Abstract
Resveratrol (trans‐3,4′,5‐trihydroxystilbene) belongs to the family of natural phytoalexins. Resveratrol first came to our attention in 1992, following reports of the cardioprotective effects of red wine. Thereafter, resveratrol was shown to exert antioxidant, anti‐inflammatory, anti‐proliferative, and angio‐regulatory effects against atherosclerosis, ischaemia, and cardiomyopathy. This article critically reviews the current findings on the molecular basis of resveratrol‐mediated cardiovascular benefits, summarizing the broad effects of resveratrol on longevity regulation, energy metabolism, stress resistance, exercise mimetics, circadian clock, and microbiota composition. In addition, this article also provides an update, both preclinically and clinically, on resveratrol‐induced cardiovascular protection and discusses the adverse and inconsistent effects of resveratrol reported in both preclinical and clinical studies. Although resveratrol has been claimed as a master anti‐aging agent against several age‐associated diseases, further detailed mechanistic investigation is still required to thoroughly unravel the therapeutic value of resveratrol against cardiovascular diseases at different stages of disease development.
Linked Articles
This article is part of a themed section on The Pharmacology of Nutraceuticals. To view the other articles in this section visit http://onlinelibrary.wiley.com/doi/10.1111/bph.v177.6/issuetoc
Abbreviations
- AMPK
5′ AMP‐activated protein kinase
- BMAL1
brain and muscle Arnt‐like protein‐1
- CLOCK
circadian locomotor output cycles kaput
- CVD
cardiovascular disease
- eNOS
endothelial NOS
- FMD
flow‐mediated dilatation
- FOXO
Forkhead box O
- HCAECs
human coronary arterial endothelial cells
- HUVEC
human umbilical vein endothelial cell
- KLF2
Krüppel‐like factor 2
- LKB1
liver kinase B1
- NOX
NADPH oxidase
- Nrf2
nuclear factor‐E2‐related factor‐2
- SIRT1
sirtuin 1
- SMC
smooth muscle cell
- UCP2
uncoupling protein 2
1. INTRODUCTION
http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=8741 (trans‐3,4′,5‐trihydroxystilbene) is a hydroxylated derivative of stilbene, falling into the family of natural phytoalexins. Resveratrol exists in either trans‐ or cis‐isomeric forms, but only trans‐resveratrol is responsible for extending life expectancy and producing cardioprotective benefits. The cis‐resveratrol is generally present at much lower contents in food sources and is believed to be less biologically active than the trans‐isoform (Weiskirchen & Weiskirchen, 2016). In this review, the major focus is on trans‐resveratrol, and we use “resveratrol” to denote its trans‐isoform.
In 1992, researchers were expressing the first real interest in the compound resveratrol owing to the “French paradox” (Renaud & deLorgeril, 1992). By activating http://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=2707 (SIRT1), one of the SIRT family of deacetylases which are claimed to act as longevity regulators, resveratrol is considered to be a potent anti‐aging agent. Over the years, as reported by many basic science investigations and over 240 clinical trials, resveratrol exerts beneficial effects against chronic diseases, including cardiovascular diseases (CVDs), diabetes mellitus, hypertension, Alzheimer's disease, liver diseases and kidney diseases, and carcinomas, such as breast cancer, colorectal cancer, and myeloma (Singh et al., 2019). Among these disorders, aging is likely to be the most common risk factor.
Aging generally refers to the gradual loss of physiological integrity, resulting in progressive decline of body function and higher vulnerability to chronic diseases and death. Aging is often associated with the accumulation of ROS, increased inflammatory lesions, dysregulated cell proliferation, and altered angiogenesis (López‐otín, Blasco, Partridge, Serrano, & Kroemer, 2013), underlying the onset and progression of different pathologies. Moreover, autophagy overtly declines during aging, leading to the accumulation of dysfunctional organelles and misfolded protein aggregates. Decreased autophagy exacerbates dysfunction of various organs, particularly the heart (W. Zhang, Huang, et al., 2017; Y. Zhang, Wang, et al., 2017).
In the cardiovascular system, aging is undoubtedly one of the most important determinants of different disorders. In 2015, there were almost 423 million CVD incidents and 18 million CVD deaths, in which ischaemic heart disease remained the leading cause of global CVD mortality (Roth et al., 2017). In spite of the gradual increase in life expectancy, CVDs will still remain the leading cause of human mortality (~40%) worldwide till 2030 (North & Sinclair, 2012), generating enormous economic burdens on society. Importantly, a 2012 clinical trial covering 1,000 individuals stated that resveratrol intake through wine consumption could improve blood lipid profiles and fasting blood glucose (Zamora‐Ros et al., 2012). As resveratrol has long been regarded as an anti‐aging compound, its associated antioxidant, anti‐inflammatory, anti‐proliferative, angio‐regulatory, and autophagy‐enhancing properties might account for its cardioprotective effects.
More importantly, further studies revealed that resveratrol treatment was associated with lower expression of inflammation markers, such as the intercellular adhesion molecules (ICAMs) and https://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=821, in endothelial cells and lower diastolic blood pressure in the systemic circulation, accounting for lower risks of ischaemic stroke and hypertension (Berman, Motechin, Wiesenfeld, & Holz, 2017). In addition to the SIRT1 pathway, resveratrol also regulates other signalling involving http://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=1540 and http://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=594 in the cardiovascular system (Ruderman et al., 2010). Recent studies reveal that resveratrol could modulate the composition gut microbiota and, eventually, alter host metabolite profile for cardiovascular health (Chaplin, Carpéné, & Mercader, 2018). Evidence is also available that resveratrol modulates the biological circadian rhythm (I. Park, Lee, Kim, & Kim, 2014), a central paradigm that may affect development of CVDs. These findings all suggest the diverse beneficial effects of resveratrol towards promoting cardiovascular health. However, resveratrol intake is also associated with certain side effects, although severe adverse effects are not often recorded.
Overall, the purpose of this review article is threefold: (a) to highlight the cardioprotective effects of resveratrol in the context of longevity regulation, energy metabolism, stress resistance, exercise mimetics, circadian clock, and microbiota composition, (b) to elaborate the cardioprotective effects of resveratrol in terms of its antioxidant, anti‐inflammatory, anti‐proliferative, and angioregulatory properties, and (c) to reflect the potential negative outcomes and inconsistent actions of resveratrol usage in both preclinical and clinical findings.
2. MOLECULAR BASIS OF RESVERATROL ACTION
2.1. Resveratrol‐mediated beneficial effects on the cardiovascular system
The broad effects of resveratrol on many cardiovascular target cells make it a feasible option for cardioprotection, readily available in food and drink (Table 1). Over the past 70 years, research groups have been working intensively and extensively to unravel the molecular network of resveratrol action (Figure 1). Upon entering the bloodstream, resveratrol is readily absorbed by vascular endothelial cells through both passive diffusion and https://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=915‐mediated active transport, for its intracellular action (Z. Chen, Shentu, Wen, Johnson, & Shyy, 2013; M. Chen, Yi, et al., 2013). Apart from this first‐line guardian of the vascular wall (i.e., endothelial cells), resveratrol can also readily act on monocytes and M1/M2 macrophages for modulation of inflammatory profiles (Schwager et al., 2017), on vascular smooth muscle cells (SMCs) for regulation of proliferation and apoptosis (Poussier et al., 2005), and on cardiomyocytes for counteracting high oxidative stress (Y. Li et al., 2013).
Table 1.
The function of resveratrol in the cardiovascular system
| Cell type | Function | Reference |
|---|---|---|
| Endothelial cells | Inflammation↓ | (Berman et al., 2017), (Schwager, Richard, Widmer, & Raederstorff, 2017), (Sangwung et al., 2017), (Cicha et al., 2009) |
| Endothelial function↑ | (Wallerath et al., 2002), (Mattagajasingh et al., 2007), (Cheang et al., 2018), (Wong et al., 2013), (Magyar et al., 2012) | |
| Oxidative stress↓ | (W. Zhang, Huang, et al., 2017; Y. Zhang, Wang, et al., 2017), (Liu et al., 2015), (Sangwung et al., 2017), (J. Li, Yu, Ying, Shi, & Wang, 2017), (Cheang et al., 2014) | |
| Autophagy↑ | (Liu et al., 2015), (Vion et al., 2017) | |
| Elongation↑ | (Cicha et al., 2011) | |
| Apoptosis↓ | (J. Li et al., 2017) | |
| Mitochondrial mass↓ | (Csiszar et al., 2009) | |
| Mitochondrial ROS↓ | (Zhou et al., 2014) | |
| eNOS uncoupling↓ | (Xia et al., 2010) | |
| Over‐proliferation↓ | (Yao et al., 2013) | |
| Re‐endothelialization↑ | (Yurdagul et al., 2014) | |
| Angiogenesis↓ | (Fukuda et al., 2006), (Simão et al., 2012) | |
| Vascular SMCs | Hyperplasia↓ | (Poussier, Cordova, Becquemin, & Sumpio, 2005), (Thompson, Martin, & Rzucidlo, 2014), (Yurdagul et al., 2014), (Hwang et al., 2016) |
| Differentiation↑ | (Thompson et al., 2014) | |
| Senescence↓ | (E. N. Kim, Kim, et al., 2018; T. T. Kim, Parajuli, et al., 2018) | |
| Fibrosis↓ | (E. N. Kim, Kim, et al., 2018; T. T. Kim, Parajuli, et al., 2018) | |
| Migration↓ | (Hwang et al., 2016) | |
| Cardiomyocytes | Oxidative stress↓ | (Y. Li et al., 2013), (Bagul, Deepthi, Sultana, & Banerjee, 2015), (Diao et al., 2018), (B. Wang et al., 2014) |
| Cardiac hypertrophy↓ | (Bagul et al., 2015), (Thandapilly et al., 2011), (Gélinas et al., 2018), (Ma et al., 2017), (Matsumura et al., 2018), (Gan et al., 2014) | |
| Apoptosis↓ | (C. J. Chen et al., 2009), (Diao et al., 2018), (B. Wang et al., 2014), (Varma Penumathsa et al., 2006) | |
| Inflammation↓ | (C. Zhang et al., 2012), (Planavila, Iglesias, Giralt, & Villarroya, 2011), (Gupta, DiPette, & Supowit, 2014) | |
| Mitochondrial elongation↓ | (Ren et al., 2017) | |
| Mitochondrial biogenesis↑ | (Biala et al., 2010), (Ma et al., 2017) | |
| Cardiac remodelling↓ | (Biala et al., 2010) | |
| Dysfunctional autophagic flux↓ | (B. Wang et al., 2014) | |
| Cardiac remodelling↑ | (B. Wang et al., 2014) | |
| Cardiac fibrosis↓ | (G. Wang, Song, Zhao, Li, & Liu, 2018), (Diao et al., 2018), (C. Chen et al., 2019) | |
| Ischaemia/reperfusion ischaemia↓ | (Deng, Wang, He, Xu, & Xie, 2017) | |
| Autophagy↑ | (B. Wang et al., 2014) | |
| Cardiac angiogenesis↑ | (Fukuda et al., 2006) | |
| Monocytes/macrophages | Inflammation↓ | (Schwager et al., 2017), (S. Y. Park et al., 2017), (Buttari et al., 2014) |
| M2 polarization↑ | (S. Y. Park et al., 2017) | |
| Monocyte‐to‐macrophage differentiation↓ | (Vasamsetti et al., 2016) | |
| Macrophage infiltration↓ | (Gupta et al., 2014) | |
| Foam cell formation↓ | (Dong et al., 2014), (Berrougui, Grenier, Loued, Drouin, & Khalil, 2009) |
Figure 1.
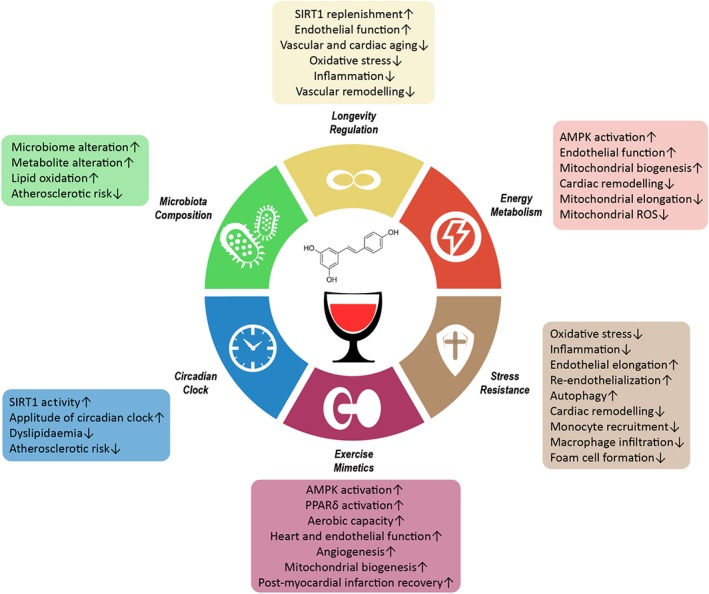
The cardiovascular benefits of resveratrol. The diagram outlines the six major cardiovascular benefits of resveratrol. Resveratrol exerts its effects in the context of longevity regulation, energy metabolism, stress resistance, exercise mimetic, circadian clock, and microbiota composition. Corresponding positive outcomes are listed
2.2. Longevity regulation
Known to extend lifespan of different model organisms (e.g., Saccharomyces cerevisiae, Caenorhabditis elegans, Drosophila melanogaster, Nothobranchius fuzeri, and obese Mus musculus), resveratrol is a noted activator of SIRT1, the http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=2451‐dependent protein deacetylase that regulates aging, transcription, proliferation, apoptosis, and inflammation. During aging, gradual loss of SIRT1 expression in endothelial cells, vascular SMCs, and cardiomyocytes initiates both vascular and cardiac aging (Favero, Franceschetti, Rodella, & Rezzani, 2015). As a pharmacological mimic of calorie restriction, resveratrol activates SIRT1 for the modulation of enzyme activity, protein phosphorylation, and transcription factor function (Figure 2). Collectively, these findings underlie the vasodilatory, antioxidant, anti‐inflammatory, anti‐apoptotic, and anti‐senescence properties of resveratrol in the cardiovascular system.
Figure 2.
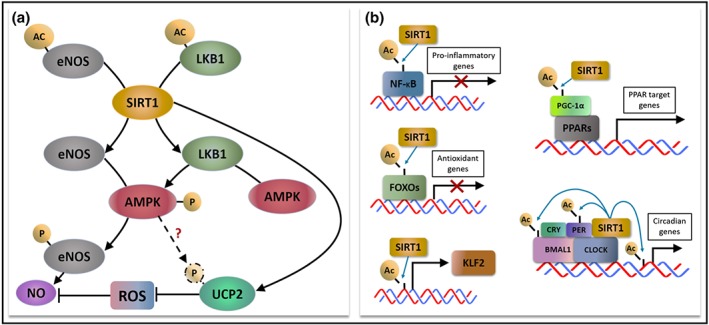
The deacetylating action of resveratrol‐activated SIRT1. (a) SIRT1 deacetylates and hence enhances the activity of eNOS, which also undergoes phosphorylation by AMPK for NO production. SIRT1 deacetylates LKB1 to promote AMPK phosphorylation for downstream eNOS activation. SIRT1 directly activates and may indirectly activate UCP2 through AMPK to counteract oxidative stress. (b) SIRT1 modulates transcriptional activity by directly deacetylating the transcription factors themselves (e.g., NF‐κB and FOXOs), epigenetically acting as a histone deacetylase for transcription factors (e.g., KLF2), indirectly deacetylating the co‐activator of the transcription factor complex (e.g., PPARs) or the combination of above mechanisms (e.g., CLOCK:BMAL1)
2.2.1. Enzyme activity modulation
Resveratrol benefits endothelial function by affecting activities of enzymes that are crucial to maintaining cardiovascular health (Figure 2a). In 2002, Wallerath et al. were the first to show that resveratrol up‐regulates http://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=1249 expression in HUVECs (Wallerath et al., 2002), where eNOS is the enzyme generating the unorthodox gaseous molecule NO to mediate endothelium‐dependent vasorelaxation. Also, resveratrol‐induced activation of SIRT1 also increases eNOS activity via the post‐translational deacetylation of its lysine residues (Mattagajasingh et al., 2007). NO directly functions as a scavenger of ROS, in particular, the superoxide anions (O2 −). Normal eNOS expression and activity control the NO/ROS balance in order to prevent endothelial dysfunction (Folino, Losano, & Rastaldo, 2013). As an early marker of hypertension and atherosclerosis, endothelial dysfunction increases vascular tone and prompts vascular remodelling (Ungvari et al., 2018).
In addition, SIRT1 activation contributes to the regulation of other redox enzymes including http://www.guidetopharmacology.org/GRAC/FamilyDisplayForward?familyId=993) and SOD in the cardiovascular system. Generally expressed by endothelium, vascular smooth muscle, cardiomyocytes, and adventitia, NOX is one of the main physiological sources of ROS in the cardiovascular system. Meanwhile, SOD acts as an antioxidant enzyme (Montezano & Touyz, 2014). Through diminishing and enhancing the expression and activity of NOX and SOD, respectively, SIRT1 reduces oxidative stress in endothelial cells (W. Zhang, Huang, et al., 2017; Y. Zhang, Wang, et al., 2017) and in cardiomyocytes of fructose‐induced diabetic Sprague–Dawley rats (Bagul et al., 2015). A lower oxidative stress in cardiomyocytes is associated with reduced risk of cardiac hypertrophy in diabetes (Bagul et al., 2015). Resveratrol‐induced SIRT1 activation thus provides antioxidant benefits by targeting both ROS formation and clearance.
2.2.2. Post‐translational regulation
Although SIRT1 is well‐known as a protein deacetylase, its ability to regulate phosphorylation through deacetylation of corresponding kinases enriches the cardiovascular benefits of resveratrol (Figure 2b). In particular, resveratrol can deacetylase the master kinase AMPK and the tumour suppressor http://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=2212. Resveratrol‐activated SIRT1 deacetylates LKB1 to increase AMPK phosphorylation at Thr172, which in turn phosphorylates eNOS at Ser1177 (Cacicedo et al., 2011), thus providing hints that SIRT1 can directly and indirectly increase eNOS activity through AMPK deacetylation and AMPK‐mediated eNOS phosphorylation. Similar resveratrol‐induced activation of SIRT1/AMPK axis is also present in macrophages, favouring macrophage polarization towards an anti‐inflammatory M2 phenotype (S. Y. Park et al., 2017). In brief, AMPK activation is important in maintaining an antioxidant and anti‐inflammatory interior environment for cell components of the cardiovascular system. As AMPK activity declines with age (Salminen, Kaarniranta, & Kauppinen, 2016), resveratrol can serve as a compensatory supplement for age‐related AMPK deactivation.
2.2.3. Transcriptional changes
Resveratrol‐activated SIRT1 can translocate into the nucleus to modulate functions of transcription factors. SIRT1 achieves such effects either by deacetylating transcription factor(s) directly or by epigenetically deacetylating the corresponding histone proteins (Z. Chen, Shentu, et al., 2013; M. Chen, Yi, et al., 2013; Figure 2b). Transcription factors like Forkhead box O (FOXO) proteins and the pro‐inflammatory NF‐κB can be directly deacetylated by SIRT1. Both transcription factors are extensively involved in the crosstalk between oxidative stress and inflammation (Z. Chen, Shentu, et al., 2013; M. Chen, Yi, et al., 2013). SIRT1‐mediated deacetylation activates FOXOs for subsequent up‐regulation of antioxidants including catalase, SOD, and thioredoxin‐1 (Matsushima & Sadoshima, 2015). Moreover, SIRT1‐mediated FOXO1 activation was confirmed to protect endothelial cells against oxidative stress (Liu et al., 2015). Through the SIRT1/FOXO1 axis, resveratrol defends cardiomyocytes against hypoxia‐induced apoptosis (C. J. Chen et al., 2009), which is a hallmark of ischaemic heart disease. Clinically, a human trial stated that resveratrol supplement improves ischaemic stroke recovery by reducing Levels of matrix metallopeptidase 9, a known downstream target of FOXO1 related to tissue remodelling (J. Chen, Bai, Zhao, Sui, & Xie, 2016; M. Chen, Yi, et al., 2016). Resveratrol‐induced SIRT1/FOXO cascade confers cardiovascular protection against oxidative stress and undesirable vascular remodelling, thus alleviating the progression of hypertension and atherosclerotic CVDs.
During aging, a slow increase in activation of NF‐κB underlies the chronic inflammatory response (Salminen & Kaarniranta, 2009). Resveratrol represses NF‐κB signalling through SIRT1‐mediated deacetylation of p65 of the NF‐κB complex (W. Zhang, Huang, et al., 2017; Y. Zhang, Wang, et al., 2017). Resveratrol markedly inhibits inflammation by suppressing nuclear translocation of NF‐κB in anoxia/reoxygenation‐injured cardiomyocytes (C. Zhang et al., 2012). Resveratrol also prevents NF‐κB activation to down‐regulate pro‐inflammatory cytokines in both human M1 and M2 macrophages subjected to challenge with 7‐oxo‐cholesterol (Buttari et al., 2014). These results support the protective effect of resveratrol against chronic inflammation, lowering the risk for cardiovascular events such as atherosclerosis and thrombosis during aging.
Another known SIRT1‐activated transcription factor is Krüppel‐like factor 2 (KLF2), which plays a critical role in flow‐mediated vaso‐protective phenotype in human endothelial cells. SIRT1 can epigenetically up‐regulate KLF2 levels, by histone deacetylation (Z. Chen, Shentu, et al., 2013; M. Chen, Yi, et al., 2013) and KLF2 is believed to confer vasodilatory, anti‐inflammatory, and anti‐thrombotic properties to vascular endothelium. According to a recent transcriptomic study, endothelium‐specific knockout of KLF2 in mouse primary microvascular endothelial cells up‐regulates expression of genes involved in hypoxia, angiogenesis, coagulation, unfolded protein response, and inflammatory reaction (Sangwung et al., 2017). Therefore, the SIRT1/KLF2 axis plays important roles in endothelial homeostasis.
The PPAR family comprises a collection of nuclear receptor proteins that act as transcription factors to modulate cardiovascular function. SIRT1 mediates deacetylation of https://www.guidetopharmacology.org/GRAC/CoregulatorDisplayForward?coregId=62, which binds to PPAR protein as a coactivator for downstream transcriptional regulation. Through the SIRT1/https://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=593 cascade, resveratrol exerts anti‐inflammatory and anti‐hypertrophic effects by lowering the expression of the pro‐inflammatory cytokine https://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=771 and https://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=4869 in Sprague‐Dawley rat cardiomyocytes (Planavila et al., 2011). Also, resveratrol ameliorates endothelial dysfunction in obese and diabetic mice through a SIRT1/https://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=594 pathway (Cheang et al., 2018), supporting the vaso‐protective effect of resveratrol. Resveratrol‐induced SIRT1/PPARδ signalling is a new mechanism that benefits the cardiovascular function.
SIRT1 plays a critical role in regulating the circadian clock by sensing changes in the cellular metabolic states. Better control of circadian rhythms is believed to improve health and increase longevity of organisms. SIRT1 binds to the circadian locomotor output cycles kaput (https://www.guidetopharmacology.org/GRAC/FamilyDisplayForward?familyId=872#2649): brain and muscle http://www.guidetopharmacology.org/GRAC/FamilyDisplayForward?familyId=916 heterodimer transcription factor for deacetylation, altering circadian gene expression (Hood & Amir, 2017). The circadian genes are involved in a variety of downstream pathways in different systems including the cardiovascular system. More detailed information of resveratrol‐induced circadian regulation is discussed in Section 2.6. Taken together, resveratrol‐induced SIRT1 up‐regulation compensates for the gradual SIRT1 loss in the aging cardiovascular system, thus lowering CVD risks.
2.3. Energy metabolism
2.3.1. AMPK activation
Resveratrol acts as a metabolic regulatory agent in the cardiovascular system to modulate energy homeostasis. Resveratrol alters the activity of AMPK, the master energy sensor and kinase for tight regulation of anabolic and catabolic pathways in different organs. In low energy states or low ATP/AMP ratios, AMPK increases ATP generation by increasing the expression or activity of proteins related to catabolism, while it reduces ATP consumption by turning off anabolic pathways in high energy states or high ATP/AMP ratios (Hardie, 2011). Because AMPK activity declines with age, energy homeostasis is progressive disturbed during aging. Declining sensitivity of AMPK activation increases chronic low‐grade inflammation, increases cellular stress, and raises the risk for age‐associated CVDs (Salminen et al., 2016). Resveratrol stimulates AMPK activation through direct and indirect mechanisms, to compensate for the progressive loss of AMPK activity during aging.
Indirect AMPK activation is SIRT1‐dependent and remains the dominant view of resveratrol action. As described in Section 2.2, resveratrol activates the SIRT1/LKB1/AMPK axis to facilitate downstream protein phosphorylation. However, resveratrol, at higher doses, can directly activate AMPK but in a SIRT1‐independent manner. In a SIRT1‐knockout mouse, resveratrol, given at a high dose (4 g·kg−1 diet; Price et al., 2012) was still able to activate AMPK. On the contrary, some studies stated that resveratrol can activate SIRT1 in an AMPK‐dependent manner to benefit the cardiovascular system. AMPK was even shown to phosphorylate SIRT1 at T344 (Lau, Liu, Inuzuka, & Gao, 2014). Alternatively, AMPK can increase the SIRT1 activity by increasing intracellular levels of NAD+ (Ruderman et al., 2010). Further studies are still required to understand the AMPK/SIRT1 partnership in mediating resveratrol action.
Sustained AMPK activation mediates cardiovascular benefits of resveratrol. Resveratrol treatment inhibits ROS overproduction, induced by high glucose, in bovine retinal capillary endothelial cells in an AMPK/SIRT1/PGC‐1α‐dependent fashion (J. Li et al., 2017). Resveratrol‐activated AMPK phosphorylates eNOS to improve vascular function of the superior thyroid arteries from patients with dyslipidaemia and hypertension (Carrizzo et al., 2013). In a clinical trial, daily administration of 75‐mg resveratrol for 6 weeks augmented flow‐mediated dilatation (FMD) in healthy obese individuals (Wong et al., 2013). Co‐supplementation of resveratrol with https://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=2489 improved cerebral blood flow in young male individuals (Wightman et al., 2014). These results suggest the antioxidant and vasoregulatory effects of resveratrol through acting on vascular endothelium.
Beyond endothelial cells, AMPK activation by resveratrol promotes AMPK‐NO signalling to reverse https://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=484‐induced cardiomyocyte hypertrophy in adult rats (Thandapilly et al., 2011). Aberrant SMC proliferation promotes the initiation and early progression of plaque formation. Resveratrol‐induced AMPK activation favours vascular SMC differentiation, where differentiated vascular SMCs demonstrate a slower pace of proliferation (Thompson et al., 2014). Therefore, resveratrol can limit vascular SMC proliferation to retard early plaque formation. In addition, resveratrol ameliorates monocyte‐to‐macrophage differentiation, an inflammation‐associated phenomenon with relevance to atherosclerosis, by modulating intracellular http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=6737 homeostasis via AMPK activation (Vasamsetti et al., 2016). These findings highlight the anti‐hypertrophic and anti‐atherogenic effects of resveratrol. Taken together, resveratrol works as an AMPK activator, either in a direct or indirect manner, to benefit cardiovascular function against CVDs.
2.3.2. Mitochondrial dynamics
Mitochondria function as central organelles designed for energy metabolism. Oxidative phosphorylation is a key mitochondrial process for transforming free energy to the cellular energy currency ATP. Decreased mitochondrial activity and quality are inevitable biological events during aging, potentially increasing the risk of CVDs (N. Sun, Youle, & Finkel, 2016). In virtually all the tissues, mammalian aging is associated with the progressive build‐up of mitochondrial oxidative stress. The accumulating ROS accounts for oxidative damage in macromolecules including proteins, DNA, and lipids, thus expediting aging. Dysregulated mitochondrial dynamics and mitochondrial oxidative stress are often identified in age‐related diseases, such as CVDs, metabolic syndrome, and neurocognitive disorders (Ungvari, Sonntag, de Cabo, Baur, & Csiszar, 2011). Moreover, defects in mitochondrial fission cause mitochondrial elongation, prompting senescence in aging cells (Ren et al., 2017). Notably, resveratrol acts on both mitochondrial dynamics and mitochondrial ROS in the cardiovascular system.
Resveratrol can directly bind to mitochondrial complex I (Gueguen et al., 2015) and it regulated mitochondrial dynamics by increasing mitochondrial biogenesis and by ameliorating mitochondrial elongation. In vitro, resveratrol significantly increased mitochondrial mass in human coronary arterial endothelial cells (HCAECs; Csiszar et al., 2009), suggesting that resveratrol may potentially minimize dysregulated mitochondrial biogenesis which is frequently observed in the early onset of endothelial dysfunction (Ungvari et al., 2011). Likewise, in animal studies in vivo, resveratrol induced mitochondrial biogenesis in cardiomyocytes to attenuate https://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=2504‐induced cardiac remodelling (Biala et al., 2010). More importantly, resveratrol treatment is associated with improved vascular function and increased mitochondrial number in glucose‐intolerant, elderly patients (Pollack et al., 2017). An increased mitochondrial biogenesis can ameliorate the impaired energy homeostasis of aging heart and vasculature. In senescent cardiomyocytes, resveratrol suppressed mitochondrial elongation through up‐regulating the expression of dynamin‐related protein 1 (Ren et al., 2017). The elongated and dysfunctional mitochondria eventually accumulate in cardiomyocytes, underlying the reduction in ischaemic preconditioning of aged hearts.
Resveratrol can lower mitochondrial ROS production for better energy homeostasis. In HCAECs, resveratrol reduced mitochondrial oxidative stress in a SIRT1/SOD‐dependent manner (Ungvari et al., 2009). In addition to SIRT1, resveratrol up‐regulates https://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=622)‐dependent SIRT3 transcription in endothelial cells by stimulating PGC‐1α signalling. Subsequently, SIRT3 is translocated into mitochondria to deacetylate mitochondrial enzymes related to mitochondrial ROS inhibition (Zhou et al., 2014). Increased oxidative stress in mitochondria inactivates enzymes crucial to mitochondrial metabolism, especially α‐ketoglutarate dehydrogenase, greatly limiting ATP generation (Ungvari et al., 2011). This SIRT3‐dependent signalling inhibits mitochondrial dysfunction and the latter may lead to endothelial dysfunction (Zhou et al., 2014). Moreover, transplantation of cardiac progenitor cells, previously treated by mitochondria‐targeted resveratrol delivery, into mice with cardiomyopathy reduces oxidative stress and apoptosis (Abe, Yamada, Takeda, & Harashima, 2018). Resveratrol exerts antioxidant effects against both mitochondrial and non‐mitochondrial sources of ROS, thus replenishing ATP production during aging.
2.4. Stress resistance
Any imbalance in the generation and amelioration of stress prompts the body towards a diseased state. Such imbalance progressively increases with age, resulting in higher stress. During aging, different forms of stress accumulate. The gradual build‐up of oxidative stress, progressive loss of shear stress, and prolonged stress‐induced inflammation impair cardiovascular function. The cumulative build‐up of oxidative damage (i.e., oxidative stress) on intracellular macromolecules (e.g., DNA, proteins, and lipids) stresses various cellular components of the cardiovascular system. ROS overproduction is usually linked to the onset of cardiovascular events, such as atherosclerosis, stroke, and myocardial infarction. The antioxidant effects of resveratrol can alleviate age‐related ROS overproduction.
2.4.1. Oxidative stress
Resveratrol limits oxidative stress in the cardiovascular system through a range of molecular mechanisms. As discussed in Section 2.2, resveratrol up‐regulates antioxidant genes including catalase, SOD, and thioredoxin‐1 through the SIRT1/FOXO axis. Resveratrol clearly elevated the expression of SOD isoforms (SOD1–3), catalase, and GSH peroxidase 1 in ApoE−/− mice to reverse eNOS uncoupling (Xia et al., 2010), a state when eNOS produces superoxide anions instead of NO upon high oxidative stress and associated endothelial dysfunction (C. K. Cheng, Bakar, Gollasch, & Huang, 2018). In a streptozotocin‐induced diabetic cardiomyopathy mouse model, long‐term feeding of resveratrol‐enriched diet reduced myocardial oxidative stress through SIRT1/FOXO1‐mediated defense mechanisms (B. Wang et al., 2014). Activation of SIRT‐1‐dependent signalling partly explains the antioxidant effect of resveratrol in the cardiovascular system.
Resveratrol also exerts antioxidant effects in a SIRT1‐independent manner. In quiescent states, the transcription factor, http://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=3057 is localized in the cytoplasm and is subjected to ubiquitination‐mediated degradation. In the presence of resveratrol, Nrf2 more readily translocates to the nucleus for transcriptional activation of antioxidant enzymes, such as NAD(P)H:quinone oxidoreductase 1 and https://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=1441 (Bryan, Olayanju, Goldring, & Park, 2013). A recent study links the resveratrol‐induced Nrf2 up‐regulation to the prevention of cardiac fibrosis in streptozotocin‐induced diabetic mice (G. Wang et al., 2018). Hyperactivity of the renin‐angiotensin system is associated with build‐up of oxidative stress and development of hypertension. Resveratrol can inhibit the renin‐angiotensin system activity in aging C57BL/6 mice, as reflected by reduction of serum level of angiotensin II and in aortic expression of http://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=1613, as well as suppressing the levels of aortic ROS (E. N. Kim, Kim, et al., 2018; T. T. Kim, Parajuli, et al., 2018). Resveratrol supplementation may become an alternative therapy to target the impaired antioxidant mechanisms upon the onset of hypertension.
Resveratrol‐induced AMPK activation accounts for the ROS‐lowering effect. One of the downstream targets of AMPK is the ubiquitously expressed protein, https://www.guidetopharmacology.org/GRAC/FamilyDisplayForward?familyId=207#1067, the mitochondrial transporter protein responsible for uncoupling oxygen consumption and ATP synthesis. Although the putative phosphorylation site on UCP2 remains undetermined, UCP2 dissipates metabolic energy and lowers mitochondrial membrane potential to inhibit ROS accumulation. UCP2 is a pivotal protein to counteract oxidative stress in endothelial cells of diet‐induced obese mice (Tian et al., 2012). Elevation of UCP2 expression suppresses oxidative stress and reduces both myocardial remodelling and apoptosis in resveratrol‐treated rats with cardiomyopathy (Diao et al., 2018). A recent study shows that intraperitoneal resveratrol injection protects cardiomyocytes against ischaemia/reperfusion injury through a SIRT1/UCP2‐dependent mechanism (Deng et al., 2017). Although SIRT1 directly docks to the UCP2 promoter for transcriptional activation (Olmos et al., 2013), a comprehensive mechanism underlying the interplay between AMPK, UCP2, and SIRT1 is still lacking.
These preclinical findings provide support for the claimed antioxidant effects of resveratrol in clinical studies. In a randomized trial, a daily intake of 500‐mg resveratrol for 30 days improved total antioxidant status in healthy smokers (Bo et al., 2013). Furthermore, resveratrol supplemented with other phenolics, especially caffeic acid and gallic acid, can act synergistically in enhancing the antioxidant activity (Skroza, Generalić Mekinić, Svilović, Šimat, & Katalinić, 2015). Collectively, many antioxidant mechanisms show that resveratrol decreases ROS in the cardiovascular system, protecting against the cumulative oxidative damage associated with aging.
2.4.2. Shear stress
Blood vessels experience a luminal stress due to continuous mechanical loading from blood flow and blood pressure. This mechanical stress, termed shear stress, not only plays a pivotal role in defining endothelium morphology and phenotype but also exerts vaso‐protective effects by augmenting NO release for NO/ROS homeostasis. High shear stress is caused by laminar blood flow in straight arteries. In the presence of physiologically relevant laminar flow, vascular endothelial cells align in the same direction as blood flow and express higher levels of SIRT1. In contrast, disturbed or oscillatory blood flow generally occurs in curved arteries corresponding to a lower shear stress. This pathophysiologically relevant, disturbed flow results in irregular alignment of endothelial cells, down‐regulation of SIRT1, and up‐regulation of pro‐atherogenic genes (Z. Chen et al., 2010). Aging is associated with increased oscillatory shear rate and decreased laminar shear stress, resulting in reduced SIRT1 level and NO bioavailability (Trinity et al., 2014). Subsequently, the low shear stress promotes ROS generation and vascular inflammation (Z. Chen et al., 2010), the critical paradigms of vascular aging and atherosclerosis. The SIRT1‐activating property of resveratrol allows endothelial cells to resist the accumulation of ROS induced by low shear stress and vascular inflammation.
Resveratrol is beneficial against the harmful effects of low shear stress during vascular aging. Interestingly, resveratrol exerts effects similar to those of laminar flow, on endothelial cells (Figure 3). Both laminar flow and resveratrol up‐regulate SIRT1 expression to inhibit over‐proliferation of endothelial cells upon early onset of atherosclerosis. Laminar flow and disturbed flow oppositely regulate endothelial cell growth to partly account for their anti‐ and pro‐atherogenic properties respectively (Yao et al., 2013). Furthermore, both resveratrol and laminar flow inhibit recruitment of monocytic cells to atherosclerosis‐prone regions, thus attenuating the inflammatory response (Cicha et al., 2009). These findings suggest that laminar shear stress and resveratrol share similar atheroprotective actions and that resveratrol can serve as an extrinsic supplement to replenish SIRT1 loss during aging.
Figure 3.
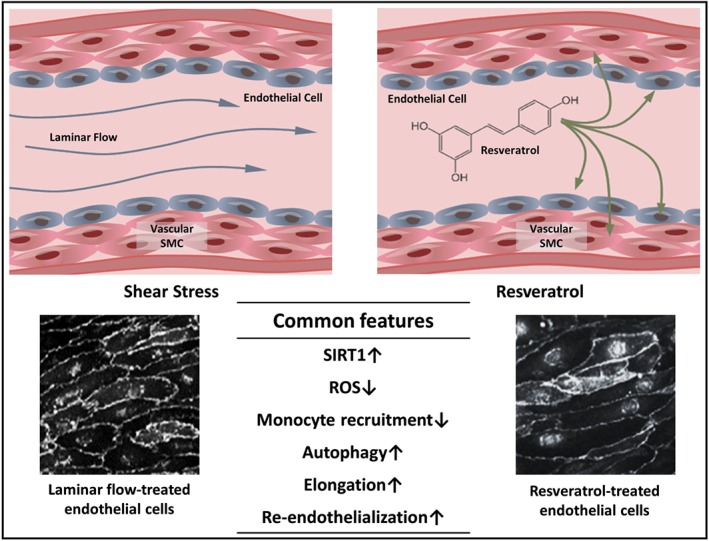
Common endothelial benefits induced by laminar shear stress and resveratrol. Both shear stress and resveratrol trigger SIRT1 up‐regulation, ROS level down‐regulation, inhibition of monocyte recruitment, increased autophagy, endothelial elongation, and improved re‐endothelialization in endothelial cells. Similar elongation patterns are observed in phase contrast images of laminar flow‐treated (reproduced with permission; Copyright 2017, Development; Poduri et al., 2017) and resveratrol‐treated endothelial cells (reproduced with permission; Copyright 2011, Journal of Atherosclerosis and Thrombosis; Cicha et al., 2011)
A low shear stress corresponds to a high oxidative stress owing to augmented oxidase activities and diminished superoxide scavenging capacity. Resveratrol produces a similar oxidative stress‐lowering effect as the atheroprotective laminar flow. In the context of redox homeostasis, both resveratrol and laminar flow can promote autophagy, the self‐digestion process responsible for degradation of damaged organelles and misfolded proteins in endothelial cells, by AMPK‐ and SIRT/FOXO‐dependent cascades. A dysregulated autophagy causes improper alignment of endothelial cells, endothelial dysfunction and increases the severity of atherosclerotic lesions (Vion et al., 2017). In addition to endothelial cells, resveratrol can enhance autophagy in hearts of diabetic mice, resulting in diminished cardiac oxidative stress and apoptosis (B. Wang et al., 2014). Similarly, resveratrol repressed the decreased autophagy during aging to increase resistance against age‐related oxidative stress.
Both resveratrol and laminar flow can trigger morphological and structural changes in endothelial cells to provide cardioprotective benefits. Resveratrol elongates endothelial cells, causing them less likely to detach from the vascular wall and become part of the developing thrombotic plug (Cicha et al., 2011). Likewise, exposure to laminar flow causes endothelial cell elongation and provides anti‐inflammatory benefits (Poduri et al., 2017; Figure 3). An elongated morphology of endothelial cells is associated with a better vascular homeostasis. Furthermore, both resveratrol and laminar flow facilitate re‐endothelialization, an important regrowth process of endothelial cells after arterial injury. After angioplasty, hyperplasia of vascular SMC causes restenosis of the coronary artery. Resveratrol restrains vascular SMC overgrowth and enhances re‐endothelialization, thus greatly reducing the risk of thrombosis (Yurdagul et al., 2014). This is consisitent with another study showing that upstream shear stress is important for facilitating re‐endothelialization to avoid neointimal hyperplasia (Liu et al., 2017). Further studies may focus on the synergistic effect of resveratrol and laminar shear stress in resisting age‐related cardiovascular events.
2.4.3. Stress‐induced inflammation
Inflammaging refers to the development of chronic and low‐grade inflammation during aging, which contributes to the increasing risks of age‐related CVDs. The chronic accumulation of endogenous cell debris, erroneous macromolecules, and oxidative stress byproducts results in sustained activation of the innate immune system (Franceschi, Garagnani, Parini, Giuliani, & Santoro, 2018). Upon aging, the accumulating oxidative stress and the diminishing shear stress even reinforce the inflammatory responses to accelerate the onset and progression of inflammation‐associated cardiovascular complications, especially atherosclerotic CVDs. Resveratrol, by exerting antioxidant effects to attenuate ROS accumulation and by eliciting anti‐inflammatory effects, may ameliorate inflammaging.
Resveratrol intake can limit pro‐inflammatory cytokine profile in different cell components of the cardiovascular system. Resveratrol down‐regulates the expression of https://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=6757 and IL‐1β in https://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=5074‐stimulated HCAECs (F. C. Huang et al., 2017). In neonatal cardiomyocytes, resveratrol suppresses LPS‐induced up‐regulation of CCL2 (Planavila et al., 2011). Lower expression of chemokines inhibits infiltration of large numbers of monocytes, which differentiate to excessive macrophages causing tissue damage, rather than tissue repair, in endothelium and myocardium (Shahid, Lip, & Shantsila, 2018). More specifically, lower expression of chemokines can attenuate monocyte adhesion onto endothelium, which is the initial event of atherogenesis. Resveratrol inhibited macrophage and mast cell infiltration in pressure overloaded hearts of C57/BL6 mice (Gupta et al., 2014). Such anti‐inflammatory properties of resveratrol allow it to attenuate cardiac dysfunction and remodelling, two outcomes accelerating the progression to heart failure.
The anti‐inflammatory effect of resveratrol is consistent in both preclinical and clinical findings. In addition to cell‐specific cytokine levels, resveratrol also limits the levels of serum cytokines. Daily consumption of 8‐mg resveratrol over one year, in 75 patients undergoing primary CVD prevention, improved the serum inflammatory profile, characterized by down‐regulated TNF‐α levels and https://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=4998/https://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=4975 ratios (Tomé‐Carneiro, Gonzálvez, Larrosa, García‐Almagro, et al., 2012). A recent meta‐analysis also correlated resveratrol supplementation with the down‐regulation of inflammatory biomarkers, especially C‐reactive protein and TNF‐α, in patients with metabolic syndrome (Tabrizi et al., 2018). Through down‐regulation of pro‐inflammatory cytokine profile, resveratrol might be an inflammaging alleviator in the cardiovascular system.
In addition to the major cardiovascular cell components, resveratrol also acts on immune cells to protect cardiovascular function indirectly. Through modulation of GSH homeostasis, resveratrol suppressed the differentiation of monocyte to macrophages, which is an initial stage in progression of atherosclerosis (Vasamsetti et al., 2016). By massive uptake of oxLDL, the lipid‐laden macrophages develop into foam cells, which participate in inflammation and tissue remodelling inside the plaque. Resveratrol can inhibit this process by limiting lipid accumulation through PPARγ and PPARα signalling (Dong et al., 2014). in vitro data also indicate that resveratrol triggers apolipoprotein A1‐mediated cholesterol efflux in macrophages (Berrougui et al., 2009). Therefore, resveratrol prevents cholesterol accumulation in and increases cholesterol clearance from macrophages. These findings support the anti‐inflammatory and anti‐atherogenic property of resveratrol on immune cells.
2.5. Exercise mimetics
Regular physical activity benefits metabolic and cardiovascular function by lowering cardiovascular risks of developing hypertension, diabetes mellitus, obesity, metabolic syndrome, and dyslipidaemia. Regular exercise in old adults is associated with lower risks of coronary artery disease, haemorrhage stroke, ischaemic stroke, and atherosclerosis (S. J. Cheng et al., 2013). The term “exercise mimetic” refers to those pharmacological compounds which, to a certain extent, mimic the central and systemic benefits of exercise. These mimetics are particularly beneficial to people subjected to severe injury, serious illness, and/or aging‐associated frailty, who have difficulty in performing regular exercise. Resveratrol could be one of such mimetics to protect the heart and vasculature.
2.5.1. Shear stress substitute
One of the most significant features of exercise training is the increased shear stress of flowing blood on the luminal endothelial cells. The elevated shear stress explains the beneficial signalling events such as AMPK activation, SIRT1 up‐regulation, reduced eNOS uncoupling, enhanced eNOS activity, and increased expression of ROS scavengers (Schuler, Adams, & Goto, 2013). Collectively, these events account for the reduced ROS level, improved endothelial function, and diminished arterial stiffness after chronic exercise (Schuler et al., 2013). As resveratrol can mimic the beneficial effects of increased shear stress as described in Section 2.4, it might serve as a potential exercise mimetic. Resveratrol supplement provides an option to compensate for the declined AMPK activity and SIRT1 expression level in the vasculature during aging.
2.5.2. AMPK activator
Another main feature of exercise mimetic is AMPK activation at different tissues and organs. During exercise, AMPK is activated in organs including skeletal muscle, liver, and adipose tissue. Exercise promotes AMPK activation in both vasculature and heart (Zaha & Young, 2012). In general, exercise depletes the energy currency ATP in the cells comparable to calorie restriction. AMPK is consequently activated to boost the ATP/AMP ratio (Ke, Xu, Li, Luo, & Huang, 2018). Resveratrol can bring about cardiovascular benefits comparable to http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=4779, well‐known as an AMPK activator and another potential exercise mimetic. For instance, both resveratrol and metformin share analogous beneficial effects in counteracting oxidative stress in endothelial cells by activating AMPK (Cheang et al., 2014). They both can attenuate cardiac hypertrophy by activating AMPK (Gélinas et al., 2018). In a previous study, resveratrol and metformin may synergistically lower hepatic lipid contents and systolic blood pressure in Type 2 diabetes patients (Timmers et al., 2016). Hence, resveratrol might be another option of exercise mimetic and an add‐on agent for other pharmacological treatments, to benefit the cardiovascular function.
2.5.3. Aerobic capacity and cardiac function
Exercise training contributes to the improved aerobic capacity and heart function, which guarantee cardiovascular benefits in aging populations and reduce the risks of CVDs like myocardial hypertrophy, cardiac fibrosis, and eventual heart failure. Animal experiments have highlighted the cardiac protective effects of resveratrol. In an overload‐induced cardiac hypertrophic mouse, resveratrol supplement significantly suppressed cardiac fibrosis (C. Chen et al., 2019). Furthermore, resveratrol treatment shares similar beneficial outcomes as aerobic exercise training in improving mitochondrial biogenesis to alleviate cardiac hypertrophy (Ma et al., 2017). Resveratrol in combination with statins is more effective in counteracting cardiomyocyte apoptosis in hypercholesterolemic rats (Varma Penumathsa et al., 2006).
Available experimental data would suggest beneficial effects of resveratrol on aerobic capacity. Resveratrol supplement was reported to alleviate aerobic capacity loss in ApoE−/− mice (Tomayko, Cachia, Chung, & Wilund, 2014). More importantly, resveratrol intake exerts synergistic effects to promote health in combination with exercise. Mice subjected to resveratrol supplement and anaerobic training are associated with elevated tissue glycogen, muscle growth, and aerobic capacity (Kan et al., 2018). In a clinical study, a higher aerobic fitness correlates with lower CVD risk (Fernström, Fernberg, Eliason, & Hurtig‐Wennlof, 2017).
2.5.4. Post‐myocardial infarction recovery
Physical activity post‐myocardial infarction provides cardiovascular benefits. Appropriate amount of exercise post‐infarction critically reduces mortality rate and improves cardiac function (Garza, Wason, & Zhang, 2015). Similar to exercise, resveratrol treatment remarkably alleviates cardiac function and hypertrophy in rats post‐infarction. Resveratrol prevents cytochrome P450 1B1 from producing cardiotoxic metabolites of hydroxyeicosatetraenoic acid (Matsumura et al., 2018). In addition to cardiac health, resveratrol supplement post‐infarction is also beneficial to the vasculature. In a clinical trial of 40 post‐infarction patients, a 3‐month daily administration of 10‐mg resveratrol improved both FMD and left ventricle diastolic function (Magyar et al., 2012). Both preclinical and clinical studies highlight the potential of resveratrol to act as an exercise mimetic post‐myocardial infarction.
2.5.5. Angiogenesis
Exercise induces angiogenesis. Aging is accompanied by diminished angiogenesis in endothelial cells and muscle tissues and, hence, lower levels of angiogenic factors (Kwak, Lee, Zhang, & Song, 2018). Exercise promotes angiogenesis by increasing shear stress in vasculature and by up‐regulating VEGF (Garza et al., 2015). Angiogenesis post‐infarction is particularly crucial to compensatory hypertrophy for better replacement of damaged tissue. In a rat model, a 10‐week treadmill training protocol favourably enhances cardiac angiogenesis post‐infarction (Leosco et al., 2008). Importantly, resveratrol treatment raised cardiac capillary density via a VEGF‐dependent manner in rats post‐infarction (Fukuda et al., 2006). In another study, resveratrol was shown to activate eNOS through the MAPK/ERK cascade, for subsequent NO‐mediated up‐regulation of VEGF and matrix metalloproteinases, in cerebral endothelial cells (Simão et al., 2012). These results suggest that resveratrol can be a candidate exercise mimetic to favour blood flow recovery after myocardial infarction and stroke.
2.5.6. PPARδ activator
Narkar et al. provided crucial data to indicate that PPARδ activators could be exercise mimetics (Narkar et al., 2008). PPARδ is closely involved in lipid absorption, muscle endurance, insulin sensitivity, and atherogenic inflammation suppression. Resveratrol is one of such activators. PPARδ was previously shown to be required to ameliorate endothelial dysfunction in exercise‐trained diabetic mice (Cheang et al., 2017). Similarly, resveratrol improves endothelial function in a PPARδ‐dependent manner (Cheang et al., 2018). In addition, both https://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=2687, a PPARδ receptor agonist, and resveratrol inhibit oxLDL‐induced migration and proliferation of vascular SMCs, probably through the SIRT1/PPARδ signalling transduction as well (Hwang et al., 2016). Resveratrol and PPARδ activators share common effects in ameliorating endothelial dysfunction and atherosclerotic risk. Being the active activator of three critical molecules (i.e., SIRT1, AMPK, and PPARδ), resveratrol could potentially mimic the cardioprotective effects of exercise to some extent.
2.6. Circadian clock
The behaviour and physiology of human body are tightly regulated by an internal circadian rhythm, which sensitively anticipates solar day‐associated environmental alterations. The clock mechanism can be interpreted as the network of transcriptional–translational feedback loops which alter the expression patterns of central clock components in a rhythmic fashion over approximately 24‐hr. The CLOCK and BMAL1 gene products serve as the constituents of the heterodimer which acts as the fundamental transcription factor of the primary feedback loop. The CLOCK:BMAL1 heterodimer complex is responsible for the rhythmic expressional regulation of thousands of downstream genes to regularly define the oscillatory pattern of vast biological functions (Trott & Menet, 2018).
The cardiovascular system is also under the tight regulation of the circadian clock. Chronic dysregulation of circadian rhythm is believed to increase the cardiovascular risk (Takeda & Maemura, 2011). Comparing to younger adults, older adults demonstrate a faster circadian cycle with dampened peak (Hood & Amir, 2017). Such alterations in circadian rhythms might be closely related to the increased risk of CVDs. An epidemiological study suggests that certain severe cardiovascular events demonstrates temporal dependency. For instance, the occurrence of sudden cardiac arrest, myocardial infarction, ventricular arrhythmias, and stroke was more frequent in the morning (Thosar, Butler, & Shea, 2018). Therefore, any pharmacological agents that can target circadian rhythm might be a novel therapeutic candidate for treatment of CVDs.
As stated in Section 2.2, resveratrol is a known SIRT1 activator. The NAD+‐dependent deacetylase SIRT1 binds to the CLOCK protein of the core clock component CLOCK:BMAL1 for modulation of circadian gene expression (Hood & Amir, 2017). SIRT1 regulates the deacetylation of BMAL1 in CLOCK:BMAL1 complex and PER2 in the repressive PER2:CRY1 complex. The latter heterodimer functions in close association with CLOCK:BMAL1 complex for down‐regulation of its transcriptional activity (Asher et al., 2008). SIRT1 also deacetylases H3K9 for epigenetic circadian control (Figure 2b; Masri, 2015). Aging‐associated SIRT1 down‐regulation accounts for reduced amplitudes of all CLOCK:BMAL1‐driven rhythms, influencing a wide range of physiological events. Hence, resveratrol supplement may replenish age‐related SIRT1 loss and restore the repression on PER2:CRY1 complex. This provides hints that resveratrol may potentially rejuvenate the circadian cycle in older adults to lower CVD risks.
Resveratrol can be a dietary option to regulate circadian rhythm of cardiovascular system for reducing CVD risk. In vitro, resveratrol altered circadian rhythm in neonatal rat cardiomyocytes (du Pré et al., 2017), implying its therapeutic potential against CVDs such as cardiac hypertrophy and myocardial infarction which are related to dysregulated myocardial circadian clock (Durgan & Young, 2010). In vivo, resveratrol triggered the SIRT1/CLOCK:BMAL1 axis to restore lipid metabolism rhythmic disorder in livers of high‐fat diet‐fed mice (L. Sun et al., 2015). Also, resveratrol reversed high‐fat diet‐induced lipogenesis and changes in the expression of reverse erythroblastosis virus α, another transcriptional repressor of BMAL1 (Miranda et al., 2013). Dysregulated lipid metabolism due to altered hepatic circadian clock may be potentially atherogenic. These preclinical studies provide partial explanations for the results of a meta‐analysis, based on 681 adults, that resveratrol reduces plasma total cholesterol levels in obese adults (H. Huang et al., 2016). Future studies may focus on whether resveratrol acts on the circadian cycles of other cell components of the cardiovascular system under pathological conditions.
2.7. Microbiota composition
In recent years, host–gut microbiota interaction have drawn increasing attention in studies of the pathophysiology of major chronic diseases. Although the causal relationship remains largely elusive, aging is often associated with alterations in gut microbiome, and sometimes such alterations can be problematic (Nagpal et al., 2018). There is a tight linkage between maladaptation in intestinal microbiota composition (i.e., dysbiosis) and the pathologies of various diseases, including Type 2 diabetes, cancer, chronic kidney disease, and CVDs (Tang, Kitai, & Hazen, 2017). For instance, altered stool microbial composition has been confirmed in patients with unstable plaques as opposed to those with stable plaques (Karlsson et al., 2012). One may reverse the undesirable microbiome alteration by artificial means to promote cardiovascular health. Administration of Lactobacillus plantarum improves left ventricular function and reduces myocardial hypertrophy in rats post‐myocardial infarction (Gan et al., 2014). Notably, resveratrol may also exert cardioprotective effects by acting on gut microbiome.
Before entering the bloodstream by passive diffusion or by interacting with membrane transporters, resveratrol can be readily metabolized by intestinal microbiota and such resveratrol metabolites favour the intestinal microbiota towards a more healthy phenotype (Carrera‐Quintanar et al., 2018). In rodents, resveratrol‐induced changes in microbiota composition are associated with decreased body weight gain, reduced adiposity, improved insulin sensitivity, and restored blood pressure (Bird, Raederstorff, Weber, & Steinert, 2017). As noted, these parameters are important factors for CVD risk. More importantly, a 12‐week resveratrol supplementation in overweight male individuals shows lower abundance of Bacteroidetes and Faecalibacterium prausnitzii. Such microbiome alteration is associated with beneficial outcomes including the up‐regulation of lipid oxidation and mitochondrial oxidative capacity (Most, Penders, Lucchesi, Goossens, & Blaak, 2017). Resveratrol supplement alters gut microbiota composition for a more favourable metabolic profile, which may partly explain its cardiovascular benefits.
The resveratrol‐altered microbiota composition may modulate the host metabolite profile to elicit cardiovascular benefits. A 0.4% resveratrol supplementation given to ApoE−/− mice markedly remodelled the gut microbiome to limit trimethylamine‐N‐oxide production, where plasma trimethylamine‐N‐oxide level correlates with atherosclerosis development (J. Chen, Bai, et al., 2016; M. Chen, Yi, et al., 2016). More interestingly, fecal microbiome transplants from resveratrol‐fed mice alleviated hyperglycaemia in obese mice and lower systolic blood pressure in hypertensive mice (E. N. Kim, Kim, et al., 2018; T. T. Kim, Parajuli, et al., 2018). However, more work is needed to unravel more alterations in the microbiota and to understand the metabolic and biological function of such alterations.
3. ADVERSE EFFECTS OF RESVERATROL
3.1. Dual pattern of resveratrol
Although severe adverse outcomes of resveratrol usage in humans are rarely reported, the dual pattern of resveratrol action may account for certain adverse effects of resveratrol. Resveratrol has been considered as a natural antioxidant for decades, but resveratrol might also act as a potent pro‐oxidant under certain circumstances. At higher pH, due to the presence of hydroxyl anions or bicarbonate ions, resveratrol is more likely to be auto‐oxidized to generate 4′‐phenoxyl radical and semiquinones, causing cellular oxidative stress (Salehi et al., 2018). In addition, depending on the timing of resveratrol administration, resveratrol might behave differently. One study has found that resveratrol treatment during the dark hours was more likely to be antioxidant, while that administered during the light hours was more likely to be pro‐oxidant, resulting in higher lipoperoxidation level in the heart, kidney, and liver (Gadacha et al., 2009). Therefore, more attention should be paid to the time of treatment in studies on resveratrol, particularly those involving circadian rhythm.
Resveratrol exhibits structural similarity to the synthetic oestrogen, http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=2801. It is therefore to be expected that resveratrol can act as either agonist or antagonist for oestrogen receptors in different systems. Because such agonist and antagonist properties are concentration‐dependent, resveratrol shows a biphasic behaviour in different studies. Resveratrol can act as an anti‐oestrogen by inhibiting estradiol binding to oestrogen receptor at concentrations comparable to those required for oestrogen‐mediated biological outcomes (Gehm, McAndrews, Chien, & Jameson, 1997). In contrast, in vitro, resveratrol treatment can exert a pro‐estrogenic action in mammary cancer cells in the absence of oestrogen (Bhat et al., 2001). Therefore, the pro‐estrogenic activity of resveratrol might potentially aggravate tumourigenesis in oestrogen‐dependent breast and prostate cancers. Extra caution is required when condsidering resveratrol for clinical trials and human use.
3.2. Interaction with other medications
The interaction between resveratrol and other medications should not be overlooked. In some cases, such interaction might provide add‐on or synergistic benefits. As stated, a combined therapy of resveratrol and statins is more effective in inhibiting apoptosis in cardiomyocytes (Varma Penumathsa et al., 2006). On the other hand, undesirable interactions would adversely affect the efficacy and clearance of other pharmacological agents. Resveratrol inhibited the activity of the hepatic enzyme cytochrome https://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=1337, which is essential in metabolizing various drugs including cholesterol‐lowering statins, immunosuppressive pharmaceuticals for patients after transplantation, and chemotherapeutic agents. Undesirable resveratrol administration in combination with these drugs may lower their efficacy. In addition, undesirable cytotoxic compounds might be generated by isozymes of cytochrome P450 3A4, resulting in more drug toxicity (Chow et al., 2010). Resveratrol may also exacerbate drug toxicity by dysregulating drug clearance. For instance, resveratrol inhibits the activity of another hepatic enzyme cytochrome P450 https://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=1326, involved in the clearance of anticoagulants, COX‐2 inhibitors, and non‐steroidal anti‐inflammatory drugs (Chow et al., 2010). Caution should be exercised when administering resveratrol with other medications, to humans.
4. CONFLICTING EFFECTS OF RESVERATROL
Inconsistent effects of resveratrol have been reported in preclinical and clinical studies. It seems that challenges are still present during the translation of preclinical results to health benefits in clinical trials. Certain studies affirm the clinical benefits of resveratrol supplement in improving FMD, lowering systolic blood pressure, and reducing LDL cholesterol levels (Zordoky, Robertson, & Dyck, 2015). For example, a 6‐month daily consumption of 350‐mg resveratrol‐enriched grape supplement lowered levels of oxLDL and apolipoprotein‐B in 75 statin‐treated patients (Tomé‐Carneiro, Gonzálvez, Larrosa, Yáñez‐Gascón, et al., 2012). However, resveratrol supplements were not always effective in improving metabolic profile. Notably, a recent meta‐analysis on randomized clinical trials covering more than 700 adults did not find pronounced differences in LDL‐C and HDL‐C levels after resveratrol treatment (Haghighatdoost & Hariri, 2018). In order to narrow the knowledge gap between animal studies and human trials, the following aspects can be considered.
4.1. Pharmacokinetics and biotransformation of resveratrol
Upon oral administration of resveratrol, more than 70% of resveratrol is absorbed based on previous urinary excretion data. The extremely low bioavailability of unmodified resveratrol in the systemic circulation is attributed to the rapid metabolism mediated by gut microbiota before absorption and by endogenous metabolic enzymes after absorption. Most of the modified resveratrol exists as glucuronated, sulfated, and hydroxylated metabolites. Normally, the peak circulation levels of these resveratrol metabolites is achieved 1 hr post‐administration, implying a rapid distribution phase. Most of the resveratrol metabolites are excreted through urine, while only trace amounts are detectable in feces (P. Wang & Sang, 2018). Notably, the modified resveratrol might perform very differently from the parent compound, so that the rapid biotransformation of resveratrol could account for the inconsistent results among different cellular, animal, and human studies.
4.2. Low bioavailability of resveratrol
In a pig study, only 0.5% of administrated resveratrol was delivered to different organs (e.g., brain, heart, lungs, kidneys, liver, pancreas, spleen, aorta tissue, and urinary bladder) 6 hr after administration (Azorín‐Ortuño et al., 2011). In another study on rats, the resveratrol metabolites became detectable in heart tissues (0.01–0.05 nmol·g−1) after a 6‐week resveratrol regimen at a dosage of 5 mg·kg−1·day−1 (Bresciani et al., 2014). The low bio‐accumulation of resveratrol in the cardiovascular system (e.g., heart and aortic tissue) is another concern. The concentration of resveratrol attained in target organs may be significantly lower than the effective doses applied in cell and animal studies. Before reaching the target cells, resveratrol might have been rapidly degraded in the gastrointestinal tract, over‐metabolized by gut microbiota, and/or overconsumed by other non‐target cells.
The low bioavailability of resveratrol in target cells limits its therapeutic efficiency. Developing effective drug delivery systems for specific transfer of resveratrol into cardiovascular cells might be a feasible solution to improve bioavailability. Besides, micronization of resveratrol can be another approach to increase its bioavailability. By reducing the particle size of pharmaceutical compounds, their physical structures are greatly modified such that better solubility, permeability, and eventually bioavailability can be achieved (Aguiar et al., 2016). Optimization of drug delivery of resveratrol may resolve the lack of consistency between preclinical and clinical findings.
4.3. Distinct experimental settings
The gap between preclinical and clinical findings may also be attributed to the distinct experimental settings. In particular, many cell culture and rodent experiments were prevention studies while human trials were more likely to be progression studies. The recruited participants of the human studies are often patients in certain disease states such as metabolic syndrome, Type 2 diabetes mellitus, hypertension, and non‐alcoholic fatty liver disease. Notably, the initiation and progression of diseases often involve different pathways, and the organs of the patients might have already been affected or even severely damaged by such diseases. As a result, resveratrol may fail to activate related pathways to significantly rescue or repair the dysfunctional organs. Furthermore, in rodent studies, the animals are sometimes treated by resveratrol for months, which is comparable to decades in humans. However, the majority of recent clinical investigations are limited to a much shorter timescale of 1 to 6 months. Studies of long‐term resveratrol use should be considered. Nevertheless, we should not be too optimistic when observing positive outcomes in animal models.
4.4. Inter‐individual difference
Inter‐individual difference might also account for the inconsistent outcomes of resveratrol‐related studies. Gender is always one of the most critical biological factors in testing drug responses. Gender differences in resveratrol metabolism have been observed in human (Dellinger, Gomez Garcia, & Meyskens, 2014). In addition, a larger proportion of resveratrol clinical studies only sampled male individuals from Western countries (Zordoky et al., 2015). This may also overlook the contribution of ethnicity to differences of resveratrol‐induced effects among Westerners and non‐Westerners. Recently, the intestinal microbiota have been shown to plays an active role in metabolizing resveratrol or resveratrol precursors to control the bioavailability of resveratrol derivatives (P. Wang & Sang, 2018). Notably, remarkable inter‐individual differences in resveratrol metabolism have been observed in human intestinal microbiota, probably due to different microbiota composition (Bode et al., 2013). Several clinical studies involved subjects associated with obesity, metabolic syndrome, and/or diabetes (Zordoky et al., 2015), and these subjects are more likely to develop dysbiosis. Furthermore, the intestinal microbiota composition depends greatly on the geographical locations and the dietary habits of particular individuals (Senghor, Sokhna, Ruimy, & Lagier, 2018). Hence, it is reasonable to postulate that Westerners and non‐Westerners would show different intestinal responses to resveratrol. We may need to consider the effects of dysbiosis when in any analysis of the effects and efficacy of resveratrol.
5. FUTURE PERSPECTIVES: UNADDRESSED ISSUES
In the context of molecular mechanisms, several questions remain to be addressed in further investigations. Earlier studies have indicated that both AMPK and SIRT1 can regulate each other for downstream signalling cascades (Ruderman et al., 2010). Interestingly, resveratrol is still able to induce AMPK activation in SIRT1‐knockout mice (Price et al., 2012), implying that resveratrol may directly activate AMPK independently of the SIRT1/LKB1/AMPK axis. The downstream events have already been extensively studied, and, therefore, new studies should focus on the upstream events about how resveratrol affects the AMPK/SIRT1 interaction or partnership.
As AMPK activation greatly depends upon the cellular energy level, it is reasonable to postulate that resveratrol may be involved in cellular events that alter the AMP/ATP and ADP/ATP ratios. Hence, cellular events like glycolysis, fatty acid metabolism, amino acid metabolism, and mitochondrial oxidative phosphorylation may be the potential upstream targets of resveratrol‐induced AMPK activity. Exercise elevates the AMP/ATP ratio for subsequent AMPK activation. As resveratrol may act as an exercise mimetic, the exercise‐induced cellular energy utilization might provide crucial hints for further characterization of pharmacological actions of resveratrol. Besides, the above‐described cellular events are also tightly related to NAD metabolism and any resulting alterations in the NAD/NADH ratio may also contribute to SIRT1 activation by resveratrol. Extensive study is still required to uncover the elusive mechanisms of resveratrol‐mediated AMPK and SIRT1 activation in the context of cellular energy homeostasis.
UCP2, the downstream molecule of both AMPK and SIRT1, plays important roles in counteracting oxidative stress during pathogenesis of CVDs. However, comprehensive information on the interplay among AMPK, SIRT1, and UCP2 is still lacking. As the precise phosphorylation site on UCP2 remains unclear, it is possible that AMPK or SIRT1 might transcriptionally or post‐transcriptionally regulate UCP2. L. Wang et al. discovered the active participation of Hippo pathway proteins (i.e., YAP and TAZ) in mechanotransduction of endothelial cells upon shear stress (L. Wang et al., 2016). As resveratrol was previously shown to target YAP/TAZ signalling in cancer cells through AMPK activation (Jiang et al., 2016), it may also target the YAP/TAZ cascade in endothelial cells to induce similar effects as laminar shear stress.
The circadian clock plays pivotal roles in numerous physiological events. Only until recently, have researchers become aware of the effects of dysregulated circadian clock in development of CVDs (Thosar et al., 2018). As noted, nearly all the cells possess their own self‐sustained circadian clocks. It is, therefore, of significance to identify more potential cell targets for resveratrol‐induced circadian regulation in the cardiovascular system. Future investigations shall aim to determine whether resveratrol can rejuvenate the circadian rhythms of aging population and whether such alterations are beneficial to cardiovascular health.
The inconsistent findings between preclinical and clinical studies might be attributable to poor distribution of resveratrol to the desired tissues/cells. Although in vitro resveratrol treatment exhibits remarkable beneficial outcomes in many cell types, the low resveratrol bioavailability during in vivo studies (e.g., animal experiments and human trials) might limit its therapeutic potential. This generates the need to develop a better drug delivery system to raise resveratrol concentrations at target tissues or cells in order to optimize therapeutic effects. Several nano‐approaches such as nanoparticles and liposomes have been used to deliver resveratrol into selected tissues to achieve optimal cardioprotective effects and to minimize side effects (Neves, Martins, Segundo, & Reis, 2016). In a Phase I clinical trial, micronization of resveratrol enhanced the plasma resveratrol levels by 3.6‐fold and increased the resveratrol delivery to livers in patients with hepatic metastases (Howells et al., 2011). Nanocarriers and/or micronized resveratrol might be potential solutions to overcome low bioavailability of resveratrol in vivo. Further studies are required for optimization of these delivery approaches. In contrast to resveratrol administration alone, the synergistic effects of resveratrol in combination with other supplements or exercise should also be under consideration.
In addition, in order to better resolve the inconsistency between preclinical and clinical findings, efforts ought to be made in future explorations to minimize the effects of inter‐individual difference (e.g., age, gender, region, and microbiota composition). Importantly, the inter‐individual difference of microbiota composition may affect the metabolism of resveratrol. Prior to regular resveratrol supplement, the intestinal microbiome of patients might need some restoration or normalization in order to optimize the therapeutic effects of resveratrol.
6. CONCLUDING REMARKS
The recognition of the cardioprotective benefits of resveratrol in 1992, exemplified by the “French paradox”, initiated a range of studies in an attempt to uncover the molecular basis of resveratrol action. Beneficial to our cardiovascular system, resveratrol exerts a number of favourable effects on longevity regulation, energy metabolism, stress resistance, exercise mimetics, circadian clock, and microbiota composition. Notably, the dual pattern of resveratrol action, particularly in terms of oxidative stress and oestrogen action, and inter‐individual difference among patients, especially the diverse intestinal microbiota composition, should not be overlooked, as we unravel the clinical importance of resveratrol. Further efforts are still required to broaden our understanding towards the underlying mechanistic network and to narrow the knowledge gap between preclinical studies and human trials of resveratrol.
6.1. Nomenclature of targets and ligands
Key protein targets and in this article are hyperlinked to corresponding entries in http://www.guidetopharmacology.org, the common portal for data from IUPHAR/BPS Guide to PHARMACOLOGY (Harding et al., 2018), and are permanently archived in the Concise Guide to PHARMACOLOGY 2017/18 (Alexander, Cidlowski et al., 2017; Alexander, Fabbro et al., 2017; Alexander, Kelly et al., 2017a, b).
CONFLICT OF INTEREST
The authors declare no competing interests.
ACKNOWLEDGEMENTS
The authors acknowledge funding from the Health and Medical Research Fund (Grant 13140871) and CUHK Direct Grant (Grant 2018.086).
Cheng CK, Luo J‐Y, Lau CW, Chen Z‐Y, Tian XY, Huang Y. Pharmacological basis and new insights of resveratrol action in the cardiovascular system. Br J Pharmacol. 2020;177:1258–1277. 10.1111/bph.14801
REFERENCES
- Abe, J. , Yamada, Y. , Takeda, A. , & Harashima, H. (2018). Cardiac progenitor cells activated by mitochondrial delivery of resveratrol enhance the survival of a doxorubicin‐induced cardiomyopathy mouse model via the mitochondrial activation of a damaged myocardium. Journal of Controlled Release, 269, 177–188. 10.1016/J.JCONREL.2017.11.024 [DOI] [PubMed] [Google Scholar]
- Aguiar, G. P. S. , Boschetto, D. L. , Chaves, L. M. P. C. , Arcari, B. D. , Piato, A. L. , Oliveira, J. V. , & Lanza, M. (2016). Trans‐resveratrol micronization by SEDS technique. Industrial Crops and Products, 89, 350–355. 10.1016/J.INDCROP.2016.04.047 [DOI] [Google Scholar]
- Alexander, S. P. , Cidlowski, J. A. , Kelly, E. , Marrion, N. V. , Peters, J. A. , Faccenda, E. , … Davies, J. A. (2017). The concise guide to PHARMACOLOGY 2017/18: Nuclear hormone receptors. British Journal of Pharmacology, 174, S208–S224. 10.1111/bph.13880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander, S. P. , Fabbro, D. , Kelly, E. , Marrion, N. V. , Peters, J. A. , Faccenda, E. , … Davies, J. A. (2017). The concise guide to PHARMACOLOGY 2017/18: Enzymes. British Journal of Pharmacology, 174, S272–S359. 10.1111/bph.13877 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander, S. P. H. , Kelly, E. , Marrion, N. V. , Peters, J. A. , Faccenda, E. , Harding, S. D. , … CGTP Collaborators (2017a). THE CONCISE GUIDE TO PHARMACOLOGY 2017/18: Other proteins. British Journal of Pharmacology, 174, S1–S16. 10.1111/bph.13882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander, S. P. , Kelly, E. , Marrion, N. V. , Peters, J. A. , Faccenda, E. , Harding, S. D. , … Davies, J. A. (2017b). The concise guide to PHARMACOLOGY 2017/18: Transporters. British Journal of Pharmacology, 174, S360–S446. 10.1111/bph.13883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asher, G. , Gatfield, D. , Stratmann, M. , Reinke, H. , Dibner, C. , Kreppel, F. , … Schibler, U. (2008). SIRT1 regulates circadian clock gene expression through PER2 deacetylation. Cell, 134(2), 317–328. 10.1016/j.cell.2008.06.050 [DOI] [PubMed] [Google Scholar]
- Azorín‐Ortuño, M. , Yáñez‐Gascón, M. J. , Vallejo, F. , Pallarés, F. J. , Larrosa, M. , Lucas, R. , … Espín, J. C. (2011). Metabolites and tissue distribution of resveratrol in the pig. Molecular Nutrition & Food Research, 55(8), 1154–1168. 10.1002/mnfr.201100140 [DOI] [PubMed] [Google Scholar]
- Bagul, P. K. , Deepthi, N. , Sultana, R. , & Banerjee, S. K. (2015). Resveratrol ameliorates cardiac oxidative stress in diabetes through deacetylation of NFkB‐p65 and histone 3. The Journal of Nutritional Biochemistry, 26(11), 1298–1307. 10.1016/J.JNUTBIO.2015.06.006 [DOI] [PubMed] [Google Scholar]
- Berman, A. Y. , Motechin, R. A. , Wiesenfeld, M. Y. , & Holz, M. K. (2017). The therapeutic potential of resveratrol: A review of clinical trials. Npj Precision Oncology, 1(1), 35 10.1038/s41698-017-0038-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berrougui, H. , Grenier, G. , Loued, S. , Drouin, G. , & Khalil, A. (2009). A new insight into resveratrol as an atheroprotective compound: Inhibition of lipid peroxidation and enhancement of cholesterol efflux. Atherosclerosis, 207(2), 420–427. 10.1016/j.atherosclerosis.2009.05.017 [DOI] [PubMed] [Google Scholar]
- Bhat, K. P. , Lantvit, D. , Christov, K. , Mehta, R. G. , Moon, R. C. , & Pezzuto, J. M. (2001). Estrogenic and antiestrogenic properties of resveratrol in mammary tumor models. Cancer Research, 61(20), 7456–7463. [PubMed] [Google Scholar]
- Biala, A. , Tauriainen, E. , Siltanen, A. , Shi, J. , Merasto, S. , Louhelainen, M. , … Mervaala, E. (2010). Resveratrol induces mitochondrial biogenesis and ameliorates Ang II‐induced cardiac remodeling in transgenic rats harboring human renin and angiotensinogen genes. Blood Pressure, 19(3), 196–205. 10.3109/08037051.2010.481808 [DOI] [PubMed] [Google Scholar]
- Bird, J. K. , Raederstorff, D. , Weber, P. , & Steinert, R. E. (2017). Cardiovascular and antiobesity effects of resveratrol mediated through the gut microbiota. Advances in Nutrition (Bethesda, Md.), 8(6), 839–849. 10.3945/an.117.016568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bo, S. , Ciccone, G. , Castiglione, A. , Gambino, R. , de Michieli, F. , Villois, P. , … Cassader, M. (2013). Anti‐inflammatory and antioxidant effects of resveratrol in healthy smokers a randomized, double‐blind, placebo‐controlled, cross‐over trial. Current Medicinal Chemistry, 20(10), 1323–1331. 10.2174/0929867311320100009 [DOI] [PubMed] [Google Scholar]
- Bode, L. M. , Bunzel, D. , Huch, M. , Cho, G. S. , Ruhland, D. , Bunzel, M. , … Kulling, S. E. (2013). In vivo and in vitro metabolism of trans‐resveratrol by human gut microbiota. The American Journal of Clinical Nutrition, 97(2), 295–309. 10.3945/ajcn.112.049379 [DOI] [PubMed] [Google Scholar]
- Bresciani, L. , Calani, L. , Bocchi, L. , Delucchi, F. , Savi, M. , Ray, S. , … del Rio, D. (2014). Bioaccumulation of resveratrol metabolites in myocardial tissue is dose‐time dependent and related to cardiac hemodynamics in diabetic rats. Nutrition, Metabolism and Cardiovascular Diseases, 24(4), 408–415. 10.1016/j.numecd.2013.09.008 [DOI] [PubMed] [Google Scholar]
- Bryan, H. K. , Olayanju, A. , Goldring, C. E. , & Park, B. K. (2013). The Nrf2 cell defence pathway: Keap1‐dependent and ‐independent mechanisms of regulation. Biochemical Pharmacology, 85(6), 705–717. 10.1016/J.BCP.2012.11.016 [DOI] [PubMed] [Google Scholar]
- Buttari, B. , Profumo, E. , Segoni, L. , D'Arcangelo, D. , Rossi, S. , Facchiano, F. , … Riganò, R. (2014). Resveratrol counteracts inflammation in human M1 and M2 macrophages upon challenge with 7‐oxo‐cholesterol: Potential therapeutic implications in atherosclerosis. Oxidative Medicine and Cellular Longevity, 2014, 257543–12. 10.1155/2014/257543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cacicedo, J. M. , Gauthier, M. S. , Lebrasseur, N. K. , Jasuja, R. , Ruderman, N. B. , & Ido, Y. (2011). Acute exercise activates AMPK and eNOS in the mouse aorta. American Journal of Physiology‐Heart and Circulatory Physiology, 301(4), H1255–H1265. 10.1152/ajpheart.01279.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrera‐Quintanar, L. , López Roa, R. I. , Quintero‐Fabián, S. , Sánchez‐Sánchez, M. A. , Vizmanos, B. , & Ortuño‐Sahagún, D. (2018). Phytochemicals that influence gut microbiota as prophylactics and for the treatment of obesity and inflammatory diseases. Mediators of Inflammation, 2018, 1–18. 10.1155/2018/9734845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrizzo, A. , Puca, A. , Damato, A. , Marino, M. , Franco, E. , Pompeo, F. , … Vecchione, C. (2013). Resveratrol improves vascular function in patients with hypertension and dyslipidemia by modulating NO metabolism. Hypertension, 62(2), 359–366. 10.1161/HYPERTENSIONAHA.111.01009 [DOI] [PubMed] [Google Scholar]
- Chaplin, A. , Carpéné, C. , & Mercader, J. (2018). Resveratrol, metabolic syndrome, and gut microbiota. Nutrients, 10(11), 1651 10.3390/nu10111651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheang, W. S. , Tian, X. Y. , Wong, W. T. , Lau, C. W. , Lee, S. S. T. , Chen, Z. Y. , … Huang, Y. (2014). Metformin protects endothelial function in diet‐induced obese mice by inhibition of endoplasmic reticulum stress through 5′ adenosine monophosphate–activated protein kinase–peroxisome proliferator–activated receptor δ pathway. Arteriosclerosis, Thrombosis, and Vascular Biology, 34(4), 830–836. 10.1161/ATVBAHA.113.301938 [DOI] [PubMed] [Google Scholar]
- Cheang, W. S. , Wong, W. T. , Wang, L. , Cheng, C. K. , Lau, C. W. , Ma, R. C. W. , … Tian, X. Y. (2018). Resveratrol ameliorates endothelial dysfunction in diabetic and obese mice through sirtuin 1 and peroxisome proliferator‐activated receptor δ. Pharmacological Research, 139, 384–394. 10.1016/j.phrs.2018.11.041 [DOI] [PubMed] [Google Scholar]
- Cheang, W. S. , Wong, W. T. , Zhao, L. , Xu, J. , Wang, L. , Lau, C. W. , … Huang, Y. (2017). PPARδ is required for exercise to attenuate endoplasmic reticulum stress and endothelial dysfunction in diabetic mice. Diabetes, 66(2), 519–528. 10.2337/db15-1657 [DOI] [PubMed] [Google Scholar]
- Chen, C. , Zou, L. X. , Lin, Q. Y. , Yan, X. , Bi, H. L. , Xie, X. , … Li, H. H. (2019). Resveratrol as a new inhibitor of immunoproteasome prevents PTEN degradation and attenuates cardiac hypertrophy after pressure overload. Redox Biology, 20, 390–401. 10.1016/j.redox.2018.10.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, C. J. , Yu, W. , Fu, Y. C. , Wang, X. , Li, J. L. , & Wang, W. (2009). Resveratrol protects cardiomyocytes from hypoxia‐induced apoptosis through the SIRT1–FoxO1 pathway. Biochemical and Biophysical Research Communications. Academic Press, 378(3), 389–393. 10.1016/J.BBRC.2008.11.110 [DOI] [PubMed] [Google Scholar]
- Chen, J. , Bai, Q. , Zhao, Z. , Sui, H. , & Xie, X. (2016). Resveratrol improves delayed r‐tPA treatment outcome by reducing MMPs. Acta Neurologica Scandinavica, 134(1), 54–60. 10.1111/ane.12511 [DOI] [PubMed] [Google Scholar]
- Chen, M. , Yi, L. , Jin, X. , Xie, Q. , Zhang, T. , Zhou, X. , … Mi, M. T. (2013). Absorption of resveratrol by vascular endothelial cells through passive diffusion and an SGLT1‐mediated pathway. The Journal of Nutritional Biochemistry, 24(11), 1823–1829. 10.1016/j.jnutbio.2013.04.003 [DOI] [PubMed] [Google Scholar]
- Chen, M. , Yi, L. , Zhang, Y. , Zhou, X. , Ran, L. , Yang, J. , … Mi, M. T. (2016). Resveratrol attenuates trimethylamine‐n‐oxide (TMAO)‐induced atherosclerosis by regulating tmao synthesis and bile acid metabolism via remodeling of the gut microbiota. MBio, 7(2), e02210–e02215. 10.1128/mBio.02210-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, Z. , Peng, I. C. , Cui, X. , Li, Y. S. , Chien, S. , & Shyy, J. Y. J. (2010). Shear stress, SIRT1, and vascular homeostasis. Proceedings of the National Academy of Sciences, 107(22), 10268–10273. 10.1073/pnas.1003833107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, Z. , Shentu, T. P. , Wen, L. , Johnson, D. A. , & Shyy, J. Y. J. (2013). Regulation of SIRT1 by oxidative stress‐responsive miRNAs and a systematic approach to identify its role in the endothelium. Antioxidants & Redox Signaling, 19(13), 1522–1538. 10.1089/ars.2012.4803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng, C. K. , Bakar, H. A. , Gollasch, M. , & Huang, Y. (2018). Perivascular adipose tissue: The sixth man of the cardiovascular system. Cardiovascular Drugs and Therapy, 32(5), 481–502. 10.1007/s10557-018-6820-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng, S. J. , Yu, H. K. , Chen, Y. C. , Chen, C. Y. , Lien, W. C. , Yang, P. Y. , & Hu, G. C. (2013). Physical activity and risk of cardiovascular disease among older adults. International Journal of Gerontology, 7(3), 133–136. 10.1016/J.IJGE.2013.03.001 [DOI] [Google Scholar]
- Chow, H. H. S. , Garland, L. L. , Hsu, C. H. , Vining, D. R. , Chew, W. M. , Miller, J. A. , … Alberts, D. S. (2010). Resveratrol modulates drug‐ and carcinogen‐metabolizing enzymes in a healthy volunteer study. Cancer Prevention Research (Philadelphia, Pa.), 3(9), 1168–1175. 10.1158/1940-6207.CAPR-09-0155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cicha, I. , Beronov, K. , Ramirez, E. L. , Osterode, K. , Goppelt‐Struebe, M. , Raaz, D. , … Garlichs, C. D. (2009). Shear stress preconditioning modulates endothelial susceptibility to circulating TNF‐α and monocytic cell recruitment in a simplified model of arterial bifurcations. Atherosclerosis, 207(1), 93–102. 10.1016/j.atherosclerosis.2009.04.034 [DOI] [PubMed] [Google Scholar]
- Cicha, I. , Regler, M. , Urschel, K. , Goppelt‐Struebe, M. , Daniel, W. G. , & Garlichs, C. D. (2011). Resveratrol inhibits monocytic cell chemotaxis to MCP‐1 and prevents spontaneous endothelial cell migration through Rho kinase‐dependent mechanism. Journal of Atherosclerosis and Thrombosis, 18(12), 1031–1042. 10.5551/jat.8136 [DOI] [PubMed] [Google Scholar]
- Csiszar, A. , Labinskyy, N. , Pinto, J. T. , Ballabh, P. , Zhang, H. , Losonczy, G. , … Ungvari, Z. (2009). Resveratrol induces mitochondrial biogenesis in endothelial cells. American Journal of Physiology. Heart and Circulatory Physiology, 297(1), H13–H20. 10.1152/ajpheart.00368.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dellinger, R. W. , Gomez Garcia, A. M. , & Meyskens, F. L. Jr. (2014). Differences in the glucuronidation of resveratrol and pterostilbene: Altered enzyme specificity and potential gender differences. Drug Metabolism and Pharmacokinetics, 29(2), 112–119. 10.2133/dmpk.DMPK-13-RG-012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng, M. , Wang, D. , He, S. , Xu, R. , & Xie, Y. (2017). SIRT1 confers protection against ischemia/reperfusion injury in cardiomyocytes via regulation of uncoupling protein 2 expression. Molecular Medicine Reports, 16(5), 7098–7104. 10.3892/mmr.2017.7452 [DOI] [PubMed] [Google Scholar]
- Diao, J. , Wei, J. , Yan, R. , Fan, G. , Lin, L. , & Chen, M. (2018). Effects of resveratrol on regulation on UCP2 and cardiac function in diabetic rats. Journal of Physiology and Biochemistry., 75, 39–51. 10.1007/s13105-018-0648-7 [DOI] [PubMed] [Google Scholar]
- Dong, W. , Wang, X. , Bi, S. , Pan, Z. , Liu, S. , Yu, H. , … Zhang, W. (2014). Inhibitory effects of resveratrol on foam cell formation are mediated through monocyte chemotactic protein‐1 and lipid metabolism‐related proteins. International Journal of Molecular Medicine, 33(5), 1161–1168. 10.3892/ijmm.2014.1680 [DOI] [PubMed] [Google Scholar]
- Durgan, D. J. , & Young, M. E. (2010). The cardiomyocyte circadian clock: Emerging roles in health and disease. Circulation Research, 106(4), 647–658. 10.1161/CIRCRESAHA.109.209957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Favero, G. , Franceschetti, L. , Rodella, L. F. , & Rezzani, R. (2015). Sirtuins, aging, and cardiovascular risks. Age (Dordrecht, Netherlands), 37(4), 9804 10.1007/s11357-015-9804-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernström, M. , Fernberg, U. , Eliason, G. , & Hurtig‐Wennlof, A. (2017). Aerobic fitness is associated with low cardiovascular disease risk: The impact of lifestyle on early risk factors for atherosclerosis in young healthy Swedish individuals—The lifestyle, biomarker, and atherosclerosis study. Vascular Health and Risk Management, 13, 91–99. 10.2147/VHRM.S125966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folino, A. , Losano, G. , & Rastaldo, R. (2013). Balance of nitric oxide and reactive oxygen species in myocardial reperfusion injury and protection. Journal of Cardiovascular Pharmacology, 62(6), 567–575. 10.1097/FJC.0b013e3182a50c45 [DOI] [PubMed] [Google Scholar]
- Franceschi, C. , Garagnani, P. , Parini, P. , Giuliani, C. , & Santoro, A. (2018). Inflammaging: A new immune–metabolic viewpoint for age‐related diseases. Nature Reviews Endocrinology, 14(10), 576–590. 10.1038/s41574-018-0059-4 [DOI] [PubMed] [Google Scholar]
- Fukuda, S. , Kaga, S. , Zhan, L. , Bagchi, D. , Das, D. K. , Bertelli, A. , & Maulik, N. (2006). Resveratrol ameliorates myocardial damage by inducing vascular endothelial growth factor‐angiogenesis and tyrosine kinase receptor Flk‐1. Cell Biochemistry and Biophysics, 44(1), 043–050. 10.1385/CBB:44:1:043 [DOI] [PubMed] [Google Scholar]
- Gadacha, W. , Ben‐Attia, M. , Bonnefont‐Rousselot, D. , Aouani, E. , Ghanem‐Boughanmi, N. , & Touitou, Y. (2009). Resveratrol opposite effects on rat tissue lipoperoxidation: Pro‐oxidant during day‐time and antioxidant at night resveratrol opposite effects on rat tissue lipoperoxidation: Pro‐oxidant during day‐time and antioxidant at night. Redox Report, 14(4), 154–158. 10.1179/135100009X466131 [DOI] [PubMed] [Google Scholar]
- Gan, X. T. , Ettinger, G. , Huang, C. X. , Burton, J. P. , Haist, J. V. , Rajapurohitam, V. , … Karmazyn, M. (2014). Probiotic administration attenuates myocardial hypertrophy and heart failure after myocardial infarction in the rat. Circulation: Heart Failure, 7(3), 491–499. 10.1161/CIRCHEARTFAILURE.113.000978 [DOI] [PubMed] [Google Scholar]
- Garza, M. A. , Wason, E. A. , & Zhang, J. Q. (2015). Cardiac remodeling and physical training post myocardial infarction. World Journal of Cardiology, 7(2), 52–64. 10.4330/wjc.v7.i2.52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gehm, B. D. , McAndrews, J. M. , Chien, P. Y. , & Jameson, J. L. (1997). Resveratrol, a polyphenolic compound found in grapes and wine, is an agonist for the estrogen receptor. Proceedings of the National Academy of Sciences of the United States of America, 94(25), 14138–14143. 10.1073/pnas.94.25.14138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gélinas, R. , Mailleux, F. , Dontaine, J. , Bultot, L. , Demeulder, B. , Ginion, A. , … Bertrand, L. (2018). AMPK activation counteracts cardiac hypertrophy by reducing O‐GlcNAcylation. Nature Communications, 9(1), 374 10.1038/s41467-017-02795-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gueguen, N. , Desquiret‐Dumas, V. , Leman, G. , Chupin, S. , Baron, S. , Nivet‐Antoine, V. , … Procaccio, V. (2015). Resveratrol directly binds to mitochondrial complex I and increases oxidative stress in brain mitochondria of aged mice. PLoS ONE. Edited by J. Zhang, 10(12), e0144290 10.1371/journal.pone.0144290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta, P. K. , DiPette, D. J. , & Supowit, S. C. (2014). Protective effect of resveratrol against pressure overload‐induced heart failure. Food Science & Nutrition, 2(3), 218–229. 10.1002/fsn3.92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haghighatdoost, F. , & Hariri, M. (2018). Effect of resveratrol on lipid profile: An updated systematic review and meta‐analysis on randomized clinical trials. Pharmacological Research, 129, 141–150. 10.1016/j.phrs.2017.12.033 [DOI] [PubMed] [Google Scholar]
- Hardie, D. G. (2011). AMP‐activated protein kinase: An energy sensor that regulates all aspects of cell function. Genes & Development, 25(18), 1895–1908. 10.1101/gad.17420111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harding, S. D. , Sharman, J. L. , Faccenda, E. , Southan, C. , Pawson, A. J. , Ireland, S. , … NC‐IUPHAR (2018). The IUPHAR/BPS guide to PHARMACOLOGY in 2018: Updates and expansion to encompass the new guide to IMMUNOPHARMACOLOGY. Nucleic Acids Research, 46(D1), D1091–D1106. 10.1093/nar/gkx1121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hood, S. , & Amir, S. (2017). The aging clock: Circadian rhythms and later life. The Journal of Clinical Investigation, 127(2), 437–446. 10.1172/JCI90328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howells, L. M. , Berry, D. P. , Elliott, P. J. , Jacobson, E. W. , Hoffmann, E. , Hegarty, B. , … Gescher, A. J. (2011). Phase I randomized, double‐blind pilot study of micronized resveratrol (SRT501) in patients with hepatic metastases—Safety, pharmacokinetics, and pharmacodynamics. Cancer Prevention Research (Philadelphia, Pa.), 4(9), 1419–1425. 10.1158/1940-6207.CAPR-11-0148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang, F. C. , Kuo, H. C. , Huang, Y. H. , Yu, H. R. , Li, S. C. , & Kuo, H. C. (2017). Anti‐inflammatory effect of resveratrol in human coronary arterial endothelial cells via induction of autophagy: Implication for the treatment of Kawasaki disease. BMC Pharmacology and Toxicology, 18(1), 3 10.1186/s40360-016-0109-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang, H. , Chen, G. , Liao, D. , Zhu, Y. , Pu, R. , & Xue, X. (2016). The effects of resveratrol intervention on risk markers of cardiovascular health in overweight and obese subjects: A pooled analysis of randomized controlled trials. Obesity Reviews, 17(12), 1329–1340. 10.1111/obr.12458 [DOI] [PubMed] [Google Scholar]
- Hwang, J. S. , Ham, S. A. , Yoo, T. , Lee, W. J. , Paek, K. S. , Lee, C. H. , & Seo, H. G. (2016). Sirtuin 1 mediates the actions of peroxisome proliferator‐activated receptor on the oxidized low‐density lipoprotein‐triggered migration and proliferation of vascular smooth muscle cells. Molecular Pharmacology, 90(5), 522–529. 10.1124/mol.116.104679 [DOI] [PubMed] [Google Scholar]
- Jiang, Z. , Chen, X. , Chen, K. , Sun, L. , Gao, L. , Zhou, C. , … Ma, J. (2016). YAP inhibition by resveratrol via activation of AMPK enhances the sensitivity of pancreatic cancer cells to gemcitabine. Nutrients, 8(10). 10.3390/nu8100546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kan, N. W. , Lee, M. C. , Tung, Y. T. , Chiu, C. C. , Huang, C. C. , & Huang, W. C. (2018). The synergistic effects of resveratrol combined with resistant training on exercise performance and physiological adaption. Nutrients, 10(10), 1360 10.3390/nu10101360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karlsson, F. H. , Fåk, F. , Nookaew, I. , Tremaroli, V. , Fagerberg, B. , Petranovic, D. , … Nielsen, J. (2012). Symptomatic atherosclerosis is associated with an altered gut metagenome. Nature Communications, 3, 1245 10.1038/ncomms2266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ke, R. , Xu, Q. , Li, C. , Luo, L. , & Huang, D. (2018). Mechanisms of AMPK in the maintenance of ATP balance during energy metabolism. Cell Biology International, 42(4), 384–392. 10.1002/cbin.10915 [DOI] [PubMed] [Google Scholar]
- Kim, E. N. , Kim, M. Y. , Lim, J. H. , Kim, Y. , Shin, S. J. , Park, C. W. , … Choi, B. S. (2018). The protective effect of resveratrol on vascular aging by modulation of the renin–angiotensin system. Atherosclerosis, 270, 123–131. 10.1016/J.ATHEROSCLEROSIS.2018.01.043 [DOI] [PubMed] [Google Scholar]
- Kim, T. T. , Parajuli, N. , Sung, M. M. , Bairwa, S. C. , Levasseur, J. , Soltys, C. L. M. , … Dyck, J. R. B. (2018). Fecal transplant from resveratrol‐fed donors improves glycaemia and cardiovascular features of the metabolic syndrome in mice. American Journal of Physiology‐Endocrinology and Metabolism, 315(4), E511–E519. 10.1152/ajpendo.00471.2017 [DOI] [PubMed] [Google Scholar]
- Kwak, S. E. , Lee, J. H. , Zhang, D. , & Song, W. (2018). Angiogenesis: Focusing on the effects of exercise in aging and cancer. Journal of Exercise Nutrition & Biochemistry, 22(3), 21–26. 10.20463/jenb.2018.0020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau, A. W. , Liu, P. , Inuzuka, H. , & Gao, D. (2014). SIRT1 phosphorylation by AMP‐activated protein kinase regulates p53 acetylation. American Journal of Cancer Research, 4(3), 245–255. [PMC free article] [PubMed] [Google Scholar]
- Leosco, D. , Rengo, G. , Iaccarino, G. , Golino, L. , Marchese, M. , Fortunato, F. , … Rengo, F. (2008). Exercise promotes angiogenesis and improves β‐adrenergic receptor signalling in the post‐ischaemic failing rat heart. Cardiovascular Research, 78(2), 385–394. 10.1093/cvr/cvm109 [DOI] [PubMed] [Google Scholar]
- Li, J. , Yu, S. , Ying, J. , Shi, T. , & Wang, P. (2017). Resveratrol prevents ROS‐induced apoptosis in high glucose‐treated retinal capillary endothelial cells via the activation of AMPK/Sirt1/PGC‐1 α pathway. Oxidative Medicine and Cellular Longevity, 2017, 1–10. 10.1155/2017/7584691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, Y. , Zhu, W. , Tao, J. P. , Xin, P. , Liu, M. Y. , Li, J. B. , & Wei, M. (2013). Resveratrol protects cardiomyocytes from oxidative stress through SIRT1 and mitochondrial biogenesis signaling pathways. Biochemical and Biophysical Research Communications, 438(2), 270–276. 10.1016/J.BBRC.2013.07.042 [DOI] [PubMed] [Google Scholar]
- Liu, J. , Bi, X. , Chen, T. , Zhang, Q. , Wang, S. X. , Chiu, J. J. , … Jiang, F. (2015). Shear stress regulates endothelial cell autophagy via redox regulation and Sirt1 expression. Cell Death & Disease, 6(7), e1827 10.1038/cddis.2015.193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, J. , Peng, Y. , Lai, J. , Gao, W. , Song, A. , & Zhang, G. (2017). Fluid upstream shear stress of rabbit aortic stenosis inhibits neointimal hyperplasia by promoting endothelization after balloon injury. BMC Cardiovascular Disorders, 17(1), 273 10.1186/s12872-017-0690-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- López‐otín, C. , Blasco, M. A. , Partridge, L. , Serrano, M. , & Kroemer, G. (2013). The hallmarks of aging. Cell, 153(6), 1194–1217. 10.1016/j.cell.2013.05.039.The [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma, S. , Feng, J. , Zhang, R. , Chen, J. , Han, D. , Li, X. , … Cao, F. (2017). SIRT1 activation by resveratrol alleviates cardiac dysfunction via mitochondrial regulation in diabetic cardiomyopathy mice. Oxidative Medicine and Cellular Longevity, 2017, 4602715–15. 10.1155/2017/4602715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magyar, K. , Halmosi, R. , Palfi, A. , Feher, G. , Czopf, L. , Fulop, A. , … Szabados, E. (2012). Cardioprotection by resveratrol: A human clinical trial in patients with stable coronary artery disease. Clinical Hemorheology and Microcirculation, 50(3), 179–187. 10.3233/CH-2011-1424 [DOI] [PubMed] [Google Scholar]
- Masri, S. (2015). Sirtuin‐dependent clock control: New advances in metabolism, aging and cancer. Current Opinion in Clinical Nutrition and Metabolic Care, 18(6), 521–527. 10.1097/MCO.0000000000000219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumura, N. , Takahara, S. , Maayah, Z. H. , Parajuli, N. , Byrne, N. J. , Shoieb, S. M. , … Dyck, J. R. B. (2018). Resveratrol improves cardiac function and exercise performance in MI‐induced heart failure through the inhibition of cardiotoxic HETE metabolites. Journal of Molecular and Cellular Cardiology, 125, 162–173. 10.1016/j.yjmcc.2018.10.023 [DOI] [PubMed] [Google Scholar]
- Matsushima, S. , & Sadoshima, J. (2015). The role of sirtuins in cardiac disease. American Journal of Physiology. Heart and Circulatory Physiology, 309(9), H1375–H1389. 10.1152/ajpheart.00053.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattagajasingh, I. , Kim, C. S. , Naqvi, A. , Yamamori, T. , Hoffman, T. A. , Jung, S. B. , … Irani, K. (2007). SIRT1 promotes endothelium‐dependent vascular relaxation by activating endothelial nitric oxide synthase. Proceedings of the National Academy of Sciences of the United States of America, 104(37), 14855–14860. 10.1073/pnas.0704329104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miranda, J. , Portillo, M. P. , Madrid, J. A. , Arias, N. , Macarulla, M. T. , & Garaulet, M. (2013). Effects of resveratrol on changes induced by high‐fat feeding on clock genes in rats. British Journal of Nutrition, 110(8), 1421–1428. 10.1017/S0007114513000755 [DOI] [PubMed] [Google Scholar]
- Montezano, A. C. , & Touyz, R. M. (2014). Reactive oxygen species, vascular Noxs, and hypertension: Focus on translational and clinical research. Antioxidants & Redox Signaling, 20(1), 164–182. 10.1089/ars.2013.5302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Most, J. , Penders, J. , Lucchesi, M. , Goossens, G. H. , & Blaak, E. E. (2017). Gut microbiota composition in relation to the metabolic response to 12‐week combined polyphenol supplementation in overweight men and women. European Journal of Clinical Nutrition, 71(9), 1040–1045. 10.1038/ejcn.2017.89 [DOI] [PubMed] [Google Scholar]
- Nagpal, R. , Mainali, R. , Ahmadi, S. , Wang, S. , Singh, R. , Kavanagh, K. , … Yadav, H. (2018). Gut microbiome and aging: Physiological and mechanistic insights. Nutrition and Healthy Aging, 4(4), 267–285. 10.3233/NHA-170030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narkar, V. A. , Downes, M. , Yu, R. T. , Embler, E. , Wang, Y. X. , Banayo, E. , … Evans, R. M. (2008). AMPK and PPARδ agonists are exercise mimetics. Cell, 134(3), 405–415. 10.1016/j.cell.2008.06.051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neves, A. R. , Martins, S. , Segundo, M. A. , & Reis, S. (2016). Nanoscale delivery of resveratrol towards enhancement of supplements and nutraceuticals. Nutrients, 8(3), 131 10.3390/nu8030131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- North, B. J. , & Sinclair, D. A. (2012). The intersection between aging and cardiovascular disease. Circulation Research, 110(8), 1097–1108. 10.1161/CIRCRESAHA.111.246876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olmos, Y. , Sánchez‐Gómez, F. J. , Wild, B. , García‐Quintans, N. , Cabezudo, S. , Lamas, S. , & Monsalve, M. (2013). SirT1 regulation of antioxidant genes is dependent on the formation of a FoxO3a/PGC‐1α complex. Antioxidants & Redox Signaling, 19(13), 1507–1521. 10.1089/ars.2012.4713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park, I. , Lee, Y. , Kim, H. D. , & Kim, K. (2014). Effect of resveratrol, a SIRT1 activator, on the interactions of the CLOCK/BMAL1 complex. Endocrinology and Metabolism, 29, 379–387. 10.3803/EnM.2014.29.3.379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park, S. Y. , Lee, S. W. , Lee, S. Y. , Hong, K. W. , Bae, S. S. , Kim, K. , & Kim, C. D. (2017). SIRT1/adenosine monophosphate‐activated protein kinase α signaling enhances macrophage polarization to an anti‐inflammatory phenotype in rheumatoid arthritis. Frontiers in Immunology, 8, 1135 10.3389/fimmu.2017.01135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Planavila, A. , Iglesias, R. , Giralt, M. , & Villarroya, F. (2011). Sirt1 acts in association with PPAR to protect the heart from hypertrophy, metabolic dysregulation, and inflammation. Cardiovascular Research, 90(2), 276–284. 10.1093/cvr/cvq376 [DOI] [PubMed] [Google Scholar]
- Poduri, A. , Chang, A. H. , Raftrey, B. , Rhee, S. , van, M. , & Red‐Horse, K. (2017). Endothelial cells respond to the direction of mechanical stimuli through SMAD signaling to regulate coronary artery size. Development (Cambridge, England), 144(18), 3241–3252. 10.1242/dev.150904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollack, R. M. , Barzilai, N. , Anghel, V. , Kulkarni, A. S. , Golden, A. , O'Broin, P. , … Crandall, J. P. (2017). Resveratrol improves vascular function and mitochondrial number but not glucose metabolism in older adults. The Journals of Gerontology: Series a, 72(12), 1703–1709. 10.1093/gerona/glx041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poussier, B. , Cordova, A. C. , Becquemin, J. P. , & Sumpio, B. E. (2005). Resveratrol inhibits vascular smooth muscle cell proliferation and induces apoptosis. Journal of Vascular Surgery, 42(6), 1190–1190.e14. 10.1016/j.jvs.2005.08.014 [DOI] [PubMed] [Google Scholar]
- du Pré, B. C. , Dierickx, P. , Crnko, S. , Doevendans, P. A. , Vos, M. A. , Geijsen, N. , … van Laake, L. W. (2017). Neonatal rat cardiomyocytes as an in vitro model for circadian rhythms in the heart. Journal of Molecular and Cellular Cardiology, 112, 58–63. 10.1016/J.YJMCC.2017.08.009 [DOI] [PubMed] [Google Scholar]
- Price, N. L. , Gomes, A. P. , Ling, A. J. Y. , Duarte, F. V. , Martin‐Montalvo, A. , North, B. J. , … Sinclair, D. A. (2012). SIRT1 is required for AMPK activation and the beneficial effects of resveratrol on mitochondrial function. Cell Metabolism, 15(5), 675–690. 10.1016/j.cmet.2012.04.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren, X. , Chen, L. , Xie, J. , Zhang, Z. , Dong, G. , Liang, J. , … Luo, P. (2017). Resveratrol ameliorates mitochondrial elongation via Drp1/Parkin/PINK1 signaling in senescent‐like cardiomyocytes. Oxidative Medicine and Cellular Longevity, 2017, 4175353–20. 10.1155/2017/4175353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renaud, S. , & deLorgeril, M. (1992). Wine, alcohol, platelets, and the French paradox for coronary heart disease. The Lancet, 339(8808), 1523–1526. 10.1016/0140-6736(92)91277-F [DOI] [PubMed] [Google Scholar]
- Roth, G. A. , Johnson, C. , Abajobir, A. , Abd‐Allah, F. , Abera, S. F. , Abyu, G. , … Murray, C. (2017). Global, regional, and national burden of cardiovascular diseases for 10 causes, 1990 to 2015. Journal of the American College of Cardiology, 70(1), 1–25. 10.1016/j.jacc.2017.04.052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruderman, N. B. , Julia Xu, X. , Nelson, L. , Cacicedo, J. M. , Saha, A. K. , Lan, F. , & Ido, Y. (2010). AMPK and SIRT1: A long‐standing partnership? American Journal of Physiology. Endocrinology and Metabolism, 298(4), E751–E760. 10.1152/ajpendo.00745.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salehi, B. , Mishra, A. P. , Nigam, M. , Sener, B. , Kilic, M. , Sharifi‐Rad, M. , … Sharifi‐Rad, J. (2018). Resveratrol: A double‐edged sword in health benefits. Biomedicine, 6(3). 10.3390/biomedicines6030091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salminen, A. , & Kaarniranta, K. (2009). NF‐κB signaling in the aging process. Journal of Clinical Immunology, 29(4), 397–405. 10.1007/s10875-009-9296-6 [DOI] [PubMed] [Google Scholar]
- Salminen, A. , Kaarniranta, K. , & Kauppinen, A. (2016). Age‐related changes in AMPK activation: Role for AMPK phosphatases and inhibitory phosphorylation by upstream signaling pathways. Ageing Research Reviews, 28, 15–26. 10.1016/j.arr.2016.04.003 [DOI] [PubMed] [Google Scholar]
- Sangwung, P. , Zhou, G. , Nayak, L. , Chan, E. R. , Kumar, S. , Kang, D. W. , … Jain, M. K. (2017). KLF2 and KLF4 control endothelial identity and vascular integrity. JCI Insight, 2(4), e91700 10.1172/jci.insight.91700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuler, G. , Adams, V. , & Goto, Y. (2013). Role of exercise in the prevention of cardiovascular disease: Results, mechanisms, and new perspectives. European Heart Journal, 34(24), 1790–1799. 10.1093/eurheartj/eht111 [DOI] [PubMed] [Google Scholar]
- Schwager, J. , Richard, N. , Widmer, F. , & Raederstorff, D. (2017). Resveratrol distinctively modulates the inflammatory profiles of immune and endothelial cells. BMC Complementary and Alternative Medicine, 17(1), 1–12. 10.1186/s12906-017-1823-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senghor, B. , Sokhna, C. , Ruimy, R. , & Lagier, J. C. (2018). Gut microbiota diversity according to dietary habits and geographical provenance. Human Microbiome Journal, 7–8, 1–9. 10.1016/J.HUMIC.2018.01.001 [DOI] [Google Scholar]
- Shahid, F. , Lip, G. Y. H. , & Shantsila, E. (2018). Role of monocytes in heart failure and atrial fibrillation. Journal of the American Heart Association, 7(3). 10.1161/JAHA.117.007849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simão, F. , Pagnussat, A. S. , Seo, J. H. , Navaratna, D. , Leung, W. , Lok, J. , … Lo, E. H. (2012). Pro‐angiogenic effects of resveratrol in brain endothelial cells: Nitric oxide‐mediated regulation of vascular endothelial growth factor and metalloproteinases. Journal of Cerebral Blood Flow and Metabolism: Official Journal of the International Society of Cerebral Blood Flow and Metabolism, 32(5), 884–895. 10.1038/jcbfm.2012.2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh, A. P. , Singh, R. , Verma, S. S. , Rai, V. , Kaschula, C. H. , Maiti, P. , & Gupta, S. C. (2019). Health benefits of resveratrol: Evidence from clinical studies. Medicinal Research Reviews.. 10.1002/med.21565 [DOI] [PubMed] [Google Scholar]
- Skroza, D. , Generalić Mekinić, I. , Svilović, S. , Šimat, V. , & Katalinić, V. (2015). Investigation of the potential synergistic effect of resveratrol with other phenolic compounds: A case of binary phenolic mixtures. Journal of Food Composition and Analysis, 38, 13–18. 10.1016/J.JFCA.2014.06.013 [DOI] [Google Scholar]
- Sun, L. , Wang, Y. , Song, Y. , Cheng, X. R. , Xia, S. , Rahman, M. R. T. , … le, G. (2015). Resveratrol restores the circadian rhythmic disorder of lipid metabolism induced by high‐fat diet in mice. Biochemical and Biophysical Research Communications, 458(1), 86–91. 10.1016/J.BBRC.2015.01.072 [DOI] [PubMed] [Google Scholar]
- Sun, N. , Youle, R. J. , & Finkel, T. (2016). The mitochondrial basis of aging. Molecular Cell, 61(5), 654–666. 10.1016/j.molcel.2016.01.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabrizi, R. , Tamtaji, O. R. , Lankarani, K. B. , Mirhosseini, N. , Akbari, M. , Dadgostar, E. , … Asemi, Z. (2018). The effects of resveratrol supplementation on biomarkers of inflammation and oxidative stress among patients with metabolic syndrome and related disorders: A systematic review and meta‐analysis of randomized controlled trials. Food & Function, 9(12), 6116–6128. 10.1039/C8FO01259H [DOI] [PubMed] [Google Scholar]
- Takeda, N. , & Maemura, K. (2011). Circadian clock and cardiovascular disease. Journal of Cardiology, 57(3), 249–256. 10.1016/j.jjcc.2011.02.006 [DOI] [PubMed] [Google Scholar]
- Tang, W. H. W. , Kitai, T. , & Hazen, S. L. (2017). Gut microbiota in cardiovascular health and disease. Circulation Research, 120(7), 1183–1196. 10.1161/CIRCRESAHA.117.309715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thandapilly, S. J. , Louis, X. L. , Yang, T. , Stringer, D. M. , Yu, L. , Zhang, S. , … Netticadan, T. (2011). Resveratrol prevents norepinephrine induced hypertrophy in adult rat cardiomyocytes, by activating NO‐AMPK pathway. European Journal of Pharmacology, 668(1–2), 217–224. 10.1016/J.EJPHAR.2011.06.042 [DOI] [PubMed] [Google Scholar]
- Thompson, A. M. , Martin, K. A. , & Rzucidlo, E. M. (2014). Resveratrol induces vascular smooth muscle cell differentiation through stimulation of SirT1 and AMPK. PLoS ONE. Edited by M. Nurminskaya, 9(1), e85495 10.1371/journal.pone.0085495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thosar, S. S. , Butler, M. P. , & Shea, S. A. (2018). Role of the circadian system in cardiovascular disease. Journal of Clinical Investigation, 128(6), 2157–2167. 10.1172/JCI80590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian, X. Y. , Wong, W. T. , Xu, A. , Lu, Y. , Zhang, Y. , Wang, L. , … Huang, Y. (2012). Uncoupling protein‐2 protects endothelial function in diet‐induced obese mice. Circulation Research, 110(9), 1211–1216. 10.1161/CIRCRESAHA.111.262170 [DOI] [PubMed] [Google Scholar]
- Timmers, S. , de Ligt, M. , Phielix, E. , van de Weijer, T. , Hansen, J. , Moonen‐Kornips, E. , … Schrauwen, P. (2016). Resveratrol as add‐on therapy in subjects with well‐controlled type 2 diabetes: A randomized controlled trial. Diabetes Care, 39(12), 2211–2217. 10.2337/dc16-0499 [DOI] [PubMed] [Google Scholar]
- Tomayko, E. J. , Cachia, A. J. , Chung, H. R. , & Wilund, K. R. (2014). Resveratrol supplementation reduces aortic atherosclerosis and calcification and attenuates loss of aerobic capacity in a mouse model of uremia. Journal of Medicinal Food, 17(2), 278–283. 10.1089/jmf.2012.0219 [DOI] [PubMed] [Google Scholar]
- Tomé‐Carneiro, J. , Gonzálvez, M. , Larrosa, M. , García‐Almagro, F. J. , Avilés‐Plaza, F. , Parra, S. , … Espín, J. C. (2012). Consumption of a grape extract supplement containing resveratrol decreases oxidized LDL and ApoB in patients undergoing primary prevention of cardiovascular disease: A triple‐blind, 6‐month follow‐up, placebo‐controlled, randomized trial. Molecular Nutrition & Food Research, 56(5), 810–821. 10.1002/mnfr.201100673 [DOI] [PubMed] [Google Scholar]
- Tomé‐Carneiro, J. , Gonzálvez, M. , Larrosa, M. , Yáñez‐Gascón, M. J. , García‐Almagro, F. J. , Ruiz‐Ros, J. A. , … Espín, J. C. (2012). One‐year consumption of a grape nutraceutical containing resveratrol improves the inflammatory and fibrinolytic status of patients in primary prevention of cardiovascular disease. The American Journal of Cardiology, 110(3), 356–363. 10.1016/j.amjcard.2012.03.030 [DOI] [PubMed] [Google Scholar]
- Trinity, J. D. , Groot, H. J. , Layec, G. , Rossman, M. J. , Ives, S. J. , & Richardson, R. S. (2014). Impact of age and body position on the contribution of nitric oxide to femoral artery shear rate: Implications for atherosclerosis. Hypertension (Dallas, Tex. : 1979), 63(5), 1019–1025. 10.1161/HYPERTENSIONAHA.113.02854 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trott, A. J. , & Menet, J. S. (2018). Regulation of circadian clock transcriptional output by CLOCK:BMAL1. PLoS Genetics, 14(1), e1007156 10.1371/journal.pgen.1007156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ungvari, Z. , Labinskyy, N. , Mukhopadhyay, P. , Pinto, J. T. , Bagi, Z. , Ballabh, P. , … Csiszar, A. (2009). Resveratrol attenuates mitochondrial oxidative stress in coronary arterial endothelial cells. American Journal of Physiology. Heart and Circulatory Physiology, 297(5), H1876–H1881. 10.1152/ajpheart.00375.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ungvari, Z. , Sonntag, W. E. , de Cabo, R. , Baur, J. A. , & Csiszar, A. (2011). Mitochondrial protection by resveratrol. Exercise and Sport Sciences Reviews, 39(3), 128–132. 10.1097/JES.0b013e3182141f80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ungvari, Z. , Tarantini, S. , Kiss, T. , Wren, J. D. , Giles, C. B. , Griffin, C. T. , … Csiszar, A. (2018). Endothelial dysfunction and angiogenesis impairment in the ageing vasculature. Nature Reviews Cardiology, 15(9), 555–565. 10.1038/s41569-018-0030-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varma Penumathsa, S. , Thirunavukkarasu, M. , Koneru, S. , Juhasz, B. , Zhan, L. , Pant, R. , … Maulik, N. (2006). Statin and resveratrol in combination induces cardioprotection against myocardial infarction in hypercholesterolemic rat. Journal of Molecular and Cellular Cardiology, 42(3), 508–516. 10.1016/j.yjmcc.2006.10.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasamsetti, S. B. , Karnewar, S. , Gopoju, R. , Gollavilli, P. N. , Narra, S. R. , Kumar, J. M. , & Kotamraju, S. (2016). Resveratrol attenuates monocyte‐to‐macrophage differentiation and associated inflammation via modulation of intracellular GSH homeostasis: Relevance in atherosclerosis. Free Radical Biology and Medicine, 96, 392–405. 10.1016/j.freeradbiomed.2016.05.003 [DOI] [PubMed] [Google Scholar]
- Vion, A. C. , Kheloufi, M. , Hammoutene, A. , Poisson, J. , Lasselin, J. , Devue, C. , … Rautou, P. E. (2017). Autophagy is required for endothelial cell alignment and atheroprotection under physiological blood flow. Proceedings of the National Academy of Sciences, 114(41), E8675–E8684. 10.1073/PNAS.1702223114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallerath, T. , Deckert, G. , … Ternes, T. , Anderson, H. , Li, H. , Witte, K. , & Förstermann, U. (2002). Resveratrol, a polyphenolic phytoalexin present in red wine, enhances expression and activity of endothelial nitric oxide synthase. Circulation, 106, 1652–1658. 10.1161/01.CIR.0000029925.18593.5C [DOI] [PubMed] [Google Scholar]
- Wang, B. , Yang, Q. , Sun, Y. , Xing, Y. F. , Wang, Y. B. , Lu, X. T. , … Zhao, Y. X. (2014). Resveratrol‐enhanced autophagic flux ameliorates myocardial oxidative stress injury in diabetic mice. Journal of Cellular and Molecular Medicine, 18(8), 1599–1611. 10.1111/jcmm.12312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, G. , Song, X. , Zhao, L. , Li, Z. , & Liu, B. (2018). Resveratrol prevents diabetic cardiomyopathy by increasing Nrf2 expression and transcriptional activity. BioMed Research International, 2018, 2150218–13. 10.1155/2018/2150218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, L. , Luo, J. Y. , Li, B. , Tian, X. Y. , Chen, L. J. , Huang, Y. , … Huang, Y. (2016). Integrin‐YAP/TAZ‐JNK cascade mediates atheroprotective effect of unidirectional shear flow. Nature, 540(7634), 579–582. 10.1038/nature20602 [DOI] [PubMed] [Google Scholar]
- Wang, P. , & Sang, S. (2018). Metabolism and pharmacokinetics of resveratrol and pterostilbene. BioFactors, 44(1), 16–25. 10.1002/biof.1410 [DOI] [PubMed] [Google Scholar]
- Weiskirchen, S. , & Weiskirchen, R. (2016). Resveratrol: How much wine do you have to drink to stay healthy? Advances in Nutrition (Bethesda, Md.), 7(4), 706–718. 10.3945/an.115.011627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wightman, E. L. , Reay, J. L. , Haskell, C. F. , Williamson, G. , Dew, T. P. , & Kennedy, D. O. (2014). Effects of resveratrol alone or in combination with piperine on cerebral blood flow parameters and cognitive performance in human subjects: A randomised, double‐blind, placebo‐controlled, cross‐over investigation. British Journal of Nutrition, 112(2), 203–213. 10.1017/S0007114514000737 [DOI] [PubMed] [Google Scholar]
- Wong, R. H. X. , Berry, N. M. , Coates, A. M. , Buckley, J. D. , Bryan, J. , Kunz, I. , & Howe, P. R. C. (2013). Chronic resveratrol consumption improves brachial flow‐mediated dilatation in healthy obese adults. Journal of Hypertension, 31(9), 1819–1827. 10.1097/HJH.0b013e328362b9d6 [DOI] [PubMed] [Google Scholar]
- Xia, N. , Daiber, A. , Habermeier, A. , Closs, E. I. , Thum, T. , Spanier, G. , … Li, H. (2010). Resveratrol reverses endothelial nitric‐oxide synthase uncoupling in apolipoprotein E knockout mice. The Journal of Pharmacology and Experimental Therapeutics, 335(1), 149–154. 10.1124/jpet.110.168724 [DOI] [PubMed] [Google Scholar]
- Yao, Q. P. , Qi, Y. X. , Zhang, P. , Cheng, B. B. , Yan, Z. Q. , & Jiang, Z. L. (2013). SIRT1 and Connexin40 mediate the normal shear stress‐induced inhibition of the proliferation of endothelial cells co‐cultured with vascular smooth muscle cells. Cellular Physiology and Biochemistry: International Journal of Experimental Cellular Physiology, Biochemistry, and Pharmacology, 31(2–3), 389–399. 10.1159/000343376 [DOI] [PubMed] [Google Scholar]
- Yurdagul, A. , Kleinedler, J. J. , McInnis, M. C. , Khandelwal, A. R. , Spence, A. L. , Orr, A. W. , & Dugas, T. R. (2014). Resveratrol promotes endothelial cell wound healing under laminar shear stress through an estrogen receptor‐α‐dependent pathway. American Journal of Physiology‐Heart and Circulatory Physiology, 306(6), H797–H806. 10.1152/ajpheart.00892.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaha, V. G. , & Young, L. H. (2012). AMP‐activated protein kinase regulation and biological actions in the heart. Circulation Research, 111(6), 800–814. 10.1161/CIRCRESAHA.111.255505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zamora‐Ros, R. , Urpi‐Sarda, M. , Lamuela‐Raventós, R. M. , Martínez‐González, M. Á. , Salas‐Salvadó, J. , Arós, F. , … Andres‐Lacueva, C. (2012). High urinary levels of resveratrol metabolites are associated with a reduction in the prevalence of cardiovascular risk factors in high‐risk patients. Pharmacological Research, 65(6), 615–620. 10.1016/j.phrs.2012.03.009 [DOI] [PubMed] [Google Scholar]
- Zhang, C. , Lin, G. , Wan, W. , Li, X. , Zeng, B. , Yang, B. , & Huang, C. (2012). Resveratrol, a polyphenol phytoalexin, protects cardiomyocytes against anoxia/reoxygenation injury via the TLR4/NF‐κB signaling pathway. International Journal of Molecular Medicine, 29(4), 557–563. 10.3892/ijmm.2012.885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, W. , Huang, Q. , Zeng, Z. , Wu, J. , Zhang, Y. , & Chen, Z. (2017). Sirt1 inhibits oxidative stress in vascular endothelial cells. Oxidative Medicine and Cellular Longevity, 2017, 7543973 10.1155/2017/7543973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, Y. , Wang, C. , Zhou, J. , Sun, A. , Hueckstaedt, L. K. , Ge, J. , & Ren, J. (2017). Complex inhibition of autophagy by mitochondrial aldehyde dehydrogenase shortens lifespan and exacerbates cardiac aging. Biochimica et Biophysica Acta. Molecular Basis of Disease, 1863(8), 1919–1932. 10.1016/j.bbadis.2017.03.016 [DOI] [PubMed] [Google Scholar]
- Zhou, X. , Chen, M. , Zeng, X. , Yang, J. , Deng, H. , Yi, L. , & Mi, M. T. (2014). Resveratrol regulates mitochondrial reactive oxygen species homeostasis through Sirt3 signaling pathway in human vascular endothelial cells. Cell Death & Disease, 5(12), e1576–e1576. 10.1038/cddis.2014.530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zordoky, B. N. M. , Robertson, I. M. , & Dyck, J. R. B. (2015). Preclinical and clinical evidence for the role of resveratrol in the treatment of cardiovascular diseases. Biochimica et Biophysica Acta ‐ Molecular Basis of Disease, 1852, 1155–1177. 10.1016/J.BBADIS.2014.10.016 [DOI] [PubMed] [Google Scholar]


