Abstract
Objectives:
We sought to investigate older patients’ ability to correctly and efficiently dose multi-drug regimens over nearly a decade and to explore factors predicting declines in medication self-management.
Design:
Longitudinal cohort study funded by the National Institutes on Aging
Setting:
One academic internal medicine clinic and six community health centers.
Participants:
Beginning in 2008, 900 English-speaking adults ages 55–74 were enrolled in the study, completing a baseline (T1) assessment. To date, 303 participants have completed the same assessment 9 years post-baseline (T4).
Measurements:
At T1, subjects were given a standardized, seven-drug regimen and asked to demonstrate how they would take medicine over 24 hours. The number of dosing errors made and times per day that a participant would take medicine were recorded. Health literacy was measured via the Newest Vital Sign and cognitive decline by the Mini Mental State Exam.
Results:
Participants on average made 2.9 dosing errors (SD: 2.5, range: 0–21) out of 21 potential errors at T1 and 5.0 errors (SD: 2.1, range: 1–18, p<0.001) at T4. In a multivariate model, limited literacy (β: 0.69, 95% CI: [0.18,1.20], p=0.01), meaningful cognitive decline (β :1.72, 95% CI: [0.70,2.74], p=0.01), number of chronic conditions (β: 0.21, 95% CI: [0.07,0.34], p=0.01), and number of baseline dosing errors (β: −0.76, 95% CI: [−0.85,−0.67], p<0.001) were significant, independent predictors of changes in dosing errors. Most patients overcomplicated their daily medication schedule; no sociodemographic characteristics were predictive of poor regimen organization in multivariate models. In a multivariate model, there were no significant predictors of changes in regimen consolidation over time, except regimen consolidation at T1.
Conclusions:
Older patients frequently overcomplicated drug regimens, and increasingly made more dosing errors over 9 years of follow-up. Patients with limited literacy, cognitive decline, and those with multi-morbidity were at greatest risk for errors.
Keywords: health literacy, medication safety, polypharmacy
INTRODUCTION
An estimated 90% of individuals age 65 and older in the United States are living with two or more chronic conditions requiring ongoing medical care.1 The increasing prevalence of older adults with multi-morbidity has led to a greater proportion of older patients challenged with polypharmacy,2 commonly defined as being prescribed five or more medications.3 Polypharmacy has increased roughly 3-fold over the past two decades. Among adults ages 45 to 64, almost one in five now take five or more prescription (Rx) medications, while more than 40% of individuals over 65 are self-managing complex, multi-drug regimens.4
Polypharmacy increases older patients’ risks for adverse drug events (ADEs), inadequate adherence, and worse chronic disease outcomes.5,6 The exact mechanisms through which polypharmacy leads to worse outcomes among older patients have been debated,7 however, patient confusion surrounding how to safely organize and take complex, multi-drug regimens is likely to be a key, root cause of medication errors and unintentional non-adherence. A number of cross-sectional studies have shown that many patients, particularly those who are older and those with limited health literacy skills, have difficulty understanding Rx instructions for use and are likely to overcomplicate daily Rx regimens, increasing the risk for poor adherence and ADEs.8–10 These challenges are often compounded as the complexity of a patient’s Rx regimen increases.8
What has not been as extensively studied is how patients’ ability to self-manage and organize complex Rx regimens changes over time, especially among older patients who are likely to face increased health demands due to aging and cognitive decline. The ability of older patients to take medication regimens correctly and efficiently has major ramifications on health, quality of life, and independence; understanding factors that may affect a decline in this skillset is therefore essential. In this study, we utilized a National Institutes on Aging (NIA) cohort study to investigate how older patients’ ability to correctly interpret, dose, and organize a standardized, seven-drug medication regimen changed over nearly a decade. We also sought to explore individual-level factors predicting declines in these medication self-management skills over time.
METHODS
This study utilizes data from a longitudinal NIA study (“Health Literacy and Cognitive Function among Older Adults”; R01AG030611; PI: Wolf), referred to as ‘LitCog.’ LitCog is a prospective cohort study of English-speaking older adults that examines changes in literacy skills and cognitive function over time and how these factors affect patient performance on common healthcare tasks.11
Participants
LitCog participants were originally enrolled in the cohort study from August 2008 through October 2010 from one academic general internal medicine clinic and six federally qualified health centers in Chicago, IL. Eligibility criteria included: 1) age 55–74, 2) English-speaking, 3) two or more visits at a study clinic within the past year, and 4) no severe cognitive, visual, or hearing impairment that would preclude study participation or consent.
Participants were recruited via a multi-stage process. First, potentially eligible patients were identified via medical record queries at participating clinic sites. These patients were sent a letter describing the study; the letter included a toll-free number that patients could call to opt out of being contacted by research personnel. Patients who did not opt out were contacted by trained research assistants (RAs), who introduced the study, verified eligibility, and scheduled an in-person interview. A complete description of these procedures has been published previously.11 Study procedures were approved by the Northwestern University Institutional Review Board.
Procedure
After study enrollment, participants were invited to complete two structured, in-person interviews, 7–10 days apart. Each interview lasted 2 hours and participants were compensated $100 total for their time. Following baseline interviews (referred to as T1), patients were invited to participate in additional assessments roughly 3 years (T2), 6 years (T3), and 9 years (T4) post-baseline. Batteries included measures of demographic, personal, and health characteristics as well as an extensive cognitive battery and assessments of performance on everyday health activities. A full description of study measures has been published previously.11 Data for analyses described herein are from T1 and T4 interviews; relevant measures are detailed below.
Main Measures
Predictor Variables
Health literacy and cognitive decline were the main predictor variables of interest for this study. To measure health literacy, trained RAs administered the Newest Vital Sign (NVS), an assessment of literacy and numeracy skills, to patients.12 This measure asks patients to read and interpret information presented on a standard nutrition facts label. The NVS was scored according to published guidelines.12 NVS scores at baseline were used to classify participants as having either a ‘high likelihood of limited literacy,’ ‘a possibility of limited literacy,’ or ‘adequate literacy’ skills (hereafter referred to as ‘low,’ ‘marginal,’ or ‘adequate’ literacy skills, respectively).12 Participants in the low and marginal categories were combined into a ‘limited literacy’ category for analyses.
Participants also completed the Mini Mental State Exam (MMSE) at both T1 and T4.13 The MMSE is a brief, standardized measure that has been used extensively in research and clinical settings to measure multiple domains of cognitive function in adults. A meaningful cognitive decline between T1 and T4 was defined as a decrease in MMSE raw score by 4 or more points according to published guidelines.14
Outcome Measures
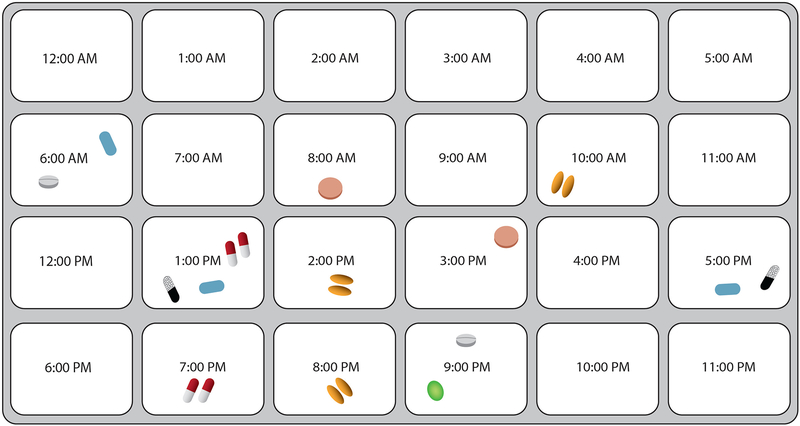
Two outcomes – number of dosing errors and regimen consolidation – were the focus of these analyses. Both variables were measured using a standardized assessment utilized by our team in multiple studies.8,15,16 Specifically, patients were given a hypothetical, multi-drug regimen, which consisted of seven Rx pill bottles with mock-up labels displaying different dosing instructions (see Table 1). Each Rx bottle contained simulated pills. Patients were also provided with a medication dosing tray, which contained 24 slots each labeled with one hour of the day (i.e. 11:00am). RAs reviewed these materials with participants and, after a brief orientation, asked them to use the dosing tray and ‘pills’ to demonstrate how they would dose out each medicine in the regimen over the course of 24 hours (see Figure 1). This assessment method has been effectively utilized in prior studies.8,15,16
Table 1.
Drug Regimen Instructions.
| Pimvampicillin 700mg | TAKE 1 TABLET BY MOUTH TWICE DAILY FOR 10 DAYS |
| Disopyramide 400mg | TAKE 2 TABLETS BY MOUTH EVERY 12 HOURS |
| Zoplicone 7.5mg | TAKE 1 TABLET BY MOUTH AT BEDTIME |
| Colesevelam 1875mg | TAKE 1 TABLET TWICE DAILY WITH MEALS AND LIQUID |
| Trimipramine 100mg | TAKE 1 TABLET BY MOUTH 3 TIMES DAILY |
| Cephalothin 500mg | TAKE 1 TABLET BY MOUTH 3 TIMES DAILY |
| Pinaverium 50mg | TAKE 2 TABLETS BY MOUTH 3 TIMES DAILY WITH FOOD AND WATER |
Figure 1.
Example of Regimen Dosing and Consolidation Exercise
The number of errors made by participants during the dosing exercise was calculated by reviewing the number of pills participants placed in each tray compartment for each medication. We determined whether there were any errors in: dose (number of pills taken), frequency (number of times medication is taken per day), and/or spacing (number of hours between doses). The number of dosing errors made by a participant was analyzed as a count variable; change in dosing errors between T1 and T4 was calculated as a count variable by subtracting the number of dosing errors at T1 from those at T4.
Regimen consolidation was also coded as a count variable using data from the dosing exercise. Its value was equivalent to the number of compartments utilized by participants in the dosing tray; this was intended to represent the number of times per day that a participant indicated they would take medication over the course of 24 hours. Only those patients who demonstrated correct frequency (i.e. number of times per day that a medication should be taken) for each medication in the regimen were included in these analyses to avoid categorizing participants who incorrectly took medication at too few times per day as ‘consolidating’ their regimen. The regimen consolidation variable was meant to broadly represent how well participants are able to organize and simplify a complex drug regimen. Given the label instructions for each medication, the standardized, 7-drug regimen could be organized so that medication was taken at only 3 distinct times over 24 hours.
Other Covariates
Participants were asked to self-report their race/ethnicity, age, sex, household income, and level of educational attainment. They were also asked to report whether they had ever been told by a doctor that they had any of the following chronic conditions: cardiovascular disease, chronic heart failure, diabetes, asthma, stroke, chronic bronchitis, hypertension, high cholesterol, arthritis, cancer and depression.
Analysis Plan
Simple descriptive statistics were calculated for variables measuring participants’ sociodemographic and health characteristics. We compared the mean number of dosing errors at T1 and T4 using a paired t-test. Bivariate analyses were conducted to examine the relationship between participant characteristics and study outcomes. We then conducted generalized linear modeling to assess the change in number of dosing errors from T1 to T4, adjusting for relevant baseline variables, including patient demographics theorized to influence dosing ability and regimen consolidation (e.g. age, sex, race/ethnicity, educational attainment), literacy levels, meaningful cognitive decline, and number of chronic conditions. Other relevant covariates related to study methodology (i.e. time elapsed between T1 and T4 interviews, number of baseline dosing errors at T1) were also included in multivariate models to reflect the study protocol. Similar analyses and modeling with the same covariates were conducted to examine the outcome of regimen consolidation. Analyses were conducted using STATA 15.1 (College Station, TX).
RESULTS
Sample Characteristics
A total of 900 patients completed T1 interviews in 2008–2010. Of these, 303 patients to date have participated in the ongoing T4 interviews and have complete data from the T1 and T4 medication dosing exercises. Compared to participants who completed the T4 interview, participants who were lost to follow-up by T4 had a significantly higher mean number of dosing errors at T1 (mean: 3.99, SD: 3.5 vs. mean: 2.89, SD: 2.5, p<0.001); they were also significantly older, less educated, had lower health literacy, were members of racial/ethnic minority groups, had lower income, and had a greater number of chronic conditions.
On average, patients in this sample were 62.6 years old at study enrollment and predominately female (72.9%; see Table 2). About one-third of patients identified as racial/ethnic minorities, 38.3% had limited literacy skills according to the NVS, and 21.8% had a household income of less than $25,000 per year. At T1, patients in the sample self-reported an average of 2.3 chronic conditions. Almost all participants (98%) had no cognitive impairment and the remaining 2% had mild cognitive impairment according to the MMSE. By T4, patients in this sample had an average of 3.2 chronic conditions; 96% had no cognitive impairment, 3.6% had mild cognitive impairment, and 1 participant had severe cognitive impairment. A total of 13 participants (4.3%) experienced meaningful cognitive decline according to the MMSE between T1 and T4.
Table 2.
Study Sample Characteristics
| Variables | Participants (N=303) |
|---|---|
| Age, mean (SD) | 62.6 (5.4) |
| Male, n (%) | 82 (27.1) |
| Race, n (%) | |
| White | 188 (62.5) |
| African American/Black | 91 (30.2) |
| Other | 122 (7.3) |
| Literacy Level, n (%) | |
| Limited/ Marginal | 116 (38.3) |
| Adequate | 187 (61.7) |
| Educational attainment, n (%) | |
| Grade 12 or GED (HS graduate) | 48 (15.8) |
| 1–3 years College/Technical Degree | 54 (17.8) |
| College 4 years (College graduate) | 70 (23.1) |
| Graduate degree | 131 (43.2) |
| Meaningful Decline in Cognitive Function, n (%) | |
| Yes | 13 (4.3) |
| No | 290 (95.7) |
| # of Chronic Conditions, mean (SD) | 2.3 (1.6) |
Outcomes
Dosing Errors
Participants on average made 2.9 dosing errors (SD: 2.5, range: 0–21) out of 21 potential errors at T1 and 5.0 errors at T4 (SD: 2.05, range: 1–18, p<0.001). At T1, among all participants, 16.8% made at least one frequency error, 33% made at least one dose error, and 82.5% made at least one spacing error (categories not mutually exclusive). The distribution of these errors was similar at T4.
In bivariate analyses, significant differences in total dosing errors made were found by race, educational attainment, and literacy skills at both T1 and T4; no differences were found by age or sex (Table 3). There was also a significant association between number of chronic conditions and total dosing errors at both T1 and T4 (β: 0.27, 95% CI: [0.09, 0.45], p=0.004 and β: 0.35, 95% CI: [0.20, 0.49], p<0.001, respectively). Specifically, those with lower literacy skills, who identified as Black/African-American, who had more chronic conditions, and had lower educational attainment were more likely to make dosing errors. Patients who experienced meaningful cognitive decline from T1 to T4 were also significantly more likely to make more dosing errors on average at T4 than those who did not experience decline (mean: 7.85, SD: 3.1 vs. mean: 4.90, SD: 1.9, p<0.001). There were no statistically significant relationships found between any demographic or health-related variables under study and change in dosing errors over time in bivariate analyses.
Table 3.
Bivariate Associations with Number of Dosing Errors and Regimen Consolidation
| Variables | # Dosing Errors (n=303) | Regimen Consolidation (n=199) | ||||
|---|---|---|---|---|---|---|
| T1 | T4 | T4–T1 | T1 | T4 | T4–T1 | |
| Age | ||||||
| <60 | 3.07 (2.9) | 5.08 (2.4) | 2.01 (3.0) | 6.02 (1.6) | 6.23 (1.5) | 0.22 (1.9) |
| 60–64 | 2.72 (2.0) | 5.15 (2.0) | 2.43 (2.3) | 5.86 (1.6) | 6.07 (1.5) | 0.21 (2.1) |
| 65–69 | 2.84(2.3) | 4.79 (1.8) | 1.96 (2.3) | 6.21 (1.9) | 6.77 (2.4) | 0.56 (1.9) |
| 70+ | 2.87 (2.7) | 5.05 (2.2) | 2.18 (1.4) | 5.90 (1.2) | 5.93 (1.7) | 0.03 (1.8) |
| Literacy | ||||||
| Limited/ Marginal | 3.96 (3.2) | 6.05 (2.8) | 2.09 (3.2) | 6.34 (1.9) | 6.39 (1.9) | 0.05 (2.1) |
| Adequate | 2.23 (1.7) | 4.40 (1.1) | 2.17 (1.9) | 5.85 (1.4) | 6.22 (1.7) | 0.37 (1.9) |
| Sex | ||||||
| Male | 2.90 (3.2) | 4.82 (1.6) | 1.91 (2.7) | 6.25 (1.7) | 6.25 (1.6) | 0.00 (1.9) |
| Female | 2.89 (2.2) | 5.11 (2.2) | 2.23 (2.4) | 5.90 (1.6) | 6.28 (1.9) | 0.38 (2.0) |
| Race | ||||||
| White | 2.16 (1.7) | 4.52 (1.2) | 2.36 (2.0) | 5.89 (1.4) | 6.11 (1.5) | 0.23 (1.8) |
| Black | 4.18 (3.2) | 5.86 (2.6) | 1.68 (2.9) | 6.51 (2.0) | 6.59 (2.4) | 0.08 (2.3) |
| Other | 3.86 (2.9) | 6.09 (3.7) | 2.23 (4.0) | 5.17 (0.9) | 6.33 (1.8) | 1.17 (1.9) |
| Educational Attainment | ||||||
| High School or Less | 4.75 (4.0) | 6.46 (3.2) | 1.71 (3.6) | 6.57 (1.9) | 6.29 (2.4) | −0.29 (2.9) |
| Some College | 3.33 (2.5) | 5.11 (1.7) | 1.78 (1.9) | 6.26 (1.7) | 6.32 (1.8) | 0.06 (2.0) |
| College Graduate | 2.49 (1.8) | 5.00 (2.3) | 2.51 (2.7) | 5.89 (1.7) | 6.47 (1.9) | 0.57 (2.4) |
| Graduate Degree | 2.24 (1.8) | 4.50 (1.1) | 2.25 (2.0) | 5.85 (1.5) | 6.16 (1.6) | 0.31 (1.5) |
Bolded text indicates results that are statistically significant at p<.05
In a multivariate model, limited literacy (β :0.69, 95% CI: [0.18,1.20], p=0.01), meaningful cognitive decline (β: 1.72, 95% CI: 0.70–2.74, p=0.01), number of chronic conditions (β: 0.21, 95% CI: [0.07,0.34], p=0.01), and number of baseline dosing errors (β: −0.76, 95% CI: [−0.85,−0.67], p<0.001) were significant, independent predictors of changes in dosing errors over 9 years. (Table 4). Age, race, sex, and time elapsed between T1 and T4 were not significantly associated with changes in dosing errors.
Table 4:
Multivariate Model of Changes in Total Number of Dosing Errors between T1 and T4
| Variable | ||
|---|---|---|
| Beta (95 % CI) | p- value | |
| Male | −0.24 (−0.71, 0.23) | 0.32 |
| Age | ||
| <60 | REF | -- |
| 60–64 | 0.22 (−0.28, 0.73) | 0.39 |
| 65–70 | −0.20 (−0.75, 0.36) | 0.49 |
| 70+ | −0.19 (−0.86, 0.47) | 0.57 |
| Race | ||
| Black | 0.20 (−0.35, 0.75) | 0.48 |
| Other/Unreported | 0.58 (−0.23, 1.39) | 0.16 |
| White | REF | -- |
| Educational Attainment | ||
| Grade 12 or GED (HS graduate) | 0.42 (−0.30, 1.13) | 0.25 |
| 1–3 Years of College/Technical Degree | −0.30 (−0.93, 0.34) | 0.36 |
| College 4 years (College graduate) | 0.30(−0.23, 0.84) | 0.27 |
| Graduate Degree | REF | -- |
| Limited/Marginal HL | 0.69 (0.18, 1.20) | 0.01 |
| Meaningful Decline in Cognitive Function | 1.72 (0.70, 2.74) | 0.001 |
| # of Chronic Conditions | 0.21 (0.07, 0.34) | 0.003 |
| Time Between Interviews (years) | −0.20 (−0.65, 0.26) | 0.40 |
| # of Dosing Errors at T1 | −0.76 (−0.85, −0.67) | <0.001 |
Bolded text indicates results that are statistically significant at p<.05
Regimen Consolidation
At T1, participants indicated that they would take medication an average of 6.0 times per day (SD: 1.6, range: 4–13) and at T4 participants indicated that they would take medication an average of 6.3 times per day (SD: 1.8, range: 3–14). The change in number of times a participant dosed out the regimen between T1 and T4 was statistically significant (p=.03). No patients at T1 and 1.0% of patients at T4 indicated that they would consolidate their regimen to be taken at 3 times per day, the simplest organization allowed according to labeling instructions.
In bivariate analyses, significant differences in the average number of times per day participants would take their medication were found at T1 by participants’ literacy skills and race/ethnicity (Table 3), but no other variables under study. Those with lower literacy skills and who identified as Black or African-American were more likely to dose the regimen a greater number of times per day. At T4, no participant characteristics were significantly associated with regimen consolidation. In a multivariate model controlling for relevant covariates, there were no significant, independent predictors of changes in regimen consolidation over time, except the number of times a participant dosed out the regimen at T1 (β: −0.70, 95% CI: [−0.86, −0.54], p<0.001; Table 4).
DISCUSSION
Results indicate that dosing errors and poor regimen consolidation are likely to be common among older adults and that these medication self-management skills decline significantly over time. On average, patients made approximately 3 errors in dosing a multi-drug regimen at baseline, which increased to 5 errors nearly a decade later. While the potential severity of such errors can vary greatly based upon the exact context, these findings nonetheless demonstrate that older patients are likely to have difficulty taking complex drug regimens appropriately. This could have serious ramifications for medication adherence and safety. Findings from multivariate models indicate that patients with limited literacy, those experiencing cognitive decline, and those with multi-morbidity are at greatest risk for increases in dosing errors over time. Other sociodemographic factors, such as age, race/ethnicity, and sex were not significant, independent predictors.
Findings related to regimen consolidation among older patients are similarly concerning. Bivariate analyses revealed that older adults with lower literacy skills and those who are members of racial/ethnic minority groups are likely to overcomplicate regimen dosing by taking medication a greater number of times per day than necessary, placing them at increased risk of medication errors, ADEs and poor adherence.17–19 While the 7-drug regimen provided to patients could be consolidated to be taken 3 times per day, patients on average dosed the regimen 6 times a day; only 1 percent of patients simplified the regimen to its optimal schedule. Like regimen dosing, regimen consolidation also declined significantly over time. However, we found no significant, independent predictors of this phenomenon beyond a patients’ regimen consolidation skills at baseline.
There are limitations to this study that should be noted. First and foremost, the regimen dosing and consolidation assessments were hypothetical in nature and not based upon patients’ actual regimens. It is possible that patients would pay greater attention to dosing and consolidating their own medications in actual use. However, it is also plausible that daily distractions could lead patients to commit more errors and to overcomplicate their own daily regimens more than indicated in the structured interview setting. Second, only community-dwelling adults without severe cognitive, visual or hearing impairment were recruited into the LitCog study. LitCog participants were, on average, comparatively healthy. This affects the generalizability of results to all older adults; it could also mean that our study participants were less familiar with managing complex drug regimens than the average older adult. As is common in longitudinal studies, those lost to follow-up differed significantly from those included in analyses; they were more likely to be socioeconomically disadvantaged and to commit more dosing errors at baseline. As such, our results may underestimate dosing errors over time. Underestimation may also have occurred as many older adults are prescribed more complex drug regimens then the one tested in this study. Finally, patients were provided with the same dosing scenario at multiple interview time points over approximately 9 years. It is therefore possible, although unlikely, that there could be a learning effect in regimen dosing and consolidation; if this occurred, it would have biased results towards the null.
Conclusions
Findings emphasize the importance of routine counseling for older patients on safe and appropriate medication use. As medication self-management skills are likely to decline over time, it is important for healthcare providers to regularly assess older patients’ medication use and to ensure patients are taking complex regimens accurately. The member of the healthcare team who conducts this counseling will need to be identified based upon the unique resources and workflow of each clinic practice. Results from this study also show that older adults have difficulty organizing complex regimens to the most efficient daily schedule, which can have severe ramifications on medication adherence and safety. Counseling sessions should include working with patients to organize and plan medication use throughout the day to promote medication adherence and safety.
ACKNOWLEDGMENTS
Sponsor’s Role National Institute of Aging (R01 AG030611; PI: Wolf) supported this work. The findings and conclusions in this paper do not represent the official views or opinions of the National Institute of Aging. The funder was not involved in the design, analysis of interpretation of study findings.
Funding Sources: This work was supported by the National Institute of Aging (R01AG030611; PI: Wolf).
Footnotes
Conflict of Interest
Stacy Bailey has served as a consultant to Merck, Sharp & Dohme Corp, Northwestern University/Gordon and Betty Moore Foundation, Pfizer, Inc and Luto LLC for work unrelated to this manuscript. She has also received funding support via her institution from Merck, Sharp & Dohme Corp and Eli Lilly and Company. Michael Wolf has served as a consultant to Merck, Sharp & Dohme Corp, Abbvie, Vivus, Inc., Luto LLC, Pfizer, Inc, Anheuser Busch Imbev, DenverHealth, and Teva Pharmaceuticals for work unrelated to this manuscript. He also has received funding support via his institution from Merck, Sharp & Dohme Corp, Eli Lilly and Company, Abbvie, and UnitedHealthcare. Other authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript.
Prior Presentations: This study was presented at the Society of General Internal Medicine Annual Meeting on May 11, 2019 in Washington, DC. It has been accepted for presentation at the International Conference on Communication in Healthcare on October 29, 2019.
REFERENCES
- 1.King DE, Xiang J, Pilkerton CS. Multimorbidity trends in United States adults, 1988–2014. J Am Board Fam Med. 2018;31(4):503–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fabbri E, Zoli M, Gonzalez-Freire M, Salive ME, Studenski SA, Ferrucci L. Aging and multimorbidity: New tasks, priorities, and frontiers for integrated gerontological and clinical research. J Am Med Dir Assoc. 2015;16(8):640–647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Masnoon N, Shakib S, Kalisch-Ellett L, Caughey GE. What is polypharmacy? A systematic review of definitions. BMC Geriatr. 2017;17(1):230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.National Center for Health Statistics. Health, United States, 2016: With Chartbook on Long-term Trends in Health. Hyattsville, MD; 2017. [PubMed] [Google Scholar]
- 5.Maher RL, Hanlon J, Hajjar ER. Clinical consequences of polypharmacy in elderly. Expert Opin Drug Saf. 2014;13(1):57–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fried TR, O’Leary J, Towle V, et al. Health outcomes associated with polypharmacy in community-dwelling older adults: a systematic review. J Am Geriatr Soc. 2014;62(12):2261–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fried TR, Mecca MC. Medication appropriateness in vulnerable older adults: Healthy skepticism of appropriate polypharmacy. J Am Geriatr Soc. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wolf MS, Curtis LM, Waite K, et al. Helping patients simplify and safely use complex prescription regimens. Arch Intern Med. 2011;171(4):300–05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Davis TC, Federman AD, Bass PF, et al. Improving patient understanding of prescription drug label instructions. JGIM. 2009;24(1):57–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wolf MS, Davis TC, Tilson HH, et al. Misunderstanding of prescription drug warning labels among patients with low literacy. Am J Health Sys Pharm. 2006;63(11):1048–55. [DOI] [PubMed] [Google Scholar]
- 11.Wolf MS, Curtis LM, Wilson EA, et al. Literacy, cognitive function, and health: Results of the LitCog Study. JGIM. 2012;27(10):1300–07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Weiss BD, Mays MZ, Martz W, et al. Quick assessment of literacy in primary care: the newest vital sign. Ann Fam Med. 2005;3(6):514–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12(3):189–98. [DOI] [PubMed] [Google Scholar]
- 14.Hensel A, Angermeyer MC, Riedel-Heller SG. Measuring cognitive change in older adults: reliable change indices for the Mini-Mental State Examination. J Neurol Neurosurg Psychiatry. 2007;78(12):1298–03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wolf MS, Davis TC, Curtis LM, et al. Effect of standardized, patient-centered label instructions to improve comprehension of prescription drug use. Med Care. 2011;49(1):96–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bailey SC, Sarkar U, Chen AH, et al. Evaluation of language concordant, patient-centered drug label instructions. JGIM. 2012;27(12):1707–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schneider APH, Gaedke MA, Garcez A, et al. Effect of characteristics of pharmacotherapy on non-adherence in chronic cardiovascular disease: A systematic review and meta-analysis of observational studies. Int J Clin Pract. 2018;72(1). [DOI] [PubMed] [Google Scholar]
- 18.Lavan AH, Gallagher P. Predicting risk of adverse drug reactions in older adults. Ther Adv Drug Saf. 2016;7(1):11–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Willson MN, Greer CL, Weeks DL. Medication regimen complexity and hospital readmission for an adverse drug event. Ann Pharmacother. 2014;48(1):26–32. [DOI] [PubMed] [Google Scholar]