- Ganan, M. , Collins, M. , Rastall, R. , Hotchkiss, A. T. , Chau, H. K. , Carrascosa, A. V. , & Martinez‐Rodriguez, A. J. (2010). Inhibition by pectic oligosaccharides of the invasion of undifferentiated and differentiated Caco‐2 cells by Campylobacter jejuni . International Journal of Food Microbiology, 137, 181–185. 10.1016/j.ijfoodmicro.2009.12.007 [DOI] [PubMed] [Google Scholar]
- Georgiou, N. A. , Garssen, J. , & Witkamp, R. F. (2011). Pharma‐nutrition interface: The gap is narrowing. European Journal of Pharmacology, 651, 1–8. 10.1016/j.ejphar.2010.11.007 [DOI] [PubMed] [Google Scholar]
- Gill, H. S. , Rutherfurd, K. J. , & Cross, M. L. (2001). Dietary probiotic supplementation enhances natural killer cell activity in the elderly: An investigation of age‐related immunological changes. Journal of Clinical Immunology, 21, 264–271. 10.1023/a:1010979225018 [DOI] [PubMed] [Google Scholar]
- Gill, H. S. , Rutherfurd, K. J. , Prasad, J. , & Gopal, P. K. (2000). Enhancement of natural and acquired immunity by Lactobacillus rhamnosus (HN001), Lactobacillus acidophilus (HN017) and Bifidobacterium lactis (HN019). The British Journal of Nutrition, 83, 167–176. [DOI] [PubMed] [Google Scholar]
- Goehring, K. C. , Marriage, B. J. , Oliver, J. S. , Wilder, J. A. , Barrett, E. G. , & Buck, R. H. (2016). Similar to those who are breastfed, infants fed a formula containing 2′‐fucosyllactose have lower inflammatory cytokines in a randomized controlled trial. The Journal of Nutrition, 146, 2559–2566. 10.3945/jn.116.236919 [DOI] [PubMed] [Google Scholar]
- Gonia, S. , Tuepker, M. , Heisel, T. , Autran, C. , Bode, L. , & Gale, C. A. (2015). Human milk oligosaccharides inhibit Candida albicans invasion of human premature intestinal epithelial cells. The Journal of Nutrition, 145, 1992–1998. 10.3945/jn.115.214940 [DOI] [PubMed] [Google Scholar]
- Goodridge, H. S. , Wolf, A. J. , & Underhill, D. M. (2009). β‐glucan recognition by the innate immune system. Immunological Reviews, 230, 38–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gopalakrishnan, A. , Clinthorne, J. F. , Rondini, E. A. , McCaskey, S. J. , Gurzell, E. A. , Langohr, I. M. , … Fenton, J. I. (2012). Supplementation with galacto‐oligosaccharides increases the percentage of NK cells and reduces colitis severity in Smad3‐deficient mice. The Journal of Nutrition, 142, 1336–1342. 10.3945/jn.111.154732 [DOI] [PubMed] [Google Scholar]
- Gul, K. , Singh, A. K. , & Jabeen, R. (2016). Nutraceuticals and functional foods: The foods for the future world. Critical Reviews in Food Science and Nutrition, 56, 2617–2627. [DOI] [PubMed] [Google Scholar]
- Hao, M. , Yuan, X. , Cheng, H. , Xue, H. , Zhang, T. , Zhou, Y. , & Tai, G. (2013). Comparative studies on the anti‐tumor activities of high temperature‐ and pH‐modified citrus pectins. Food & Function, 4, 960–971. 10.1039/c3fo30350k [DOI] [PubMed] [Google Scholar]
- Harding, S. D. , Sharman, J. L. , Faccenda, E. , Southan, C. , Pawson, A. J. , Ireland, S. , … NC‐IUPHAR (2018). The IUPHAR/BPS Guide to PHARMACOLOGY in 2018: Updates and expansion to encompass the new guide to IMMUNOPHARMACOLOGY. Nucleic Acids Research, 46, D1091–D1106. 10.1093/nar/gkx1121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart, A. L. , Lammers, K. , Brigidi, P. , Vitali, B. , Rizzello, F. , Gionchetti, P. , … Stagg, A. J. (2004). Modulation of human dendritic cell phenotype and function by probiotic bacteria. Gut, 53, 1602–1609. 10.1136/gut.2003.037325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- He, Y. , Lawlor, N. T. , & Newburg, D. S. (2016). Human milk components modulate toll‐like receptor‐mediated inflammation. Advances in Nutrition, 7, 102–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He, Y. , Liu, S. , Kling, D. E. , Leone, S. , Lawlor, N. T. , Huang, Y. , … Newburg, D. S. (2016). The human milk oligosaccharide 2′‐fucosyllactose modulates CD14 expression in human enterocytes, thereby attenuating LPS‐induced inflammation. Gut, 65, 33–46. 10.1136/gutjnl-2014-307544 [DOI] [PubMed] [Google Scholar]
- He, Y. , Liu, S. , Leone, S. , & Newburg, D. S. (2014). Human colostrum oligosaccharides modulate major immunologic pathways of immature human intestine. Mucosal Immunology, 7, 1326–1339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermes, R. G. , Manzanilla, E. G. , Martin‐Orue, S. M. , Perez, J. F. , & Klasing, K. C. (2011). Influence of dietary ingredients on in vitro inflammatory response of intestinal porcine epithelial cells challenged by an enterotoxigenic Escherichia coli (K88). Comparative Immunology, Microbiology and Infectious Diseases, 34, 479–488. [DOI] [PubMed] [Google Scholar]
- Hinnebusch, B. F. , Meng, S. F. , Wu, J. T. , Archer, S. Y. , & Hodin, R. A. (2002). The effects of short‐chain fatty acids on human colon cancer cell phenotype are associated with histone hyperacetylation. Journal of Nutrition, 132, 1012–1017. [DOI] [PubMed] [Google Scholar]
- Holscher, H. D. , Bode, L. , & Tappenden, K. A. (2017). Human milk oligosaccharides influence intestinal epithelial cell maturation in vitro. Journal of Pediatric Gastroenterology and Nutrition, 64, 296–301. [DOI] [PubMed] [Google Scholar]
- Holscher, H. D. , Davis, S. R. , & Tappenden, K. A. (2014). Human milk oligosaccharides influence maturation of human intestinal Caco‐2Bbe and HT‐29 cell lines. The Journal of Nutrition, 144, 586–591. [DOI] [PubMed] [Google Scholar]
- Hormann, N. , Brandao, I. , Jackel, S. , Ens, N. , Lillich, M. , Walter, U. , & Reinhardt, C. (2014). Gut microbial colonization orchestrates TLR2 expression, signaling and epithelial proliferation in the small intestinal mucosa. PLoS ONE, 9, e113080 10.1371/journal.pone.0113080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jang, S. O. , Kim, H. J. , Kim, Y. J. , Kang, M. J. , Kwon, J. W. , Seo, J. H. , … Hong, S. J. (2012). Asthma prevention by Lactobacillus rhamnosus in a mouse model is associated with CD4+CD25+Foxp3+ T cells. Allergy, Asthma & Immunology Research, 4, 150–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jantscher‐Krenn, E. , Lauwaet, T. , Bliss, L. A. , Reed, S. L. , Gillin, F. D. , & Bode, L. (2012). Human milk oligosaccharides reduce Entamoeba histolytica attachment and cytotoxicity in vitro. The British Journal of Nutrition, 108, 1839–1846. 10.1017/S0007114511007392 [DOI] [PubMed] [Google Scholar]
- Jiang, X. , Fang, L. , Wu, H. , Mei, X. , He, F. , Ding, P. , & Liu, R. (2017). TLR2 regulates allergic airway inflammation and autophagy through PI3K/Akt signaling pathway. Inflammation, 40, 1382–1392. 10.1007/s10753-017-0581-x [DOI] [PubMed] [Google Scholar]
- Jones, J. M. (2014). CODEX‐aligned dietary fiber definitions help to bridge the ‘fiber gap’. Nutrition Journal, 13, 34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kittana, H. , Quintero‐Villegas, M. I. , Bindels, L. B. , Gomes‐Neto, J. C. , Schmaltz, R. J. , Segura Munoz, R. R. , … Ramer‐Tait, A. E. (2018). Galactooligosaccharide supplementation provides protection against Citrobacter rodentium‐induced colitis without limiting pathogen burden. Microbiology‐Sgm, 164, 154–162. 10.1099/mic.0.000593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Kivit, S. , Kraneveld, A. D. , Knippels, L. M. , van Kooyk, Y. , Garssen, J. , & Willemsen, L. E. (2013). Intestinal epithelium‐derived galectin‐9 is involved in the immunomodulating effects of nondigestible oligosaccharides. Journal of Innate Immunity, 5, 625–638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleessen, B. , Hartmann, L. , & Blaut, M. (2003). Fructans in the diet cause alterations of intestinal mucosal architecture, released mucins and mucosa‐associated bifidobacteria in gnotobiotic rats. British Journal of Nutrition, 89, 597–606. [DOI] [PubMed] [Google Scholar]
- Koh, A. , De Vadder, F. , Kovatcheva‐Datchary, P. , & Backhed, F. (2016). From dietary fiber to host physiology: Short‐chain fatty acids as key bacterial metabolites. Cell, 165, 1332–1345. 10.1016/j.cell.2016.05.041 [DOI] [PubMed] [Google Scholar]
- Koleva, P. T. , Valcheva, R. S. , Sun, X. , Ganzle, M. G. , & Dieleman, L. A. (2012). Inulin and fructo‐oligosaccharides have divergent effects on colitis and commensal microbiota in HLA‐B27 transgenic rats. The British Journal of Nutrition, 108, 1633–1643. 10.1017/S0007114511007203 [DOI] [PubMed] [Google Scholar]
- Kong, C. , Elderman, M. , Cheng, L. , de Haan, B. J. , Nauta, A. , & de Vos, P. (2019). Modulation of intestinal epithelial glycocalyx development by human milk oligosaccharides and non‐digestible carbohydrates. Molecular Nutrition & Food Research, 63, e1900303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuntz, S. , Kunz, C. , & Rudloff, S. (2009). Oligosaccharides from human milk induce growth arrest via G2/M by influencing growth‐related cell cycle genes in intestinal epithelial cells. The British Journal of Nutrition, 101, 1306–1315. 10.1017/S0007114508079622 [DOI] [PubMed] [Google Scholar]
- Lehmann, S. , Hiller, J. , van Bergenhenegouwen, J. , Knippels, L. M. , Garssen, J. , & Traidl‐Hoffmann, C. (2015). In vitro evidence for immune‐modulatory properties of non‐digestible oligosaccharides: Direct effect on human monocyte derived dendritic cells. PLoS ONE, 10, e0132304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, M. , van Esch, B. C. A. M. , Henricks, P. A. J. , Folkerts, G. , & Garssen, J. (2018). The anti‐inflammatory effects of short chain fatty acids on lipopolysaccharide‐ or tumor necrosis factor α‐stimulated endothelial cells via activation of GPR41/43 and inhibition of HDACs. Frontiers in Pharmacology, 9, 533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, P. J. , Xia, J. L. , Nie, Z. Y. , & Shan, Y. (2016). Pectic oligosaccharides hydrolyzed from orange peel by fungal multi enzyme complexes and their prebiotic and antibacterial potentials. LWT ‐ Food Science and Technology, 69, 203–210. [Google Scholar]
- Lievin, V. , Peiffer, I. , Hudault, S. , Rochat, F. , Brassart, D. , Neeser, J. R. , & Servin, A. L. (2000). Bifidobacterium strains from resident infant human gastrointestinal microflora exert antimicrobial activity. Gut, 47, 646–652. 10.1136/gut.47.5.646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin, A. E. , Autran, C. A. , Szyszka, A. , Escajadillo, T. , Huang, M. , Godula, K. , … Bode, L. (2017). Human milk oligosaccharides inhibit growth of group B Streptococcus . Journal of Biological Chemistry, 292, 11243–11249. 10.1074/jbc.M117.789974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindsay, J. O. , Whelan, K. , Stagg, A. J. , Gobin, P. , Al‐Hassi, H. O. , Rayment, N. , … Forbes, A. (2006). Clinical, microbiological, and immunological effects of fructo‐oligosaccharide in patients with Crohn's disease. Gut, 55, 348–355. 10.1136/gut.2005.074971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llewellyn, S. R. , Britton, G. J. , Contijoch, E. J. , Vennaro, O. H. , Mortha, A. , Colombel, J. F. , … Faith, J. J. (2018). Interactions between diet and the intestinal microbiota alter intestinal permeability and colitis severity in mice. Gastroenterology, 154, 1037–1046 e1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Louis, P. , & Flint, H. J. (2017). Formation of propionate and butyrate by the human colonic microbiota. Environmental Microbiology, 19, 29–41. [DOI] [PubMed] [Google Scholar]
- Luna, R. A. , & Foster, J. A. (2015). Gut brain axis: Diet microbiota interactions and implications for modulation of anxiety and depression. Current Opinion in Biotechnology, 32, 35–41. 10.1016/j.copbio.2014.10.007 [DOI] [PubMed] [Google Scholar]
- Macfarlane, S. , & Macfarlane, G. T. (2003). Regulation of short‐chain fatty acid production. The Proceedings of the Nutrition Society, 62, 67–72. [DOI] [PubMed] [Google Scholar]
- Madsen, K. , Cornish, A. , Soper, P. , McKaigney, C. , Jijon, H. , Yachimec, C. , … de Simone, C. (2001). Probiotic bacteria enhance murine and human intestinal epithelial barrier function. Gastroenterology, 121, 580–591. 10.1053/gast.2001.27224 [DOI] [PubMed] [Google Scholar]
- Makki, K. , Deehan, E. C. , Walter, J. , & Backhed, F. (2018). The impact of dietary fiber on gut microbiota in host health and disease. Cell Host & Microbe, 23, 705–715. 10.1016/j.chom.2018.05.012 [DOI] [PubMed] [Google Scholar]
- Mandadzhieva, T. , Ignatova‐Ivanova, T. , Kambarev, S. , Iliev, I. , & Ivanova, I. (2011). Utilization of different prebiotics by Lactobacillus spp. and Lactococcus spp. Biotechnology & Biotechnological Equipment, 25, 117–120. [Google Scholar]
- Mao, W. A. , Sun, Y. Y. , Mao, J. Y. , Wang, L. , Zhang, J. , Zhou, J. , … Ye, Y. (2016). Inhibitory effects of Angelica polysaccharide on activation of mast cells. Evidence‐Based Complementary and Alternative Medicine, 2016, 6063475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marinelli, L. , Martin‐Gallausiaux, C. , Bourhis, J. M. , Béguet‐Crespel, F. , Blottière, H. M. , & Lapaque, N. (2019). Identification of the novel role of butyrate as AhR ligand in human intestinal epithelial cells. Scientific Reports, 9, 643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maslowski, K. M. , & Mackay, C. R. (2011). Diet, gut microbiota and immune responses. Nature Immunology, 12, 5–9. [DOI] [PubMed] [Google Scholar]
- Maslowski, K. M. , Vieira, A. T. , Ng, A. , Kranich, J. , Sierro, F. , Yu, D. , … Mackay, C. R. (2009). Regulation of inflammatory responses by gut microbiota and chemoattractant receptor GPR43. Nature, 461, 1282–1286. 10.1038/nature08530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer, E. A. , Tillisch, K. , & Gupta, A. (2015). Gut/brain axis and the microbiota. The Journal of Clinical Investigation, 125, 926–938. 10.1172/JCI76304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendis, M. , Leclerc, E. , & Simsek, S. (2016). Arabinoxylan hydrolyzates as immunomodulators in lipopolysaccharide‐induced RAW264.7 macrophages. Food & Function, 7, 3039–3045. 10.1039/c6fo00500d [DOI] [PubMed] [Google Scholar]
- Mendis, M. , Leclerc, E. , & Simsek, S. (2017). Arabinoxylan hydrolyzates as immunomodulators in Caco‐2 and HT‐29 colon cancer cell lines. Food & Function, 8, 220–231. 10.1039/c6fo00866f [DOI] [PubMed] [Google Scholar]
- Messaoudi, M. , Lalonde, R. , Violle, N. , Javelot, H. , Desor, D. , Nejdi, A. , … Cazaubiel, J. M. (2011). Assessment of psychotropic‐like properties of a probiotic formulation (Lactobacillus helveticus R0052 and Bifidobacterium longum R0175) in rats and human subjects. The British Journal of Nutrition, 105, 755–764. 10.1017/S0007114510004319 [DOI] [PubMed] [Google Scholar]
- Mikkelsen, M. S. , Jespersen, B. M. , Mehlsen, A. , Engelsen, S. B. , & Frøkiær, H. (2014). Cereal β‐glucan immune modulating activity depends on the polymer fine structure. Food Research International, 62, 829–836. [Google Scholar]
- Muanprasat, C. , Wongkrasant, P. , Satitsri, S. , Moonwiriyakit, A. , Pongkorpsakol, P. , Mattaveewong, T. , … Chatsudthipong, V. (2015). Activation of AMPK by chitosan oligosaccharide in intestinal epithelial cells: Mechanism of action and potential applications in intestinal disorders. Biochemical Pharmacology, 96, 225–236. 10.1016/j.bcp.2015.05.016 [DOI] [PubMed] [Google Scholar]
- Nepelska, M. , Cultrone, A. , Béguet‐Crespel, F. , Le Roux, K. , Doré, J. , Arulampalam, V. , & Blottière, H. M. (2012). Butyrate produced by commensal bacteria potentiates phorbol esters induced AP‐1 response in human intestinal epithelial cells. PLoS ONE, 7, e52869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newburg, D. S. , Ko, J. S. , Leone, S. , & Nanthakumar, N. N. (2016). Human milk oligosaccharides and synthetic galactosyloligosaccharides contain 3′‐, 4‐, and 6′‐galactosyllactose and attenuate inflammation in human T84, NCM‐460, and H4 cells and intestinal tissue ex vivo. The Journal of Nutrition, 146, 358–367. 10.3945/jn.115.220749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ngatu, N. R. , Tanaka, M. , Ikeda, M. , Inoue, M. , Kanbara, S. , & Nojima, S. (2017). Sujiaonori‐derived algal biomaterials inhibit allergic reaction in allergen‐sensitized RBL‐2H3 cell line and improve skin health in humans. Journal of Functional Biomaterials, 8, E37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen, T. T. , Kim, J. W. , Park, J. S. , Hwang, K. H. , Jang, T. S. , Kim, C. H. , & Kim, D. (2016). Identification of oligosaccharides in human milk bound onto the toxin A carbohydrate binding site of Clostridium difficile . Journal of Microbiology and Biotechnology, 26, 659–665. 10.4014/jmb.1509.09034 [DOI] [PubMed] [Google Scholar]
- Noll, A. J. , Gourdine, J. P. , Yu, Y. , Lasanajak, Y. , Smith, D. F. , & Cummings, R. D. (2016). Galectins are human milk glycan receptors. Glycobiology, 26, 655–669. 10.1093/glycob/cww002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olano‐Martin, E. , Williams, M. R. , Gibson, G. R. , & Rastall, R. A. (2003). Pectins and pectic‐oligosaccharides inhibit Escherichia coli O157:H7 Shiga toxin as directed towards the human colonic cell line HT29. FEMS Microbiology Letters, 218, 101–105. [DOI] [PubMed] [Google Scholar]
- Ortega‐Gonzalez, M. , Ocon, B. , Romero‐Calvo, I. , Anzola, A. , Guadix, E. , Zarzuelo, A. , … Martínez‐Augustin, O. (2014). Nondigestible oligosaccharides exert nonprebiotic effects on intestinal epithelial cells enhancing the immune response via activation of TLR4‐NFκB. Molecular Nutrition & Food Research, 58, 384–393. 10.1002/mnfr.201300296 [DOI] [PubMed] [Google Scholar]
- Peng, L. , Li, Z.‐R. , Green, R. S. , Holzman, I. R. , & Lin, J. (2009). Butyrate enhances the intestinal barrier by facilitating tight junction assembly via activation of AMP‐activated protein kinase in Caco‐2 cell monolayers. The Journal of Nutrition, 139, 1619–1625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson, L. W. , & Artis, D. (2014). Intestinal epithelial cells: Regulators of barrier function and immune homeostasis. Nature Reviews. Immunology, 14, 141–153. [DOI] [PubMed] [Google Scholar]
- Qi, X. , Al‐Ghazzewi, F. H. , & Tester, R. F. (2018). Dietary fiber, gastric emptying, and carbohydrate digestion: A mini‐review. Starch ‐ Stärke, 70, 1700346. [Google Scholar]
- Quintero, M. , Maldonado, M. , Perez‐Munoz, M. , Jimenez, R. , Fangman, T. , Rupnow, J. , … Hutkins, R. (2011). Adherence inhibition of Cronobacter sakazakii to intestinal epithelial cells by prebiotic oligosaccharides. Current Microbiology, 62, 1448–1454. [DOI] [PubMed] [Google Scholar]
- Rabbani, G. H. , Ahmed, S. , Hossain, I. , Islam, R. , Marni, F. , Akhtar, M. , & Majid, N. (2009). Green banana reduces clinical severity of childhood shigellosis: A double‐blind, randomized, controlled clinical trial. The Pediatric Infectious Disease Journal, 28, 420–425. 10.1097/INF.0b013e31819510b5 [DOI] [PubMed] [Google Scholar]
- Ramirez‐Farias, C. , Slezak, K. , Fuller, Z. , Duncan, A. , Holtrop, G. , & Louis, P. (2009). Effect of inulin on the human gut microbiota: Stimulation of Bifidobacterium adolescentis and Faecalibacterium prausnitzii . The British Journal of Nutrition, 101, 541–550. 10.1017/S0007114508019880 [DOI] [PubMed] [Google Scholar]
- Rescigno, M. (2011). The intestinal epithelial barrier in the control of homeostasis and immunity. Trends in Immunology, 32, 256–264. 10.1016/j.it.2011.04.003 [DOI] [PubMed] [Google Scholar]
- Ruiz‐Palacios, G. M. , Cervantes, L. E. , Ramos, P. , Chavez‐Munguia, B. , & Newburg, D. S. (2003). Campylobacter jejuni binds intestinal H(O) antigen (Fucα1, 2Galβ1, 4GlcNAc), and fucosyloligosaccharides of human milk inhibit its binding and infection. The Journal of Biological Chemistry, 278, 14112–14120. [DOI] [PubMed] [Google Scholar]
- Sahasrabudhe, N. M. , Beukema, M. , Tian, L. , Troost, B. , Scholte, J. , Bruininx, E. , … de Vos, P. (2018). Dietary fiber pectin directly blocks toll‐like receptor 2‐1 and prevents doxorubicin‐induced ileitis. Frontiers in Immunology, 9, 383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuelsen, A. B. , Rieder, A. , Grimmer, S. , Michaelsen, T. E. , & Knutsen, S. H. (2011). Immunomodulatory activity of dietary fiber: Arabinoxylan and mixed‐linked beta‐glucan isolated from barley show modest activities in vitro. International Journal of Molecular Sciences, 12, 570–587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sancho, E. , Batlle, E. , & Clevers, H. (2003). Live and let die in the intestinal epithelium. Current Opinion in Cell Biology, 15, 763–770. 10.1016/j.ceb.2003.10.012 [DOI] [PubMed] [Google Scholar]
- Schilderink, R. , Verseijden, C. , & de Jonge, W. J. (2013). Dietary inhibitors of histone deacetylases in intestinal immunity and homeostasis. Frontiers in Immunology, 4, 226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroeder, B. O. , Birchenough, G. M. H. , Stahlman, M. , Arike, L. , Johansson, M. E. V. , Hansson, G. C. , & Bäckhed, F. (2018). Bifidobacteria or fiber protects against diet‐induced microbiota‐mediated colonic mucus deterioration. Cell Host & Microbe, 23, 27–40 e27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schumacher, G. , Bendas, G. , Stahl, B. , & Beermann, C. (2006). Human milk oligosaccharides affect P‐selectin binding capacities: In vitro investigation. Nutrition, 22, 620–627. 10.1016/j.nut.2005.12.009 [DOI] [PubMed] [Google Scholar]
- Segain, J. P. , Raingeard de la Bletiere, D. , Bourreille, A. , Leray, V. , Gervois, N. , Rosales, C. , … Galmiche, J. P. (2000). Butyrate inhibits inflammatory responses through NFκB inhibition: Implications for Crohn's disease. Gut, 47, 397–403. 10.1136/gut.47.3.397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Servin, A. L. (2004). Antagonistic activities of lactobacilli and bifidobacteria against microbial pathogens. FEMS Microbiology Reviews, 28, 405–440. 10.1016/j.femsre.2004.01.003 [DOI] [PubMed] [Google Scholar]
- Shah, M. M. , Saio, M. , Yamashita, H. , Tanaka, H. , Takami, T. , Ezaki, T. , & Inagaki, N. (2012). Lactobacillus acidophilus strain L‐92 induces CD4+CD25+Foxp3+ regulatory T cells and suppresses allergic contact dermatitis. Biological & Pharmaceutical Bulletin, 35, 612–616. 10.1248/bpb.35.612 [DOI] [PubMed] [Google Scholar]
- Shoaf, K. , Mulvey, G. L. , Armstrong, G. D. , & Hutkins, R. W. (2006). Prebiotic galactooligosaccharides reduce adherence of enteropathogenic Escherichia coli to tissue culture cells. Infection and Immunity, 74, 6920–6928. 10.1128/IAI.01030-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silk, D. B. , Davis, A. , Vulevic, J. , Tzortzis, G. , & Gibson, G. R. (2009). Clinical trial: The effects of a trans‐galactooligosaccharide prebiotic on faecal microbiota and symptoms in irritable bowel syndrome. Alimentary Pharmacology & Therapeutics, 29, 508–518. 10.1111/j.1365-2036.2008.03911.x [DOI] [PubMed] [Google Scholar]
- Sinclair, H. R. , de Slegte, J. , Gibson, G. R. , & Rastall, R. A. (2009). Galactooligosaccharides (GOS) inhibit Vibrio cholerae toxin binding to its GM1 receptor. Journal of Agricultural and Food Chemistry, 57, 3113–3119. 10.1021/jf8034786 [DOI] [PubMed] [Google Scholar]
- Singh, N. , Gurav, A. , Sivaprakasam, S. , Brady, E. , Padia, R. , Shi, H. , … Ganapathy, V. (2014). Activation of Gpr109a, receptor for niacin and the commensal metabolite butyrate, suppresses colonic inflammation and carcinogenesis. Immunity, 40, 128–139. 10.1016/j.immuni.2013.12.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith, P. M. , Howitt, M. R. , Panikov, N. , Michaud, M. , Gallini, C. A. , Bohlooly, Y. M. , … Garrett, W. S. (2013). The microbial metabolites, short‐chain fatty acids, regulate colonic Treg cell homeostasis. Science, 341, 569–573. 10.1126/science.1241165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- So, D. , Whelan, K. , Rossi, M. , Morrison, M. , Holtmann, G. , Kelly, J. T. , … Campbell, K. L. (2018). Dietary fiber intervention on gut microbiota composition in healthy adults: A systematic review and meta‐analysis. The American Journal of Clinical Nutrition, 107, 965–983. 10.1093/ajcn/nqy041 [DOI] [PubMed] [Google Scholar]
- Sun, X. , Yang, Q. , Rogers, C. J. , Du, M. , & Zhu, M. J. (2017). AMPK improves gut epithelial differentiation and barrier function via regulating Cdx2 expression. Cell Death and Differentiation, 24, 819–831. 10.1038/cdd.2017.14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki, T. , Yoshida, S. , & Hara, H. (2008). Physiological concentrations of short‐chain fatty acids immediately suppress colonic epithelial permeability. British Journal of Nutrition, 100, 297–305. 10.1017/S0007114508888733 [DOI] [PubMed] [Google Scholar]
- Tang, C. , Kamiya, T. , Liu, Y. , Kadoki, M. , Kakuta, S. , Oshima, K. , … Iwakura, Y. (2015). Inhibition of dectin‐1 signaling ameliorates colitis by inducing Lactobacillus‐mediated regulatory T cell expansion in the intestine. Cell Host & Microbe, 18, 183–197. 10.1016/j.chom.2015.07.003 [DOI] [PubMed] [Google Scholar]
- Tap, J. , Furet, J. P. , Bensaada, M. , Philippe, C. , Roth, H. , Rabot, S. , … Leclerc, M. (2015). Gut microbiota richness promotes its stability upon increased dietary fibre intake in healthy adults. Environmental Microbiology, 17, 4954–4964. 10.1111/1462-2920.13006 [DOI] [PubMed] [Google Scholar]
- Tarr, A. J. , Galley, J. D. , Fisher, S. E. , Chichlowski, M. , Berg, B. M. , & Bailey, M. T. (2015). The prebiotics 3′Sialyllactose and 6′Sialyllactose diminish stressor‐induced anxiety‐like behavior and colonic microbiota alterations: Evidence for effects on the gut‐brain axis. Brain, Behavior, and Immunity, 50, 166–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorburn, A. N. , McKenzie, C. I. , Shen, S. , Stanley, D. , Macia, L. , Mason, L. J. , … Mackay, C. R. (2015). Evidence that asthma is a developmental origin disease influenced by maternal diet and bacterial metabolites. Nature Communications, 6, 7320 10.1038/ncomms8320 [DOI] [PubMed] [Google Scholar]
- Tramontano, M. , Andrejev, S. , Pruteanu, M. , Klünemann, M. , Kuhn, M. , Galardini, M. , … Patil, K. R. (2018). Nutritional preferences of human gut bacteria reveal their metabolic idiosyncrasies. Nature Microbiology, 3, 514–522. 10.1038/s41564-018-0123-9 [DOI] [PubMed] [Google Scholar]
- Trompette, A. , Gollwitzer, E. S. , Yadava, K. , Sichelstiel, A. K. , Sprenger, N. , Ngom‐Bru, C. , … Marsland, B. J. (2014). Gut microbiota metabolism of dietary fiber influences allergic airway disease and hematopoiesis. Nature Medicine, 20, 159–166. 10.1038/nm.3444 [DOI] [PubMed] [Google Scholar]
- Turnbaugh, P. J. , Ridaura, V. K. , Faith, J. J. , Rey, F. E. , Knight, R. , & Gordon, J. I. (2009). The effect of diet on the human gut microbiome: A metagenomic analysis in humanized gnotobiotic mice. Science Translational Medicine, 1, 6ra14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uraipan, S. , Brigidi, P. , & Hongpattarakere, T. (2014). Antagonistic mechanisms of synbiosis between Lactobacillus plantarum CIF17AN2 and green banana starch in the proximal colon model challenged with Salmonella Typhimurium. Anaerobe, 28, 44–53. 10.1016/j.anaerobe.2014.05.002 [DOI] [PubMed] [Google Scholar]
- Verma, M. , Hontecillas, R. , Tubau‐Juni, N. , Abedi, V. , & Bassaganya‐Riera, J. (2018). Challenges in personalized nutrition and health. Frontiers in Nutrition, 5, 117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Videla, S. , Vilaseca, J. , Antolin, M. , Garcia‐Lafuente, A. , Guarner, F. , Crespo, E. , … Malagelada, J. R. (2001). Dietary inulin improves distal colitis induced by dextran sodium sulfate in the rat. The American Journal of Gastroenterology, 96, 1486–1493. 10.1111/j.1572-0241.2001.03802.x [DOI] [PubMed] [Google Scholar]
- Vishu Kumar, A. B. , Varadaraj, M. C. , Gowda, L. R. , & Tharanathan, R. N. (2005). Characterization of chito‐oligosaccharides prepared by chitosanolysis with the aid of papain and Pronase, and their bactericidal action against Bacillus cereus and Escherichia coli . The Biochemical Journal, 391, 167–175. 10.1042/BJ20050093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogt, L. , Ramasamy, U. , Meyer, D. , Pullens, G. , Venema, K. , Faas, M. M. , … de Vos, P. (2013). Immune modulation by different types of β2→1‐fructans is toll‐like receptor dependent. PLoS ONE, 8, e68367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogt, L. M. , Elderman, M. E. , Borghuis, T. , de Haan, B. J. , Faas, M. M. , & de Vos, P. (2017). Chain length‐dependent effects of inulin‐type fructan dietary fiber on human systemic immune responses against hepatitis‐B. Molecular Nutrition & Food Research, 61(10). Article ID 1700171. [DOI] [PubMed] [Google Scholar]
- Vogt, L. M. , Meyer, D. , Pullens, G. , Faas, M. M. , Venema, K. , Ramasamy, U. , … de Vos, P. (2014). Toll‐like receptor 2 activation by β2→1‐fructans protects barrier function of T84 human intestinal epithelial cells in a chain length‐dependent manner. The Journal of Nutrition, 144, 1002–1008. [DOI] [PubMed] [Google Scholar]
- Vogt, L. M. , Sahasrabudhe, N. M. , Ramasamy, U. , Meyer, D. , Pullens, G. , Faas, M. M. , … de Vos, P. (2016). The impact of lemon pectin characteristics on TLR activation and T84 intestinal epithelial cell barrier function. Journal of Functional Foods, 22, 398–407. [Google Scholar]
- Wang, X. , Li, Y. , Yang, X. , & Yao, J. (2013). Astragalus polysaccharide reduces inflammatory response by decreasing permeability of LPS‐infected Caco2 cells. International Journal of Biological Macromolecules, 61, 347–352. [DOI] [PubMed] [Google Scholar]
- Wismar, R. , Brix, S. , Frokiaer, H. , & Laerke, H. N. (2010). Dietary fibers as immunoregulatory compounds in health and disease. Annals of the new York Academy of Sciences, 1190, 70–85. [DOI] [PubMed] [Google Scholar]
- Wu, Q. , Martin, R. J. , Rino, J. G. , Jeyaseelan, S. , Breed, R. , & Chu, H. W. (2007). A deficient TLR2 signaling promotes airway mucin production in Mycoplasma pneumoniae‐infected allergic mice. American Journal of Physiology. Lung Cellular and Molecular Physiology, 292, L1064–L1072. [DOI] [PubMed] [Google Scholar]
- Yarden, Y. (2001). The EGFR family and its ligands in human cancer: Signalling mechanisms and therapeutic opportunities. European Journal of Cancer, 37(Suppl 4), S3–S8. [DOI] [PubMed] [Google Scholar]
- Yu, D. C. W. , Waby, J. S. , Chirakkal, H. , Staton, C. A. , & Corfe, B. M. (2010). Butyrate suppresses expression of neuropilin I in colorectal cell lines through inhibition of Sp1 transactivation. Molecular Cancer, 9, 276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yun, C. H. , Estrada, A. , Van Kessel, A. , Park, B. C. , & Laarveld, B. (2003). β‐Glucan, extracted from oat, enhances disease resistance against bacterial and parasitic infections. FEMS Immunology and Medical Microbiology, 35, 67–75. 10.1016/S0928-8244(02)00460-1 [DOI] [PubMed] [Google Scholar]
- Zehra, S. , Khambati, I. , Vierhout, M. , Mian, M. F. , Buck, R. , & Forsythe, P. (2018). Human milk oligosaccharides attenuate antigen‐antibody complex induced chemokine release from human intestinal epithelial cell lines. Journal of Food Science, 83, 499–508. 10.1111/1750-3841.14039 [DOI] [PubMed] [Google Scholar]
- Zenhom, M. , Hyder, A. , de Vrese, M. , Heller, K. J. , Roeder, T. , & Schrezenmeir, J. (2011). Prebiotic oligosaccharides reduce proinflammatory cytokines in intestinal Caco‐2 cells via activation of PPARγ and peptidoglycan recognition protein 3. The Journal of Nutrition, 141, 971–977. 10.3945/jn.110.136176 [DOI] [PubMed] [Google Scholar]
- Zenhom, M. , Hyder, A. , Kraus‐Stojanowic, I. , Auinger, A. , Roeder, T. , & Schrezenmeir, J. (2011). PPARγ‐dependent peptidoglycan recognition protein 3 (PGlyRP3) expression regulates proinflammatory cytokines by microbial and dietary fatty acids. Immunobiology, 216, 715–724. 10.1016/j.imbio.2010.10.008 [DOI] [PubMed] [Google Scholar]
- Zheng, J. , Yuan, X. , Cheng, G. , Jiao, S. , Feng, C. , Zhao, X. , … Liu, H. (2018). Chitosan oligosaccharides improve the disturbance in glucose metabolism and reverse the dysbiosis of gut microbiota in diabetic mice. Carbohydrate Polymers, 190, 77–86. 10.1016/j.carbpol.2018.02.058 [DOI] [PubMed] [Google Scholar]


