Abstract
As our knowledge of the various roles of the gut microbiota in the maintenance of homeostasis grows and as we learn how a disrupted microbiota may contribute to disease, therapeutic strategies that target our microbial fellow‐travellers become ever more attractive. Most appealing are those interventions that seek to modify or supplement our diet through the addition of nutraceuticals. We now know that our diet, whether in the short or long term, is a major modifier of microbiota composition and function. Of the various nutraceuticals, two categories, prebiotics and probiotics, have received the greatest attention in basic research and product development. While our understanding of the impacts of prebiotics and probiotics on the indigenous microbiota and host biology have been described in great detail in vitro and in animal models, the clinical literature leaves much to be desired. While many claims have been made, few are supported by high quality clinical trials.
Linked Articles
This article is part of a themed section on The Pharmacology of Nutraceuticals. To view the other articles in this section visit http://onlinelibrary.wiley.com/doi/10.1111/bph.v177.6/issuetoc
Abbreviations
- ATCC
American Type Culture Collection
- B.
Bifidobacterium
- CDAD
Clostridioides difficile‐associated disease
- DSHEA
Dietary Supplement Health and Education Act
- EFSA
European Food Safety Authority
- FODMAP
fermentable oligosaccharides, disaccharides, monosaccharides and polyols
- FOS
fructose oligosaccharides
- GG
Gorbach and Goldin
- GOSs
Galacto‐oligosaccharides
- ISAPP
International Scientific Association for Prebiotics and Probiotics
- L.
Lactobacillus
- S.
Streptococcus
- SCFA
short‐chain fatty acids
- TREG
regulatory T cells
1. INTRODUCTION
The term “nutraceutical,” a combination of the words nutrition and pharmaceutical, is commonly attributed to DeFelice who defined a nutraceutical as, “a food (or part of a food) that provides medical or health benefits, including the prevention and/or treatment of a disorder or disease” (Brower, 1998). Later, Kalra (2003) suggested that a nutraceutical was differentiated from a functional food by the ability of the former to “aid in the prevention and/or treatment of disease or disorder.” Put more simply, a nutraceutical has been defined as “a food or part of a food that provides benefits to health in addition to its nutritional content” (Santini, Tenore, & Novellino, 2017). In reality, distinctions between dietary supplements, functional foods and nutraceuticals have remained indistinct though the various products included in these categories have been subjected to increasing regulatory scrutiny in recent years (Pereira, Barros, & Ferreira, 2017; Swann, 2016). For regulatory agencies, the essential distinction seems to lie between food and drug; any substance that claims to treat or prevent a disease or disorder will, in most jurisdictions, be regarded as a drug and subjected to the rigors of the regulatory processes that precede the approval of any drug. In the United States, the Food and Drug Administration regulates both finished dietary supplement products and dietary ingredients and regulated dietary supplements under a different set of regulations than those covering “conventional” foods and drug products: the Dietary Supplement Health and Education Act of 1994 (commonly referred to as DSHEA; https://www.fda.gov/food/dietary-supplements—accessed May 29, 2019). In Europe, the European Food Safety Authority (EFSA) oversees the regulation of dietary supplements (http://www.efsa.europa.eu/—accessed May 29, 2019). In both regions, the whole category of dietary supplements has come under more scrutiny of late with particular attention being paid to any suggestion that a product might have a health benefit. Interestingly, the term nutraceutical does not appear to have been defined by either regulatory body. Santini and colleagues, who have written extensively on the subject (Daliu, Santini, & Novellino, 2019; Santini et al., 2017; Santini & Novellino, 2018 Santini et al., 2018), stress the need for a universal definition of a nutraceutical which they envision as occupying the middle ground between food and drug. They suggest the following steps towards definition of a nutraceutical:
appropriate target identification,
safety assessment,
a clear understanding of the mechanism of action,
efficacy assessment substantiated by clinical studies,
an evaluation of possible unwanted side effects, and
an evaluation of possible interactions with other products (e.g. food, food supplements and drugs) (Santini et al., 2018)
These seem quite appropriate for a product that seeks to exert a health benefit but, for now, remain aspirational. One could envision their incorporation in whole or in part into a future regulatory definition of a nutraceutical. The details of these regulatory issues which are undoubtedly in a state of flux are beyond the scope of this review which will focus on how substances that may be found within the categories of nutraceutical, functional food or dietary supplement may influence the microbiota and, thereby, exert a therapeutic benefit. Given the vast range of substances that could be encompassed within the term nutraceutical, I have chosen to limit my discussion to certain dietary components and supplements such as prebiotics, probiotics and synbiotics as the vast majority of the extant literature relates to these areas. Before considering the interventions, let us first consider the primary biological target, the microbiome.
2. THE GUT MICROBIOME—A TARGET FOR THERAPEUTIC MANIPULATION
Strictly speaking, the term microbiome refers to the collection of genomes from all microorganisms in a given environment while the term microbiota refers to all the microorganisms found in the environment. They are often, however, used interchangeably. The gut microbiome and its implications in health and disease have rapidly evolved over the last two decades to become one of the hottest areas of medical research. The advent and widespread application of, first, high‐throughput sequencing technology and, second, and more recently, metagenomics, metabolomics, meta‐transcriptomics and other “omics” have not only facilitated the enumeration of the microbial species that inhabit the human gut but also provided predictions of microbial properties and their potential impact on the host, as well as measurements of biologically active microbial products (O'Toole & Felmer, 2016). These technological developments have spawned a host of studies describing changes in the microbiome in disease states and prompted enthusiasm for a role for microbiome analysis in diagnosis, prognosis or treatment selection. Interesting though these observations may be, they remain, for the most part, associations and well‐documented examples of truly causative microbial signatures remain singularly rare (Quigley, 2017a). Microbiome science has also revealed the therapeutic potential of the microbiome. While some microbiome‐modulating strategies have been used on an empiric basis for decades if not centuries, recent research has begun to identify the various mechanisms whereby interventions, such as prebiotics and probiotics, might actually provide benefit (Quigley, 2019).
3. WHAT DO WE KNOW?
The nature and the importance of the complex interactions between the microbiome and its host are now well recognized and the contributions of this commensal relationship to the health of the host increasingly appreciated. Accordingly, one can predict how any disruption of this relationship might lead to pathological consequences for the host. Two well‐defined clinical entities provide a vivid illustration of disrupted microbiome‐host interactions: Clostridioides difficile‐associated disease (CDAD) and Helicobacter pylori infection. The former serves as a dramatic reminder of the consequences of iatrogenic disruptions of a microbiome that, when intact, serves to protect us against pathogens (Britton & Young, 2012; Schäffler & Breitrück, 2018), and the latter exemplifies how host genome, bacterial properties and the immune response conspire to produce various disease phenotypes (Noto & Peek, 2017; Thorell, Lehours, & Vale, 2017; Waskito, Salama, & Yamaoka, 2018).
Many other studies have described associations between an altered microbiome and various gastrointestinal and systemic diseases and disorders. The term “dysbiosis” has been frequently used to refer to such apparently abnormal microbiota signatures and has entered the general lexicon in a manner that assumes clarity of definition. This term may have gained currency, but it is far from satisfactory as it presumes that we know what constitutes a “normal” microbiome. While some common patterns have been described at a higher‐order level between subjects in the general population in some studies (Arumugam et al., 2011), such is the variability between subjects and the impact of various personal and environmental factors at the levels of species and strain, that it seems premature to use the term “dysbiosis” to describe any human study. Furthermore, most human studies, regrettably, share one or more of the following limitations (Quigley, 2017a):
Such is the heterogeneity in bacterial populations between and within patient populations that it is still not possible to state with certainty what is normal in any given population.
Most studies are single point in time, rather than longitudinal, rendering it impossible to account for fluctuations in disease activity as well as the confounding impact of therapy. In other words, in can be nigh impossible to decide whether a given microbial signature represents state or trait in relation to a given disease. Only longitudinal studies with sampling at multiple time points can aid in making this distinction.
Diet, likely to be altered in many disease states, can significantly modify the microbiome (in both the long and short terms) and has not been accounted for in many of studies.
For obvious reasons of convenience, most human studies have been based on the analysis of faecal samples, an approach that disregards variations in bacterial populations along the length of the gastrointestinal tract and may fail to represent those bacterial populations that reside close to or adherent to the mucosa. Though more challenging in terms of access, studies of the juxta‐mucosal microbiome in the colon have revealed significant difference in health and disease from those studies where microbiome analyses were based on stool samples (Parthasarathy et al., 2016; Ringel et al., 2015; Shobar et al., 2016). Even more logistically challenging are studies of the small intestinal microbiome; here also, new data are appearing (Saffouri et al., 2019). Given that, for most nutrients, their digestion and absorption take place principally in the proximal small intestine (also the site of transport or absorption of most pharmaceuticals) and that the more distal small intestine is abundantly endowed in immune tissues, an understanding of the small intestinal microbiome is of critical importance.
4. DIET AND THE MICROBIOME
Any discussion of functional foods, nutraceuticals or dietary supplements should address the impact of food per se and potential interactions with a therapeutic intervention (in this case, one that impacts on the microbiome).
Several studies have amply illustrated the impact of diet on the microbiome. First, studies comparing geographically diverse communities have ascribed differences in faecal microbial profiles to lifelong dietary habits (Yatsunenko et al., 2012) and, second, differences in microbial fingerprints within communities or populations with similar demographics have been attributed to variable dietary habits (Arumugam et al., 2011; Claesson et al., 2012; Falony et al., 2016; Zhernakova, Kurilshikov, Bonder, Tigchelaar, & Schirmer M …, 2016; exemplified by Claesson et al., 2012; Figure 1). If sufficiently drastic, more short‐ to medium‐term changes in diet (e.g. high protein, low fermentable oligosaccharides, disaccharides or monosaccharides and polyol's [FODMAP's], high vs. low fibre or gluten‐free) can also impact on microbiome composition (Clarke et al., 2014; Hansen et al., 2018; Saffouri et al., 2019; Sanz, 2010; Sloan et al., 2018). The adoption of other diets that exclude a single dietary component (e.g., lactose‐, fructose‐ and sorbitol‐free diets), or involve more extensive modifications (e.g. the Mediterranean or “palaeo” diet), are also likely to alter the composition of microbiota.
Figure 1.
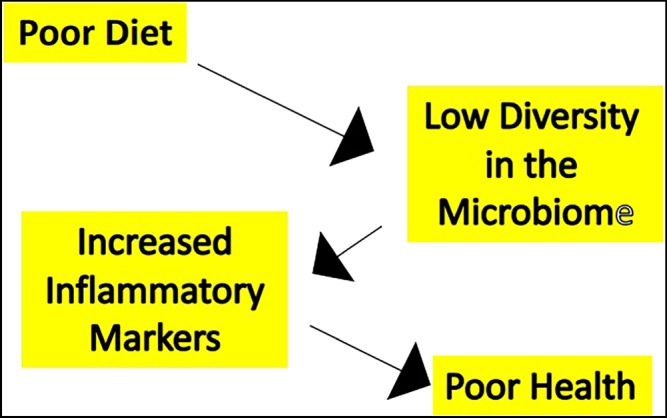
Interactions between diet and health in the elderly. In a study of various cohorts of elderly subjects and healthy younger adults, bacterial diversity diminished in parallel with diversity of diet and was associated with elevations in markers of systemic inflammation (pro‐inflammatory cytokines) and deterioration in overall health status and increasing frailty (Claesson et al., 2012)
The Mediterranean diet refers to a diet that involves daily consumption of vegetables, fruits, whole grains and healthy fats; weekly intake of fish, poultry, beans and eggs; moderate portions of dairy products; and limited intake of red meat. The Palaeo diet includes lean meats, fish, fruits, vegetables, nuts and seeds and limits dairy products, legumes, and grains. These contrast with what is often referred to as the “Western diet” which involves high intakes of https://en.wikipedia.org/wiki/Red_meat, https://en.wikipedia.org/wiki/Processed_meat, https://en.wikipedia.org/wiki/Convenience_food, https://en.wikipedia.org/wiki/Butter, https://en.wikipedia.org/wiki/Fried_foods, high‐fat https://en.wikipedia.org/wiki/Dairy_product, https://en.wikipedia.org/wiki/Chicken_eggs, https://en.wikipedia.org/wiki/Refined_grain, https://en.wikipedia.org/wiki/Potatoes, https://en.wikipedia.org/wiki/Maize, corn syrup and https://en.wikipedia.org/wiki/High-sugar_drink. The plant‐based diets are thought to provide protection against a number of chronic diseases, including cardiovascular disease, through various mechanisms, including the antioxidant effects of plant polyphenols (Pandey & Rizvi, 2009)
Of the aforementioned diets, the low FODMAP diet has gained a significant foothold in the management of irritable bowel syndrome (IBS; Dionne et al., 2018) and may also be of benefit to some affected by inflammatory bowel disease (Colombel, Shin, & Gibson, 2019) and celiac disease (Yoosuf, 2019). Given the prebiotic effects of oligosaccharides (discussed later) on important components of the normal microbiome, such as lactobacilli and bifidobacteria, as well as their metabolic products, such as short‐chain fatty acids (SCFAs), the long‐term effects of the low FODMAP diet were of some concern. Changes in the faecal microbiome have, indeed, been described in relation to this diet with a reduction in bifidobacteria being most notable (Halmos et al., 2015; Huaman et al., 2018; McIntosh et al., 2017; Sloan et al., 2018; Staudacher et al., 2017); the clinical impact of these changes in the long term, in particular, remain unclear. The literature on the low FODMAP diet does provide an example of the potential benefits of a nutraceutical intervention; in this case a probiotic. In a placebo‐controlled study of the effects of a low FODMAP diet on IBS symptoms and the faecal microbiota, 104 subjects with IBS where randomized to either a sham or a low FODMAP diet with or without a multistrain probiotic. In comparison to the sham diet, the abundance of Bifidobacterium species was decreased in subjects on the low FODMAP diet alone but was replenished when supplemented with the probiotic product (Staudacher et al., 2017).
It is undoubted that interactions between diet, the indigenous microbiome and interventions that modulate the microbiome will continue to represent a major focus of future research (Zmora, Suez, & Elinav, 2019)
5. PREBIOTICS
While the definition of a nutraceutical may still be a work in progress, there has been considerable consensus on the definition of both a prebiotic and a probiotic. Over the past two decades, the most au courant and widely used definition of a prebiotic has been that first promulgated in 1995: “a non‐digestible food ingredient that beneficially affects the host by selectively stimulating the growth and/or activity of one or a limited number of bacteria in the colon” (Gibson & Roberfroid, 1995). This was updated and slightly modified in 2016 by a panel of experts convened by the International Scientific Association for Probiotics and Prebiotics (ISAPP) to “a substrate that is selectively utilized by host microorganisms conferring health benefit” (Gibson et al., 2017). The fundamental concept behind a prebiotic is that it is a non‐viable substance that is indigestible by our repertoire of digestive enzymes but can be digested by bacterial enzymes to provide nutrition to bacteria and, in so doing, generate important products, such as short chain fatty acid (SCFAs) and gases, such as hydrogen and methane (Bouhnik et al., 1996; Cook & Sellin, 1998; Davis, Martinez, Walter, & Hutkins, 2010). Selectivity is stressed in both definitions and has become the Holy Grail in current prebiotic research as investigators seek to identify molecules which have very targeted effects on specific bacterial species and, thus, can not only optimize benefit but also avoid promoting the growth of potential pathogens. In contrast to fibres, such as cellulose, pectins and xylans, which promote the growth of many microorganisms in the gut, prebiotics primarily stimulate the proliferation of Lactobacillus and Bifidobacterium species; this is not to say that other species are unaffected; both Faecalibacterium prausnitzii and Akkermansia muciniphilia have been shown to respond to prebiotics (Gibson et al., 2017; Hutkins et al., 2016; Vandeputte et al., 2017). Others have proposed a much broader and inclusive definition of a prebiotic which is less preoccupied with selectivity; Bindels, Delzenne, Cani, and Walter (2015), for example, defined a prebiotic as “a nondigestible compound that, through its metabolization by microorganisms in the gut, modulates composition and/or activity of the gut microbiota thus conferring a beneficial physiologic effect on the host.” These authors also expanded the metabolic impact of prebiotics beyond fermentation by alluding to potentially beneficial effects mediated through other mechanisms (Bindels et al., 2015) and even extended the range of prebiotics to non‐carbohydrates adding “resistant starch, pectin, arabinoxylan, whole grains, various dietary fibers and noncarbohydrates that exert their action through a modulation of the gut microbiota” (Bindels et al., 2015). These opinions, thought provoking though they might be, remain somewhat contrarian and the mainstream continues to focus on selectivity of effects and their mediation through bacterial fermentation. While an absolute definition of selectivity is not available, it is clear that a prebiotic should stimulate the growth of some bacteria but not all and certainly not pathogens (Gibson et al., 2017; Hutkins et al., 2016).
The relationship between fibres and prebiotics deserves mention. Some fibres and soluble fibres, in particular, and in the appropriate host can exert prebiotic effects, whereas others, including insoluble fibres, cannot. In contrast, as prebiotics (as conventionally defined) are typically “carbohydrate polymers that are neither digested nor absorbed in the human small intestine” (Codex Alimentarius Committee, 2010), most prebiotics can be classified as fibres (Holscher, 2017).
Regardless of where one comes down in terms of the precise definition of a prebiotic, all would agree that, in contrast to probiotics (as we will see), the link between an intervention (in this case a prebiotic), a change in the microbiome and a physiological or pharmacological effect is self‐evident. This was recently exemplified by a study of the effects of inulin‐type fructans in adults with constipation where the prebiotic, as expected, induced a relative bloom of Bifidobacterium spp. and Anaerostipes spp. but also led to a decrease in the abundance of Bilophila, the latter effect correlating with a beneficial change in stool consistency (Vandeputte et al., 2017). To quote directly from the ISAPP document:
A prebiotic, in addition to having a selective effect on microorganisms, must also evoke a net health benefit. The guiding principles are that microorganisms affected and metabolites produced are considered to be beneficial and linked to a defined health aspect. (Gibson et al., 2017)
Substances with prebiotic effects may be found in some plants such as onions, garlic, bananas, chicory root and Jerusalem artichokes but, typically, are present at low levels. More biologically active and selective prebiotics include galacto‐oligosaccharides (GOSs), fructo‐oligosaccharides (FOSs), oligofructose, chicory fibre and inulin. Human milk oligosaccharides are important prebiotics provided to breast‐fed infants which promote the proliferation of bifidobacteria and, in this manner, have been linked to a number of health benefits.
In contrast to probiotics where clinical research has focused, in large part, on gastrointestinal ailments, research in humans with prebiotics has concentrated on metabolic effects. Among the health benefits that have been demonstrated for prebiotics include the promotion of calcium absorption, reduction of blood sugar and acceleration of colon transit (Gibson et al., 2017). Such physiological benefits have positive effects on osteoporosis, diabetes and colorectal cancer, respectively. In fact, the EFSA in positive opinion statements acknowledged that “increased consumption of native chicory inulin can increase stool frequency” and that “consumption of foods containing non‐digestible carbohydrates instead of sugars [such as inulin and FOS] induces a lower blood glucose rise after meals compared to those containing sugars” (for details, see Gibson et al., 2017; Hutkins et al., 2016).
This is not to say that evidence of beneficial gastrointestinal effects of prebiotics is lacking. For example, the effects of the aforementioned chicory inulin on gut motility and sensation have been studied in man, albeit with variable results (Azpiroz et al., 2017; Slavin & Feirtag, 2011), and benefits in constipation described (Micka, Siepelmeyer, Holz, Theis, & Schön, 2017). Nevertheless, this effect on bowel transit formed the basis for the only health claim approved by the EFSA for a prebiotic. In 2006, EFSA concluded on the basis of scientific evidence that “native chicory inulin” when consumed in a dose of 12 g daily “contributed to maintenance of normal defecation by increasing stool frequency.” The panel found the mechanism of action plausible and concluded that its effects represented a “beneficial physiological effect.” (https://www.efsa.europa.eu/en/efsajournal/pub/3951; accessed August 8, 2019). Most recently, Huaman et al. (2018) compared what at first sight appeared to be two very contrasting approaches: a low FODMAP diet (designed to reduce bacterial fermentation) and a prebiotic in the form of β‐GOS (a strategy that might, in theory, promote fermentation and increase gas production) in a randomized controlled trial in patients with functional gastrointestinal disorders and flatulence. As expected, the low FODMAP diet decreased while the prebiotic increased the abundance of bifidobacteria; the exact reverse effects were seen in relation to Bilophila wadsworthia (Huaman et al., 2018). Both strategies reduced symptoms to an equally significant extent with the exception of flatulence and borborygmi whose reductions did not achieve statistical significance in the group administered the prebiotic. More importantly and as previously demonstrated by the same research group (Azpiroz et al., 2017), the prebiotic did not exacerbate any of these supposedly “gas‐related” symptoms (Huaman et al., 2018). The explanation for these reassuring observations is provided by a separate study again from the same group that demonstrated that, over a period as short as 2 weeks, the microbiota adapts to GOS administration by shifting to low gas‐producing pathways (Mego et al., 2017; Mego et al., 2017).
In a study of considerable importance to population health, Paganini, Uyoga, et al. (2017) addressed the impact of a prebiotic, in this case GOS, on the adverse effects of iron supplementation on the gut microbiome in Kenyan infants. Having noted that oral iron decreased important commensals such as bifidobacteria and lactobacilli and promoted the growth of enteric pathogens, they randomized infants with iron deficiency to iron supplementation with or without GOS. Similar benefits in terms of correction of anaemia were seen in both groups, but reductions in bifidobacteria and lactobacilli and increased levels of virulence and toxin genes of pathogens and of fatty acid‐binding protein in plasma, in comparison to a control group, were seen in those not supplemented with GOS. These benefits of GOS supplementation have the potential to prevent the predilection to not only enteric but also respiratory tract infections seen in this population with iron supplementation and attributed to its effects on the gut microbiome (Paganini et al., 2019; Paganini, Uyoga, & Zimmermann, 2016).
Linkage between a clinical benefit of a prebiotic, oligofructose‐enriched inulin, and an effect on the microbiome has also been demonstrated in a study of overweight and obese children (Nicolucci, Hume, Martínez, Mayengbam, et al., 2017). The prebiotic altered gut microbiota by selectively increasing bifidobacteria, an effect associated with a significant reduction in body weight, percent body fat and serum levels of the pro‐inflammatory cytokine https://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=4998 (IL‐6). Interestingly, a similar effect of GOS on bifidobacteria was observed in a study among obese pre‐diabetic adults but, in this instance, no metabolic benefits ensued (Canfora et al., 2017).
6. PROBIOTICS
There is no shortage of products claiming to incorporate probiotics—the range of formulations purporting to confer probiotic effects seems to be limited only by the imagination of the manufacturer. Most fail to fulfil even the most basic aspects of quality control and fewer still have studies to support a health benefit which, by definition, a probiotic should confer (Hill et al., 2014). This brings us to a critical issue—the definition of a probiotic.
6.1. The definition of a probiotic and its implications
Anecdotes suggest that substances that may well have exerted what would nowadays have be regarded as probiotic effects have been in existence for centuries. The first definition of a probiotic was provided by Lilly & Stillwell, 1965 as “substances secreted by one microorganism which stimulate (in contrast to antibiotics) the growth of another”. This definition of a probiotic was subsequently expanded to “a preparation of, or a product containing viable, defined microorganisms in sufficient numbers, which alter the microflora (by implantation or colonization) in a compartment of the host and by that exert beneficial health effects on the host” (Schrezenmeir & Vrese, 2001). The widely quoted Food and Agriculture Organization of the United Nations/World Health Organization definition is more succinct in defining probiotics as being “live microorganisms which when administered in adequate amounts confer a health benefit on the host” (http://www.who.int/foodsafety/publications/fs_management/en/probiotics.pdf—accessed June 17, 2019). The International Scientific Association for Probiotics and Prebiotics (ISAPP) recently revisited and endorsed the Food and Agriculture Organization of the United Nations/World Health Organization definition and went on to list four categories of compounds or products that contain live microorganisms and are intended for human use and addressed their regulatory implications (Hill et al., 2014):
6.1.1. Live or active cultures
These products, such as yogurts, simply claim that they contain live and active cultures but, unless evidence is provided that they confer a health benefit (which some do), this descriptor should not be taken to imply probiotic activity.
6.1.2. Probiotic in food or supplement without a health claim
Such products state that they “contain probiotics.” They should be safe and provide evidence of a general health benefit in man. In some jurisdictions, the use of the term “probiotic” has been regarded as an implied health claim (based on the aforementioned definitions of a probiotic) and, therefore, forbidden in the absence of acceptable evidence of a health benefit in some European countries (http://www.fsai.ie/faq/probiotic_health_claims.html—accessed June 17, 2019). This is, perhaps, the starkest reminder of the implications of a given definition.
6.1.3. Probiotic in food or supplement with a specific health claim
This category requires that the product has demonstrated convincing evidence of a specific health claim such as “reinforces the body's natural defenses.” For example, in Europe, the European Food Safety Authority (EFSA) requires the following evidence to support a health claim (Salminen & van Loveren, 2012; van Loveren, Sanz, & Salminen, 2012):
Characterization of the strain or each of the strains in a probiotic mix or combination
Identification of the health relationship that is considered as a beneficial physiological effect to the target population (i.e. the general population or a defined part of it)
Demonstration of health effects in a normal healthy population.
Few probiotics have met these requirements.
6.1.4. Probiotic drug
Here the probiotic is used to treat or prevent a specific disease. In the US, and elsewhere, this is now categorized as a drug (defined as an article intended for use in the diagnosis, cure, mitigation, treatment, or prevention of disease) and must satisfy all the regulatory requirements to be approved as such.
Scarcely any probiotics have even attempted to go down this route.
The current definition of a probiotic has significant ramifications. Two issues deserve special emphasis: the focus on “live” organisms and the insistence on conferring “a health benefit on the host”. Firstly, while it is acknowledged that studies in a number of animal models have demonstrated efficacy for killed bacteria, or even bacterial products or components (sometimes referred to as post‐biotics), in generating a number of anti‐inflammatory and anti‐infective effects (Giron & Quigley, 2018; Shanahan & Collins, 2010), this strategy has not, as yet, been widely employed in human studies though there is certainly considerable interest in the therapeutic potential of biologically active moieties that span the gamut from live to dead organisms and onto the products or components of microorganisms—referred to as pharmabiotics (Giron & Quigley, 2018; Zorzela, Ardestani, McFarland, & Vohra, 2017). Whither the definition of a probiotic? Only time will tell. The second part of these definitions is even more critical. The insistence on a demonstrable health benefit can only be interpreted in one way: properly powered, appropriately conducted, randomized, double blind, controlled (by placebo or valid comparator) clinical trials in relevant patient populations—a rarity in the probiotic literature.
For now, regulatory approaches leave much to the discretion of the consumer who is rightly bewildered by the vast array of “probiotic” products bearing various, usually unsupported, claims. This is not a satisfactory situation for the consumer, the health care provider, or, in the long run, for the probiotic industry. Right now, those who endeavour to produce high‐quality probiotics supported by clinical data risk being lost in the noise of a largely unregulated market. There can be but one outcome—more regulation, a scenario that is already emerging in Europe (http://www.fsai.ie/faq/probiotic_health_claims.html—accessed June 17, 2019; Salminen & van Loveren, 2012; Binnendijk & Rijkers, 2013)
6.2. Quality control
This should be a fundamental aspect of any product on sale to the general public. Though the following guidelines for the evaluation of probiotics in food were proposed as far back as 2002 (ftp://ftp.fao.org/es/esn/food/wgreport2.pdf—accessed June 17, 2019), they still provide a valid template for quality control in the probiotic field:
6.2.1. Identification of the bacterium at genome level
Identification of the genus and species of the probiotic strain by using a combination of phenotypic and genotypic tests as clinical evidence suggesting that the health benefits of probiotics maybe strain specific.
The complete genomes of several probiotic strains have now been sequenced. Some, inevitably, have even been reclassified (Lewis et al., 2016; Quigley, 2017b). Knowledge of the genome also facilitates batch‐by‐batch testing of product to ensure consistency. This requirement, like many others, is still honoured more in the breach than the observance yet should be absolutely fundamental to the characterization of every probiotic organism.
6.2.2. In vitro testing to delineate the mechanism of the probiotic effect
In the decades since the publication of these guidelines, there have been extensive studies of the in vitro and in vivo properties and biological effects of a host of putative probiotic strains. Such studies have identified a number of effects of relevance to gastrointestinal health and disease, including effects on motility, visceral sensation, components of the gut barrier, immune responses and the microbiota–gut–brain axis (Quigley, 2019).
6.2.3. Substantiation of the clinical health benefit of probiotic agents with human trials
This remains an absolutely fundamental principle.
Additionally, safety assessment of the probiotic should, at a minimum, determine:
Patterns of antimicrobial drug resistance
Metabolic activities
Side effects noted in humans during trials and after marketing
Toxin production and haemolytic potential if the probiotic strain is known to possess these properties
Lack of infectivity in animal models
By its very definition, it stands to reason that an effective probiotic must not only be live for the duration of its shelf life but must remain viable until it reaches its potential site of action—usually the small intestine or colon. In other words, it must be capable of surviving (in adequate numbers) the various assaults of gastric acid, bile and digestive enzymes. Here again, many products on our shelves fall short. A quality product should specify exactly what strain or strains it contains and should not extrapolate claims from similar but not identical strains—for some biological effects strain specificity is absolute.
In general, the safety record of probiotics is good (Didari, Solki, Mozaffari, Nikfar, & Abdollahi, 2014; Doron & Snydman, 2015) though, as pointed out in one review, the rigor of the literature on the safety of probiotics leaves much to be desired (Sanders et al., 2010)
6.3. Mechanisms of action
In the early days of probiotic research, the assumption was that probiotic effects were mediated through the impact of the administered microorganisms on the gut microbiome of the host, a perception that persists among the lay public as “good bugs outnumbering bad bugs.” Now that we know the actual numbers of bacteria (not to mind viruses and other microorganisms) that inhabit the human gut, one can contrast them with what can be achieved through the administration of a probiotic in either single‐strain or multi‐strain format (typically, 108 to 1010 colony forming units), the naiveté of this concept is clear. It should come as no surprise, therefore, that studies of the administration of probiotics on the composition of the faecal microbiota have demonstrated, at best, modest effects (Charbonneau, Gibb, & Quigley, 2013; Kolmeder et al., 2016). A series of studies from the Elinav group have recently suggested that interactions between administered probiotics and the commensal microbiota of the host may be more complex, with the host microbiota presenting colonization resistance to a probiotic, an effect that varied between subjects, anatomical region and probiotic strain (Suez et al, 2018; Zmora et al., 2018). Given what we know of how bacteria interact and preserve niches in the intact gut, these observations should not come as a surprise and may usher in a new more personalized era of bacterio‐therapy (Suez, Zmora, & Elinav, 2019; Suez, Zmora, Segal, & Elinav, 2019). It has also been suggested, but not independently confirmed, that probiotic–microbiota interactions could impair microbiota reconstitution following its depletion by an antibiotic (Suez, Zmora et al., 2018). All would agree that a probiotic will disappear from the gastrointestinal tract soon after its administration has ceased (Grond et al., 2019).
An example of a more subtle interaction between an administered probiotic and the host microbiota is provided by our knowledge of how a probiotic inhibits the growth of potentially pathogenic bacteria. This can be achieved through the creation of a more acidic milieu that is inimical to pro‐inflammatory bacteria, by competing with pathogens for adhesion, producing bacteriocins, (intrinsic “antibiotics”) and anti‐toxins while simultaneously promoting the growth of beneficial species such as lactobacilli and bifidobacteria (Al Kassaa, Hober, Hamze, Chihib, & Drider, 2014; Rea, Alemayehu, Ross, & Hill, 2013; Surendran Nair, Amalaradjou, & Venkitanarayanan, 2017).
The lack of a consistent body of data indicating significant impacts of an administered probiotic on the microbiota (Chua et al., 2017; Kankainen et al., 2009; Kristensen et al., 2016; Medina, Pinto, Ovalle, Thomson, & Garrido, 2017) should not be taken as indicative of a lack of biological effects. Indeed, effects of an orally administered probiotic on the systemic immune system (Groeger et al., 2013; Konieczna, Akdis, Quigley, Shanahan, & O'Mahony, 2012) and on brain responses (Pinto‐Sanchez et al., 2017; Wang, Braun, Murphy, & Enck, 2019) have, indeed, been demonstrated in human studies. Clinically relevant protective effects of a probiotic against acetylsalicylic acid‐induced intestinal injury have also been demonstrated in the absence of any discernible change in the gut microbiota (Mortensen et al., 2019). These and other observations indicate that probiotic effects are subtle and not readily discernible by the more widely available high‐throughput sequencing platforms. Some may be very local, occurring at or close to the mucosal surface and in those areas of the intestine that for reasons of access remain largely unexplored. These terra incognita are now being explored (Saffouri et al., 2019); such studies may ultimately reveal the intricacies of probiotic–microbe–host interactions where they really matter—site of action.
So if probiotics do not, on the basis of available evidence, impose significant, predictable compositional changes on the microbiota, how do they exert any biological effects? Not surprisingly, given the source of many effective strains, probiotic actions in the gut are now seen to reprise interactions between the commensal microbiota, the mucosa and the gut‐associated immune system (illustrated on Figure 2). A substantial literature attests to the anti‐inflammatory effects of probiotics (Frei, Akdis, & O'Mahony, 2015; Plaza‐Diaz, Gomez‐Llorente, Fontana, & Gil, 2014; Yan & Polk, 2011) and their engagement with the mucosal immune system via https://www.guidetopharmacology.org/GRAC/FamilyDisplayForward?familyId=316 to promote a regulatory T cell (TREG) response, rather than an inflammatory pathway. (de Kivit, Tobin, Forsyth, Keshavarzian, & Landay, 2014; Dwivedi, Kumar, Laddha, & Kemp, 2016; Konieczna, Akdis, et al., 2012). Some individual bacteria with potent anti‐inflammatory effects, such as Faecalibacterium prausnitzii, are in development as probiotics for the treatment of inflammatory bowel disease (Ferreira‐Halder, Faria, & Andrade, 2017).
Figure 2.
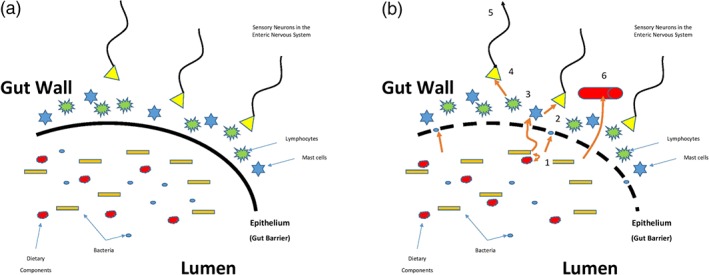
Interactions between the microbiota, dietary components and host. (a) Health. Bacteria, bacterial products or metabolites generated in the lumen by interactions between digesta and the microbiome underpin a symbiotic relationship with the host. Metabolic products generated by microbiota‐food interactions are available to the host. The host immune response to commensal species does not generate an inflammatory but rather a regulatory T‐cell response with the generation of anti‐inflammatory cytokines. Gut barrier integrity is retained. (b) Disease. When this relationship breaks down bacteria, bacterial products or metabolites gain access to the submucosal compartment via a more permeable epithelium or via transcytosis (1) where they activate mast cells and engage with lymphocytes leading to the release of proteases and cytokines/chemokines (2,3) respectively. As well as inducing an inflammatory response, these molecules also activate sensory afferents leading to local reflex responses (4) and/or central transmission (5). Simultaneously, bacterial products and inflammatory mediators may gain access to the portal and systemic circulations via the submucosal vasculature (6)
Other biological effects of probiotics include the restoration of gut barrier integrity (Hiippala et al., 2018), modulation of gut sensation and motility (Miller, Zimmermann, & Ouwehand, 2016; Theodorou, Ait Belgnaoui, Agostini, & Eutamene, 2014) and even impacts on the brain through the so‐called microbiome–gut–brain axis (Kim, Yun, Oh, & Choi, 2018).
With the recognition that the microbiome exerts a number of important metabolic effects, the ability of administered probiotics to modulate metabolic pathways came to be explored with those involved in the production of SCFAs and the deconjugation of bile acids attracting particular attention. Human data are limited, but some evidence has emerged to suggest that clinical benefits could accrue (Li et al., 2016; Plovier & Cani, 2017).
Exciting as these effects of probiotic are, it must be conceded that most of these positive and potentially important biological effects of probiotics have been demonstrated in a variety of animal models and translation to human disease has been far from consistent. This should not come as a great surprise as animal models rarely recapitulate the complete human disease phenotype and the doses of probiotics administered in these animal models are mostly in excess of that which is feasible in man.
6.4. Clinical studies
-
1
Healthy adults
Many individuals consume probiotics on a regular basis in the expectation that it will promote gut and general health. Apart from a beneficial impact on the duration and severity of the common cold, evidence for probiotic augmentation of health or prevention of disease among healthy subjects is lacking (Khalesi et al., 2019).
-
2
Gastrointestinal diseases and disorders
While there have been several studies of the impact of various probiotic formulations on a host of gastrointestinal conditions, the resultant literature leaves much to be desired. Tremendous variability between studies (even in the same disease or disorder) in relation to study population, trial design, measured endpoints, duration and product dosage and formulation often, as Ford et al. (2014) found in their systematic review of probiotics in IBS, render conclusions regarding the optimal probiotic impossible. This is especially frustrating as this same review concluded that probiotics, in general, were effective in IBS. Trials in many other gastrointestinal disorders are less numerous and often equally inconclusive. That high‐quality trials can be completed and yield positive and clinically meaningful results is amply illustrated by the recent study of the impact of a synbiotic on infections among high‐risk infants in India (Panigrahi et al., 2017). This study also illustrates the challenges posed: To demonstrate a significant effect of a synbiotic (simply a combination of a prebiotic and a probiotic) preparation on their primary outcome, sepsis, or death within the first 60 days of life among infants born in rural India, they randomized 4,556 infants to receive either Lactobacillus plantarum ATCC‐202195 plus an FOS or placebo (Panigrahi et al., 2017). This logistically complex and very expensive approach was essential in order to provide adequate power to demonstrate a clinically meaningful reduction in sepsis and death with the intervention. A comprehensive critical review of all studies of probiotics across the complete range of gastrointestinal indications is beyond the scope of this review. Fortunately, the 2017 World Gastroenterology Organization Global Guideline on Probiotics and Probiotics provides an up‐to‐date, peer‐reviewed, and evidence‐based assessment of the clinical value of probiotics in adults and children (http://www.worldgastroenterology.org/UserFiles/file/guidelines/probiotics-and-prebiotics-english-2017.pdf—accessed June 18, 2019). They identified level 1 evidence to support the use of probiotics in the following indications in adults and listed probiotic strains or formulations appropriate for each indication:
- Prevention of antibiotic‐associated diarrhea in various clinical settings:
- Yogurt with Lactobacillus (L.) casei DN114, L. bulgaricus, and Streptococcus (S.) thermophilus
- The combination of L. acidophilus CL1285 and L. casei (Bio‐K+ CL1285)
- L. rhamnosus GG
- Saccharomyces boulardii cncm i‐745
- Reduction of side effects related to eradication therapy for Helicobacter pylori:
- Saccharomyces boulardii cncm i‐745
- Prevention of post‐operative sepsis in those undergoing elective gastrointestinal surgeries:
- L. acidophilus
- L. plantarum
- Bifidobacterium longum 88
- Maintenance of remission in pouchitis:
- VSL #3
- Reducing symptoms related to lactose maldigestion:
- Yogurt with live cultures of L. delbrueckii subsp. bulgaricus and S. thermophilus
Several systematic reviews have also been published on the use of probiotics in these and other indications. Though of variable methodological rigor (Dong, Teng, Wei, Gao, & Wang, 2016), most concluded that the evidence supporting efficacy in a given indication was, at best, moderate and usually weak. Furthermore, these same reviews usually failed to identify a strain or combination of strains that was/were most effective in a particular clinical situation.
7. IMPACT OF OTHER DIETARY SUPPLEMENTS ON THE GUT MICROBIOME
Mention has already been made of the impact of iron supplementation on the gut microbiome in children (Paganini et al., 2016). Data on the impact of other micronutrients on the microbiota are scant (Biesalski, 2016). Deficiencies of vitamins A and D have been linked to a negative impact on intestinal microbial populations, effects linked to impaired gut barrier function and the promotion of pro‐inflammatory responses (Cantorna, Snyder, & Arora, 2019). Vitamin D deficiency has been linked with inflammatory bowel disease and its supplementation advocated for anti‐inflammatory effects (Fletcher, Cooper, Ghosh, & Hewison, 2019). Interactions between other vitamins and microbiota–host interplay have also been described (Yoshii, Hosomi, Sawane, & Kunisawa, 2019; Zhu et al., 2019); here again, the impact on inflammation has been of particular interests. Curcumin, another dietary additive with anti‐inflammatory properties, also impacts on the microbiota (Kali, Bhuvaneshwar, Charles, & Seetha, 2016) and it is undoubted that many other dietary components and supplements interact in one way or another with the gut microbiome or its interface with the host immune system or metabolic pathways.
8. CONCLUSIONS
The importance of the gut microbiota in homeostasis in health and in the pathogenesis of disease is now evident. The many interactions between the gut microbiota and so many aspects of mammalian physiology are revealed in elegant animal studies, and some have been confirmed in human studies. Enthusiasm for microbiota‐modulating approaches, so commonly effective in various animal models, is, therefore, understandable. Expectations have been raised but often not met—a cursory understanding of the complexities that underpin human disease and the heterogeneity of any particular phenotype in the population should have prepared us for such disappointment. Impressive and clear‐cut results in simplified models of human disease in mice and rats do not necessarily (or, indeed, seldom) translate to man. Human studies of prebiotics and probiotics have also been hampered by clinical trial designs that are all too frequently substandard in terms of quality. Information on the impact of other nutraceuticals is scant, but they are likely to also have an impact. As the regulatory climate evolves, greater rigor will inevitably follow, and high‐quality clinical trials will become de rigeur for all health and wellness claims. We can look forward to the emergence of completely characterized and appropriately formulated products from the laboratory and their rigorous testing against clearly defined and clinically relevant endpoints, whether in health or disease.
CONFLICT OF INTEREST
E.M.M.Q. has served as a consultant to Alimentary Health, Atlantia, Axon Pharma, Biocodex, and Salix, has received research support from 4D Pharma, Vibrant, and Zealand Pharma, and holds equity in Alimentary Health.
Quigley EMM. Nutraceuticals as modulators of gut microbiota: Role in therapy. Br J Pharmacol. 2020;177:1351–1362. 10.1111/bph.14902
REFERENCES
- Al Kassaa, I. , Hober, D. , Hamze, M. , Chihib, N. E. , & Drider, D. (2014). Antiviral potential of lactic acid bacteria and their bacteriocins. Probiotics Antimicrob Proteins., 6, 177–185. [DOI] [PubMed] [Google Scholar]
- Arumugam, M. , Raes, J. , Pelletier, E. , Le Paslier, D. , Yamada, T. , … Bork, P. (2011). Enterotypes of the human gut microbiome. Nature, 473, 174–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azpiroz, F. , Molne, L. , Mendez, S. , Nieto, A. , Manichanh, C. , Mego, M. , … Guarner, F. (2017). effect of chicory‐derived inulin on abdominal sensations and bowel motor function. Journal of Clinical Gastroenterology, 51, 619–625. 10.1097/MCG.0000000000000723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biesalski, H. K. (2016). Nutrition meets the microbiome: Micronutrients and the microbiota. Annals of the new York Academy of Sciences, 1372, 53–64. [DOI] [PubMed] [Google Scholar]
- Bindels, L. B. , Delzenne, N. M. , Cani, P. D. , & Walter, J. (2015). Towards a more comprehensive concept for prebiotics. Nature Reviews. Gastroenterology & Hepatology, 12, 303–310. [DOI] [PubMed] [Google Scholar]
- Binnendijk, K. H. , & Rijkers, G. T. (2013). What is a health benefit? An evaluation of EFSA opinions on health benefits with reference to probiotics. Benef Microbes., 4, 223–230. [DOI] [PubMed] [Google Scholar]
- Bouhnik, Y. , Flourie, B. , Riottot, M. , Riottot, M. , Bisetti, N. , … Rambaud, J. C. (1996). Effects of fructo‐oligosaccharides ingestion on fecal bifidobacteria and selected metabolic indexes of colon carcinogenesis in healthy humans. Nutrition and Cancer, 26, 21–29. [DOI] [PubMed] [Google Scholar]
- Britton, R. A. , & Young, V. B. (2012). Interaction between the intestinal microbiota and host in Clostridium difficile colonization resistance. Trends in Microbiology, 20, 313–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brower, V. (1998). Nutraceuticals: Poised for a healthy slice of the healthcare market? Nature Biotechnology, 16, 728–731. [DOI] [PubMed] [Google Scholar]
- Canfora, E. E. , van der Beek, C. M. , Hermes, G. D. A. , Goossens, G. H. , Jocken, J. W. E. , … Blaak, E. E. (2017). Supplementation of diet with galacto‐oligosaccharides increases bifidobacteria, but not insulin sensitivity, in obese prediabetic individuals. Gastroenterology, 153, 87–97. [DOI] [PubMed] [Google Scholar]
- Cantorna, M. T. , Snyder, L. , & Arora, J. (2019). Vitamin A and vitamin D regulate the microbial complexity, barrier function, and the mucosal immune responses to ensure intestinal homeostasis. Critical Reviews in Biochemistry and Molecular Biology, 54, 184–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charbonneau, D. , Gibb, R. D. , & Quigley, E. M. (2013). Fecal excretion of bifidobacterium infantis 35624 and changes in fecal microbiota after eight weeks of oral supplementation with encapsulated probiotic. Gut Microbes, 4, 201–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chua, M. C. , Ben‐Amor, K. , Lay, C. , Neo, A. G. E. , Chiang, W. C. , … Chongsrisawat, V. (2017). Effect of synbiotic on the gut microbiota of cesarean delivered infants: a randomized, double‐blind, multicenter study. Journal of Pediatric Gastroenterology and Nutrition, 65, 102–106. [DOI] [PubMed] [Google Scholar]
- Claesson, M. J. , Jeffery, I. B. , Conde, S. , Power, S. E. , O'Connor, E. M. , … O'Toole, P. W. (2012, 2012). Gut microbiota composition correlates with diet and health in the elderly. Nature, 488, 178–184. [DOI] [PubMed] [Google Scholar]
- Clarke, S. F. , Murphy, E. F. , O'Sullivan, O. , Lucey, A. J. , Humphreys, M. , … Cotter, P. D. (2014). Exercise and associated dietary extremes impact on gut microbial diversity. Gut, 63, 1913–1920. [DOI] [PubMed] [Google Scholar]
- Codex Alimentarius Committee (2010). Guidelines on nutrition labelling CAC/GL 2‐1985 as last amended 2010. Joint FAO/WHO Food Standards Programme, Secretariat of the Codex Alimentarius Commission. Rome, Italy: FAO. [Google Scholar]
- Colombel, J. F. , Shin, A. , & Gibson, P. R. (2019). AGA clinical practice update on functional gastrointestinal symptoms in patients with inflammatory bowel disease: Expert review. Clinical Gastroenterology and Hepatology, 17, 380–390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook, S. I. , & Sellin, J. H. (1998). Review article: short chain fatty acids in health and disease. Alimentary Pharmacology & Therapeutics, 12, 499–507. [DOI] [PubMed] [Google Scholar]
- Daliu, P. , Santini, A. , & Novellino, E. (2019). From pharmaceuticals to nutraceuticals: Bridging disease prevention and management. Expert Review of Clinical Pharmacology, 12, 1–7. [DOI] [PubMed] [Google Scholar]
- Davis, L. M. , Martinez, I. , Walter, J. , & Hutkins, R. (2010). A dose dependent impact of prebiotic galactooligosaccharides on the intestinal microbiota of healthy adults. International Journal of Food Microbiology, 144, 285–292. [DOI] [PubMed] [Google Scholar]
- de Kivit, S. , Tobin, M. C. , Forsyth, C. B. , Keshavarzian, A. , & Landay, A. L. (2014). Regulation of intestinal immune responses through TLR activation: Implications for pro‐ and prebiotics. Frontiers in Immunology, 18(5), 60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Didari, T. , Solki, S. , Mozaffari, S. , Nikfar, S. , & Abdollahi, M. (2014). A systematic review of the safety of probiotics. Expert Opinion on Drug Safety, 13, 227–239. [DOI] [PubMed] [Google Scholar]
- Dionne, J. , Ford, A. C. , Yuan, Y. , Chey, W. D. , Lacy, B. E. , … Moayyedi, P. (2018). A systematic review and meta‐analysis evaluating the efficacy of a gluten‐free diet and a low FODMAPs diet in treating symptoms of irritable bowel syndrome. The American Journal of Gastroenterology, 113, 1290–1300. [DOI] [PubMed] [Google Scholar]
- Dong, J. , Teng, G. , Wei, T. , Gao, W. , & Wang, H. (2016). Methodological quality assessment of meta‐analyses and systematic reviews of probiotics in inflammatory bowel disease and pouchitis. PLoS ONE, 11, e0168785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doron, S. , & Snydman, D. R. (2015). Risk and safety of probiotics. Clinical Infectious Diseases, 60(Suppl 2), S129–S134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dwivedi, M. , Kumar, P. , Laddha, N. C. , & Kemp, E. H. (2016). Induction of regulatory T cells: A role for probiotics and prebiotics to suppress autoimmunity. Autoimmunity Reviews, 15, 379–392. [DOI] [PubMed] [Google Scholar]
- Falony, G. , Joossens, M. , Vieira‐Silva, S. , Wang, J. , Darzi, Y. , … Raes, J. (2016). Population‐level analysis of gut microbiome variation. Science, 352, 560–564. [DOI] [PubMed] [Google Scholar]
- Ferreira‐Halder, C. V. , Faria, A. V. S. , & Andrade, S. S. (2017). Action and function of faecalibacterium prausnitzii in health and disease. Best Practice & Research. Clinical Gastroenterology, 31, 643–648. [DOI] [PubMed] [Google Scholar]
- Fletcher, J. , Cooper, S. C. , Ghosh, S. , & Hewison, M. (2019). The Role of vitamin D in inflammatory bowel disease: Mechanism to management. Nutrients, 11, E1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford, A. C. , Quigley, E. M. , Lacy, B. E. , Lembo, A. J. , Saito, Y. A. , … Moayyedi, P. (2014). Efficacy of prebiotics, probiotics, and synbiotics in irritable bowel syndrome and chronic idiopathic constipation: systematic review and meta‐analysis. The American Journal of Gastroenterology, 109, 1547–1561. [DOI] [PubMed] [Google Scholar]
- Frei, R. , Akdis, M. , & O'Mahony, L. (2015). Prebiotics, probiotics, synbiotics, and the immune system: Experimental data and clinical evidence. Current Opinion in Gastroenterology, 31, 153–158. [DOI] [PubMed] [Google Scholar]
- Gibson, G. R. , Hutkins, R. , Sanders, M. E. , Prescott, S. L. , Reimer, R. A. , … Reid, G. (2017). The International Scientific Association for Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of prebiotics. Nature Reviews. Gastroenterology & Hepatology, 14, 491–502. [DOI] [PubMed] [Google Scholar]
- Gibson, G. R. , & Roberfroid, M. B. (1995). Dietary modulation of the human colonic microbiota. Introducing the concept of prebiotics. The Journal of Nutrition, 125, 1401–1412. [DOI] [PubMed] [Google Scholar]
- Giron, F. , & Quigley, E. M. M. (2018). Pharmabiotic manipulation of the microbiota in gastrointestinal disorders: A clinical perspective. J Neurogastroenterol Motil., 24, 355–366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groeger, D. , O'Mahony, L. , Murphy, E. F. , Bourke, J. F. , Dinan, T. G. , … Quigley, E. M. (2013). Bifidobacterium infantis 35624 modulates host inflammatory processes beyond the gut. Gut Microbes, 4, 325–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grond, K. , Perreau, J. M. , Loo, W. T. , Spring, A. J. , Cavanaugh, C. M. , & Hird, S. M. (2019). Longitudinal microbiome profiling reveals impermanence of probiotic bacteria in domestic pigeons. PLoS ONE, 14, e0217804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halmos, E. P. , Christophersen, C. T. , Bird, A. R. , Shepherd, S. J. , Gibson, P. R. , & Muir, J. G. (2015, 2015). Diets that differ in their FODMAP content alter the colonic luminal microenvironment. Gut, 64, 93–100. [DOI] [PubMed] [Google Scholar]
- Hansen, L. B. S. , Roager, H. M. , Søndertoft, N. B. , Gøbel, R. J. , Kristensen, M. , … Pedersen, O. (2018). A low‐gluten diet induces changes in the intestinal microbiome of healthy Danish adults. Nature Communications, 9, 4630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiippala, K. , Jouhten, H. , Ronkainen, A. , Hartikainen, A. , Kainulainen, V. , … Satokari, R. (2018). https://www.ncbi.nlm.nih.gov/pubmed/30060606. Nutrients, 10, pii: E988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill, C. , Guarner, F. , Reid, G. , Gibson, G. R. , Merenstein, D. J. , … Sanders, M. E. (2014). Expert consensus document. The International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nature Reviews. Gastroenterology & Hepatology, 11, 506–514. [DOI] [PubMed] [Google Scholar]
- Holscher, H. D. (2017). Dietary fiber and prebiotics and gastrointestinal microbiota. Gut Microbes, 8, 172–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huaman, J. W. , Mego, M. , Manichanh, C. , Cañellas, N. , Cañueto, D. , … Azpiroz, F. (2018). Effects of prebiotics vs a diet low in FODMAPs in patients with functional gut disorders. Gastroenterology, 155, 1004–1007. [DOI] [PubMed] [Google Scholar]
- Hutkins, R. W. , Krumbeck, J. A. , Bindels, L. B. , Cani, P. D. , Fahey, G. Jr. , … Sanders, M. E. (2016). Prebiotics: Why definitions matter. Current Opinion in Biotechnology, 37, 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kali, A. , Bhuvaneshwar, D. , Charles, P. M. , & Seetha, K. S. (2016). Antibacterial synergy of curcumin with antibiotics against biofilm producing clinical bacterial isolates. J Basic Clin Pharmacol, 7, 93–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalra, E. K. (2003). Nutraceutical—Definition and introduction. AAPS PharmSci, 5, E25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kankainen, M. , Paulin, L. , Tynkkynen, S. , von Ossowski, I. , Reunanen, J. , … de Vos, W. M. (2009). Comparative genomic analysis of Lactobacillus rhamnosus GG reveals pili containing a human‐mucus binding protein. Proceedings of the National Academy of Sciences of the United States of America, 106, 17193–17198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khalesi, S. , Bellissimo, N. , Vandelanotte, C. , Williams, S. , Stanley, D. , & Irwin, C. (2019). A review of probiotic supplementation in healthy adults: Helpful or hype? European Journal of Clinical Nutrition, 73, 24–37. [DOI] [PubMed] [Google Scholar]
- Kim, N. , Yun, M. , Oh, Y. J. , & Choi, H. J. (2018). Mind‐altering with the gut: Modulation of the gut‐brain axis with probiotics. Journal of Microbiology, 56, 172–182. [DOI] [PubMed] [Google Scholar]
- Kolmeder, C. A. , Salojarvi, J. , Ritari, J. , de Been, M. , Raes, J. , … de Vos, W. M. (2016). Faecal metaproteomic analysis reveals a personalized and stable functional microbiome and limited effects of a probiotic intervention in adults. PLoS ONE, 11, e0153294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konieczna, P. , Akdis, C. A. , Quigley, E. M. , Shanahan, F. , & O'Mahony, L. (2012). Portrait of an immunoregulatory Bifidobacterium. Gut Microbes, 3, 261–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konieczna, P. , Groeger, D. , Ziegler, M. , Frei, R. , Ferstl, R. , … O'Mahony, L. (2012). Bifidobacterium infantis 35624 administration induces Foxp3 T regulatory cells in human peripheral blood: Potential role for myeloid and plasmacytoid dendritic cells. Gut, 61, 354–366. [DOI] [PubMed] [Google Scholar]
- Kristensen, N. B. , Bryrup, T. , Allin, K. H. , Nielsen, T. , Hansen, T. H. , & Pedersen, O. (2016). Alterations in fecal microbiota composition by probiotic supplementation in healthy adults: A systematic review of randomized controlled trials. Genome Medicine, 8, 52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis, Z. T. , Shani, G. , Masarweh, C. F. , Popovic, M. , Frese, S. A. , … Mills, D. A. (2016). Validating bifidobacterial species and subspecies identity in commercial probiotic products. Pediatric Research, 79, 445–452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, C. , Li, X. , Han, H. , Cui, H. , Peng, M. , … Wang, Z. (2016). Effect of probiotics on metabolic profiles in type 2 diabetes mellitus: A meta‐analysis of randomized, controlled trials. Medicine (Baltimore), 95, e4088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lilly, D. M. , & Stillwell, R. H. (1965). Probiotics. Growth promoting factors produced by micro‐organisms. Science, 147, 747–748. [DOI] [PubMed] [Google Scholar]
- McIntosh, K. , Reed, D. E. , Schneider, T. , Dang, F. , Keshteli, A. H. , … Vanner, S. (2017). FODMAPs alter symptoms and the metabolome of patients with IBS: A randomised controlled trial. Gut, 66, 1241–1251. [DOI] [PubMed] [Google Scholar]
- Medina, D. A. , Pinto, F. , Ovalle, A. , Thomson, P. , & Garrido, D. (2017). Prebiotics mediate microbial interactions in a consortium of the infant gut microbiome. International Journal of Molecular Sciences, 18, 2095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mego, M. , Accarino, A. , Tzortzis, G. , Vulevic, J. , Gibson, G. , … Azpiroz, F. (2017). Colonic gas homeostasis: Mechanisms of adaptation following HOST‐G904 galactooligosaccharide use in humans. Neurogastroenterology and Motility, 29(9). [DOI] [PubMed] [Google Scholar]
- Mego, M. , Manichanh, C. , Accarino, A. , Campos, D. , Pozuelo, M. , … Azpiroz, F. (2017). Metabolic adaptation of colonic microbiota to galactooligosaccharides: a proof‐of‐concept‐study. Alimentary Pharmacology & Therapeutics, 45, 670–680. [DOI] [PubMed] [Google Scholar]
- Micka, A. , Siepelmeyer, A. , Holz, A. , Theis, S. , & Schön, C. (2017). Effect of consumption of chicory inulin on bowel function in healthy subjects with constipation: A randomized, double‐blind, placebo‐controlled trial. International Journal of Food Sciences and Nutrition, 68, 82–89. [DOI] [PubMed] [Google Scholar]
- Miller, L. E. , Zimmermann, A. K. , & Ouwehand, A. C. (2016, 2016). Contemporary meta‐analysis of short‐term probiotic consumption on gastrointestinal transit. World Journal of Gastroenterology, 22, 5122–5131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mortensen, B. , Murphy, C. , O'Grady, J. , Lucey, M. , Elsafi, G. , … Buckley, M. (2019). Bifidobacterium breve bif195 protects against small‐intestinal damage caused by acetylsalicylic acid in healthy volunteers. Gastroenterology. 157;637–646. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- Nicolucci, A. C. , Hume, M. P. , Martínez, I. , Mayengbam, S. , … Reimer, R. A. (2017). Prebiotics reduce body fat and alter intestinal microbiota in children who are overweight or with obesity. Gastroenterology, 153, 711–722. [DOI] [PubMed] [Google Scholar]
- Noto, J. M. , & Peek, R. M. Jr. (2017). The gastric microbiome, its interaction with Helicobacter pylori, and its potential role in the progression to stomach cancer. PLoS Pathogens, 13, e1006573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Toole, P. W. , & Felmer, B. (2016). Studying the microbiome: “Omics” made accessible. Seminars in Liver Disease, 36, 1–6. [DOI] [PubMed] [Google Scholar]
- Paganini, D. , Uyoga, M. A. , Kortman, G. A. M. , Cercamondi, C. I. , Winkler, H. C. , … Zimmermann, M. B. (2019). Iron‐containing micronutrient powders modify the effect of oral antibiotics on the infant gut microbiome and increase post‐antibiotic diarrhoea risk: a controlled study in Kenya. Gut, 68, 645–653. [DOI] [PubMed] [Google Scholar]
- Paganini, D. , Uyoga, M. A. , & Zimmermann, M. B. (2016). Iron fortification of foods for infants and children in low‐income countries: Effects on the gut microbiome, gut inflammation, and diarrhea. Nutrients, 8, E494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandey, K. B. , & Rizvi, S. I. (2009). Plant polyphenols as dietary antioxidants in human health and disease. Oxidative Medicine and Cellular Longevity, 2, 270–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panigrahi, P. , Parida, S. , Nanda, N. C. , Satpathy, R. , Pradhan, L. , … Gewolb, I. H. (2017). A randomized synbiotic trial to prevent sepsis among infants in rural India. Nature, 548, 407–412. [DOI] [PubMed] [Google Scholar]
- Parthasarathy, G. , Chen, J. , Chen, X. , Chia, N. , O'Connor, H. M. , … Bharucha, A. E. (2016). Relationship between microbiota of the colonic mucosa vs feces and symptoms, colonic transit, and methane production in female patients with chronic constipation. Gastroenterology, 150, 367–379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereira, C. , Barros, L. , & Ferreira, I. C. F. R. (2017). Dietary Supplements: Foods, Medicines, or Both? A Controversial Designation with Unspecific Legislation. Current Pharmaceutical Design, 23, 2722–2730. [DOI] [PubMed] [Google Scholar]
- Pinto‐Sanchez, M. I. , Hall, G. B. , Ghajar, K. , Nardelli, A. , Bolino, C. , … Bercik, P. (2017). Probiotic Bifidobacterium longum ncc3001 reduces depression scores and alters brain activity: A pilot study in patients with irritable bowel syndrome. Gastroenterology, 153, 448–459. [DOI] [PubMed] [Google Scholar]
- Plaza‐Diaz, J. , Gomez‐Llorente, C. , Fontana, L. , & Gil, A. (2014). Modulation of immunity and inflammatory gene expression in the gut, in inflammatory diseases of the gut and in the liver by probiotics. World Journal of Gastroenterology, 20, 15632–15649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plovier, H. , & Cani, P. D. (2017). Microbial impact on host metabolism: Opportunities for novel treatments of nutritional disorders? Microbiol Spectr., 5 10.1128/microbiolspec.BAD-0002-2016 [DOI] [PubMed] [Google Scholar]
- Quigley, E. M. M. (2017a). Gut microbiome as a clinical tool in gastrointestinal disease management: Are we there yet? Nature Reviews. Gastroenterology & Hepatology, 14, 315–320. [DOI] [PubMed] [Google Scholar]
- Quigley, E. M. M. (2017b). Bifidobacteria as Probiotic organisms. An introduction In Floch M. H., Ringel Y., & Walker M. A. (Eds.), The microbiota in gastrointestinal pathophysiology (pp. 125–126). London: Elsevier Academic Press. [Google Scholar]
- Quigley, E. M. M. (2019). Prebiotics and Probiotics in Digestive Health. Clinical Gastroenterology and Hepatology, 17, 333–344. [DOI] [PubMed] [Google Scholar]
- Rea, M. C. , Alemayehu, D. , Ross, R. P. , & Hill, C. (2013). Gut solutions to a gut problem: Bacteriocins, probiotics and bacteriophage for control of Clostridium difficile infection. Journal of Medical Microbiology, 62, 1369–1378. [DOI] [PubMed] [Google Scholar]
- Ringel, Y. , Maharshak, N. , Ringel‐Kulka, T. , Wolber, E. A. , Sartor, R. B. , & Carroll, I. M. (2015). High throughput sequencing reveals distinct microbial populations within the mucosal and luminal niches in healthy individuals. Gut Microbes, 6, 173–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saffouri, G. B. , Shields‐Cutler, R. R. , Chen, J. , Yang, Y. , Lekatz, H. R. , … Kashyap, P. C. (2019). Small intestinal microbial dysbiosis underlies symptoms associated with functional gastrointestinal disorders. Nature Communications, 10, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salminen, S. , & van Loveren, H. (2012, 2012). Probiotics and prebiotics: Health claim substantiation. Microbial Ecology in Health and Disease, 23, 40–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders, M. E. , Akkermans, L. M. , Haller, D. , Hammerman, C. , Heimbach, J. , … Vaughan, E. (2010). Safety assessment of probiotics for human use. Gut Microbes, 1, 164–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santini, A. , Cammarata, S. M. , Capone, G. , Ianaro, A. , Tenore, G. C. , … Novellino, E. (2018). Nutraceuticals: Opening the debate for a regulatory framework. British Journal of Clinical Pharmacology, 84, 659–672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santini, A. , & Novellino, E. (2018). Nutraceuticals—shedding light on the grey area between pharmaceuticals and food. Expert Review of Clinical Pharmacology, 11, 545–547. [DOI] [PubMed] [Google Scholar]
- Santini, A. , Tenore, G. C. , & Novellino, E. (2017). Nutraceuticals: A paradigm of proactive medicine. European Journal of Pharmaceutical Sciences, 96, 53–61. [DOI] [PubMed] [Google Scholar]
- Sanz, Y. (2010). Effects of a gluten‐free diet on gut microbiota and immune function in healthy adult humans. Gut Microbes, 1, 135–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schäffler, H. , & Breitrück, A. (2018). Clostridium difficile—From Colonization to Infection. Frontiers in Microbiology, 9, 646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schrezenmeir, J. , & de Vrese, M. (2001). Probiotics, prebiotics, and synbiotics—Approaching a definition. The American Journal of Clinical Nutrition, 73(suppl), 361S–364S. [DOI] [PubMed] [Google Scholar]
- Shanahan, F. , & Collins, S. M. (2010). Pharmabiotic manipulation of the microbiota in gastrointestinal disorders, from rationale to reality. Gastroenterology Clinics of North America, 39, 721–726. [DOI] [PubMed] [Google Scholar]
- Shobar, R. M. , Velineni, S. , Keshavarzian, A. , Swanson, G. , DeMeo, M. T. , … Mutlu, E. A. (2016). The effects of bowel preparation on microbiota‐related metrics differ in health and in inflammatory bowel disease and for the mucosal and luminal microbiota compartments. Clinical and Translational Gastroenterology, 7, e143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slavin, J. , & Feirtag, J. (2011). Chicory inulin does not increase stool weight or speed up intestinal transit time in healthy male subjects. Food & Function, 2, 72–77. [DOI] [PubMed] [Google Scholar]
- Sloan, T. J. , Jalanka, J. , Major, G. A. D. , Krishnasamy, S. , Pritchard, S. , … Spiller, R. C. (2018). A low FODMAP diet is associated with changes in the microbiota and reduction in breath hydrogen but not colonic volume in healthy subjects. PLoS ONE, 13, e0201410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staudacher, H. M. , Lomer, M. C. E. , Farquharson, F. M. , Louis, P. , Fava, F. , … Whelan, K. (2017). A diet low in FODMAPs reduces symptoms in patients with irritable bowel syndrome and a probiotic restores bifidobacterium species: A randomized controlled trial. Gastroenterology, 153, 936–947. [DOI] [PubMed] [Google Scholar]
- Suez, J. , Zmora, N. , & Elinav, E. (2019). Probiotics in the next‐generation sequencing eraGut Microbes. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suez, J. , Zmora, N. , Segal, E. , & Elinav, E. (2019). The pros, cons, and many unknowns of probiotics. Nature Medicine, 25, 716–729. [DOI] [PubMed] [Google Scholar]
- Suez, J. , Zmora, N. , Zilberman‐Schapira, G. , Mor, U. , Dori‐Bachash, M. , … Elinav, E. (2018). Post‐Antibiotic gut mucosal microbiome reconstitution is impaired by probiotics and improved by autologous FMT. Cell, 174, 1406–1423. [DOI] [PubMed] [Google Scholar]
- Surendran Nair, M. , Amalaradjou, M. A. , & Venkitanarayanan, K. (2017). Antivirulence properties of probiotics in combating microbial pathogenesis. Advances in Applied Microbiology, 98, 1–29. [DOI] [PubMed] [Google Scholar]
- Swann, J. P. (2016). The history of efforts to regulate dietary supplements in the USA. Drug Testing and Analysis, 8, 271–282. [DOI] [PubMed] [Google Scholar]
- Theodorou, V. , Ait Belgnaoui, A. , Agostini, S. , & Eutamene, H. (2014). Effect of commensals and probiotics on visceral sensitivity and pain in irritable bowel syndrome. Gut Microbes, 5, 430–436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorell, K. , Lehours, P. , & Vale, F. F. (2017). Genomics of Helicobacter pylori. Helicobacter, 22(Suppl 1). 10.1111/hel.12409. [DOI] [PubMed] [Google Scholar]
- van Loveren, H. , Sanz, Y. , & Salminen, S. (2012). Health claims in Europe: Probiotics and prebiotics as case examples. Annual Review of Food Science and Technology, 3, 247–261. [DOI] [PubMed] [Google Scholar]
- Vandeputte, D. , Falony, G. , Vieira‐Ailva, S. , Wang, J. , Sailer, M. , … Raes, J. (2017). Prebiotic inulin‐type fructans induce specific changes in the human gut microbiota. Gut, 66, 1968–1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, H. , Braun, C. , Murphy, E. F. , & Enck, P. (2019). Bifidobacterium longum 1714™ strain modulates brain activity of healthy volunteers during social stress. The American Journal of Gastroenterology. 114;1152–1162. [epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waskito, L. A. , Salama, N. R. , & Yamaoka, Y. (2018). Pathogenesis of Helicobacter pylori infection. Helicobacter, 23(Suppl 1), e12516. [DOI] [PubMed] [Google Scholar]
- Yan, F. , & Polk, D. B. (2011). Probiotics and immune health. Current Opinion in Gastroenterology, 27, 496–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yatsunenko, T. , Rey, F. E. , Manary, M. J. , Trehan, I. , Dominguez‐Bello, M. G. , … Gordon, J. I. (2012). Human gut microbiome viewed across age and geography. Nature, 486, 222–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoosuf, S. (2019). Makharia GK (2019). Evolving Therapy for Celiac Disease. Front Pediatr., 7, 193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshii, K. , Hosomi, K. , Sawane, K. , & Kunisawa, J. (2019). Metabolism of dietary and microbial vitamin B family in the regulation of host immunity. Frontiers in Nutrition, 6, 48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhernakova, A. , Kurilshikov, A. , Bonder, M. J. , Tigchelaar, E. F. , & Schirmer M …, F. (2016). Population‐based metagenomics analysis reveals markers for gut microbiome composition and diversity. Science, 352, 565–569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu, X. , Xiang, S. , Feng, X. , Wang, H. , Tian, S. , … Han, J. (2019). Impact of cyanocobalamin and methylcobalamin on inflammatory bowel disease and the intestinal microbiota composition. Journal of Agricultural and Food Chemistry, 67, 916–926. [DOI] [PubMed] [Google Scholar]
- Zmora, N. , Suez, J. , & Elinav, E. (2019). You are what you eat: Diet, health and the gut microbiota. Nature Reviews. Gastroenterology & Hepatology, 16, 35–56. [DOI] [PubMed] [Google Scholar]
- Zmora, N. , Zilberman‐Schapira, G. , Suez, J. , Mor, U. , Dori‐Bachash, M. , … Elinav, E. (2018). Personalized gut mucosal colonization resistance to empiric probiotics is associated with unique host and microbiome features. Cell, 174, 1388–1405. [DOI] [PubMed] [Google Scholar]
- Zorzela, L. , Ardestani, S. K. , McFarland, L. V. , & Vohra, S. (2017). Is there a role for modified probiotics as beneficial microbes: A systematic review of the literature. Benef Microbes. 2017, 8, 739–754. [DOI] [PubMed] [Google Scholar]


