Abstract
Stem cell transplantation holds great promise as a potential treatment for currently incurable retinal degenerative diseases that cause poor vision and blindness. Recently, safety data have emerged from several Phase I/II clinical trials of retinal stem cell transplantation. These clinical trials, usually run in partnership with academic institutions, are based on sound preclinical studies and are focused on patient safety. However, reports of serious adverse events arising from cell therapy in other poorly regulated centers have now emerged in the lay and scientific press. While progress in stem cell research for blindness has been greeted with great enthusiasm by patients, scientists, doctors and industry alike, these adverse events have raised concerns about the safety of retinal stem cell transplantation and whether patients are truly protected from undue harm. The aim of this review is to summarize and appraise the safety of human retinal stem cell transplantation in the context of its potential to be developed into an effective treatment for retinal degenerative diseases.
1. Introduction
Inherited and age-related retinal degeneration are the main cause of currently untreatable blindness worldwide (Verbakel et al., 2018; Wong et al., 2014). Over 30 million people worldwide are affected by various forms of retinal degeneration (https://nei.nih.gov/eyedata). Inherited forms of retinal degeneration often start manifesting in childhood, whereas non-exudative or dry age related macular degeneration (dAMD), an acquired retinal degenerative disease, typically affect older individuals. Both inherited and acquired retinal degeneration can significantly impair vision, with the eventual occurrence of severe vision loss or complete blindness in many affected individuals.
The recent approval of gene therapy with Luxturna™ (voretigene neparvovec-rzyl) for inherited retinal degeneration caused by mutations in the RPE65 gene provides hope for a potential cure for at least the recessively inherited forms of this disease that affect a relatively small number of patients (Apte, 2018). Unfortunately there are no FDA-approved gene- or stem cell-based therapies yet that target other genetic subtypes of inherited retinal degeneration, dAMD, or exudative AMD (Singh and MacLaren, 2018). Furthermore in many cases, the irreversible loss of photoreceptors in later stages would make gene therapy ineffective.
Therefore, regenerative medicine in the form of cellular replacement therapy for retinal degenerative diseases holds great promise, as the same therapeutic agent can be used irrespective of the underlying genetic or acquired cause (Pan et al., 2013). Modern stem cell technologies have yielded clinical grade cellular therapies under investigation for retinal degenerations from both human embryonic stem cells (hESCs) and induced pluripotent stem cells (iPSCs) (Drukker et al., 2006; Odorico et al., 2001; Polak and Bishop, 2006; Takahashi et al., 2007; Yu et al., 2007). Sadly, however, some have exploited public enthusiasm for stem cell-based therapies by performing treatments with almost no scientific validation (Kuriyan et al., 2017; Turner and Knoepfler, 2016).
The promising success of research in legitimate stem cell therapies has increased public awareness, enthusiasm, and hope amongst patients with a number of different debilitating diseases not only in ophthalmology but also in other medical specialties. Almost universally, such hope has taken on the flavor of hype. In 2014, a group of European orthopedic surgeons and scientists published a list of ethical concerns as regards the rapidly expanding worldwide field of regenerative medicine in orthopedics (Niemansburg et al., 2014):
“The experts worried about the hype of [regenerative medicine], which is partially caused by the drive for profit of industries and by the high expectations of researchers for providing a definite cure for diseases that are now intractable. [The experts] were worried that these secondary interests [could] cause a lack of scientific integrity in [regenerative medicine] research. One of the consequences could be… improper design of clinical studies. Many observed that a consequence of the hype and profit drive in orthopedic surgery is that interventions [could be] commercialized too soon without proper research beforehand.”
This projection has been realized recently in unproven “stem cell” therapy for retinal diseases. This review aims to summarize and discuss the safety of human retinal stem cell transplantation in the context of its therapeutic potential.
1.1. Advantages of the eye as a target organ
The eye, and specifically the retina, are an excellent target for cellular replacement therapies for several reasons. First, the transplantation site and cells can be monitored directly via clinical examination and high resolution retinal imaging devices. There are also many clinical measures of visual function, including visual acuity, contrast sensitivity, microperimetry, electrophysiology and dark adaptation. Second, the small size of the intraocular tissues permits the use of smaller volumes and numbers of replacement cells compared to other bodily organs. Third, surgical accessibility to the eye and the retina permits the delivery of cells in suspension or as sheets on a scaffolding material that could promote the survival of transplanted cells (Tomita et al., 2005). Finally, unlike other central nervous system structures, the retina contains a non-synaptic layer—the retinal pigment epithelium (RPE)—which may be more amenable to cellular transplantation than photoreceptors or retinal ganglion cells that require synaptic connections to adjacent cells (Strauss, 2005).
1.2. Stem cell treatment strategies
Stem cell-based therapies are envisioned as potential treatments, or perhaps even cures, for several currently untreatable forms of retinal degeneration (Bharti, 2018; Jha and Bharti, 2015). Two distinct approaches of stem cell-based therapies are being developed:
1) Transient dosing strategy that uses multipotent stem cells or ocular progenitor cells, that are delivered in a non-polarized fashion, to provide non-selective neuroprotective and/or immune-modulatory factors to likely provide a treatment effect that is time-limited by the viability and secretory capacity of the transplanted cells;
2) Permanent implantation strategy that uses pluripotent stem cell-derived retinal photoreceptor and/or RPE cells, aiming to replace the same class(es) of atrophied cells (Song and Bharti, 2016). Based on the kind of damage ensued in a given disease state, these two approaches are being tested for different disease indications and at different disease stages.
Here we provide an overview of both these approaches as a context within which to discuss the safety of retinal stem cell therapy.
1.2.1. Transient-dosing strategy
The idea of using a transient dose of stem cells and their derivatives as potential treatment for retinal degenerative diseases follows from earlier studies on the use of neuroprotective factors in the eye. Ciliary neurotrophic factor (CNTF) has been widely studied for its protective effects on the retina, particularly the photoreceptors (Wen et al., 2012). Injections of CNTF and other neuroprotective factors (e.g. glial-derived neurotrophic factor or GDNF) demonstrated photoreceptor protective effects in animal models of retinal degeneration (Chong et al., 1999; Frasson et al., 1999). Encapsulated CNTF implants were considered safe in phase I clinical trials in patients with retinitis pigmentosa, macular telangiectasia type 2, and geographic atrophy in dAMD, and are currently being tested in phase II clinical trials (Chew et al., 2015; Kauper et al., 2012; Sieving et al., 2006). For RP, there was no therapeutic benefit of CNTF administration in the short term (12 months) in terms of visual acuity or visual field sensitivity (Birch et al., 2013). Long term observation indicated greater visual field loss in treated eyes compared to explanted eyes or sham eyes through 42 months. Over 60-96 months, there was no evidence of therapeutic benefit of CNTF administration for visual acuity, visual field sensitivity, or retinal structure in treated subjects (Birch et al., 2016).
This work also led to the hypothesis that perhaps a broader sub-set of neuroprotective factors secreted by stem cells and their derivatives will have a stronger therapeutic effect in the eye. Previous and current efforts have tested multiple different cell types in preclinical animal models and in phase I/IIa trials (detailed review in (Singh and MacLaren, 2011), (Bharti et al., 2014), (Canto-Soler et al., 2016), (Park et al., 2017) and (Singh and MacLaren, 2018)). Umbilical cord derived stem cells (clinicaltrials.gov identifier NCT02895815) and fetal brain derived neural progenitor stem cells (clinicaltrials.gov identifier NCT01632527) have been tested in phase I and II trials for the “dry” form of AMD. Although both these transplants were considered safe in these early stage trials, both studies have now been terminated (Clinicaltrials.gov). The reason of discontinuation of these two trials is not fully clear, but it is worth noticing that neither of these two cell types are of ocular origin. Furthermore, it is possible that dAMD is not the ideal target for this kind of cell therapy approach.
More recent efforts in this field feature the use of cell types of ocular origin, i.e. retinal progenitor cells (RPCs) isolated from fetal human eyes, to target inherited forms of RP (Klassen, 2016; Luo et al., 2014; Schmitt et al., 2009; Warfvinge et al., 2006). JCyte, a California-based company, is testing the intravitreal delivery of RPCs (clinicaltrials.gov identifier NCT03073733), whereas Reneuron, a Boston-based company, is testing the subretinal delivery of similar cells for RP (clinicaltrials.gov identifier NCT02464436). RPC transplantation as potential treatment of retinal degeneration is possibly more specific in its therapeutic mechanism as compared to bone marrow derived stem cells or neural stem cells. Preclinical evidence suggests that, at least in some cases, transplanted RPCs retain their capacity to differentiate into certain retinal cell types, albeit at a low efficiency (Baranov et al., 2014; Tucker et al., 2010; Yao et al., 2015). Thus, subretinal RPC transplantation may create a permanent tissue repair effect through actual photoreceptor regeneration.
The rather stochastic differentiation of transplanted RPCs into different cell types of the retina raises two additional challenges: the mechanism and magnitude of action may be inconsistent from patient-to-patient, and under allogeneic conditions tested in these trials, retinal cell types differentiated from RPCs may be subject to different immune surveillance as compared to the naïve RPCs. Data suggest that even autologous tissue may rarely suffer immune-mediated damage (van Meurs et al., 2018). The actual therapeutic window target for both of these cell types may need further study and optimization. If allogeneic RPCs show efficacy in patients, they may potentially provide an “off-the-shelf’ therapy for various forms of retinal degeneration.
1.2.2. Permanent implantation strategy
This approach aims to replace damaged or atrophied tissue in the retina. In addition to providing trophic support similar to stem cell suspension transplants, these replacement eye tissues are intended to restore most, if not all, tissue functions that are lost in the advanced disease stage. The idea of permanent transplantation is supported by earlier clinical studies on autologous RPE-choroid translocation surgery (Jha and Bharti, 2015). In this procedure, a small piece of RPE-choroid isolated from the retinal periphery in an AMD patient’s eye is translocated into the macula and positioned in the area of RPE atrophy (Joussen et al., 2006; Maaijwee et al., 2007a; Maaijwee et al., 2008; Maaijwee et al., 2007b). In small number of patients where this surgery worked and the transplant integrated, vision was stabilized for several years. Furthermore, this work provided critical proof-of-principle data that an RPE patch could be developed as a potential treatment for AMD.
Concurrent with these surgical advances to deliver an RPE transplant to the back of the eye (da Cruz et al., 2007), stem cell scientists were developing methods to differentiate RPE from stem cells (Bertolotti et al., 2014). Together these two developments made current day RPE transplants feasible for AMD patients. Starting with the work of Klimanskaya et al in 2004 (Klimanskaya et al., 2004) that isolated and characterized pigmented cells during spontaneous differentiation of ESCs to more recent developmentally-guided protocols, researchers have reproducible generated fully-mature and functional RPE cells from both iPSCs and ESCs (Bharti et al., 2011; Idelson et al., 2009; Klimanskaya et al., 2004; May-Simera et al., 2018; Miyagishima et al., 2016). In directed differentiation of ESCs and iPSCs, cells follow a developmental path akin to the normal embryonic development of the RPE via the optic neuro ectoderm, eye-field stage, committed RPE progenitors, immature RPE, to mature RPE (Bharti et al., 2011; Bharti et al., 2006; Meyer et al., 2009). This directed differentiation likely promotes the establishment of the precise epigenetic marks that promote the conversion of pluripotent stem cells into mature and functional RPE cells (May-Simera et al., 2018).
In contrast to ESCs and iPSCs, adult RPE stem cells (RPESCs) provide a completely different lineage-committed source for deriving RPE cells for transplantation. Recent work by Salero et al shows the presence of RPESC in cultures of RPE cells isolated from cadaver human eyes (Salero et al., 2012). Under appropriate culture conditions, these stem cells can be differentiated again into RPE cells that are currently being developed for a cell therapy for AMD patients (Blenkinsop et al., 2015; Blenkinsop et al., 2013; Davis et al., 2017; Davis et al., 2016; Saini et al., 2016; Stanzel et al., 2014). While the restricted lineage potential of adult RPESCs suggests possibly enhanced safety over that of ESC- and iPSC-derived RPE cells, adult RPESC derived RPE cells may present other challenges. For instance, recent evidence suggests that cadaveric RPE cells may retain cellular endophenotypes of aging and disease which may limit their potential as an effective therapeutic substrate (Golestaneh et al., 2017).
Multiple approaches are being pursued to develop RPE transplants and in the near future several trials will hopefully be completed using RPE transplants derived from these various stem cell types.
2. Treatment concepts and preclinical data
2.1. RPE transplantation
Two different approaches are being tested for RPE transplantation – the injection of a bolus of RPE cell suspension and the transplantation of a monolayer patch of RPE cells (Bharti et al., 2011; Bharti et al., 2014). As per the 21st Century Cures Act signed by President Obama, the monolayer patch approach may be considered an advanced cell therapy product since it requires tissue engineering to develop (Hudson and Collins, 2017).
In a landmark study, an ESC-derived RPE cell suspension was tested in a phase I/IIa trial for patients with AMD and Stargardt disease (Schwartz et al., 2012b; Schwartz et al., 2015b). After a two-year patient follow up, the investigators concluded that ESC-RPE cells were safe and did not cause any serious adverse events (Schwartz et al., 2012b; Schwartz et al., 2015b). It is, however, not clear if a suspension of RPE cells can form a confluent, polarized monolayer in the subretinal space in the transplantation zone. In fact, available evidence suggests that cells injected as suspension do not consistently form a monolayer of RPE when transplanted and survive poorly in the long-term as compared to RPE cells transplanted as a monolayer patch (Diniz et al., 2013; Hu et al., 2012). Such observations have promoted the development of a RPE-patch approach in several cases.
A patch of RPE cells is proposed as an ideal therapeutic substrate because RPE cells need to be a confluent monolayer to be able to perform their physiological functions most efficiently. Formation of tight junctions between neighboring RPE cells is required for the cells to be fully polarized with apically-located actin-based processes that interact with the photoreceptor outer segments and thus support the ability of RPE cells to perform functions including phagocytosis of photoreceptor outer segments, replenishment of visual pigment, maintenance of ionic homeostasis in the sub-retinal space, and transport of water, nutrients, and metabolites between the photoreceptors and the choroidal blood supply (Bharti et al., 2011). If an RPE transplant fails to perform most of these functions, then it is not likely to provide long-term efficacy in the eye.
Published data on three types of RPE patches have been presented so far in a small subset of patients in three independent studies (da Cruz et al., 2018; Kashani et al., 2018; Mandai et al., 2017b). An iPSC-RPE patch without any additional substrate was tested in one patient with non-treatable form of CNV (Mandai et al., 2017a); an ESC-RPE patch on a polyester substrate was tested in two patients with an acute form of CNV (da Cruz et al., 2018); and an ESC-RPE patch on a parylene substrate was tested in four patients with dAMD (Kashani et al., 2018). As per the most recent published report, all six patients maintained stable or improved vision, but the long-term efficacy of RPE patches is difficult to ascertain at this early stage and with such a small number of patients. Other studies on RPE transplantation are in progress including one by (NCT02286089, BioTime Inc. and CellCure Neurosciences Ltd.) with locations in the USA and Israel in which hESC-derived RPE is administered as a suspension in ophthalmic Balanced Salt Solution Plus. The delivery approach to the subretinal space utilizes the Orbit Subretinal Delivery System (Orbit Biomedical Ltd.) via the suprachoroidal approach (www.biotimeinc.com).
Here we discuss and review the manufacturing, preclinical, and clinical challenges associated with these approaches.
2.1.1. Manufacturing considerations of RPE suspensions and cell patches
The development of a cell therapy product requires manufacturing of the cells under current Good Manufacturing Practice (cGMP). cGMP ensures an appropriate design, proper monitoring, and control of the manufacturing process (Code of Federal Regulations CFR 21, FDA). Depending upon the source of stem cells, the manufacturing process can vary. For instance, it typically takes six months to manufacture an autologous iPSC-RPE-patch, but it may take only weeks to make RPE suspension transplant from adult RPESCs (Blenkinsop et al., 2013; Mandai et al., 2017b; Saini et al., 2016). One main challenge with developing an advanced cell therapy product like a stem cell derived RPE patch is to ensure the maintenance of appropriate cGMP conditions during the long and complex manufacturing process.
Several parameters affect the complexity of the cGMP manufacturing process. For instance, for developing an allogeneic ESC-derived RPE product, a validated and adventitious virus tested Master Cell Bank (MCB) is often created at the pluripotent stem cell stage, as was done for two recent clinical studies (da Cruz et al., 2018; Kashani et al., 2018). From the MCB stage, RPE differentiation may take another 20 weeks or more, especially when using the spontaneous differentiation process (da Cruz et al., 2018; Kashani et al., 2018; Mandai et al., 2017b). Such a long manufacturing process increases the burden in cGMP suites and increases the possibility of contamination of the cells.
The use of directed differentiation protocols in some of the ongoing efforts have reduced differentiation time to less than half of the spontaneous differentiation time, but at the same time increased the complexity of manufacturing optimization because of the need of different biologics and chemicals that will need to be cGMP-grade and well-controlled (Idelson et al., 2009).
The use of an autologous iPSC-based process as published in 2017 by Mandai et al. omits the need to make a MCB, but adds other complexities to the manufacturing process (Mandai et al., 2017b). For instance, the process of reprograming skin fibroblasts into iPSCs still is random to some extent and may not generate cells that are suitable for transplantation in patients. Mandai et al. showed that iPSCs derived from the skin fibroblasts of one AMD patient under cGMP-grade conditions yielded transplantable RPE cells, whereas the iPSCs developed from another patient had acquired mutations and hence were deemed not suitable for transplantation, as discussed in greater detail in section 5.5 below.
Other work suggests that CD34+ cells may be an alternate, and perhaps better, source to generate mutation-free iPSCs as compared to skin fibroblasts (Mack et al., 2011). This is likely due to the progenitor and non-senescent nature of CD34+ cells as compared to the dermal skin fibroblasts (Venditti et al., 1999; Zhong et al., 2014a). Further details on CD34+ cells in retinal therapy are discussed in sections that follow. Despite the relatively complex manufacturing process for Master Cell Banks of ESCs or for autologous iPSCs generation, there is considerable enthusiasm to promote the use of these two cell types in the development of ocular and non-ocular cell therapies.
2.1.2. Functional validation of the stem cell-derived RPE product
A critical consideration for developing a commercially approvable cell therapy for RPE diseases, or for any tissue regeneration target, is the need to develop at least one in vitro functional assay that can also predict the in vivo efficacy of the stem cell derived RPE product. Towards that goal, efforts are underway to functionally validate both ESC- and iPSC-derived RPE cells (Miyagishima et al., 2016; Miyagishima et al., 2017).
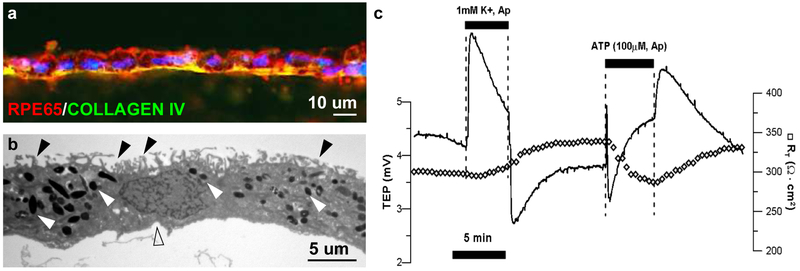
As mentioned above, the RPE functions as an intact monolayer with tight junctions that hold the cells together. Because of this property of RPE cells, most readouts of RPE functionality are performed on an intact RPE monolayer (Miyagishima et al., 2016; Miyagishima et al., 2017). This feature of RPE cells might pose a significant challenge for RPE cell suspension clinical trials. Most of these functions are lost in single cell preparations and hence it would not be easy to correlate any of the above-mentioned functions to the potency of RPE cell suspension transplants in preclinical animal models or in patients. iPSC-derived RPE monolayers acquire several molecular, structural, and functional features that resemble native human RPE (Miyagishima et al., 2016; Miyagishima et al., 2017) (Figure 1): (1) iPSC-RPE express genes and miRNAs that are specific to adult human RPE; (2) iPSC-RPE monolayers contain apically located processes, apical melanosomes, and tight junctions; (3) iPSC-RPE monolayers have a transepithelial resistance, a feature of developmentally normal tight junctions, of more than several hundred Ohms.cm2; (4) transepithelial potential, a feature of apically-basally polarized membranes, of 2-5 mV; (5) hyperpolarization response to apical low potassium stimulus; (6) intracellular calcium levels of approximately 110 nM; (7) polarized secretion of cytokines with higher vascular endothelial growth factor (VEGF) basally (choroidal side) and higher pigment epithelium derived factor apically (retinal side) and (7) ability to transport fluid (~1-5 ul/cm2/hr) from the apical towards the basal sides. This work supports the functional authentication of iPSC-RPE and provides assays that can be developed into a validated release criterion performed under cGMP conditions.
Figure 1.
Characteristics of retinal pigment epithelium (RPE) cells differentiated from human induced pluripotent stem cells (iPSC). (a) iPSC-RPE matured on a PLGA scaffold express maturity marker RPE65 and Bruch’s membrane protein COLLAGEN IV. (b) Transmission electron microscope confirms the presence of dense apical processes (black arrowheads), pigment granules (white arrowheads), and basal infoldings (clear arrowhead). (c) iPSC-RPE monolayer electrical response are similar to native RPE cells. Cells have transepithelial resistance of over 300 Ohms.cm2 and a transepithelial potential of 4.5 mV. The cultured RPE monolayer hyperpolarizes in response to low potassium and depolarizes in response to apical ATP application.
Furthermore, this functional authentication exercise performed using sixteen different iPSC lines derived from different donors provides evidence for the effect of donor-to-donor and clone-to-clone variability on functional output of iPSC-RPE. Such large dataset is also widely applicable to functionally authenticate RPE-monolayer products derived from ESCs or adult RPESCs, and will help develop commercially approvable cell therapy products for macular degeneration patients (Miyagishima et al., 2016; Sharma et al., 2017).
2.2. Photoreceptor transplantation
2.2.1. Preclinical data on photoreceptor transplantation
Sight restoration in patients blinded by the physical loss of photoreceptors has long been a goal of vision science. Pioneering studies carried out in the late 1980s early 1990s in rodent models showed the feasibility of transplanting fetal neural retinal cells as a potential strategy to regenerate photoreceptors cells (Blair and Turner, 1987; del Cerro et al., 1989; del Cerro et al., 1988; Turner and Blair, 1986; Turner et al., 1988). These studies suggested that transplanted cells might be capable of achieving some degree of integration into the host retina including formation of synaptic connections (del Cerro et al., 1991; del Cerro et al., 1989; Gouras et al., 1991).
These observations prompted some groups to undertake a similar approach for the treatment of human patients using fetal-derived retinal progenitor cells (fRPC) as a donor source. fRPC are obtained from the retina of human fetuses between 14 and 20 weeks of gestation, a time at which photoreceptor progenitors in the developing retinal neuroepithelium are exiting the cell cycle and undergoing their corresponding differentiation process (Hendrickson et al., 2008). fRPC have been transplanted into patients affected by retinitis pigmentosa (RP) and AMD, using various approaches including the transplantation of microaggregate suspensions of fRPCs, fetal neural retinal sheets consisting of an isolated photoreceptor cell layer or a full retinal component, RPE sheets, or neural retina with associated RPE (Das et al., 1999; Humayun et al., 2000; Radtke et al., 2008).
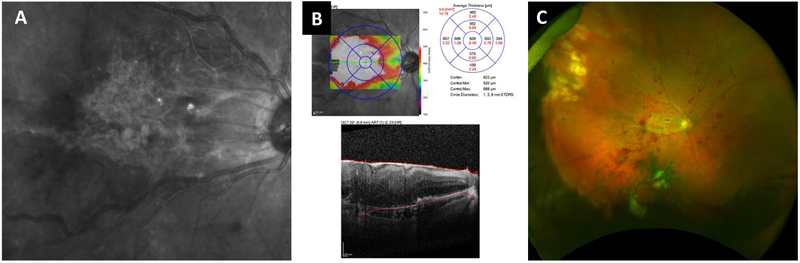
A critical outcome from these studies is the apparent lack of adverse effects (reviewed in (Seiler and Aramant, 2012a)). In addition, as reported in (Radtke et al., 2008) regarding a phase II clinical trial in which fetal retina/RPE sheets were transplanted on 10 RP and AMD patients, this treatment led to a certain level of short-term visual improvement, as assessed by EDTRS visual acuity scores up to 12 months after surgery (Figure 2). However, long-term benefits were not observed, except in the case of one patient that maintained visual improvement at a six-year follow-up. It is uncertain whether the improvement was due to a trophic effect of the implant or to functional integration of the transplanted cells.
Figure 2. Allogeneic fetal retina and retinal pigment epithelium (RPE) transplantation.
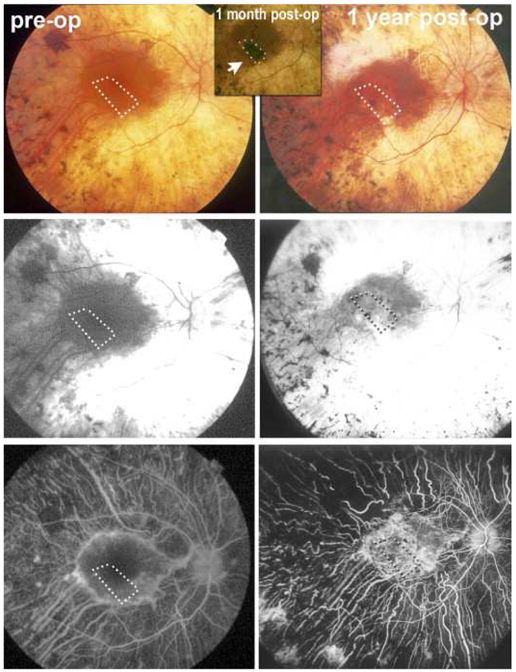
Images from a study subject with retinitis pigmentosa (RP) who was treated in a clinical trial of allogeneic retina and RPE transplantation (reprinted from Radtke ND, Aramant RB, Petry HM, Green PT, Pidwell DJ, Seiler MJ. Vision improvement in retinal degeneration patients by implantation of retina together with retinal pigment epithelium. Am J Ophthalmol 2008; 146(2): 172-82, with permission from Elsevier)(Radtke et al., 2008). Ten subjects with RP or age-related macular degeneration were included in this study. Donor tissue, comprising 2mm2 to 5mm2 sheets neural retina together with the adjacent RPE layer, was obtained from human fetal eyes of 10–15 weeks’ gestational age. Preoperative retinal images are shown in the left column (top to bottom: color fundus photograph, early-phase fluorescein angiogram, late phase fluorescein angiogram) and corresponding 1-year postoperative images on the right. The absence of fluorescein dye leakage in the region of the transplant (dotted box) was taken to imply evidence of the absence of clinical rejection of the grafts, that were all non-matched, despite the lack of immunosuppression. Four patients showed visual acuity improvements that exceeded that of the non-operated fellow eye, including one subject with 20/800 baseline vision sustained 20/200 vision for more than five years after surgery. No surgical complications occurred.
On the other hand, a Phase I/II, open-label, prospective study of the safety and tolerability of subretinally transplanted human fRPCs in patients with RP is currently underway (NCT02464436, ClinicalTrials.gov). This is a dose escalation study in which participants with RP will receive a single uniocular subretinal implantation of one of three doses of a suspension of fRPC with a follow up period of 1 year post-injection. This specific study is presented and discussed in more detail later on this review.
The advent of pluripotent stem cells, initially ESC and more recently iPSC, provided a promising alternative source for attempting photoreceptor regeneration trough cell transplantation (Jayakody et al., 2015). Within the last decade, significant progress has been made in identifying appropriate culture conditions to induce both ESC and iPSC to follow a retinal differentiation pathway. Two-dimensional stepwise differentiation protocols designed to mimic the sequential induction steps that take place in the embryo during the development of the retina demonstrated that these cells are capable of differentiating into several of the major retinal cell types, including photoreceptors ((Hirami et al., 2009; Lamba et al., 2006; Osakada et al., 2008) among others). Subsequently, it was shown that under appropriate three-dimensional (3D) culture conditions, ESC and iPSC are capable of differentiating into self-organizing 3D retinal tissue with the different major retinal cell types arranged in their proper layers (Meyer et al., 2011; Nakano et al., 2012; Phillips et al., 2012; Zhong et al., 2014b). Furthermore, in an important breakthrough, it was demonstrated that photoreceptor cells in these stem cell-derived 3D retinal tissues can achieve an advanced degree of maturation including the formation of outer segments, expression of phototransduction proteins, and response to light (Zhong et al., 2014b).
A significant number of studies have focused on subretinal transplantation of a suspension of either mouse or human ESC/iPSC-derived photoreceptor precursors into rodent models of photoreceptor degeneration (Barnea-Cramer et al., 2016; Decembrini et al., 2014; Gagliardi et al., 2019; Gasparini et al., 2019; Gonzalez-Cordero et al., 2017; Gonzalez-Cordero et al., 2013; Homma et al., 2013; Lakowski et al., 2015; Lamba et al., 2009; Lamba et al., 2010; Santos-Ferreira et al., 2016b; Singh et al., 2013; Zhu et al., 2018). Among these, earlier studies showed similar results to those obtained with fRPC, suggesting that ESC/iPSC-derived photoreceptor precursors were capable of integrating into the host retina, achieving morphological and functional differentiation similar to native photoreceptors, and restoration of visual function at least to a certain extent (Barnea-Cramer et al., 2016; Decembrini et al., 2014; Gonzalez-Cordero et al., 2013; Homma et al., 2013; Lamba et al., 2009; Santos-Ferreira et al., 2016b).
Later landmark studies uncovered that a low proportion of the transplanted cells may integrate into the host retina but the majority undergo a mechanism of intercellular material exchange with host photoreceptors (Pearson et al., 2016; Santos-Ferreira et al., 2016a; Singh et al., 2014; Singh et al., 2016). This transfer occurs independent of the source of donor photoreceptors, can be mediated by both rods and cones and is bidirectional between donor and host cells (Ortin-Martinez et al., 2017; Singh et al., 2016; Waldron et al., 2018). These observations raise the need for re-evaluating and re-interpreting previous photoreceptor transplantation studies, as well as carefully characterizing the relative contribution to function restoration from material transfer and donor integration in future studies.
More recently, taking advantage of the availability of ESC/iPSC-derived 3D retinal tissue, several groups have tested the feasibility of transplanting ESC/iPSC-derived retinal sheets (Assawachananont et al., 2014; Iraha et al., 2018; Mandai et al., 2017a; Shirai et al., 2016), building on data from primary transplants (Seiler and Aramant, 2012b) These studies showed that both mouse and human ESC/iPSC-derived retinal grafts are capable of surviving for different periods of time (up to 6 months) upon transplantation in the subretinal space of end-stage photoreceptor degeneration animal models, and differentiate into a range of retinal cell types, including photoreceptors, bipolar, amacrine and ganglion cells. In particular, photoreceptor cells achieved a relatively advanced degree of maturation as evidenced by expression of rod and cone opsins, synaptic proteins, and the formation of inner and outer segments (IS/OS) (Assawachananont et al., 2014; Iraha et al., 2018). These studies also revealed that the ESC/iPSC-derived grafts failed to maintain a properly laminated organization, developing instead a relatively disorganized histoarchitecture with formation of rosettes - reminiscent of those observed in dysplastic and degenerating retinas- with the photoreceptor inner segment (IS)/ outer segment (OS) inward and inner retinal cells outward (Assawachananont et al., 2014; Iraha et al., 2018; Mandai et al., 2017a; Shirai et al., 2016). In some instances, labeling of pre- and postsynaptic components suggested the establishment of direct contact between host bipolar cells and graft photoreceptor cells, although conclusive evidence of functional circuit restoration is still lacking (Iraha et al., 2018).
Intriguingly, some of these studies have shown the feasibility of recording light responses from rd1 mice transplanted with ESC/iPSC-derived retinal sheets by ex vivo micro-electroretinography (mERG) and ganglion cell recordings using a multiple-electrode array system (MEA) (Iraha et al., 2018; Mandai et al., 2017a). In these reports, mERG recordings were obtained from the grafted area, although not consistently and showing a- and b-waves of irregular pattern and amplitudes much smaller than those of wild-type retina. Similarly, ON responses were recorded from ganglion cells, although again not consistently.
The mechanisms underlying these light responses is however not entirely clear. Although rd1 mice exhibit severe retinal degeneration with rod loss, cone cells remain for a long period of time after rods have completely degenerated. Notably, remaining cones – even when severely compromised – may be functionally rescued upon transplantation of rods through a glucose dependent mechanism (Wang et al., 2016). Since the studies involving ESC/iPSC grafts in rd1 mice were not designed to discriminate between rod vs cone-triggered light response, it is not possible to rule out a rescue effect on the host’s remaining cones by the grafts. Another important consideration is the fact that both, a/b-wave-like responses and ganglion cell ON responses, can still be elicited from the non-transplanted degenerating rd1 retina from third order neurons even in the absence of photoreceptor-driven components (Fujii et al., 2016).
2.2.2. Current research gaps
The range of research gaps and challenges in the pursuit of functional photoreceptor regeneration upon transplantation can be better comprehended in the context of the diseases potentially amenable to this treatment. The most immediate targets for photoreceptor regenerative therapies are AMD and inherited retinal dystrophies such as RP and Stargardt disease (Levin et al., 2017; Zarbin, 2016). AMD is the leading cause of irreversible blindness in the developed world with the dry form of AMD accounting for nearly 90% of patients affected by this condition (Evans and Syed, 2013; Hanus et al., 2016). dAMD is a complex, multifactorial disease, involving genetic and environmental factors (Sobrin and Seddon, 2014). Furthermore, which component in the photoreceptor/RPE/Bruch’s membrane/choriocapillaris complex is primarily affected, remains unclear (Bhutto and Lutty, 2012).
However, the early stages of dAMD are characterized by impaired function of the RPE, which, in turn, leads to the death of rod photoreceptor cells within the parafoveal region of the macula followed by cone loss (Bhutto and Lutty, 2012; Curcio, 2001). Currently, there are no therapies to prevent or cure dAMD (Evans and Syed, 2013; Hanus et al., 2016). The lack of preventive treatments has led to an increasing number of patients with advanced stages of AMD, a condition known as geographic atrophy (GA), which is responsible for 20% of all cases of legal blindness (Hanus et al., 2016).
RP is the most common cause of hereditary blindness worldwide, affecting approximately 1 in 3,000 to 1 in 4,000 people (Dias et al., 2017; Hamel, 2006; Hartong et al., 2006; Wert et al., 2014). RP represents a clinically and genetically heterogeneous group of inherited retinal disorders, in which the primary loss of rod photoreceptors leads to subsequent degeneration of cones, and eventually atrophy of the retinal pigmented epithelium (RPE) (Dias et al., 2017; Hamel, 2006; Hartong et al., 2006; Wert et al., 2014). Typically, RP begins in the mid-periphery, and progresses over several years eventually also affecting central vision (Dias et al., 2017; Hamel, 2006; Wert et al., 2014). Ultimately complete blindness may occur, and patients affected by RP could benefit from improvement in either central or peripheral vision. Stargardt disease is a recessively inherited macular dystrophy with an estimated prevalence of 1 in 8,000 to 1 in 10,000 people (Sears et al., 2017). The pathology begins in the parafoveal region of the macula with progressive involvement into the foveal region, eventually leading to loss of central vision in both eyes and legal blindness. Over time, this disorder leads to degeneration of photoreceptors and of the RPE accompanied by progressive vision loss (Sears et al., 2017).
Although AMD, RP, and Stargardt disease have different underlying causes and different demographics, at their end-stage they show common abnormalities including loss of photoreceptors cells and dysfunctional RPE (Dias et al., 2017; Evans and Syed, 2013; Hamel, 2006; Hanus et al., 2016; Hartong et al., 2006; Sears et al., 2017; Wert et al., 2014). Within this framework, we will discuss here some of the fundamental questions that still remain unanswered.
2.2.3. Transplanting photoreceptors vs RPE vs both
As outlined above, in conditions such as AMD, RP, and SD, both photoreceptors and RPE cells are compromised (Dias et al., 2017; Evans and Syed, 2013; Hamel, 2006; Hanus et al., 2016; Sears et al., 2017). Considering the critical role that RPE plays in maintaining the health and function of photoreceptor cells (Bhutto and Lutty, 2012; Handa, 2012; Handa et al., 2017), it is conceivable that replacing only the affected photoreceptors would not lead to a long-term positive effect, as the regenerated photoreceptors would still lack the support of healthy RPE. On the other hand, replacing only the diseased RPE may not be sufficient to fully rescue the remaining but already compromised photoreceptors in the host retina. This raises the possibility of the need for transplanting both photoreceptors and RPE, either simultaneously or sequentially, in order to efficiently restore vision in these patients.
Addressing these important issues will require a systematic analysis of the alternative scenarios, accounting not only for the cell type(s) being transplanted, but within the context of the different diseases, and perhaps most importantly, the stages of disease progression. It is plausible that no single approach will succeed under all conditions, but rather different transplantation approaches may prove most efficacious depending on the disease and stage of progression.
2.2.4. Transplanting rods vs cones
Given the predominance of rod production over cones either from donor tissue or stem cell cultures under current methods, and data from animal models suggesting that rods may be capable of integrating more readily than cones, rod replacement might be more attainable in the short term (Gamm et al., 2015). The ability to regenerate rods through transplantation would be primarily applicable to retinal dystrophies such as RP, particularly if the intervention is done at relatively early disease stages when transplanted rods may achieve better integration and function while also exerting a protective effect upon remaining cones (Gamm et al., 2015).
One important caveat however is that treatment of RP and allied diseases would require regeneration of large retinal areas, the feasibility of which is yet to be determined. On the other hand, degenerative conditions affecting the macula (e.g. AMD and SD) might require transplantation of cone photoreceptors predominantly. Few studies to date have been focused on the regeneration of cones, mostly due to the challenges for producing large quantities of these cells (Gamm et al., 2015), and they have thus far shown very low levels of integration (Decembrini et al., 2017; Lakowski et al., 2010; Smiley et al., 2016). Of note, these transplantations have been done in the rodent rod-enriched retina, which may be less advantageous for the functional integration of cones than a cone enriched retina with a maculalike structure. In support of this possibility, recent studies have shown a relatively larger, though still suboptimal, number of donor cone cells integrating into the host retina of the Nrl−/− and Prph2rd2/rd2 transgenic mice, which retinas are composed largely of cones rather than rods (Waldron et al., 2018), suggesting that a cone-enriched environment may better support cone integration. This points to the need for alternative animal models that are closer to the physiology and anatomy of the human eye, including the presence of a macula-like structure, and capable of emulating critical aspects of human degenerative diseases, the urgency of which has been highlighted by the recent NEI initiative focused on the development of translation-enabling animal models to evaluate survival and integration of photoreceptor and ganglion cells (RFA-EY-17-003).
Considering that the viability of cone functional integration upon transplantation remains to be determined, such animal models could also play a key role in evaluating the feasibility of rod transplantation and regeneration within the macula as an alternative strategy. As is well known, the macula contains two subregions with distinctly different photoreceptor content: a small cone-dominated fovea, only 0.8 mm in diameter, and a surrounding rod-dominated parafovea (Curcio, 2001). In both AMD and Stargardt disease macular rods are affected earlier and more severely than cones, and the loss of rods eventually leads to subsequent cone loss (Curcio, 2001; Sears et al., 2017). It is then conceivable that rods transplanted within the macula might achieve functional integration and provide meaningful visual improvement, even if not enough to restore high acuity vision.
2.2.5. Transplanting cell suspensions vs tissue explants
In most cases, preclinical studies to date have utilized a suspension of dissociated photoreceptor cells. Although these studies have shown some promising results, as outlined above, the transplanted cells most generally failed to survive or to become functionally integrated to a degree necessary to achieve meaningful restoration of visual function (Canto-Soler et al., 2016; Gamm et al., 2015; Zarbin, 2016). With the advent of stem cell-derived three-dimensional retinal technology comes the opportunity to address the feasibility of photoreceptor transplantation as a pre-organized tissue rather than isolated single cells.
A reasonable expectation is that this approach might provide the transplanted photoreceptors with an improved physical and physiological microenvironment that would in turn have a positive impact on their ability to survive and become functionally integrated. This area of research is however still in its infancy, and the few studies published to date, as discussed above, highlight some of the emerging challenges and limitation this strategy presents.
2.2.6. Achieving functional local circuit integration
Independent of the transplantation approach, one of the most fundamental challenges that still needs to be addressed is that of promoting synaptogenesis and recreating functional circuits to a degree capable of restoring meaningful vision. As discussed above, immunohistochemical and ultrastructural studies have been used as the main evidence of the ability of transplanted rods and cones to establish synaptic connections with bipolar cells within the host retina.
However, in light of the newly identified mechanism of cellular material transfer (discussed in detail in section 2.6 below) demonstrating that most, if not all, the photoreceptors assumed to be of transplantation origin were actually host photoreceptors connected to host bipolar cells (Ortin-Martinez et al., 2017; Pearson et al., 2016; Santos-Ferreira et al., 2016a; Singh et al., 2014; Singh et al., 2016; Waldron et al., 2018), a careful re-evaluation of previous results is required. On the other hand, studies aimed at photoreceptor replacement in the end-stage rd1 mouse with a zero anatomical and functional baseline, have shown expression of rod specific synaptic proteins at the terminals of donor-derived rods and the corresponding postsynaptic proteins in host bipolar cells at sites of contact with donor cells (Singh et al., 2013). These structural observations were correlated with pupil light responses consistent with visual function improvement arising directly from transplanted rod precursors, rather than indirectly through host cone rescue (Singh et al., 2013).
Though encouraging, behavioral studies are still needed to determine whether these observations do correlate with meaningful vision restoration. All in all, there is still a critical gap in knowledge regarding the mechanisms underlying photoreceptor circuit development, particularly those that regulate specificity in the synaptic connectivity of both rods and cones during normal development (Gamm et al., 2015). Addressing this gap could help in the identification of factors that could be used to promote appropriate wiring of transplanted rod and cone photoreceptors as well as re-wiring of the host inner retinal cells.
2.3. Umbilical stem cell transplantation
Umbilical tissue is an attractive source of cells for therapy because of the relative ease of harvest of such cells through techniques that are not highly invasive – and it is appealing to imagine a therapeutic use for what was once considered medical waste.
Human umbilical tissue includes stem and/or progenitor cells derived either from the cord tissue component or from cord blood component. Umbilical blood is considered a source of hematopoietic stem cells and the cord tissue is regarded a source of mesenchymal stem cells (MSC) that in principle have the capacity to differentiate into connective tissue lineages including bone, cartilage and fat. Interestingly, MSC have been observed to give rise to neuroglial-like cells and hepatocyte-like cells under specific culture conditions. (Lee et al., 2004) Umbilical cord blood cells show higher expansion capacity than cells derived from bone marrow or adipose tissue, but are somewhat more challenging to isolate (Kern et al., 2006).
Currently there are over 130 active interventional Phase I, II or III clinical trials using umbilical-derived cells for a variety for conditions including spinal cord injury, rheumatoid arthritis, graft- versus-host disease, stroke, heart failure, muscular dystrophy and other conditions (data from clinicaltrials.gov accessed Sept 7, 2018).
Human umbilical tissue-derived cells (hUTC) were investigated by Ray Lund, Shaomei Wang and colleagues in the context of attempting to rescue the retinal degeneration process in a small animal model (Lund et al., 2007). The donor cells were obtained by mincing and enzymatically digesting human umbilical cords and then culturing the resultant cells for 10 passages, a process that approximated to 20 population doublings). These cells also showed a population doubling capacity without incurring karyotypic changes (Lund et al., 2007). They demonstrated preservation of both photoreceptor cells and visual function when these cells were injected into the subretinal space of the Royal College of Surgeons rat at a relatively early stage of retinal degeneration. In fact, subretinal hUTC injection resulted in more extensive and robust retinal cell protection than two other expandable tissue-derived cell types, i.e. placental cells and bone marrow-derived mesenchymal cells.
The mechanism underlying this retinal protective effect was further studied in the same animal model. hUTC were found to repair RPE phagocytic dysfunction in retinal degenerations through various cellular mechanisms involving bridge molecules that promote the binding of photoreceptor outer segments to RPE, and also by the secretion of receptor tyrosine kinase ligands including brain-derived neurotrophic factor (BDNF), hepatocyte growth factor (HGF), and glial cell-derived neurotrophic factor (GDNF) (Cao et al., 2016). It is unclear if similar mechanisms could be activated though the intravitreal delivery of hUTC cells, or if these rescue pathways depend on placing hUTCs in the subretinal space in close proximity to the degenerating cells.
It is also unclear exactly how the site of intraocular placement (i.e. subretinal or intravitreal) of these cells affect their proliferation rate after transplantation; this potential concern exists for any non-neuronal or non-terminally differentiated cell type that in principle could retain a capacity for continued proliferation inside the eye after delivery. Nevertheless, umbilical cells appear to be a rich source of paracrine factors that could promote the survival and sustained function of photoreceptor and/or RPE cells in the context of retinal degenerative disease.
2.4. Human retinal progenitor cell transplantation
Retinal progenitor cells (RPCs) are a cell type found in the developing neural retina that can be grown in culture (Klassen et al., 2004b). These immature cells are mitotically active and multipotent, i.e., capable of differentiating into neurons as well as glia. RPCs are analogous to other neural progenitor cells found elsewhere in the developing central nervous system (CNS), with the caveat that they preferentially differentiate into retinal cell types such as photoreceptor neurons and Mueller glia. Furthermore, RPCs do not give rise to oligodendrocytes, a glia cell type not normally present in the retina. The reason for this is that, although myelination enhances the speed of axonal conduction, it is important for the retina to remain optically clear to subserve optimal vision.
As a type of neural progenitor, RPCs exhibit many of the now familiar characteristics of this relatively well-studied category of stem-like cells, including the potential for engraftment and long-term survival within the CNS microenvironment. Because they are mitotically active, RPCs have the potential for expansion in culture, allowing for manufacturing of an allogeneic cell product. Because RPCs generate photoreceptors, they are attractive as a potential means of repopulating the retina with those cells in patients with rod-cone dystrophy and other degenerations of the outer retina. In fact, as work has progressed, additional potential uses for RPCs as a means of delivering trophic support to ailing retinal neurons have also gained attention.
2.4.1. Preclinical development of RPC transplantation
The conceptual process leading to the clinical development of RPC transplantation began with an awareness of the lack of CNS regeneration seen in mammals that contrasted sharply with the impressive regenerative capacity seen in certain lower vertebrates, particularly fish and amphibians. Studies of those animals provided evidence that CNS tissue structures, fiber tracts, and functional circuits could often be regenerated to a surprising degree, even in adults (Arora and Sperry, 1962; Jacobson and Gaze, 1965). In mammals such is not the case, although exciting early work showed that grafts of immature neural tissue could survive transplantation to the mature brain and provide functional benefits in rodent models of neurological disease (Björklund and Stenevi, 1972; Brundin et al., 1986).
Subsequent to the initial success of Bjorklund’s group, transplantation of immature neural tissue was pursued in a variety of rodent models, including work in the visual system. It was discovered that an immature retina could be transferred as an allograft to the brain of a newborn rat where it would engraft, survive into adulthood, and send projections to visual centers in the host brainstem. (Hankin and Lund, 1987; Kirschen McLoon et al., 1981). To test whether such graft-host connections might be functional, the plan required surgically exposing the intracranial graft in juvenile animals, shining a light on the area, and looking for a pupillary light response in the host eye. However, the transplants were similar in appearance to the surrounding brain and sometimes located deep within the brain. Because stray light could also stimulate the host eye, the optic nerve was severed to eliminate this possibility. All surgery had to be done in a way that avoided damage to adjacent neural structures, including the graft and the 3rd cranial nerve. Active bleeding, however slight, could quickly block light from reaching the transplant. Furthermore, although the grafts contained photoreceptors, it was unclear whether these were functional in that they were unsupported by other tissues normally present in an eye, including the RPE and choriocapillaris.
Despite these challenges, it was demonstrated that a pupillary light reflex (PLR) could be elicited by photic stimulation of intracranial retinal transplants, indicating that a functional graft-host pathway had been established (Klassen and Lund, 1987). Further analysis revealed a relationship between the maximum magnitude of the response and the robustness of underlying graft-host innervation (Klassen and Lund, 1990a).
Taken together, these results showed that a new neural pathway could be established in the mammalian CNS that was capable of relaying luminance information over a continuous range of intensities. Less clear was how this finding might be translated into clinically relevant interventions, particularly given the even greater effort needed to replicate similar results with transplants to more mature animals (Klassen and Lund, 1990b).
Help came from the next set of game-changing results provided by work with neural progenitor cells derived by the Gage lab from the adult rat hippocampus, which were found to integrate into the neural retina of newborn rats where they appeared to differentiate into various types of retinal neurons (Takahashi et al., 1998). While the quality of the integration was impressive, the cells failed to integrate in adults and did not differentiate into photoreceptor cells.
One of these two challenges was overcome when the same hippocampal progenitor cells were transplanted into the eyes of adult rats with retinal dystrophy. It was found that the presence of an active degenerative process made an enormous difference in the behavior of the grafted cells (Young et al., 2000). During the period of photoreceptor cell death, hippocampal progenitors migrated into the retina in abundance and once again showed a remarkable capacity for integration. Notably, this behavior was no longer seen once the underlying degeneration had run its course. Apparently the grafted progenitor cells were responding to an injury signal from the host retina and remained quiescent in its absence. This was positive news from a therapeutic perspective in that it evidenced both a previously unrealized receptivity on the part of mature retina for integration of new neurons, as well as a self-regulatory capacity on the part of the progenitor cells wherein they respond only in the presence of active disease. However, once again the cells failed to differentiate into photoreceptors, the missing cell type in clinical conditions such as RP.
To overcome this last barrier it was necessary to go back to first principles: adult hippocampal progenitors, despite their considerable plasticity, might have lost the capacity to become photoreceptors early in development. Photoreceptors are a highly specialized type of neuron that is specific to the retina and pineal gland and development of the eye diverges from that of the brain at a relatively early time point. Furthermore, unlike the case in the dentate gyrus of the hippocampus, there is little evidence for RPCs in the mature retina, although Connie Cepko and colleagues had established the presence of multipotent progenitors in the developing retina (Turner and Cepko, 1988; Turner et al., 1990). The totality of these considerations led to the reasoning that it might be necessary to start with immature retinal tissue in order to obtain a cell type capable of reliably generating photoreceptors and thus having therapeutic potential in the setting of retinal degeneration.
By starting with immature retinal tissue, multipotent RPCs were successfully derived from a GFP-transgenic mouse line (Klassen et al., 2004a) as well as human donor tissue (Klassen et al., 2004a). Since RPCs are immature cells in an actively proliferative state, they can be expanded in culture in the presence of defined growth factors. Upon growth factor removal, proliferation subsides and the cells differentiate into a mixed population of neurons and glia. Following transplantation to the subretinal space, GFP+ RPCs were seen to differentiate into various retinal neurons, specifically including photoreceptor-like profiles expressing the markers recoverin and rhodopsin. A subset of such cells integrated with correct polarity into the host outer nuclear layer. RPC treatment was associated with enhanced light-induced behavioral responses, thereby providing initial proof-of-principle for use of subretinal transplantation of allogeneic RPCs as a therapeutic strategy for photoreceptor replacement in retinal degenerative diseases (Klassen et al., 2004b). The extent to which cellular materials transfer (discussed in Section 2.6 below) may have contributed to these results is unclear at this time.
There were, however, additional considerations that related to the therapeutic objective of cell replacement. Although photoreceptor integration was replicated in animals by multiple teams (Klassen et al., 2004b; Lamba et al., 2009; MacLaren et al., 2006), the efficiency of integration was generally low (West et al., 2012) and inevitably restricted to small regions of retina overlying the subretinal injection site. These issues could present serious challenges to the goal of demonstrating clinical proof-of-principle and therefore pose an avoidable risk to the therapeutic development program. Adjuncts including scaffolds or growth factors might be required to increase survival and integration efficiency. With this in mind, an alternative approach was considered, namely neuroprotection.
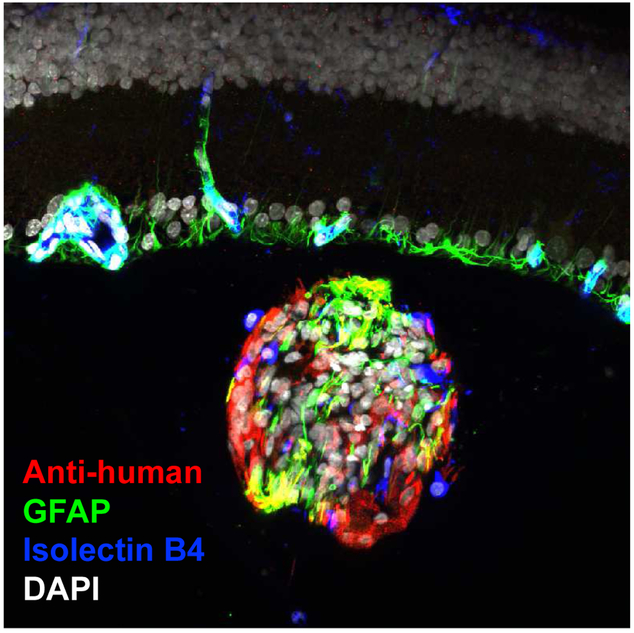
It has long been appreciated that dystrophic photoreceptors can be preserved by intravitreal injection of certain therapeutics, particularly so-called neurotrophic factors (Faktorovich et al., 1990; Lavail et al., 1992), potentially in quite low concentrations (Whiteley et al., 2001). A related approach was demonstrated in a pig optic nerve injury model (Ejstrup et al., 2010) and mesenchymal (Siqueira et al., 2015; Siqueira et al., 2011) and hematopoietic cells (Park et al., 2014) have been tested in clinical trials as discussed below in Section 5.3. Somewhat surprisingly, unmodified RPCs conferred neuroprotective effects on the photoreceptors of dystrophic rats (Yang et al., 2013). Based on the potential advantages of neuroprotection over cell replacement as a translational strategy, a novel approach combining an RPC product with intravitreal delivery method appeared to be feasible (Figure 4).
Figure 4.
Intravitreal graft of retinal progenitor cells. Cultured human RPCs, labeled with antihuman antibody (red), are seen following injection into the vitreous cavity of a rat eye. The cells are injected as a single cell suspension but subsequently aggregate in vivo to form small clusters, as seen here. These clusters are free-floating and provide neutrotrophic support to the retina (laminar structure above the graft) without the need for integration into the host tissue. The RPCs of the grafts differentiate along either neuronal or glial lineages, with the latter seen here by way of labeling for GFAP (green). Host mononuclear leukocytes investigate the donor cells, illustrated here by positivity for isolectin B4 (blue) within the graft, but do not elicit an immunological rejection response. Nuclei are labeled with DAPI (white). This image was provided by Dr. Geoffrey Lewis, UCSB.
2.4.2. Potential challenges
Advantages related to RPC transplantation come with a more challenging side as well. Fetal tissue is more developmentally mature and therefore less tumor prone than pluripotent cell lines, however, sourcing of donor tissue poses greater potential for future challenges in terms of supply lines for manufacturing, even if currently achievable. The supply issue is compounded by the self-limited expansion of RPCs, although this characteristic again diminishes the risk of uncontrolled proliferation. Nevertheless, extended passaging of RPCs is possible under hypoxic conditions (Baranov et al., 2014).
Neurotrophic benefits can be easier to achieve than cell replacement, however, the mechanism of action can be more complex and difficult to delineate. In addition, the inherent variability in a cell product, as compared to small molecules or other biologics, will necessarily entail a different set of comparability criteria during manufacturing.
2.4.3. Treatment indications
The primary indication for intravitreal RPC transplantation is RP. RP, also referred to as rod-cone dystrophy, is a heritable condition classically described as involving loss of first rods and then cone photoreceptors. It is bilateral, progressive, and currently untreatable. Although an orphan condition, RP is an important cause of blindness worldwide. Even though all current clinical activity has been directed towards RP, because retinal neurons are not regenerated in humans, there are many other conditions that might benefit from this neuroprotective approach, alone or in combination with other therapeutics.
Other retinal dystrophies come immediately to mind, as does age-related macular degeneration (AMD), particularly the atrophic type. Amelioration of photoreceptor loss following retinal detachment might also be of interest. Ongoing work in the laboratory suggests that optic nerve and retinal vascular conditions could benefit from intravitreal RPCs. Additional work will be necessary to develop clinical programs aimed at treating these conditions, but early indications are promising.
2.5. Bone marrow cell transplantation
Bone marrow contains stem cells that have been explored in preclinical and clinical studies as a potential regenerative treatment for ischemic or degenerative retinal conditions (Clinicaltrials.gov, 2018; Enzmann et al., 2009; Machalinska et al., 2009; Siqueira et al., 2011). The rationale for using stem cells in bone marrow originates from the observation that these stem cells are plastic and appear to play an important role in tissue repair and maintenance in the body (Mackie and Losordo, 2011; Rafii and Lyden, 2003). They are known to be mobilized into the systemic circulation to sites of tissue ischemia for repair (Asahara et al., 1997). By harvesting the stem cells from bone marrow and administering the cells directly into the eye, the repair potential of these cells may be maximized by bypassing the systemic circulation and delivering a high number of effector stem cells directly to the target tissue or organ. Most of the regenerative effects of these stem cells appear to be via paracrine mechanisms (Park et al., 2017). There is evidence that at least some of these cells engraft into tissue suggestive of tissue replacement as well (Caballero et al., 2007; Park et al., 2012). There is also evidence that these cells can fuse with cells in the retina to become retinal progenitor cells (Sanges et al., 2016)Thus the mechanism of action of these cells may be diverse depending on the cell of interest and the damaged tissue to be repaired.
2.5.1. Bone marrow derived cell types
Bone marrow contains a relatively high concentration of stem cells in the body and is a good source of these adult stem cells. However, bone marrow consists of a very heterogeneous mixture of cells and it is important to note the major cells in bone marrow are blood cells (Park et al., 2017). The stem cells of interest that can have regenerative potential constitute less than 0.1% of the total cells harvested from bone marrow. One of the challenges in using stem cells in bone marrow for tissue regeneration is identifying and harvesting the ideal target stem cell or cells for regenerative treatment.
There are different cells that have been harvested from bone marrow and explored for tissue regeneration. They include mononuclear cells, hematopoietic stem cells/CD34+ cells and mesenchymal stem cells. Confusion can result as these different cells have all been called “bone marrow stem cells”. Many studies do not clearly differentiate the various different cells isolated from bone marrow. Often, the reader needs to review the methodology of cell isolation to determine which bone marrow stem cell was investigated in preclinical or clinical research.
2.5.2. Mononuclear cells
Mononuclear cells are a mixture of cells isolated after Ficoll density gradient separation of the bone marrow aspirate. This procedure removes most of the erythrocytes and polymorphonuclear cells. The resulting mononuclear cell fraction is a mixture of lymphoid, myeloid, erythroid and stem cell population. It consists mostly of lymphocytes and monocytes and contains < 0.2% hematopoietic stem cells (i.e. CD34+ cells in human) and even fewer mesenchymal stem cells (Pang et al., 2011; Posel et al., 2012). This cell mixture has been used as “bone marrow stem cell” therapy for many preclinical and clinical studies since this cell mixture can be easily obtained from the bone marrow aspirate with minimal cost and effort.
A preclinical study using bone marrow mononuclear cells injected intravitreally showed long term incorporation of some of these cells in the retina following intravitreal injection in rat eyes with retinal injury (Tomita et al., 2002). Early clinical studies conducted in Germany and Brazil showed autologous intravitreal injection of mononuclear cells from bone marrow was well-tolerated in eyes with retinal degeneration or ischemia (Jonas et al., 2010; Siqueira et al., 2011).
However, visual benefit was modest to none. Improvement in cystoid macular edema associated with RP or retinal vein occlusion has been observed following intravitreal injection of mononuclear cells (Siqueira et al., 2015; Siqueira et al., 2013). More recently, the same group reported improvement in mean visual acuity and macular sensitivity in 10 eyes with dAMD following intravitreal mononuclear cell therapy with no adverse effects (Cotrim et al., 2017). The therapeutic effect is stated to be based on CD34+ cells present in the mononuclear cell fraction. In fact, as stated previously, there are relatively few CD34+ cells present in human mononuclear cells fraction of bone marrow (< 0.2%). Clinical trials using mononuclear cell fraction of cells from bone marrow or from peripheral blood after mobilization of cells to treat ischemic cardiomyopathy have shown no significant safety concerns. Efficacy results have been variable and felt to be correlated with number CD34+ cells present in the mononuclear cell fraction used (Mackie and Losordo, 2011; Vrtovec et al., 2013).
2.5.3. Hematopoietic stem cells
Hematopoietic stem cells are also harvested from bone marrow and often identified by the cell surface marker, CD34 in humans. These stem cells are plastic and have been used for allogeneic bone marrow transplantation for many years in clinical practice to treat hematologic disorders (Goodell et al., 2015. These CD34+ stem cells also have been explored in clinical trial as potential therapy for ischemic cardiomyopathy since the CD34 cell surface marker also identifies endothelial progenitor cells (Mackie and Losordo, 2011; Vrtovec et al., 2013). In fact, human CD34+ cells are believed to be mobilized into the systemic circulation from bone marrow in response to tissue ischemia and thought to play an important role in tissue revascularization (Asahara et al., 1997).
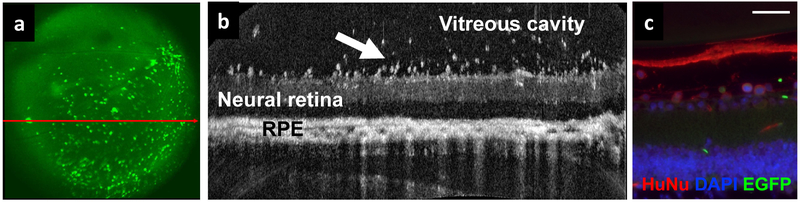
There is phase II clinical trial evidence in support of this autologous therapy in treating ischemic and non-ischemic cardiomyopathy (Quyyumi et al., 2017; Vrtovec et al., 2013). Based on preclinical and clinical studies, the effects of CD34+ cells on ischemic cardiomyopathy appear to be via a combination of paracrine trophic effects and direct engraftment of the cells into the damaged vascular endothelium (Vrtovec et al., 2013). A synergistic effect of this combined mechanism is believed to result. No safety concerns have been noted associated with intracoronary infusion of these autologous cells. Autologous hematopoietic stem cells from bone marrow have been injected intravitreally into murine eyes with hereditary retinal degeneration and shown to have a protective effect (Otani et al., 2004). Since the injected cells were only found in the retinal vasculature and not in the photoreceptor layer, a paracrine mechanism was speculated. Intravitreal injection of human CD34+ cells from bone marrow in mice with hereditary retinal degeneration resulted in rapid dramatic homing of the cells to the retinal surface which can be visualized using in vivo retinal imaging (Figure 5). The cell treatment was associated with dramatic molecular changes in the degenerating retina in mice with concurrent systemic immunosuppression administered to avoid rejection of human cells (Moisseiev et al., 2016). Expression of genes that control apoptosis and photoreceptor maintenance and transduction were significantly affected. Human CD34+ cells have been injected intravitreally in immune deficient murine eyes with retinal vasculopathy (Caballero et al., 2007; Park et al., 2012). Homing and integration of these human cells into the damaged retinal vascular wall with possible repair has been demonstrated short-term and long-term.
Figure 5.
Intravitreal injection of human CD34+ stem cells from bone marrow in rd1 mice with retinal degeneration results in rapid homing and integration of these human cells to the surface layers of the retina (Moisseiev et al., 2016). The mouse was immunosuppressed with tacrolimus and rapamycin to avoid rejection of human cells. (A) Scanning laser ophthalmoscope (SLO) fundus image shows fluorescence from EGFP-labeled human CD34+ cells that have homed to the retina. (B) Simultaneous b-scan optical coherence tomography (OCT) imaging of the retina shows cells integrating into the retinal surface 1 week after intravitreal injection (arrow). RPE: retinal pigment epithelium. (C) Immunohistochemical analysis using anti-human nuclei monoclonal antibody (HuNu, red) shows human cells (identified by ring-shaped staining) within the superficial layers of the retina (scale bar, 50μm). (SLO and OCT images courtesy of Pengfei Zhang, PhD, Robert J. Zawadzki, PhD; immunohistochemical staining and images courtesy of Sharon Olten).
These hematopoietic stem cells do not readily replicate and cannot be expanded in culture (Park et al., 2017). This feature of hematopoietic stem cells limits the number of cells that can be administered for regenerative treatment. However, this feature of CD34+ stem cells theoretically makes them less teratogenic and potentially safer for clinical applications. Long-term preclinical studies have shown no safety concerns following intravitreal injection of human CD34+ cells from bone marrow in NOD-SCID mice with acute retinal ischemic injury (Park et al., 2012). There was no abnormal proliferation of the human cells in the eye or systemically following the intravitreal injection of human CD34+ cells.
Based on the promising preclinical safety and efficacy profile of intravitreal injection of bone marrow hematopoietic stem cells/human CD34+ stem cells, an early phase clinical trial has been initiated by investigators at the University of California Davis exploring intravitreal injection of autologous CD34+ stem cells isolated from bone marrow as treatment for ischemic and degenerative retinal conditions (Park et al., 2014). This clinical trial will be discussed in more detail later in this article along with an update on the status of this study.
2.5.4. Mesenchymal stem cells
Mesenchymal stem cells from bone marrow are harvested by growing bone marrow aspirate in tissue culture. Mesenchymal stem cells constitute < 0.1% of the cells in bone marrow, but these cells adhere to plastic, grow and expand readily in tissue culture (Park et al., 2017).These mesenchymal stem cells are characterized by different cell surface markers from hematopoietic stem cells and are different cells. Mesenchymal stem cells were first isolated from bone marrow in 1968 but similar type of cells have been isolated from other tissue such as adipose tissue, muscle, Wharton’s jelly, amniotic fluid, and umbilical cord blood (Friedenstein et al., 1968; Park, 2016). Preclinical studies indicate that these cells have some plasticity and can differentiate into other cells of mesenchymal origin. They have limited capacity to differentiate into cells of ectodermal or endodermal origin. Most of the regenerative effects of these cells are via paracrine effects. They have been reported to secrete various neurotrophic and angiogenic factors, such as ciliary neurotrophic factor (CNTF), vascular endothelial growth factor and fibroblast growth factor (Caplan and Dennis, 2006).
The appealing features of mesenchymal stem cells that make them a target for cell therapy for tissue regeneration are that they can be easily expanded in culture and allogeneic use is potentially possible. Mesenchymal stem cells express very low levels of HLA class 1 antigen and do not express any HLA class 2 antigen. Preclinical studies show allogeneic therapy is possible but these cells are not completely immune privileged (Ryan et al., 2005). Furthermore, these cells may modulate the immune system in unexpected way since both pro and anti-inflammatory effects of mesenchymal stem cell have been reported in preclinical models following cell administration (Bernardo and Fibbe, 2013; Galderisi and Giordano, 2014; Le Blanc, 2006). The potential proinflammatory effect of these cells may limit the clinical applications of these cells.
Another limitation in exploring mesenchymal stem cells as regenerative treatment is the heterogeneity of these cells among laboratories (Bara et al., 2014). This is because the cells harvested can change depending on culture condition. The International Society for Cellular Therapy established that mesenchymal stem cells must have >95% positive cell surface markers for CD105, CD73, and CD90 and >95% negative for CD45, CD34, CD14 or CD11b, CD79α or CD19, and HLA-DR (Dominici et al., 2006). Despite these criteria, heterogeneity persists.
2.5.5. Advantages and potential concerns
The cellular heterogeneity and potential safety issues limit the clinical applications of mesenchymal stem cells as treatment for retinal disease despite extensive preclinical research showing potential efficacy. Cultured mesenchymal stem cells have been injected intravitreally or subretinally in animal models of retinal degeneration or ischemia. Preclinical studies indicate that the subretinal administration of mesenchymal stem cells can have protective effects in eyes with retinal degeneration (Arnhold et al., 2007; Tzameret et al., 2014). The protective effect is less obvious following intravitreal administration of the cells in eyes with retinal degeneration(Tzameret et al., 2014). For retinal ischemia, intravitreal administration of mesenchymal stem cells has been shown to have a protective effect in preclinical studies (Li et al., 2009). Some of these injected mesenchymal stem cells integrate into the retinal surface and stimulate gliosis while others can form a cellular clump in the vitreous cavity (Li et al., 2009; Tzameret et al., 2014). In NOD-SCID mice, intravitreal administration of cultured human mesenchymal stem cells resulted in abnormal cellular proliferation, leading to vitreous haze and retinal traction which was visualized using in vivo retinal imaging (Park et al., 2017). These observations raise safety concerns regarding intravitreal administration of these cells. Recently, similar safety concerns have been reported with the intravitreal injection of autologous mesenchymal stem cells in an early clinical trial for retinitis pigmentosa (Satarian et al., 2017).
There are multiple types of cells that have been isolated from bone marrow and explored for tissue regeneration. The advantages of using bone marrow stem cells for tissue regeneration are many. They include the ease of accessibility of these cells, the ease of delivery of these cells to the target tissue given the homing ability of the cells and paracrine effects, autologous cell therapy that is possible which avoids the issue of immune rejection, lack of ethical issues, and broad potential clinical applications given paracrine effects of the cell therapy.
The potential pitfalls or limitations of using bone marrow stem cells are the potential heterogeneous nature of the cell product, limited ability to expand some isolated cells such as CD34+/ hematopoietic stem cells, and host factors that may affect the repair potential of the isolated cells (Park et al., 2017; Vrtovec et al., 2016). At the current time, stem cell therapy from bone marrow remains an investigational treatment for retinal disorders.
2.6. Cytoplasmic transfer between donor and recipient cells
It was previously assumed that, in the context of photoreceptor precursor transplantation, the transplanted exogenous photoreceptor precursor cells would migrate into the recipient retinal tissue and thereby augment the total number of photoreceptor cell bodies (or soma) in the recipient.
However, in light of recent data (Ortin-Martinez et al., 2017; Pearson et al., 2016; Santos-Ferreira et al., 2016a; Singh et al., 2014; Singh et al., 2016; Waldron et al., 2018), it is now clear that transfer of cytoplasmic materials between transplanted cells and recipient cells is the more common mechanism of cellular regeneration, rather than somatic integration.
It should be noted that this phenomenon has been observed to occur if at least two conditions are met. Firstly, the recipient must not be completely degenerate – i.e., there must be sufficient numbers of recipient photoreceptor cells remaining that can act as ‘transfer partners’ with the transplanted cells. In the setting of end-stage retinal degeneration in the recipient, when almost all photoreceptor cells have degenerated in regions of retinal atrophy (Singh et al., 2013), the transplanted cells have no ‘transfer partners’ and therefore cellular materials transfer is not thought to occur to a significant extent. Secondly, the phenomenon has been observed only when the donor photoreceptor cells are prepared as an enzymatically or mechanically dissociated cell suspension of individual cells or cell clumps. The precise mechanism of the transfer of materials, and the reason for the promotion of this process by donor cell dissociation, remain unclear.
Cellular materials transfer between photoreceptor cells could involve direct membrane fusion (Jahn et al., 2003; Jahn and Scheller, 2006) or other methods of intercellular trafficking including exosomes (Théry et al., 2002; Valadi et al., 2007). The occurrence of this phenomenon between other retinal cell types, for example RPE cells, or inner retinal neurons, has not been comprehensively studied at this time.
Interestingly, materials transfer between endogenous bone marrow stem cells and retinal Müller glial cells has also been observed in vivo (Lluis and Cosma, 2010; Sanges et al., 2016). This process appears to prompt the reprogramming of Müller glial cells into retinal progenitor cells which can then differentiate into non-photoreceptor cell types, namely ganglion cells and amacrine cells. The data indicate that retinal cell fate can be modulated through the process of cellular materials transfer.
Therefore, safety concerns could arise from changes in cell fate or in cell cycle regulation that could occur following material transfer between retinal and/or non-retinal cell types. However, definitive data regarding these concerns have not yet been published. It is not yet known if cellular materials transfer between photoreceptor cells or RPE cells could give rise to adverse safety outcomes involving uncontrolled proliferation. Further experiments using cell lines or animal models are required in order to address this question.
2.7. Immune considerations
A very helpful feature of intraocular RPC transplantation emerged from the observation that allogeneic neural progenitor cells were well tolerated in rodent models, without the need for immunosuppression. This tolerance was consistent with results from the previous work with intracranial neural tissue transplantation, described earlier (Klassen and Lund, 1987, 1990a). The anterior chamber of the eye and the brain have long been described as “immune privileged” sites in terms of transplantation and this phenomenon could have contributed to the tolerance seen here. Despite this, the immunogenicity of the cells themselves was still in question. Therefore, the issue was examined in detail in mice, where it was shown that cultured murine neural progenitors did not express MHC class I or class II antigens and were tolerated as allografts even following transplantation to the kidney capsule, a conventional (non-privileged) site (Hori et al., 2003).
Further work revealed that although expression of MHC class I antigens by cultured neural progenitors varied by species (Hori et al., 2003; Klassen et al., 2003; Klassen et al., 2001; Klassen et al., 2004b), the tolerance for these types of cells as allografts was a generalizable feature that was replicated in rats (Young et al., 2000), pigs (Klassen et al., 2007a), and cats (Klassen et al., 2007b). In contrast to allografts, it was found that xenografts, although sometimes successful (Van Hoffelen et al., 2003), were frequently rejected, particularly between discordant species (Warfvinge et al., 2006; Warfvinge et al., 2005); human BPC to pig (Warfvinge et al., 2011). Nevertheless, the available evidence suggested that allografts of human RPCs would be tolerated in patients, although animal testing of human cells could not adequately evaluate this hypothesis. More on point, reports from overseas trials appeared to support the tolerance of intraocular allogeneic neural progenitors in humans (Liu et al., 2017). If confirmed, this would present a significant advantage for RPC transplantation over alternate cell-based approaches requiring immune suppression.
Recent work regarding the need for immune suppression in allogeneic subretinal transplants has shown that there was poor survival of allogeneic iPSC-RPE cells following transplantation into the subretinal space of rhesus macaques (McGill et al., 2018). Transplanted cells that were detectable 4 days after surgery were no longer detectable at 3 weeks. An early T-cell response at 4 days had later converted to a B-cell response by 3 weeks. These data indicate that immune rejection likely contributed to poor subretinal cell survival following allogeneic RPE cell transplantation when immune suppression was not employed. Data from the minipig model of subretinal allogeneic iPSC-RPE cell transplantation also indicated that the innate immune response was elicited (Sohn et al., 2015). This information challenges the view that the subretinal space affords sufficient immune privilege to support allogeneic grafts. Strategies to consider as alternatives therefore include autologous transplantation, immunological matching or chronic immune suppression.
3. Regulatory framework for safety
The only US Food and Drug Administration (FDA) approved stem cell therapies are seven human progenitor cell cord blood products, all used for unrelated donor cell transplantation and for a broad range of hematopoietic diseases (FDA, 2017b). A sterile cord blood collection unit for collection of umbilical cord blood from either vaginal birth or cesarean section is also FDA approved (FDA, 2016). Allogenic bone marrow transplantation, which is known to include stem cells is not regulated by the FDA but is regulated by the Health Resources and Services Administration, the National Marrow Donor Program, and the Foundation for Accreditation of Cellular Therapy at the University of Nebraska Medical Center (University of Nebraska Medical Center, 2014). Additionally, a multibillion dollar industry in the U.S. in regenerative medicine already exists charging patients directly for unproven “stem cell” therapies for a broad range of diseases (Berger et al., 2016).
These “stem cell” therapies are neither approved by the FDA nor provided in the context of a clinical trial registered with the FDA. Practitioners often offer such therapies without appropriate experience in treating the disease of concern, and circumventing much of traditional, mainstream medicine and FDA or other regulation. A 2016 study found 187 unique “stem cell” clinic websites offering non-FDA approved “stem cell” interventions at 215 clinics (Berger et al., 2016). At least 40 companies provide “stem cell” therapies in the US for ocular diseases, including intravitreal, retrobulbar, or topical deliveries (Nirwan et al., 2019). The most common ocular condition for which therapy is offered is macular degeneration.
Literature targeted at potential patients often overstates the known efficacy and safety of the body’s own “stem cell” populations in curing diseases, while paying only cursory attention to the paucity of data (Berman, 2015). Mainstream medicine’s skepticism of the purported benefits of currently available, unproven “stem cell” therapy is often described as uninformed or dishonest. While anecdotes of success are frequently given, minimal discussion of serious health risks from unproven “stem cell” therapies are provided. Third party payers do not pay for these therapies, and patients are typically required to pay for them directly as fee for service or patient-funded research. The “stem cell” clinics employ direct-to-consumer advertisements using websites that emphasize patient testimonials. This can result in patients not being properly informed of the potential complications and the paucity of rigorous peer-reviewed publications demonstrating efficacy data for these procedures.
The American Academy of Ophthalmology issued a clinical statement in 2016 emphasizing that there are no FDA-approved stem cell therapies for ocular diseases and that the risks of treatments at these stem cell clinics is not known (American Academy of Ophthalmology, 2016). Stem cell products are regulated by the Center for Biologics Evaluation and Research as biologics within the FDA. Under sections 351 and 361 of the Public Health Service Act, human cell biologic therapies are divided into low risk and high risk. In 1997, the FDA provided the following clarification on the regulation of stem cell therapies that are considered low risk:
FDA would not require premarket review and approval for cellular and tissue-based products that are minimally manipulated, are used for homologous function, do not contain non-cell/non-tissue components, and are for structural or reproductive use. Such products raise relatively limited clinical safety and effectiveness concerns, and thus would not be subject to premarket submission of clinical data. Additionally, as a policy matter the agency would not require premarket submission of clinical data for cellular or tissue-based products that are minimally manipulated, are used for homologous function, do not contain non-cell/non-tissue components, and are for metabolic use, when they are to be used autologously or in a close blood relative of the donor… Examples of such products would include heart valve and dura mater transplants, vein grafts, tendons to repair or replace tendons, autologous or family use of peripheral or cord blood stem cells for hematopoietic reconstitution, and human gametes (sperm and eggs), zygotes, and embryos intended for insemination, fertilization, or transfer (FDA, February 28 1997).
Tissues and cells that are highly processed, used for other than their normal function, combined with non-tissue components or used for metabolic purposes are considered high risk. High risk human cell biologic therapies are required to demonstrate efficacy and safety and file an investigational new drug application (IND) with the FDA. Low risk therapies are not. “Stem cell” clinics that use autologous stem cells contend that the cells are minimally manipulated cells and applied for homologous use, so they do not fall under strict regulatory oversight. FDA draft guidance statements narrowing the definition of minimal manipulation (FDA, 2017d) and clarifying homologous use (FDA, 2017c) were created in order to eliminate any doubt that the use of autologous “stem cells” is high risk and should fall under the regulatory oversight of the FDA. Homologous use means the repair, reconstruction, replacement, or supplementation of a recipient's cells or tissues with a human cell, tissue, or cellular and tissue-based product (HCT/P) that performs the same basic function or functions in the recipient as in the donor (FDA, 2017a). Allogenic bone marrow transplantation is considered homologous use.
The FDA has posted a warning letter to the facility that treated three of the patients described above. In the accompanying press release (FDA, 2017b), FDA Commissioner Scott Gottlieb, M.D. explains that:
“Stem cell clinics that mislead vulnerable patients into believing they are being given safe, effective treatments that are in full compliance with the law are dangerously exploiting consumers and putting their health at risk.”
He goes on to say that the FDA will be increasing enforcement actions against these clinics. Recently the FDA filed two complaints in federal court seeking permanent injunctions to stop two stem cell clinics from marketing stem cell products without FDA approval and for significant deviations from current good manufacturing practice requirements (FDA, 2018). One of these clinics was performing the intravitreal injections.
The retina is particularly suited for the benefits of stem cell therapy because of the relatively easy anatomic access and physiological monitoring. However, treatment of retinal disease with unproven “stem cell” therapy, without careful gathering of efficacy and safety data, has repeatedly resulted in severe vision loss. Ethical and meticulous clinical science is paramount for the advancement of realistic stem cell therapy for retinal disease.
4. Surgical safety aspects of cell transplantation
The transplantation techniques that are employed in retinal stem cell clinical trials should have a reasonably low risk so that the safety and efficacy of the cells themselves can be evaluated properly.
Therapeutic cells can be delivered to the intravitreal space or the subretinal space. The intravitreal delivery approach is shown in Figure 7a. There are three main surgical approaches which have been employed for the delivery of RPE cells to the subretinal space.
Figure 7.
Schematic diagram depicting selected approaches of cell delivery for retinal therapy. (a) Intravitreal injection. The cells are injected as a suspension (white arrowhead) into the vitreous gel via a needle (clear arrowhead) introduced into the eye via the pars plana. The cells do not gain access to the subretinal space and remain in the vitreous. Note that the a vitrectomy procedure has not been performed here because it is not required for intravitreal delivery. (b) Subretinal injection of a cell suspension. After vitrectomy, a small-gauge needle or rigid cannula (arrow) is introduced into the eye via the pars plana. The needle or cannula is passed through a retinotomy at the injection site (white arrowhead) and the cell suspension is placed in the subretinal space near the fovea (black arrowhead). (c) Subretinal injection of a sheet of cells with or without a scaffold. suspension. After vitrectomy, a small-gauge needle or rigid cannula is introduced into the eye via the pars plana and passed through a retinotomy at the injection site (white arrowhead). The sheet construct is placed in the subretinal space near the fovea (black arrowhead). (d) Suprachoroidal cannulation. A flexible catheter (arrow) is inserted into a retinal bleb (white arrowhead) and threaded into the suprachoroidal space to the posterior pole or macula where the cell suspension is injected (black arrowhead) in the subretinal space near the fovea. A vitrectomy procedure is typically required and so the vitreous cavity shown here is devoid of gel. (Illustration by Timothy Phelps, MS, FAMI.)
The first is vitrectomy followed by delivery of a cell suspension to the subretinal space via a subretinal cannula (Figure 6 and Figure 7b) (Nagiel and Schwartz, 2018). In this technique, the retinotomy is tiny (38-gauge) and self-sealing which minimizes the risk of retinal detachment or other serious complications. The exact effect of small-gauge passage on cell survival for all donor cell types is yet to be determined, however delivery via larger-gauge needles (e.g. 30-gauge or 31-gauge) may be associated with enhanced RPE cell viability in large animal models (Scruggs et al., 2019; Wilson et al., 2017). These surgical maneuvers are familiar to most vitreoretinal surgeons.
Figure 6.
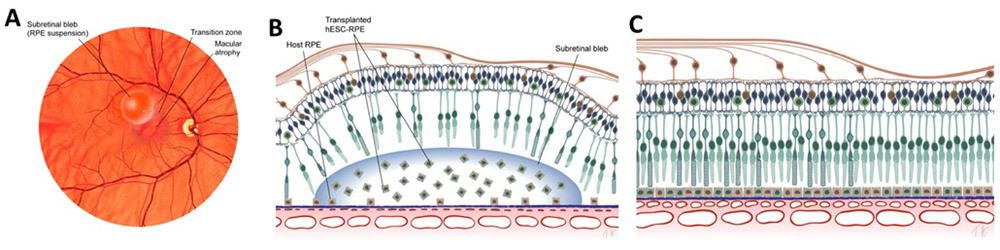
Schematic drawing of hESC-derived RPE transplanted as a suspension for macular degeneration. (A) A fixed volume of transplanted cells is delivered subretinally as a suspension via a 39-gauge injection site, or retinotomy (yellow dot). The transition zone at the border of the atrophic region was treated. (B) Schematic cross-section through the subretinal bleb at the time of delivery to a diseased retina with a suspension of transplanted hESC-derived RPE. (C) Ideally the transplanted cells survive the injection procedure, engraft on Bruch membrane, polarize, and then rescue the surviving photoreceptors. (Illustration by Timothy Hengst)
The second method is more complex and involves subretinal delivery of RPE cells grown on a scaffold (da Cruz et al., 2018; Kashani et al., 2018). The main advantage of this technique (Figure 7c) is that the delivered cells are already organized in a monolayer and polarized (Thomas et al., 2016). But in order for a scaffold of sufficient size to be delivered, a wide retinotomy must be created and the RPE sheet delivered with the use of specialized delivery device. As a result, the edges of the retinal incision must be sealed with laser and a tamponade agent such as silicone oil must be used. These additional techniques may result in a noticeable scotoma or tissue damage with insertion of the scaffold.
The third technique involves subretinal delivery of cells via a cannula delivered ab externo through the suprachoroidal space (Baldassare et al., 2017; Ho et al., 2017). This technique (Figure 7d) was utilized in a pioneering study of human umbilical tissue-derived cells for their neuroprotective effect rather than as cell replacement, as discussed in detail in section 5.2.1 below (Koh et al., 2018). In order to deliver these cells to the macula, a scleral cutdown was created and a subretinal cannula threaded through the suprachoroidal space to the macula. Although this technique avoids exposure of the cells to the vitreous cavity, there were serious surgical adverse events related to the technique such as retinal perforations and detachments, as discussed in section 5.2.2 below.
5. Review of selected clinical trial data
5.1. JCyte
RPC transplantation for the treatment of RP is in the clinical phase of development, as sponsored by the company jCyte (Newport Beach, CA). An initial phase I/IIa trial (NCT02320812) has been completed and those patients were automatically entered into an extension study. Meanwhile, a masked phase IIb trial (NCT03073733) has completed enrollment.
5.1.1. Clinical protocol
The clinical protocol is as follows. Allogeneic RPCs, originally derived and expanded from fetal donor tissue, and cryopreserved in dose-sized aliquots, are thawed and briefly re-cultured prior to use. Synchronous to this process, the patient (having already completed baseline testing) is prepared for an intraocular injection in the usual manner, including lid speculum, topical anesthesia of the ocular surface, pupillary dilation, and local antiseptic measures. The injection is performed using a low volume syringe using a 30-gauge needle in under a minute and the cells are delivered directly to the intended location, i.e., the vitreous cavity, as a single cell suspension. Apart from a brief course of topical steroids, no immunosuppression is used.
After injection, the cells remain suspended in the vitreous cavity where they rapidly aggregate into clusters and strands, as can be observed with clinical biomicroscopy. Animal data had earlier shown that the cells gradually differentiated into neurons and glia. They also express a number of known neurotrophic factors, together with other candidate factors and extracellular gene products of interest. These factors are free to diffuse throughout the vitreous and enter the retina, where multiple host cell types exhibit signs of activation, leading directly or indirectly to a neurotrophic effect at the level of the photoreceptors. In phase I/IIa study subjects with RP, intravitreal hRPC treatment was associated with improved BCVA outcomes in treated eyes versus uninjected eyes at 12 months follow up (Kuppermann et al., 2018). Results from the masked, controlled phase 2b are not yet available.
Using this approach, the intravitreal RPCs are not required to integrate into the host retina to achieve the desired treatment effect. The grafts are not vascularized and do not stimulate neovascularization. This lack of integration and vascularization may contribute to the cells gradually losing viability over time, eventually leading to elimination of the graft. Experience in animal models indicates that repeat injection of RPCs are feasible and, importantly, that prior treatment does not lead to graft rejection upon repeat dosing (Chen et al., unpublished data). Initial human data show tolerance for a repeat hRPC injection, given to the fellow eye, although functional responses to re-injection are not available currently and same-eye re-injection has not been explored.
With funding from the California Institute for Regenerative Medicine (CIRM), initial clinical product manufacturing was performed at UC Davis in 2012. The starting material was fetal retina, received via an approved tissue donation process. This was followed by formal preclinical studies of intended clinical product in rodents and pigs (the latter in collaboration with NCATS). Results were submitted to the FDA as part of an IND package in late 2014. Following successful completion of the review process in 2015, a phase I/IIa clinical trial of human retinal progenitor cells was initiated in RP (NCT02320812). The objective of this initial, single arm, open label study was to demonstrate safety and tolerability in a small group of severely affected patients, including investigation of dose escalation. Treatment consisted of a single injection of allogeneic human RPCs, under topical anesthesia, into the vitreous cavity of the worse-seeing eye.
Based on extensive prior experience with allogeneic neural and retinal progenitor cell transplantation in a range of animal species, including mice (Klassen et al., 2004b), rats (Young et al., 2000), pigs (Klassen et al., 2007a), and cats (Klassen et al., 2007b), it was expected that allogeneic RPCs would also be well tolerated in humans. Therefore, active immunosuppressive measures were not employed, although a brief course of topical steroids was used with the objective of mitigating inflammation related to the injection procedure. Similarly, the MHC status of both cell lots and trial participants was determined for research purposes, but not used to prospectively cross-match cells with recipients.
Likewise, because the goal of this intervention was a non-specific neurotrophic effect with emphasis on reviving cone function, the precise rod-related mutations underlying each patient’s RP were not used as an enrollment criterion. Genetic testing was performed on enrolled subjects for retrospective analysis, but not used as a screening tool. Similarly, sex of donor and recipient were recorded but not matched prospectively. Patients 18 years of age and older were potentially eligible and a broad range of ages were eventually enrolled, as were a number of patients with Usher syndrome. Patients were divided into two cohorts based on initial visual assessment. In the interest of safety, patients in the first cohort were more severely affected, with best-corrected visual acuity (BCVA) ranging from “hand motions” to 20/200. The follow-on cohort had somewhat better vision, ranging from 20/200 to 20/63. In either case the visual fields were severely restricted and central fixation was absent or impaired.
The initial dose tested was 0.5 million cells. Following a series of reviews by an external DSMB, the dose level was sequential raised to 1, 2, and finally 3 million cells. These dose figures could be viewed as moderate by systemic and central nervous system standards, where larger cell numbers are often thought to be necessary (Selden et al., 2013; Sezer et al., 2000). This is made possible by the relatively small confines and restricted microenvironment of the eye. In the context of other ocular projects, the greater volume of the vitreous cavity allows for delivery of larger cell numbers than can be easily accommodated under the retina.
5.1.2. Outcome data
After treatment, all patients were followed for a 12 month period with BCVA at that time point (versus fellow eye) used as the primary outcome measure. Given the relentless nature of RP, combined with the slow time course of progression, the use of BCVA was a controversial choice in that it might not be expected to demonstrate positive treatment-related changes, particularly over the relatively brief 1 year time period evaluated. Nevertheless, BCVA is a particularly well-accepted measure, familiar to the clinical sites and FDA. The need to validate alternative potential measures poses significant challenges to rapid implementation and acceptance. Ultimately, BCVA was chosen to expedite progress while additional measures were evaluated.
A total of 28 patients were enrolled in the trial (NCT02320812), which was completed in August of 2017, and the results are currently being prepared for publication. Overall, the investigational cells were well tolerated, with a favorable safety profile. The majority of adverse events (AEs) recorded were anticipated based on the injection procedure alone, such as transient ocular pain and conjunctival injection or hemorrhage. Beyond that, there were cases of mild to moderate anterior segment inflammation that responded to conventional steroid treatment. Of note, these inflammatory events were not associated with destruction of the intravitreal grafts and therefore did not constitute classical immune rejection.
There were no ocular severe adverse events (SAEs). A single systemic SAE was declared based on new onset leg pain associated with difficulty ambulating in a visually impaired subject. The case was extensively worked up and ultimately redesignated as unlikely to be treatment-related.
There were widespread subjective reports of gradual improvement in various aspects of visual function. These anecdotal reports were backed up by objective measures of BCVA. Acuity of treated eyes was superior to that of untreated eyes at both 6 and 12 month time points. Evidence of treatment effect appeared more pronounced for the highest dose level: the mean change in BCVA from pre-treatment to month 12 (treated eye minus untreated eye) was 3.64 letters for all subjects, (subgroups: 1.38 letters for the 0.5M dose group, 1.00 letter for the 1.0M dose group, 4.83 letters for the 2.0M dose group and 9.00 letters for 3.0M hRPC). BCVA was also elevated relative to baseline in the treated eyes, consistent with an actual improvement in visual resolution, as opposed to just slowing of visual decline (Kuppermann et al., 2018).
All 28 patients were entered into an extension of the phase I/IIa trial and offered a single treatment in the fellow eye. Most patients opted to get bilateral treatment. In addition, a mobility test (maze) and other assessments were introduced as part of follow up testing. Immune suppression was again not used, even though a repeat dose presents a potentially greater immune challenge, and these second grafts have also been well tolerated to date.
5.1.3. Future work
Based on the gratifying results of the phase I/IIa trial, a phase IIb clinical proof-of-concept trial (NCT03073733) was initiated with a goal of demonstrating efficacy in a masked study with a control arm. This masked study includes two dose levels and a mock injection group. The goal is to enroll sufficient numbers of patients in order to obtain final results from a minimum of 25 subjects per group for statistical purposes. Enrollment was completed in June of 2018.
From a strictly biological perspective, intravitreal RPC transplantation appears to be helpful at the level of individual patients. Since it is not yet approved for general use, further validation will be required, in association with additional steps in the translational process. These include completion of the current phase IIb clinical trial, ongoing technology transfer and scale up of manufacturing at a commercial manufacturing organization (CMO), as well as a future phase III pivotal trial using commercial product.
The use of intravitreal RPCs in RP shows considerable promise as a treatment for this devastating and otherwise untreatable condition. So far, treatment has been necessarily restricted to patients at an advanced stage of disease progression, hence with severe visual impairment. It will be interesting to see how this neurotrophic-based effect impacts patients at different stages of the disease. In particular, how effective might it be in forestalling visual decline in patients when used at an earlier stage? With repeated treatment starting early enough might it be possible to avoid progression to legal blindness? It is an intriguing possibility that begs to be explored, once an acceptable safety profile is clearly established.
Additional work can also be done at the level of the cells. As details of the mechanism of action are uncovered, strategies for enhancing potency will likely become apparent. Looking ahead, RPCs can also be genetically modified and used in combination with other cell types, substrates, or technologies. Progress with pluripotent cell lines suggests a future in which manufacturing will no longer require starting from donor tissue.
RPCs are not specific to RP as an indication. A wide range of retinal and optic nerve diseases could benefit from effective neuroprotection. Even where treatments are now available, such as exudative AMD and diabetic retinopathy, current long term clinical outcomes suggest ample room for improvement.
5.2. Palucorcel
Janssen Research and Development LLC sponsored an open-label phase I/IIa trial (clinicaltrials.gov identifier NCT01226628) to evaluate the safety and tolerability of the subretinal administration of hUTC (known as CNTO-2476) in patients with visual impairment due to GA secondary to dAMD. CNTO-2476, or palucorcel, comprises hUTCs in a proprietary formulation. The therapeutic principle behind this study was that the subretinal hUTC would preserve or promote RPE function, and possibly neural retinal function, through a paracrine mechanism (Koh et al., 2018; Lund et al., 2007). Non-injected fellow eyes served as controls.
5.2.1. Delivery protocol
The cells were cryopreserved prior to delivery. The delivery of the cells into the subretinal space was through a surgical procedure using a novel device, the iTrack model 275 microcatheter (iScience Interventional Corporation, USA) that was inserted via a peripheral scleral cutdown. This type of device was originally designed for the treatment of glaucoma by canaloplasty (Lewis et al., 2009, 2011) – it was inserted into the front of the eye and placed into Schlemm’s canal to deliver viscoelastic material directly into this space. The device includes an innovative lighted tip so that the surgeon can use this beacon to detect its position inside the eye and therefore be able to guide it appropriately.
A different model, the iTrack model 400 microcatheter, was used for suprachoroidal delivery of non-cellular medications for advanced exudative AMD in another clinical trial, and the use of this device was successfully accomplished in all eyes without significant surgical complications (Tetz et al., 2012). However it should be noted that the goal in this study (Tetz et al., 2012) was to deliver the therapeutics into the suprachoroidal space, not the subretinal space – the latter target requiring penetration of the choroid, a maneuver that in principle could increase the risk of internal bleeding as the choroid is highly vascular. The palucorcel study targeted the subretinal space.
It is useful to review the surgical procedure in detail here as this component of the study appeared to be the main source of safety concerns in this clinical trial rather than the actual cell therapy product. The authors describe a purely ab-externo procedure without vitrectomy. In this procedure, a moderate-sized (measuring approximately 3 mm long and positioned 8–11 mm posterior to the corneoscleral limbus) peripheral scleral cutdown was made, followed by choroidotomy which is an incision or penetration through the vascular choroid layer. Then, a subretinal bleb was created with a wire-tip microcannula and sodium hyaluronate viscoelastic injection (Healon, Abbott Medical Optics, USA).
5.2.2. Outcome data
A total of 35 participants underwent at least a partial surgical procedure as reported by the authors in their 2017 publication of the study results (Ho et al., 2017) and the cellular dose range was 60,000 – 300,000 cells per administration. The cell product was successfully administered in 33 subjects.
In this study, a high rate of complications related to the delivery procedure was reported: six (17.1%) retinal detachments and 13 (37.1%) retinal perforations, likely resulting in off-target delivery in these cases. The authors commented that the rates were unacceptably high. The retinal perforations were most often located anteriorly, at the site of the subretinal bleb induction. Retinal detachments are a known consequence of untreated retinal breaks or perforations, and can lead to proliferative vitreoretinopathy (PVR) in severe cases. PVR is analogous to a severe retinal scarring response which can cause recurrent retinal detachments (Machemer et al., 1991; Pastor, 1998). PVR occurred in two (5.7%) patients, raising some concern of a possible link with the injected cells. Retinal detachment and retinal perforations were considered as severe adverse effects (AE). Overall, the authors’ analysis was that the majority of AEs were reasonably judged to be related to the eye surgery or surgical delivery system (including the iScience microcatheter and ancillary devices used in the surgical procedure).
Retinal perforations could occur at anterior or posterior locations. Per the report, “if a retinal perforation posterior to the equator was observed, the investigator was not to inject palucorcel. For retinal perforations observed anterior to the equator, injection of the cells was based on the investigator's clinical judgment.” Presumably, this caution was instituted because perforations that occurred posterior to the equator could lead to off target cell product delivery into the vitreous cavity, or further retinal damage, if the injection procedure was carried out.
Initially, visualization of the inside of the eye and target surgical zone was achieved with indirect ophthalmoscopy. After the first ten subjects had received palucorcel, the investigators introduced a surgical modification consisting of ophthalmic endoscopy (Endo Optiks E2 System, Beaver Visitec, USA) that facilitated better internal visualization. They noted a reduction in the rate of retinal detachment but an increase in the rate of retinal perforation – the latter likely due to improved detection of anterior perforations as a result of the endoscopic examination. Interestingly, in some subjects, follow up retinal examination revealed the presence of catheter tracks and linear fibrosis along the route of travel of the iTrack microcatheter. The authors stated that “the iTrack microcatheter delivery system was not adequate for continued development’ (Ho et al., 2017) and that improvements in the surgical methods are in evolution.
Histological data regarding an epiretinal membrane removed from a patent that received CNTO-2476 ERM indicated the presence of both subject (self) cells and palucorcel (Spencer et al., 2017). This suggested that that reflux of CNTO-2476 into the vitreous cavity after retinotomy may have contributed to ERM and RD development in this case.
Encouragingly, no significant immune response or rejection was observed in eyes that received palucorcel, indicating that the cell product did not provoke a significant reaction even though there was no protocol-specified systemic immunosuppression included in this study. There was no tumor formation. The presence of serum antibodies were detected in several patients but these did not correlate with ocular AEs or inflammation events. In terms of visual acuity results, at month 12, the median BCVA change was 4.5 letters (range, −41 to 32) in the intervention eyes and −0.5 letters (range, −30 to 15) in the fellow control eyes. More than 30% of subjects gained ten or more letters in BCVA at months 3 though 12. These visual acuity results should be interpreted in the context of the absence of masking this study. In terms of anatomical outcomes, the intervention did not seem to alter the growth rate of GA lesions when analyzed as a group.
Overall, this study appears to describe a cell product that is reasonably safe and well-tolerated as a single-dose therapy. The cell product also has potential for positive biological effects: where the cells were contained in the subretinal space, the encouraging results suggested a advantageous effect on the retinal degenerative disease process as CNTO-2476 conferred potential visual function improvements in certain patients (Ho et al., 2017). However, the surgical delivery method could be further optimized so that sight-threatening complications are minimized as far as possible.
5.3. UC Davis
The team of researchers at the University of California Davis has successfully made the transition from bench to clinic exploring the retinal regenerative potential of intravitreal injection of human CD34+ stem cells harvested from bone marrow. This was accomplished by completing all preclinical safety studies as requested by the Food and Drug Administration (FDA) and completing a new investigational drug (IND) application for clearance from the FDA.
5.3.1. Background data
The investigators selected CD34+ cells from bone marrow as the target cell therapy since these cells are known to be mobilized into the systemic circulation from bone marrow in response to tissue injury as part of the normal repair mechanism of the body (Asahara et al., 1997; Park, 2016). They play an active role in tissue revascularization following ischemia and appear to have regenerative effects beyond ischemic injury (Mackie and Losordo, 2011; Park et al., 2017; Vrtovec et al., 2013). By introducing these repair cells directly into the eye via intravitreal injection, the goal is to enhance the normal repair mechanism of the body. The investigators adopted the idea of exploring intravitreal injection of CD34+ cells to treat retinal disorders based on the excellent safety profile of using these cells in clinical trials for cardiomyopathy and reported potential efficacy as detailed in Section 2.5.3 above.
The long-term ocular and systemic effects of intravitreal injection of human CD34+ cells from bone marrow was studied in NOD-SCID mice with acute retinal ischemia-reperfusion injury (Park et al., 2012). Some of these injected human cells were noted integrated into the mouse retinal vasculature long-term without any abnormal cellular proliferation in the eye or in any major organ of the body. An apparent normalization of the murine retinal vasculature was noted long-term.
The FDA cleared the IND application for intravitreal injection of autologous CD34+ stem cells isolated from bone marrow so that a clinical trial could be started exploring this cell therapy as a potential treatment for vision loss associated with ischemic or degenerative retinal conditions. Autologous cell therapy was chosen by the investigators since it avoids the use of systemic immunosuppression which can have significant systemic side-effects (Schwartz et al., 2015b). CD34+ cell treatment is being explored in a clinical trial for several different ischemic and degenerative retinal disorders since preclinical studies indicate that the therapeutic effects of the CD34+ stem cells are not limited to a particular disease and can have broad clinical applications (Park et al., 2017). These cells can regenerate tissue via direct engraftment and paracrine trophic effects and these effects do not appear to be disease-specific (Vrtovec et al., 2013). Preclinical studies have shown protective and potential regenerative effects of intravitreal injection of human CD34+ cells or bone marrow hematopoietic stem cells on ischemic or degenerating retina in various animal models (Caballero et al., 2007; Moisseiev et al., 2016; Otani et al., 2004; Park et al., 2012).
5.3.2. Study design
A pilot single-center clinical study was started at UC Davis exploring the safety and feasibility of intravitreal injection of CD34+ cells isolated from bone marrow (clinicaltrials.gov identifier NCT01736059). To date, the study has enrolled and treated nine eyes from nine subjects with persistent vision loss from various retinal conditions. They include hereditary or nonexudative age-related macular degeneration, retinitis pigmentosa, retinal vein occlusion and diabetic retinopathy. As a phase I study, this is an open label study designed to determine safety and feasibility of this experimental treatment. As such, only one eye with the worse visual acuity is treated with the experimental cell therapy and the vision loss in this eye has to be longstanding and moderately severe (best corrected visual acuity of 20/100 or worse or visual field constricted to within 10 degrees). Individuals on systemic immunosuppression are excluded from study enrollment since such treatment will alter the bone marrow. Pregnant women are excluded to avoid unknown risks to the fetus. Children and prisoners are also excluded since they are considered vulnerable populations.
5.3.3. Outcome data
The results of the phase I study showed that intravitreal injection of autologous CD34+ cells was feasible and not associated with major safety concerns. The findings of the first six eyes from six subjects enrolled and treated in this open-labeled prospective study has been published (Park et al., 2014). Three additional subjects have been enrolled and treated without any safety or feasibility concerns since the 2014 publication. The bone marrow aspiration was performed in an out-patient setting and under local anesthesia by an experienced hematologist. The procedure was well-tolerated by the study subjects. A desired number of CD34+ cells could be isolated from a single bone marrow aspirate. The CD34+ cell isolation and enrichment was performed under Good Manufacturing Practice (GMP)-conditions in a certified laboratory within the University of California Davis Institute for Regenerative Cures. The cells were isolated by positive selection from the monocular cell fraction of the bone marrow aspirate using a magnetic cell sorter device which is approved by the FDA for clinical applications. All harvested cells passed the release test and post-release analysis for sterility and viability. The phenotype of the harvested CD34+ cells was confirmed by flow cytometry. The isolated CD34+ cells were resuspended in a small volume of saline for intravitreal injection. The intravitreal injection was performed promptly and on the same day as the bone marrow aspiration. The injection was performed by a vitreoretinal specialist in the eye clinic. It was well-tolerated by all study subjects. No adverse effects have been noted during the six-month follow-up period of the study and during the extended follow-up as part of standard of care.
Although the phase I study is not designed to evaluate for efficacy, varying degrees of visual gain was noted in many treated eyes. In some subjects, high resolution in vivo retinal imaging instruments were used to visualize cellular changes within the retina following cell therapy. Adaptive optics-optical coherence tomography imaging showed subtle changes within the retina at a cellular level in some of the treated eyes consistent with intraretinal homing and integration of injected cells (Park et al., 2014). Among the treated subjects in the study, the subject with persistent vision loss from a central retinal vein occlusion had the greatest improvement in vision after this cell therapy. Visual acuity improvement was noted within one month following cell therapy. Marked resolution of retinal hemorrhages and microvascular changes were noted on fundus photography and fluorescein angiography by three months following CD34+ cell therapy in this eye with retinal vein occlusion, although such changes may also occur in the natural history of untreated central retinal vein occlusion. The improvement was sustained for the duration of follow-up.
5.3.4. Future work
The National Eye Institute will sponsor a phase I/II randomized prospective double-blinded sham control study to study further the safety and potential efficacy of intravitreal autologous bone marrow CD34+ cell therapy in treating retinal vein occlusion. This single-center study will be called the TRUST study (Treatment of Retinal vein occlusion Using STem cells). The study will be conducted at UC Davis. The study will be conducted under a new IND that has been cleared by the FDA and cross-referenced to the original IND for this cell therapy. Study enrollment is anticipated to start in early 2019.
The TRUST study will enroll a total of 20 eyes from 20 subjects with persistent vision loss from retinal vein occlusion. Best corrected visual acuity in the study eye must be 20/60 to counting fingers from retinal vein occlusion to qualify for enrollment. Vision loss must have persisted for over 6 months. An eye that received other standard of care therapies for retinal vein occlusion within 6 months before study enrollment is excluded. The double cross-over design of the study enables all enrolled subjects to receive CD34+ stem cell treatment by month six while maintaining masking for the duration of the study. Each subject will be followed for 24 months. The primary endpoints of this study are (1) the incidence and severity of ocular and systemic adverse events associated with the intravitreal CD34+ stem cell treatment and (2) the mean number of CD34+ cells isolated from bone marrow aspirate for intravitreal injection. The secondary endpoints of the study will include changes in retinal function and morphology following stem cell treatment as gauged by various diagnostic tests and imaging instrumentation. Although the study is small and may not powered to detect treatment efficacy, the various diagnostic tests and subjective and objective measures of retinal function provide opportunities to detect treatment trends that could be used to design a larger future clinical trial. The results of this prospective study will provide a framework for a potential larger clinical trial powered and designed to evaluate treatment efficacy.
5.3.5. Potential limitations
The potential pitfalls of this autologous CD34+ cell therapy are host factors that may affect the regenerative potential of the cells (Park et al., 2017). Chronic diseases such as diabetes mellitus and cardiovascular risk factors have been shown to affect the quality and quantity of these cells mobilized into the systemic circulation (Vrtovec et al., 2016). These host factors may also affect unmobilized CD34+ cells in bone marrow. Fortunately, research shows that the effect of these host factors on CD34+ cells can be modified; various methods are being explored by researchers (Park et al., 2017).
Another potential limitation of autologous CD34+ cell therapy is that the CD34+ cells represent a mixture of cell subclasses (Park, 2016). Research is on-going to better characterize these subclasses. Whether further isolation and expansion of a “target cell” will result in a more robust regenerative therapeutic response than using CD34+ cells is unknown and this is another area of ongoing research. Based on data from ischemic and non-ischemic cardiomyopathy clinical trials, clinical response to cell therapy appears to correlate with number of CD34+ cells administered in the cell treatment (Vrtovec et al., 2013).
5.4. Astellas
Schwartz et al. were the first to transplant hESC-derived RPE into the subretinal space of human patients with dAMD and Stargardt disease (Schwartz et al., 2012a).
Because of concerns for genetic alterations in iPSCs (Lister et al., 2011; Ohi et al., 2011), hESC were used as the source cell to differentiate into clinical grade RPE cells. The MA09 hESC line was expanded in culture and allowed to differentiate into embryoid bodies with pigmented RPE cells. The pigmented cells formed hexagonal monolayers and were tested extensively for pathogens, chromosomal rearrangements, purity, phagocytosis (functional) assays, and differentiation studies using quantitative PCR and immunostaining.
The preclinical safety and efficacy analysis was done in three animal models (Lu et al., 2009), In Royal College of Surgeons (RCS) rats and Elovl4 mutant mice, the hESC-derived RPE cells were injected subretinally to confirm integration of the transplanted human RPE in these models of retinal degeneration. Visual function and light sensitivity thresholds improved without evidence of tumor formation or inflammatory response. Furthermore, transplantation of hESC-derived RPE in National Institutes of Health III immune-deficient mice showed no evidence of teratoma formation or metastasis.
5.4.1. Study design considerations
Two important anatomic considerations were carefully incorporated into the design of the study and are worth noting here. First, although dAMD is far more prevalent than Stargardt disease, patients with the latter were incorporated into the Phase I/II trial in order to determine the effect of Bruch membrane and choriocapillaris health. In advanced dAMD, there is known to be senescence of Bruch membrane and thinning of the choriocapillaris which not only plays a role in the pathophysiology but also may impact the relative survival, engraftment, and polarization of the transplanted cells (Ardeljan and Chan, 2013). By including patients with SD, who are in general much younger than dAMD patients, the relative effect of a diseased Bruch/choriocapillaris complex can be partially addressed by studying the transplanted cells in both disorders – although it must be noted that significant choriocapillaris attenuation is known to occur in relatively young SD patients (Battaglia Parodi et al., 2017).
The second consideration was the intended delivery site of the cells. The investigators sought to treat an area adjacent to the central macula—a transition zone between the relatively healthy retina and the severely atrophic fovea (Schwartz et al., 2016). This area has already been compromised by the disease process and in some ways recapitulates the state of the macula in earlier stages of the disease. Early-stage disease may represent the eventual target for treatment with cellular therapies. It is unlikely that visual recovery of the atrophic central macula can be achieved solely with transplanted RPE cells when there is substantial atrophy of other surrounding tissues such as the choriocapillaris and photoreceptors.
5.4.2. Immunosuppression and other safety issues
A major concern with subretinal transplantation of hESC-derived RPE cells is the potential for immunologic response to the cells. This immune response could not only eliminate the transplanted cells but also incur collateral damage to neighboring host cells. The immunosuppression dose used by Schwartz et al included tacrolimus and mycophenolate mofetil one week before and up to 6 weeks after the treatment, followed by mycophenolate mofetil alone for an additional 6 weeks (Schwartz et al., 2015b). Importantly, no subretinal inflammation was observed in any of the patients treated. However, the tolerability of this immunosuppressive regimen in elderly patients was linked to the majority of systemic adverse events observed in the pilot studies. These included one urinary tract infection and two non-melanoma skin cancers. The degree to which transplant-dose immunosuppression is necessary must be analyzed in future trials. In addition, the use of autologous iPSCs or HLA-matched iPSC could be considered as potential solutions to avoid rejection and immunosuppression issues (Ardeljan and Chan, 2013; Schwartz et al., 2012a; Schwartz et al., 2015b; Schwartz et al., 2016).
There was no evidence of teratoma formation or hyperproliferation. The only serious complication was a case of Staphylococcus epidermidis endophthalmitis in a Stargardt disease patient that was noted in the immediate postoperative period and resolved with medical management. Although three eyes had preretinal pigmentation at the injection site, none of the eyes developed epiretinal membranes or vitritis.
The hESC-derived RPE delivery by Schwartz et al was performed via subretinal injection of a cellular suspension. Because this technique is the least challenging, it shifts safety and efficacy concerns to the transplanted cells rather than the surgical technique. Furthermore, the use of a scaffold introduces an additional experimental variable that reflects the material used, its dimensions, the delivery device, biodegradability, and permeability aspects (George et al., 2017).
5.4.3. Outcomes of hESC-derived RPE transplantation studies
When interpreting the results of the Phase I/II studies, it is important to keep in mind that the studies were designed as first-in-human safety studies. It was a multi-center study including nine patients with dAMD (median age: 77 years) and nine patients with Stargardt disease (median age: 50 years). There were three dose cohorts ranging from 50,000 cells to 150,000 cells. The primary endpoint was safety of the hESC-derived RPE cells in these two patient populations.
Secondary endpoints included visual acuity, visual fields, ophthalmoscopy, optical coherence tomography (OCT), fundus autofluorescence, fluorescein angiography, and electroretinography. Serial physical examinations and blood testing was performed by an internist.
The hESC-derived RPE cells were well tolerated without any adverse events related to the cells themselves. 13 out of 18 patients displayed subretinal pigmentation—potential evidence of cellular integration and survival. This pigmentation occurred at the border of the atrophy (transition zone) with some degree of thickening of the RPE layer on OCT. It was not possible to exclude that the pigmentation may have included pigment-laden macrophages. However, definitive structure-function correlations could not be performed without the use of adaptive optics imaging, microperimetry, or a labeling technique for the cells.
Visual outcomes in these pilot studies must be interpreted with caution. The study was small and included patients with advanced disease and no masked controls. Numbers were too small for meaningful statistical analysis (Hanley and Lippman-Hand, 1983). Furthermore, visual acuity measurements can be difficult in patients with advanced geographic atrophy (Sunness, 2015). However, among the nine patients with dAMD, BCVA at six months improved by up to three lines or remained stable without any eyes losing vision. Among the eight eyes of Stargardt disease patients, seven eyes gained up to three lines of vision or remained stable. One eye lost two lines of vision.
These results from the first-in-human use of hESC-derived RPE for treatment of two forms of macular degeneration are encouraging and have set the foundation for future trials. The next step is to perform randomized multicenter trials using more advanced imaging and functional measures to better ascertain efficacy. These studies should also define the ideal state of the disease in which to intervene, which may involve studying eyes with mild to moderate vision loss and no geographic atrophy.
5.5. RIKEN
5.5.1. iPSC as a source for RPE
Hirami et al. (2009) were the first to successfully produce retinal cells from mouse and human iPSCs by differentiating in vitro using a serum- and feeder-free method to avoid the risk of adverse immunologic responses and potential exposure to xenopathogens (Hirami et al., 2009). Jin et al. (2011) reported the production of intrinsic patient-specific iPSCs from patients with distinct mutations in the RP1, RP9, PRPH2, or RHO genes that recapitulated the disease feature in vitro (Jin et al., 2011). These disease modeling techniques have contributed greatly to the elucidation of the retinal degeneration pathology.
The morphological and functional features of iPSC-RPE for its clinical adaptation have been reported. Kamao et al. (2014) assessed the quality, quantity, consistency, and safety of clinical-grade hiPSC-RPE sheets (Kamao et al., 2014), and demonstrated that iPSC-RPE formed tight junctions, secreted growth factors, and showed phagocytotic ability and gene-expression patterns akin to those of native RPE. Maeda et al. showed that iPSC-RPE possesses functional visual cycle enzymes in vitro and in vivo. Moreover, they reported that transplantation of iPSC-RPE into Lrat−/− and Rpe65−/− mice rescued vision in these animals (Maeda et al., 2013). These RPE cells expressed typical RPE markers and showed secretory ability and physiological activity similar to those of native RPE in vivo and thus can be readily used as cells for transplantation. Hirami et al applied the method to human iPSCs (Hirami et al., 2009). Kamao et al. evaluated the human iPSC-RPE cell sheet as preclinical study (Kamao et al., 2017).
5.5.2. Conceptual framework for regulation and safety
For cell therapy, there are two strategies: one is replacement of damaged tissues and the other is using trophic effect of donor cells. The requirement of the donor cells will be different for each strategy. For a trophic effect, donor cells can be immature and few in number. But for replacement therapy, one should prepare sufficient numbers of fully functional cells. Several clinical trials are ongoing using intravenous injection of mesenchymal stem cells. From a regulatory perspective, those cells are treated like drugs because the route to reach to the lesion and the potential systemic influence is similar to drugs. Another style is cell therapy that requires surgery. The effects of these donor cells are usually local and therefore the procedure should be modified and improved constantly like usual surgeries.
Safety is the most important issue for the first clinical application of iPSC cell therapy. The following risks should be considered:
Cell risk – Tumorigenicity, gene mutation, contamination, etc.
Treatment risk – Complications of local and systemic immune suppression, surgical complications, donor cell reaction to the host environment, etc.
Disease risk – Deterioration risk of disease without treatment.
It is common for observers to consider only the cell risk. The small number of cells (e.g. 1×105) that are sufficient for retinal cell transplantation reduces the chance of tumor-forming cells contaminating the donor cell preparation. However, as distinct from small molecule drugs, cell therapy entails surgery and additional surgery-related complications. The reported complications include epiretinal cells or membranes with both ESC and iPSC delivery, retinal detachment and endophthalmitis with ESC-RPE delivery, diarrhea, pneumonia, protrusion of the slow release steroid as immunosuppression-related issues, and proliferative vitreoretinal retinopathy with mesenchymal cell delivery.
Contrary to the common view that somatic cells are the safest option, it can be seen that mesenchymal cells have caused the most severe complications of retinal cell therapy so far (Kuriyan et al., 2017). The safety profile of iPSCs is high because this treatment approach would not need immune suppression when applied autologously and could be the safest option to use for older patients. From these points of view, the RIKEN team applied a protocol of the first iPSC clinical research to the ethics committee in 2012, five years after the invention of human iPSCs.
The iPSC-RPE transplantation effort using human iPSCs was fast-tracked for its clinical use in patients with AMD for the following reasons: 1) the cellular functions and reproducibility of iPSC-RPE have been well described, as mentioned above; 2) the number of cells required for treatment is relatively small; 3) iPSC-RPE shows unique pigmentation during differentiation, which is useful for the identification, purification, and evaluation of these cells (Kamao et al., 2014); 4) clinical studies of human ESC-derived RPE for dry AMD had already been performed (Schwartz et al., 2012a; Schwartz et al., 2015a); and 5) RPE is an especially safe cell type and rarely forms tumors.
5.5.3. Production of iPSC-RPE for clinical application
The production of iPSC-RPE for clinical application requires strict quality control and accordance with good manufacturing practice (GMP). The GMP ministerial ordinance, issued by the Japanese government, stipulates the maintenance of both product safety and manufacturing.
In the production of RPE sheets for this clinical study, all culture reagents were traceable and secured, and a clinically usable manufacturing practice was adopted to ensure that the differentiation-induction process was suitable for clinical use. For iPSCs culture, a cell processing facility with a GMP-compliant cell regulation room was used. All procedures were checked by personnel from three divisions at the investigators’ institution: 1) the cell culture department; 2) the quality control department; and 3) the facility management department. All processes including cell culture, quality control and facility management were recorded in standard operation procedure (SOP) documents (Takahashi, 2016).
5.5.4. Cellular safety and quality assays
It is important to consider the heterogeneity that is present in a single colony of stem cells. It is well known that different stem cell lines have different characteristics. However, the truth is that different characteristics exist even amongst heterogeneous sub-populations in a single colony within the same protocol. For example, if several genetically mutated cells exist in the iPSC/ESC colony, they may proliferate faster than normal cells and will dominate in various ratios in the cell line. Thus, even in the working cells at a certain passage number that are expanded from the same master cell bank, the population is probably different over time. With this in mind, safety must be ensured with a robust protocol including a purification process for the elimination of tumor-forming cells. The investigators considered this discrepancy from the beginning of this project and ensured purification by picking up only the pigmented cell clusters at the end of cell production to cancel the developmental differences among cell lines from various patients. It is especially important to plan the autologous iPSC-derived cell transplantation, because the difficulty of generating consistently good iPSCs and the varying efficiency of RPE differentiation from patient to patient.
Two lines of patient-specific iPSCs were generated using non-integrating episomal vectors and were differentiated into RPE cells as described previously. Quality control for safety was tested as follows:
PCR virus check: negative
Immunocytochemical purity: 100%
In vivo tumorigenicity test: Negative
- Genomic analysis:
- qRT-PCR for Lin 28: negative (<0.01%)
- Karyotype: Normal
- Plasmid fragment remnant check: Whole genome sequence (WGS), qRT-PCR, capture sequence
- Copy number variation: SNP array
- Mutations in the driver genes: WGS
- Epigenetic analysis: Methylome analysis
- Purity: Single cell RT-PCR – RPE marker 100% and iPSC marker 0 %
Lin28 was negative (less than 1/50000 cells) by qRT-PCR. 100% of the cells for the first patient showed RPE markers, and 0 % showed iPSC markers by single cell RT-PCR. By microarray analysis, the 10 lines from three individuals with two different iPSC generation methods and two different RPE differentiation methods showed similar expression patterns of RPE signature genes. Cluster analysis showed those 10 lines clustered together near the cluster of fetal RPE cells, whereas RPE19 data were situated distant from those clusters. CAGE transcriptome analysis of 18,000,000 genes showed that the discrepancy between the iPSC lines and among the iPSC-RPEs were less than that between individual skin fibroblasts. Thus, the team was confident that they could make RPE cells of the same quality from different individuals; however it is difficult to make same good quality iPSCs from each individual.
The greatest risk of cell transplantation is tumor formation. There always exists the risk of gene mutation at each cell division. RPE cells were chosen as the target cells because they seldom form tumors. There is no report about a metastatic tumor derived from RPE even in familial tumor patients who have various cancers with hereditary oncogene (e.g. p53) mutations. This means even with an established oncogenic mutation, RPE cells do not generate metastatic malignant tumors. This was viewed as a big advantage for the first clinical application of iPSCs compared to other type of cells in the body.
In 2013 and 2014, the Kawamata group reported a highly sensitive method to detect residual human iPSC based on qRT-PCR (Kuroda et al., 2012) and confirmed that iPSC-RPE possessed negligible tumorigenic potential, as shown by the results of subcutaneous tumorigenicity test using Matrigel and subretinal tumorigenicity test in NOG mice and nude rats, respectively (Kanemura et al., 2014). The same group also reported that PEDF secreted from primary RPE and iPSC-RPE induced apoptosis in iPSC, indicating that the tumor-forming potential of iPSCs can be suppressed by the simultaneous transplantation of iPSC-RPE (Kanemura et al., 2013). Moreover, the inside of the eye abounds with retinoic acid, which has antitumor activity, and there has been no report of RPE tumor growth in the eye. Taken together, these findings suggested that RPE is a particularly safe cell type that seldom forms tumors, especially under a non-permissive environment for tumor formation. The safety of iPSC-RPE cells was further confirmed by conducting a series of tumorigenicity tests in immunodeficient mice for one year.
5.5.5. First clinical application of iPSC for AMD
The first clinical study of iPSC-RPE for neovascular AMD was initiated in August 2013; the patient was enrolled in November 2013 and received the iPSC-RPE sheet transplant for neovascular AMD in September 2014. The primary endpoint was the validity of the overall protocol of autologous iPSC-RPE sheet transplantation, namely, the safety of iPSC-RPE as a graft source and the procedure of transplantation of an RPE sheet prepared in vitro. The secondary endpoints of clinical efficiency including the retinal morphology and visual function after surgery was also addressed.
The patient was a 77-year-old Japanese woman diagnosed with polypoidal choroidal vasculopathy (PCV, a subtype of neovascular AMD). She had received repeated intraocular injections of an anti-VEGF drug before the surgery and presented a gradual decrease in visual acuity for 5 years.
The patient underwent surgery to remove the neovascular membrane and received transplantation of the autologous iPSC-derived RPE cell sheet under the macular area (the center of visual function), using a surgical device consisting of a custom-designed hand piece and a cannula (Kamao et al., 2017). The surgery was successful and no major bleeding or other serious adverse events were observed. After the surgery, the graft sheet initially curled on its margin, but gradually flattened and the graft area gradually expanded until 8 weeks post-surgery. At 2 years following the surgery, the transplanted sheet remained intact as detected by both fundus photography and optical coherence tomography (OCT). These findings indicated successful engraftment without rejection (Mandai et al., 2017b).
A previous report showed that autologous iPSC-derived cells can cause immune-mediated rejection in mice (Zhao et al., 2011); however, the patient in this study did not show any sign of tissue rejection even without the use of immunosuppressant drugs. Nevertheless, the RIKEN team advise caution in extrapolating the findings for other types of iPSC-based transplantation, especially because iPSC-derived RPE cells have been shown to inhibit T-cell activation (Sugita et al., 2016b). It is not clear if other iPSC-derived tissues do the same.
For the patient in this study, there was no improvement in post-operative best corrected visual acuity that was maintained at approximately 0.1 (equivalent to Snellen 20/200) throughout the follow-up period. The VFQ-25 score increased from 48.8 preoperatively to 58.3 after 1 year. Although the patient’s diagnosis and disease stage precluded significant visual gains in this safety study, BCVA was stabilized with improvement in the VFQ score, and the patient expressed satisfaction with “brighter” vision, which was probably due to the removal of the CNV membrane. She also benefited from requiring no more anti-VEGF injections after surgery up to the present.
OCT presented good retinal integrity over the graft over 1 year after the transplantation with a highly reflecting signal that may have indicated recovering photoreceptor inner/outer segments. There were no serious complications accompanying the surgical protocol. The central retinal thickness was reduced by removal of the neovascularization with no recurrence so far.
Thus far, there has been no tumor formation; however, the long-term safety of this treatment must be further assessed. It should be emphasized that the findings from a single case cannot fully clarify the risk or benefit associated with this procedure.
A CD34-positive vascular rich CNV membrane was found despite the repeated injections of anti-VEGF drugs. This, and the reappearance of fluid immediately after the cessation of anti-VEGF treatment before the surgery, suggested that CNV removal was a reasonable therapeutic decision in this case.
Photoreceptor function over the grafted RPE is still to be determined in a longer observation of the patient or in future studies. At 3 year after surgery, the transplanted sheet survived well with no sign of rejection, and no serious adverse event including tumor formation had been observed. Photoreceptors and choroidal vessels were maintained only in the area adjacent to the grafted sheet.
5.5.6. Concerns of tumor formation
The major concern about cell-based therapies including those using iPSC is possible tumorigenicity. The investigators used a framework to classify safety levels of cell types as follows:
Category I : Graft cells that disappear after several months
Category II: Graft cells that survive in the long term but proliferate only several times in vivo
Category III: Graft cells that survive in the long term and continue proliferation
For categories I and II, it is important to rule out contamination of the graft with proliferating cells, and in vivo tumorigenicity tests are most important for this purpose. For category III, that applies for the transplantation of progenitor cells themselves, genomic analyses are important because continuous cell proliferation may induce genomic aberrations after transplantation and result in tumors even with negative short-term in vivo tumorgenicity tests. Since iPSC-RPE transplantation belongs to category II, after confirmation of tumorigenicity in vivo for short duration, genetic mutations are not anticipated to pose a concern with regards to the late onset tumor formation. Strict in vivo tumorgenicity tests were performed using NOG mice, the most competent mouse strain that accepts human cells, together with a Matrigel-embedded transplantation procedure, which allows ~10000 fold higher sensitivity than conventional method. For category III, cancer genes in the donor cells should be screened because a second hit gene mutation may occur during continued cell division after transplantation in the host. Following the conceptual framework above, the RIKEN investigators were not overly concerned about genetic alterations that were detected in the iPSC-RPE of Patient 2 (see below) after the 3 negative tumorigenicity tests. However, the ethical committee recommended that the transplantation be halted.
Patient 2 was a 68-year-old male with PCV. The iPSC and iPSC-RPE of Patient 2 fulfilled all the standard requirements. Being the first clinical trial, genomic analyses including whole-genome and whole-exome sequencing were performed to assess genome integrity, focusing on alterations that could reinitiate the cell cycle in RPE cells such as the activation of protooncogenes or inactivation of tumor suppressor genes. Driver mutations in the iPSC-28 or iPSC-RPE of Patient 1 were not detected. However, for Patient 2, one copy loss of two genes on autosomes (loss of one copy of the YAF2 gene on chromosome 12 and of the SNRPN gene on chromosome15) and a deletion of another gene located on the X-chromosome (loss of the normal copy of the STS coding gene) in both the iPSC and iPSC-RPE, but not the parental fibroblasts, were detected. The cells lacked a normal copy of the STS gene because the patient was male (XY). Since high sensitivity analysis by deletion allele-targeting PCR could not detect these deletions in the founder fibroblasts, it seemed the genomic change occurred during the reprogramming. Furthermore, there have been no reports indicating that the discovered copy number alterations (CNAs) have roles in tumorigenicity. However, in our experiences, few, if any, iPSC clones possess de novo CNAs that affect protein-coding regions. Therefore, these CNAs postulated a possible alert.
Transplantation of RPE has several advantages with regard to a relatively low risk of tumorigenicity:
a small number of cells are transplanted,
no metastatic tumors originating from RPE cells have been ever reported, and
the direct observation of the grafts on the fundus or with OCT at 7-μm resolution will immediately detect unexpected proliferation
Thus, one could argue that postponing the surgery for Patient 2 because of CNAs was unduly strict. Although all the vivo tumorigenicity tests were negative for the iPSC-RPE cells prepared from Patient 2 even with those genetic changes, the transplantation was suspended because of the relatively moderate activity of the neovascular membrane and stable visual acuity under anti-VEGF therapy. Further studies and discussion are required on how to apply genomic analyses for evaluation of iPSC-derived cells for clinical use.
In this study, the first-in-man application of iPSC-derived cells was demonstrated and the study’s endpoint – validation of the therapeutic protocol from iPSC-RPE generation to clinical competency – was met. Simultaneously, challenges for the future were highlighted including the need for a consensus on the criteria for genome integrity and the possible limits large-scale practicality of autologous transplantation related to this issue. Although the study demonstrated that autologous iPSC-RPE transplantation can be done safely, the time and expense to prepare the graft and to ensure its safety were immense.
5.5.7. Allo-transplantation using iPSC-RPE
As a next step, the investigators are now pursuing allo-transplantation using iPSC-RPE for AMD patients with matched HLA. Presently, the first 6-loci homologous iPSC cell line bearing the most frequent HLA combinations are being established to cover appropriately 19% of the Japanese AMD population, based on recently published data regarding haplotypes in this specific population (Takagi et al., 2018). With this strategy, the aim is for efficient clinical trials that cover a larger number of subjects.
The host immune response to the transplanted cells must be thoroughly examined for allogeneic grafts, and the importance of HLA-matching in transplantation therapy has been demonstrated in hematopoietic stem cell transplantation (Lee et al., 2007). New laws for regenerative medicine in Japan were enforced in 2015, necessitating the closure of the initial protocol. At that time the investigators concluded that HLA 6-loci matched iPSC-RPE allogeneic transplantation would not cause immune rejection by in vitro and in vivo studies and moved to the allogeneic clinical research.
The team first investigated the presence of immune responses in in vitro and in vivo HLA-matched models. In vitro, iPSC-derived RPE cells express HLA class I/II antigens, but T-cells do not respond to HLA-A, -B, and -DRB1-matched iPSC-derived RPE cells from HLA homozygote donors (Sugita et al., 2016b). Moreover, in a mammalian in vivo model, no sign of tissue rejection was observed in MHC-matched iPSC-derived RPE allografts in the absence of immunosuppression. In contrast, immune response was detected around the graft and retinal tissue damage in the MHC-mismatched model (Sugita et al., 2016a). Based on these findings, the investigators hypothesized that iPSC-RPE from MHC homozygous donors can be used to treat retinal diseases in histocompatible recipients.
The Center for iPSC Research and Application (CiRA) of Kyoto University has provided guidance for this study including access the Japanese bone marrow bank to create iPSCs with common HLA in homozygous Japanese population. Using this resource, the team is currently performing larger, more efficient, and more informative clinical trials.
5.6. Regenerative Patch Technologies
The RIKEN study (Mandai et al., 2017b) entailed the use of a cellular sheet without an extrinsic scaffold; however, an alternative approach is to specifically address the degenerative changes in native Bruch membrane in AMD patients by including a synthetic Bruch membrane replacement as part of a composite implant. Kashani and colleagues at the University of Southern California Roski Eye Institute recently reported the interim results of a Phase 1/II clinical trial (clinicaltrials.gov identifier NCT02590692) evaluating a composite implant of stem cell derived RPE and such a synthetic substrate in the worse-seeing eye of subjects with advanced dAMD (Kashani et al., 2018). The study was sponsored by Regenerative Patch Technologies.
5.6.1. Study design and scaffold
The RPE cells derived from human ESCs (derived from the NIH–H9 line), coupled with a nonbiodegradable parylene membrane substrate, was termed the California Project to Cure Blindness–Retinal Pigment Epithelium 1 (CPCB-RPE1) (Figure 8).
Figure 8.
The California Project to Cure Blindness–Retinal Pigment Epithelium 1 (CPCB-RPE1) implant. (a) Low-magnification image of the implant that measured 3.5mm by 6.25mm comprising a synthetic parylene substrate and an overlying monolayer of RPE cells derived from human embryonic stem cells. (b) High-magnification image of the CPCB-RPE1 implant showing several ultrathin circular regions, measuring less than one micron in thickness. These regions facilitated nutrient and growth factor diffusion from the underlying choroid as depicted in the schematic in (c). (d–e) Color fundus photographs of the maculae of two representative study subjects (d, 125 days and e, 120 days post-operatively). The geographic atrophy regions in each subject are demarcated by white dashed lines and the CPCB-RPE1 implants by the black dashed lines respectively. Figure and legend adapted from Kashani AH, Lebkowski JS, Rahhal FM, et al. A bioengineered retinal pigment epithelial monolayer for advanced, dry age-related macular degeneration. Sci Transl Med 2018; 10(435).(Kashani et al., 2018) Reprinted with permission from AAAS.
The parylene membrane used in this study was just six microns thick with a nonporous anterior surface that allowed the adherence of the overlying ESC-RPE cell sheet. It also featured a pattern of circular indentations, each approximately 50 microns in diameter, that were less than one micron thick. This membrane mimicked Bruch’s membrane in its molecular exclusion characteristics. These circular regions enabled the bidirectional diffusion of solutes including nutrients and growth factors, hence supporting the survival of the overlying ESC-RPE cells after transplantation. The CPCB-RPE1 construct measured 3.5 mm × 6.25 mm and supported approximately 100,000 mature ESC-RPE cells on its surface, so theoretically it could regenerate a patch of macula measuring almost 22 mm2.
The FDA-cleared protocol included CPCB-RPE1 and a custom-designed surgical insertion forceps that was used for implantation. Protocol-specified immunosuppression was with tacrolimus from days −8 to 60 with a dose taper from day 42. The surgical procedure entailed an ab interno approach to the subretinal space though a standard pars plana vitrectomy procedure and a 1 mm retinotomy that was sealed with laser retinopexy after CPCB-RPE1 insertion.
5.6.2. Outcome data
In terms of the primary outcomes of safety and tolerability, one serious adverse event occurred and was judged to be possibly related to the surgical procedure (Kashani et al., 2018) and not the composite cell product or immunosuppression regimen. This serious adverse event was a subretinal hemorrhage that was reported during routine follow up. The authors stated that this subretinal hemorrhage had “substantially resolved” during the last follow up visit at 180 days. The subject received one injection of an anti-VEGF agent (bevacizumab), presumably aiming to treat suspected or presumed choroidal neovascular membrane formation, or macular edema, that could be associated with retinal hemorrhages.
In contrast to this one case of serious hemorrhage, mild or moderate intraoperative or postoperative hemorrhages were reported in all other subjects and these resolved spontaneously. Encouragingly, the authors did not report any uncontrolled proliferation or off target cell delivery. The latter observation indicates that the attachment of the ESC-RPE cells was likely maintained on the parylene substrate during and after the surgical implantation procedure, thus containing the stem cell derivatives in a confined location during this period.
As a Phase 1/II study, this protocol was not powered to detect changes in visual function and it should be noted that this report was on the first five patients which was a consecutive initial subset of the full cohort. However a sustained gain in visual acuity of 17 letters and visual fixation ability improvement were detected in one patient (Kashani et al., 2018). Other subjects showed stability of visual acuity over the study period. While data on the unoperated fellow eyes were included in the report as controls – and these showed stability or decline in visual acuity over time – it should be noted that these were the better-seeing eyes in all subjects and so in principle had greater potential for visual acuity decline than the study eyes.
High-resolution in vivo imaging of the anatomy of the overlying retina in subjects showed signs of possible restoration of outer retinal lamina in some regions overlying the implant. The authors interpreted these imaging data as possible photoreceptor layer restoration and/or integration with the ESC-RPE cells of the CPCB-RPE1 implant, although histological correlates are currently unavailable.
The surgical procedure under general anesthesia was initiated but not completed in one subject in whom the CPCB-RPE1 was not transplanted due to the presence of what was described as “intraoperative fibrinoid debris” in the subretinal space. The authors stated that subsequent surgeries were modified to prevent accumulation of subretinal debris (Kashani et al., 2018). Perhaps preoperative indicators of this possible hindrance could be determined based on high-resolution retinal imaging.
5.7. Pfizer
Da Cruz and colleagues in the United Kingdom conducted a Phase I/II study (clinicaltrials.gov identifier NCT01691261) of human ESC-derived RPE cells on a different synthetic basement membrane than the one used in CPCB-RPE1.
This study was sponsored by Pfizer Inc. Unique amongst other similar studies was the inclusion of subjects with exudative AMD – instead of dAMD – with evidence of good foveal fixation. Conceptually, patients with a relatively recent onset of exudative AMD will have significant numbers of remaining photoreceptor cells that could be highly amenable to rescue treatment by an RPE cellular implant, rather than photoreceptor cells that are compromised by dAMD which is a more chronic process. The outcome data from the first two enrolled subjects have been pubished (da Cruz et al., 2018).
The RPE cells were originated from the SHEF-1.3 hESC line using a spontaneous differentiation method. Notably, the 10-micron thick synthetic basement membrane was composed of polyester (polyethylene terephthalate or PET) and was porous (Sterlitech, Washington, USA). The pores were sized at 0.4 microns and were arranged at a density of 1 × 108 pores per cm2. The membrane was coated with human vitronectin. Overall, the patch measured 6 mm × 3 mm (total area 17 mm2) (da Cruz et al., 2018) and was covered with approximately 100,000 cells. The patch was cut to this size with a purpose-built punch prior to implantation.
5.7.1. Preclinical safety assays
The investigators provided in-depth information about the preclinical safety assays in their publication (da Cruz et al., 2018). The chief potential concern here was that of intraocular neoplasm formation. To address this potential safety issue, the investigators conducted mouse teratoma and in vitro cell-spiking studies. NIH III mice that were injected with undifferentiated hESCs formed teratomas. However, teratomas were not detected in mice injected with hESC-RPE cells in suspension. Interestingly, the investigators reported the presence of pigmented hESC-RPE cells that were lining the surface of the retinae and crystalline lenses of the injected eyes of certain mice after 26 weeks’ observation. It is not clear how these cells were able to migrate from the subretinal transplant location into the vitreous cavity; however, it is comforting to know that these non-subretinal cells did not form tumors in the vitreous cavity and appeared to retain their identity as relatively mature, pigmented RPE cells during this interval.
In a further experiment, undifferentiated hESCs were spiked into the RPE cell culture (from 1% to 50% dose of the total cells) at the start of the expansion phase. This spiking did not have a detectable negative impact on the resulting RPE cultures. The resultant cultures showed the typical pigmented cobblestone appearance of mature RPE cells, along with immunohistochemical evidence of premelanosome formation as a marker of differentiation. Encouragingly, the cellular pluripotency marker Tra-1-60 that is typically present in undifferentiated hESC was not detectable after just two days post-spiking. These data indicate that the RPE differentiation medium did not support undifferentiated hESC survival. So the potential contamination of the RPE cultures with undifferentiated ESC remained as a largely theoretical concern and did not engender any practical negative impact on the overall safety of the cell product in terms of uncontrolled proliferation during cell culture.
Regulatory permission – granted by the UK Medicines and Health Products Regulatory Authority, the Gene Therapy Advisory Committee (GTAC), the Moorfields Research Governance Committee and the London–West London & GTAC Research Ethics Committee – allowed for enrollment of 10 patients in total.
5.7.2. Study design and surgical protocol
The composite cellular patch including its coated synthetic basement membrane was introduced into the subretinal space through the ab interno approach using a custom surgical delivery tool. The approach entailed a standard pars plana vitrectomy, purposeful induction of a poster vitreous detachment, and protocol-specified 360° laser application that was placed presumably as prophylaxis against possible retinal detachment. The therapeutic patch was introduced into the subretinal space via a retinotomy that was secured with laser application after the completion of patch insertion. The choroidal neovascular membrane was not removed intentionally but the authors stated that they could not exclude that it was removed inadvertently. Silicone oil tamponade was employed in all cases, and therefore a second surgical procedure was necessary for silicon oil removal as is consistent with typical clinical practice.
The custom surgical tool in this case was designed to protect the composite cellular patch until its implantation into the target location. The device consisted of a handle containing a mechanism by which the patch was pushed out axially as the surgeon manipulated a wheel manually (da Cruz et al., 2018).
Regarding immunosuppression, both systemic and local agents were used. Up to 1 mg/kg oral prednisolone (up to 60 mg maximum) daily was given from days −2 or −4 to day 14 at the minimum and then tapered off. Sub-Tenon triamcinolone acetonide was administered after the patch implantation procedure. For the second procedure, the oral prednisolone regimen began at day −7 instead. Subjects also received an intravitreal implant of fluocinolone acetonide postoperatively which provided sustained intraocular (local) immunosuppression after the cessation of systemic oral prednisolone administration.
5.7.3. Outcome data
In-vivo imaging modalities were used to assess anatomical outcomes following transplantation. In the first patient, OCT and imaging showed the overlap of the native RPE and the patch in one area of the implant. The implant remained covered by the hESC–RPE cells for 12 months. Interestingly, darker pigmented areas was seen to grow into areas that were contiguous with the patch over six months, and the investigators took these to represent possible RPE cell migration away from the patch. However, the investigators judged that there was no evidence of neoplastic transformation during the period of observation. Long term data will be useful to show if the fate of these pigmented cells could change eventually if they are no longer directly confined to the PET membrane and vitronectin coating.
In terms of visual recovery, the 12-month change was +29 letters in patient one and +21 letters in patient two. These results should be interpreted with caution in the light of the absence of directly comparable control eyes in this protocol and the absence of a direct comparison with anti-VEGF monotherapy. The removal of the choroidal neovascular membrane, i.e. the chief pathological culprit in exudative AMD, was not intentionally carried out (unlike in the RIKEN trial (Mandai et al., 2017b)); nevertheless there appeared to be no recurrent choroidal neovascular membrane activity over the study period.
The investigators detected a reduction in global photoreceptor function at six months using full field electroretinography (FF-ERG) testing in both patients. This reduction persisted in patient one but recovered in patient two by the end of the observation period of 12 months. The exact causes and long-term consequences of this apparent pan-photoreceptoral disturbance are currently unclear but are of interest as a potential safety issue as the FF-ERG measures the summed function of all retinal photoreceptor cells – the majority of which herein were not affected by exudative AMD, were located distant from the implant and outside the macula, and by this result have shown a possible reduction in functionality. The macular photoreceptors over the implant, however, demonstrated functional improvements from baseline (in visual acuity, reading speed and contrast sensitivity). It is conceivable that the functional improvements detected in these macular photoreceptors could have been blunted by the apparent global reduction in photoreceptor function.
In terms of other safety signals, the investigators describe three serious adverse events, and these were judged to be unrelated to the therapeutic composite patch (da Cruz et al., 2018). Patient one experienced suture exposure requiring surface revision surgery. In patient two, a worsening of diabetic status was detected following oral prednisolone immunosuppressive therapy and a retinal detachment was detected at eight weeks postoperatively (being absent four weeks prior to detection). This detachment was associated with PVR under silicon oil tamponade and was described as a tractional retinal detachment that was distant from the implant. Surgical repair of this retinal detachment was carried out with peeling of the PVR membranes and 180° retinectomy with continued silicone oil tamponade. In a third procedure, the silicone oil was removed and the retina was noted to remain fully attached. There was an epiretinal band causing focal traction on the macular surface that was not treated. It is not currently possible to know for sure if the presence of hESC-RPE cells, either on the synthetic membrane as intended or possibly at off-target locations such as the epiretinal surface or vitreous cavity, had contributed to the PVR process, although this can be considered as a plausible mechanism of PVR development in this case.
Longer observation than 12 months – of the full cohort – will be required to fully educate peers and potential consumers on the actual and potential safety concerns of retinal detachment, PVR, macular traction, unanticipated cellular proliferation or migration and intraocular pressure increase, among other risks. Nevertheless the data indicate the feasibility of manufacturing and delivering an hESC-RPE monolayer supported on a synthetic basement membrane in patients with recent-onset exudative AMD. The success of this proposed stem cell based intervention compared to the typical current treatment of this condition with intraocular anti-VEGF medications remains to be explored.
5.8. Stem Cell Ophthalmology Treatment Study (SCOTS)
The Stem Cell Ophthalmology Treatment Study (SCOTS) may be the largest non-FDA registered, patient funded clinical trial in stem cell therapy for ocular disease. Final results have not been published.
The study involves a single-physician study treating patients in Florida or Dubai (Johnson, 2017). The trial has no sham or control arm, but it does have three different arms offering a range of intravenous, subtenons, retrobulbar, intravitreal, or subretinal injection of autologous bone marrow derived stem cells, depending on disease severity and fellow-eye status. Specific conditions listed include “retinal disease,” macular degeneration, hereditary retinal dystrophy, optic nerve disease, and glaucoma. The patients have to pay in the region of $15,000-20,000 to join the ‘clinical trial’.
The primary outcome measure as listed on clinicaltrials.gov is “best corrected visual acuity… measured with Snellen Eye Chart and the [Early Treatment Diabetic Retinopathy Study] Eye Chart when available at each post-procedure visit.” (US Clinical Trials.gov, 2018) Secondary outcome measures include visual field outcomes, although no exact form of field analysis is prespecified. The estimated enrolment is 300. The one-year study started August 2013 and is estimated to finish in August 2019. A second SCOTSII trial was registered with clinicaltrials.gov starting in 2016 (Clinicaltrials.gov, 2018). The SCOTSII study is largely similar to SCOTS but adds specific mention of OCT as a secondary outcome measure. In the press it has been reported that over 500 patients have been treated with stem cells by the physician providing these treatments (Lade, 2017), it is unclear how many of these were enrolled in the SCOTS. The SCOTS has reported outcomes in at least 5 peer-reviewed open access publications. Three case reports reveal remarkable results in three patients with idiopathic bilateral optic neuritis (Weiss et al., 2015b), relapsing optic neuropathy, (Weiss et al., 2015a) or serpiginous choroidopathy (Weiss et al., 2016a). Five cases of Leber’s hereditary optic neuropathy showed improved vision in all cases, although average visual change is not reported in the manuscript (Weiss et al., 2016b). Ten cases of bilateral non-arteritic ischemic retinopathy were reported with visual acuity benefit documented in all patients (Weiss et al., 2017).
In the lay press numerous positive outcomes have been reported (MD Stem Cells, 2018) and it has been reported that “for retinitis pigmentosa... approximately 45 percent of the patients [gained] seven or more lines of vision (Mertens, 2018).” This result has yet to be published in the peer-reviewed literature. The reported safety data seems remarkably good, with no adverse events reported in any of the 18 cases in the scientific literature. At least one case of retinal detachment has been recorded in the literature by other clinicians treating a patient after the procedure (Leung et al., 2016). This suggests that currently available reports are likely best outcome cases. Furthermore, the trial is not registered with the FDA, and thus has limited if any oversight. Ethical concerns among some participants have focused on poor informed consent with less than complete details of potential harm related to the treatment (Kemp, 2018).
Ethical and scientific issues regarding patient-funded research need to be considered. Both treating physicians and patients may not be in equipoise regarding patient outcome as relative large sums of money are involved. A selection bias may be introduced into the study by only allowing participation by those who are able to pay. Patient-funded research also raises social justice and access issues because people who are unwilling or unable to pay are excluded from participating in clinical trials that should be open to all patients that fit the selection criteria based on medical grounds (Amezcua and Nelson, 2017).
5.9. Reported adverse events outside clinical trials
The risks of these procedures, especially for retinal disease, is significant. Kuriyan et al. reported a case series of three patients who underwent bilateral, same day, intravitreal injection of autologous adipose tissue-derived “stem cells” for AMD not regulated by the FDA (Kuriyan et al., 2017).
Adipose tissue was harvested in all cases on the same day as the intravitreal injection by means of periumbilical liposuction and same day processing detailed in the report. The patients paid $5000 for each procedure. The consequences of this for-cash procedure were devastating. Five out of six eyes exhibited evidence of weakness of the crystalline lens or capsule, which may have occurred as a consequence of zonular enzymatic degradation possibly by enzymes used for “stem cell” preparation. Also, five of six treated eyes in the three patients developing epiretinal membranes and delayed severe retinal detachments (Figure 9), resulting in visual acuities ranging from 20/200 to no light perception in the better eye at one year of follow-up, in spite of multiple surgeries for retinal detachment. Visual acuities in the better seeing eye prior to the procedures had ranged from 20/30 to 20/50.
Figure 9.
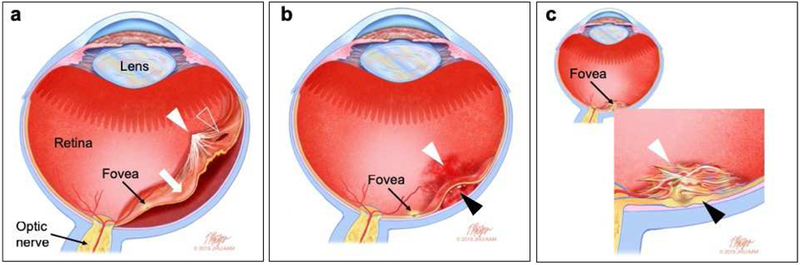
Schematic diagram depicting selected complications of retinal cell therapy. (a) Retinal detachment and proliferative vitreoretinopathy. The retina has detached (white arrow) and the detachment involves the fovea, thus severely reducing visual acuity. The detachment depicted here is associated with a proliferative vitreoretinopathy membrane (white arrowhead) and a retinal break (clear arrowhead). (b) Hemorrhage. Here, blood has collected in the vitreous cavity and on the retinal surface (vitreous cavity hemorrhage, white arrowhead) and beneath the retina (subretinal hemorrhage, black arrowhead) causing a hemorrhagic retinal detachment that threatens the fovea. (c) Epiretinal membrane. A thick epiretinal membrane (white arrowhead) has resulted in foveal thickening and distortion (black arrowhead). Epiretinal membranes can form after cell delivery regardless of whether the exogenous cells have been inadvertently placed in the epiretinal surface. with or without Complications that are not depicted in this diagram include infection (endophthalmitis), cataract, elevation of intraocular pressure and crystalline lens dislocation. (Illustration by Timothy Phelps, MS, FAMI.)
A similar case of intravitreal “stem cell” injections at a different, unaffiliated stem cell clinic with blinding complications, including severe bilateral retinal detachments has been recently reported (Saraf et al., 2017). In this case, the patient received the treatment for exudative macular degeneration and experienced vision loss from 20/200 in the right eye and 20/400 in the left, prior to the procedure, to hand motion in the right eye and light perception in the left, after the procedure. A third report documented poor outcomes including retinal detachment after subretinal injection of stem cells at a third facility (Leung et al., 2016). A fourth report documents a retinal detachment following intravitreal adipose tissue derived stem cells with vision loss from 20/50 to hand motions vision (Rong et al., 2018). The detachments detailed in these reports occurred days to weeks after the intravitreal injection and often with the appearance of epiretinal membranes and proliferative vitreoretinopathy (Figure 9 and Figure 10).
Figure 10.
Adverse events outside of clinical trials. (A) Red free retinal image (showing epiretinal membrane, ERM) and (B) OCT of the right eye of a 78-year-old woman having received intravitreal injection of adipose-derived stem cells one week prior to presentation. She suffered profound vision loss immediately following injection from 20/50 to hand motions vision. Seventeen days after injection, she developed a combined traction-rhegmatogenous retinal detachment in this eye (C). After surgical repair, her final visual acuity was counting fingers vision. The OCT and infrared images demonstrate the robust ERM after adipose-derived stem cell injection that was not there prior to injection. The data from this patient have been reported in this reference (Kuriyan et al., 2017), however none of these images were published.
Together, these cases establish that the scope of the problem with unproven “stem cell” therapy for retinal disease is not limited to one site or one disease. It is possible that the preparations of adipose-derived “stem cells” used for intravitreal injection, may include autologous fibroblasts or cells that differentiate into autologous fibroblasts (Gimble et al., 2007). Intravitreal injection of autologous fibroblasts is a classic animal model of vitreoretinopathy and retinal detachment (Agrawal et al., 2007) and may explain the multitude of retinal detachments after intravitreal “stem cell” injection. It is therefore essential for any viable intravitreal stem cell therapy to establish exactly which cell populations are injected in the eye and how these cells differentiate in the eye after injection.
6. Future Directions
The promise of stem cell therapy to preserve or restore vision in retinal degenerative diseases is finally taking shape. Whereas a decade ago, such ideas were confined to basic and translational laboratories, in the current era stem cell transplantation into the retina is finally in human clinical trials in the setting of well-run registered clinical trials with the oversight of the FDA and appropriate ethical and safety review infrastructure built in. These aspects promote the protection of study subjects from undue harm, and facilitate the dissemination of the results to the scientific community and the peer review process.
Excitingly, a variety of cell products are in evaluation – including fetal derived cells, umbilical cells and retinal cellular derivatives from pluripotent sources including ESCs and iPSCs. Hopefully, over time, we will better understand which cell source is the best therapy for the different indications of dAMD, exudative AMD, RP, Stargardt disease, and other conditions – in their early manifestations, but also in the late stages of established outer retinal atrophy. Encouragingly, a variety of cellular scaffolds are also in clinical trials evaluation and this will afford an indirect comparison with dissociated cell suspension transplants. The reader is directed to two recent comprehensive reviews (Hunt et al., 2018; Wang et al., 2018) on the subject of scaffolds in retinal regenerative treatment.
However, numerous challenges remain to be addressed. A major difficulty with the use of cellular therapies is that the disease process may affect multiple retinal cell types or arise in one cell and then affect adjacent cells. In the case of dry AMD, for example, the retinal pigment epithelium (RPE), photoreceptors, and choriocapillaris may all be affected to some degree without a clear sequential progression of cell loss (Ratnapriya and Chew, 2013; Shen et al., 2007). This poses issues for the ability of transplanted cells of a single subtype to not only restore function but also to halt ongoing degeneration. One potential solution is to use more complex multilayered grafts containing two or more cell types. However, this potential solution of using multilayered grafts to faithfully recapitulate outer retinal anatomy has not been fully explored in basic science experiments nor in clinical trials. Another alternative is to intervene earlier in the course of disease when the transplanted cells could integrate into the existing layers and engender survival of neighboring cell types. This approach may be more technically straightforward but must be weighed against the safety profile of the transplanted cells and procedure in early stage disease.
The advent of CRISPR gene editing technology (Jinek et al., 2012; Mali et al., 2013) may provide avenues to correct genetic mutations in self-derived stem cells (Burnight et al., 2018) prior to autologous transplantation. CRISPR gene editing has been used to correct disease-causing mutations in stem cell organoids of cystic fibrosis (Schwank et al., 2013), colorectal cancer (Roper et al., 2017) and retinitis pigmentosa (Deng et al., 2018). Gene editing is not yet being investigated in as a tool within clinical trials of retinal stem cell treatment. Its future use in removing disease-causing mutations in therapeutic cells may pose additional risks related to off-target mutagenesis (Fu et al., 2013) that will need to be comprehensively characterized, understood and mitigated as far as possible prior to clinical application in autologous stem cell therapy. Ex-vivo gene editing in the context of stem cell therapy may pose a lower risk level, and could be easier to assess, than in vivo gene editing.
It is difficult to overstate the grave concern that arises with the administration of scientifically unproven and unregulated retinal cell therapy products, that have not been prepared in a safe and standardized manner, to vulnerable patients who are desperate for a treatment for their condition. The publication of serious adverse events in the peer-reviewed scientific literature (Kuriyan et al., 2017; Leung et al., 2016; Saraf et al., 2017) has rightly sounded the alarm. Hopefully patients who are contemplating such treatments will have access to this important information regarding the very severe complications that can occur, including the complete and irreversible loss of vision. Physicians who encounter patients who have experienced complications after receiving retinal cell therapy with poor scientific foundations or no regulatory oversight should feel compelled to report these instances in highly visible peer-reviewed scientific journals.
It is also worthwhile to comment on how peer-reviewed published research from well-run institutional teams could prompt over-enthusiasm in the lay or generalist reader. The publication of very short-term observations of safety and possible efficacy outcomes effects could lead to scientifically unfounded over-optimism to the non-specialist audience. The publication of these early observations are typically important for continued milestone-based funding – and make for strong headlines – but do not address the clinically relevant concerns of delayed adverse events and sustained functional effects. It will be important for established groups to lead the way in the culture of scientific reporting by emphasizing outcome data that arise from cohorts of reasonable size followed for an appropriate length of time following stem cell transplantation. Long-term data more fully address concerns regarding delayed adverse events relevant to cell proliferation, such as tractional retinal detachment, and the longevity of the stabilization or improvement in vision in transplant recipients.
With the combined expertise and resources of well-established academic centers, corporate partners and sponsors, foundations, government research institutes and regulatory authorities, the future appears bright. The cutting edge progress in the development of gene- and cell-based therapeutics for the retina, together with the well-established work in the hematopoietic system, plus the recent flurry of excitement surrounding the use of CAR-T cells for cancer (Davila et al., 2014; Kalos et al., 2011), all combine to signal the advent of the age of regenerative medicine.
Figure 3.
Stem cell-based differentiation of light-sensitive photoreceptor cells in three-dimensional culture. (a-b) Human induced pluripotent stem cells differentiating in adherent conditions formed neural retinal (NR) domains expressing VSX2 that were surrounded by a retinal pigmented epithelium (RPE) domain expressing MITF. (c) These NR domains were isolated and cultured in suspension to yield three-dimensional (3D) retinal cups containing NR and retinal pigment epithelium (RPE) cells. (d) Higher magnification of a retinal cup showing the NR and RPE cells that typically formed adjacent to each other. (d) Over time, 3D retinal cups acquired the characteristic retinal lamination containing the precursors of most of the major neuronal cell types, including ganglion, amacrine and horizontal cells (PAX6), and photoreceptors (OTX2). (f-i) Relatively advanced differentiation of photoreceptors occurred in culture, with morphological and molecular differentiation of rods and cones, including expression of rod opsin (f-g), S-opsin (h), and L/M-opsin (i) in individual cells. (j) As further evidence of relatively advanced differentiation, transmission electron microscopy showed presence of inner segments containing centriole (C), basal bodies (BB) and connecting cilia (CC); an outer limiting membrane (*) was also observed. (k) Laminated outer segments discs (arrowheads) also grew in culture, indicating specific and relatively advanced photoreceptor ultrastructural differentiation. (i) Perforated-patch electrophysiological recordings showed a flash-triggered response from light-sensitive photoreceptors. Scaler bars: 100μm (a-c and e), 50μm (d), 10μm (f), 0.05μm (j-k). (Figure and legend adapted from Zhong XF, Gutierrez C, Xue T, et al. Generation of three-dimensional retinal tissue with functional photoreceptors from human iPSCs. Nature Communications 2014; 5, CC-BY license).(Zhong et al., 2014a)
Acknowledgement:
We thank Tim Phelps, MS, FAMI, Professor and Production Director, Department of Art as Applied to Medicine, Johns Hopkins University School of Medicine for the original artwork.
Funding:
MSS: Research to Prevent Blindness (to the Department), The Shulsky Foundation, The Hartwell Foundation, Foundation Fighting Blindness
SSP: National Institute of Health (UG1EY026876-01A1)
TAA: Research to Prevent Blindness, National Institutes of Health (Center Core grant, P30EY014801 and P30EY001319), the Department of Defense (W81XWH-09-1-0675), and the Klorfine Foundation (to Dr. Albini)
MVCS: National Institute of Health (R01EY022631 to Dr. Canto-Soler), BrightFocus Foundation (to Dr. Canto-Soler), The Gates Family Foundation (to Dr. Canto-Soler), The Doni Solich Family Foundation (to Dr. Canto-Soler), Research to Prevent Blindness (to the Department of Ophthalmology)
HK: California Institute for Regenerative Medicine (to Dr. Klassen via jCyte), Polly and Michael Smith Foundation (to Dr. Klassen), Geneva Matlock, MD (to Dr. Klassen), Research to Prevent Blindness (to Department of Ophthalmology)
REM: The Medical Research Council (UK), NIHR Senior Investigator Award, Oxstem Ocular and the Royal College of Surgeons of Edinburgh
MT: Center for Biosystems Dynamics Research, RIKEN
AN: The Las Madrinas Endowment in Experimental Therapeutics for Ophthalmology and an unrestricted grant to the Department of Ophthalmology at the USC Keck School of Medicine from Research to Prevent Blindness
SDS: Research to Prevent Blindness, the Price Foundation, UCLA Broad Stem Cell Research Center, and the Stein Eye Institute Clinical Research Center, UCLA Department of Ophthalmology
KB: National Eye Institute Intramural Research Program and Common Funds Award
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
- MSS: Patents on retinal cell delivery technology (Johns Hopkins University)
- SSP: None
- TAA: Consulting for Janssen Biotech, Inc.
- MVCS: None
- HK: Has an equity interest in jCyte, a company that may potentially benefit from his research results, and also serves on the company's Board. The terms of this arrangement have been reviewed and approved by the University of California, Irvine in accordance with its conflict of interest policies.
- REM: Grant funding from Oxstem Ocular Ltd and Astellas Inc.
- MT: None
- AN: None
- SDS: Grant support from Astellas, Nikon, Nidek and Verily
- KB: None
References
- Agrawal RN, He S, Spee C, Cui JZ, Ryan SJ, Hinton DR, 2007. In vivo models of proliferative vitreoretinopathy. Nat. Protoc 2, 67–77. [DOI] [PubMed] [Google Scholar]
- American Academy of Ophthalmology, 2016. Intraocular stem cell therapy. [Google Scholar]
- Amezcua L, Nelson F, 2017. Ethical Considerations of Patient-Funded Research for Multiple Sclerosis Therapeutics. Neurotherapeutics : the journal of the American Society for Experimental NeuroTherapeutics 14, 945–951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apte RS, 2018. Gene therapy for retinal degeneration. Cell 173, 5. [DOI] [PubMed] [Google Scholar]
- Ardeljan D, Chan CC, 2013. Aging is not a disease: distinguishing age-related macular degeneration from aging. Prog. Retin. Eye Res 37, 68–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnhold S, Absenger Y, Klein H, Addicks K, Schraermeyer U, 2007. Transplantation of bone marrow-derived mesenchymal stem cells rescue photoreceptor cells in the dystrophic retina of the rhodopsin knockout mouse. Graefes Arch. Clin. Exp. Ophthalmol 245, 414–422. [DOI] [PubMed] [Google Scholar]
- Arora HL, Sperry RW, 1962. Optic nerve regeneration after surgical cross-union of medial and lateral optic tracts. Am. Zool 2. [Google Scholar]
- Asahara T, Murohara T, Sullivan A, Silver M, van der Zee R, Li T, Witzenbichler B, Schatteman G, Isner JM, 1997. Isolation of putative progenitor endothelial cells for angiogenesis. Science 275, 964–967. [DOI] [PubMed] [Google Scholar]
- Assawachananont J, Mandai M, Okamoto S, Yamada C, Eiraku M, Yonemura S, Sasai Y, Takahashi M, 2014. Transplantation of embryonic and induced pluripotent stem cell-derived 3D retinal sheets into retinal degenerative mice. Stem Cell Reports 2, 662–674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldassare JS, Joseph A, Keane M, Heier JS, 2017. Subretinal delivery of cells via the suprachoroidal space: Janssen trial, in: Schwartz SD, Nagiel A, Lanza R (Eds.), Cellular Therapies for Retinal Disease. Springer International Publishing, pp. 95–104. [Google Scholar]
- Bara JJ, Richards RG, Alini M, Stoddart MJ, 2014. Concise review: Bone marrow-derived mesenchymal stem cells change phenotype following in vitro culture: implications for basic research and the clinic. Stem Cells 32, 1713–1723. [DOI] [PubMed] [Google Scholar]
- Baranov PY, Tucker BA, Young MJ, 2014. Low-oxygen culture conditions extend the multipotent properties of human retinal progenitor cells. Tissue engineering. Part A 20, 1465–1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnea-Cramer AO, Wang W, Lu SJ, Singh MS, Luo C, Huo H, McClements ME, Barnard AR, MacLaren RE, Lanza R, 2016. Function of human pluripotent stem cell-derived photoreceptor progenitors in blind mice. Sci. Rep 6, 29784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Battaglia Parodi M, Cicinelli MV, Rabiolo A, Pierro L, Bolognesi G, Bandello F, 2017. Vascular abnormalities in patients with Stargardt disease assessed with optical coherence tomography angiography. Br. J. Ophthalmol 101, 780–785. [DOI] [PubMed] [Google Scholar]
- Berger I, Ahmad A, Bansal A, Kapoor T, Sipp D, Rasko JEJ, 2016. Global distribution of businesses marketing stem cell-based interventions. Cell Stem Cell 19, 158–162. [DOI] [PubMed] [Google Scholar]
- Berman ML, E., 2015. The stem cell revolution AuthorHouse, Bloomington, Indiana. [Google Scholar]
- Bernardo ME, Fibbe WE, 2013. Mesenchymal stromal cells: sensors and switchers of inflammation. Cell Stem Cell 13, 392–402. [DOI] [PubMed] [Google Scholar]
- Bertolotti E, Neri A, Camparini M, Macaluso C, Marigo V, 2014. Stem cells as source for retinal pigment epithelium transplantation. Prog. Retin. Eye Res 42, 130–144. [DOI] [PubMed] [Google Scholar]
- Bharti K, 2018. Patching the retina with stem cells. Nat. Biotechnol 36, 311–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bharti K, Miller SS, Arnheiter H, 2011. The new paradigm: Retinal pigment epithelium cells generated from embryonic or induced pluripotent stem cells. Pigment Cell and Melanoma Research 24, 21–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bharti K, Nguyen MT, Skuntz S, Bertuzzi S, Arnheiter H, 2006. The other pigment cell: specification and development of the pigmented epithelium of the vertebrate eye. Pigment Cell Res 19, 380–394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bharti K, Rao M, Hull SC, Stroncek D, Brooks BP, Feigal E, van Meurs JC, Huang CA, Miller SS, 2014. Developing cellular therapies for retinal degenerative diseases. Invest. Ophthalmol. Vis. Sci 55, 1191–1202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhutto I, Lutty G, 2012. Understanding age-related macular degeneration (AMD): relationships between the photoreceptor/retinal pigment epithelium/Bruch's membrane/choriocapillaris complex. Mol. Aspects Med 33, 295–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birch DG, Bennett LD, Duncan JL, Weleber RG, Pennesi ME, 2016. Long-term Follow-up of Patients With Retinitis Pigmentosa Receiving Intraocular Ciliary Neurotrophic Factor Implants. Am. J. Ophthalmol 170, 10–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birch DG, Weleber RG, Duncan JL, Jaffe GJ, Tao W, Ciliary Neurotrophic Factor Retinitis Pigmentosa Study, G., 2013. Randomized trial of ciliary neurotrophic factor delivered by encapsulated cell intraocular implants for retinitis pigmentosa. Am. J. Ophthalmol 156, 283–292.e281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Björklund A, Stenevi U, 1972. Nerve growth factor: Stimulation of regenerative growth of central noradrenergic neurons. Science 175, 1251–1253. [DOI] [PubMed] [Google Scholar]
- Blair JR, Turner JE, 1987. Optimum conditions for successful transplantation of immature rat retina to the lesioned adult retina. Brain Res 433, 257–270. [DOI] [PubMed] [Google Scholar]
- Blenkinsop TA, Saini JS, Maminishkis A, Bharti K, Wan Q, Banzon T, Lotfi M, Davis J, Singh D, Rizzolo LJ, Miller S, Temple S, Stern JH, 2015. Human adult retinal pigment epithelial stem cell-derived RPE monolayers exhibit key physiological characteristics of native tissue. Invest. Ophthalmol. Vis. Sci 56, 7085–7099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blenkinsop TA, Salero E, Stern JH, Temple S, 2013. The culture and maintenance of functional retinal pigment epithelial monolayers from adult human eye. Methods Mol. Biol 945, 45–65. [DOI] [PubMed] [Google Scholar]
- Brundin P, Nilsson OG, Strecker RE, Lindvall O, Åstedt B, Björklund A, 1986. Behavioural effects of human fetal dopamine neurons grafted in a rat model of Parkinson's disease. Exp. Brain Res 65, 235–240. [DOI] [PubMed] [Google Scholar]
- Burnight ER, Giacalone JC, Cooke JA, Thompson JR, Bohrer LR, Chirco KR, Drack AV, Fingert JH, Worthington KS, Wiley LA, Mullins RF, Stone EM, Tucker BA, 2018. CRISPR-Cas9 genome engineering: Treating inherited retinal degeneration. Prog. Retin. Eye Res 65, 28–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caballero S, Sengupta N, Afzal A, Chang KH, Li Calzi S, Guberski DL, Kern TS, Grant MB, 2007. Ischemic vascular damage can be repaired by healthy, but not diabetic, endothelial progenitor cells. Diabetes 56, 960–967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canto-Soler V, Flores-Bellver M, Vergara MN, 2016. Stem cell sources and their potential for the treatment of retinal degenerations. Invest. Ophthalmol. Vis. Sci 57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao J, Murat C, An W, Yao X, Lee J, Santulli-Marotto S, Harris IR, Inana G, 2016. Human umbilical tissue-derived cells rescue retinal pigment epithelium dysfunction in retinal degeneration. Stem Cells 34, 367–379. [DOI] [PubMed] [Google Scholar]
- Caplan AI, Dennis JE, 2006. Mesenchymal stem cells as trophic mediators. J. Cell. Biochem 98, 1076–1084. [DOI] [PubMed] [Google Scholar]
- Chew EY, Clemons TE, Peto T, Sallo FB, Ingerman A, Tao W, Singerman L, Schwartz SD, Peachey NS, Bird AC, MacTel CRG, 2015. Ciliary neurotrophic factor for macular telangiectasia type 2: results from a phase 1 safety trial. Am. J. Ophthalmol 159, 659–666 e651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chong NH, Alexander RA, Waters L, Barnett KC, Bird AC, Luthert PJ, 1999. Repeated injections of a ciliary neurotrophic factor analogue leading to long-term photoreceptor survival in hereditary retinal degeneration. Invest. Ophthalmol. Vis. Sci 40, 1298–1305. [PubMed] [Google Scholar]
- Clinicaltrials.gov, 2018. Stem Cell Ophthalmology Treatment Study II (SCOTS2). U.S. National Library of Medicine [Google Scholar]
- Cotrim CC, Toscano L, Messias A, Jorge R, Siqueira RC, 2017. Intravitreal use of bone marrow mononuclear fraction containing CD34(+) stem cells in patients with atrophic age-related macular degeneration. Clin. Ophthalmol 11, 931–938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curcio CA, 2001. Photoreceptor topography in ageing and age-related maculopathy. Eye (Lond.) 15, 376–383. [DOI] [PubMed] [Google Scholar]
- da Cruz L, Chen FK, Ahmado A, Greenwood J, Coffey P, 2007. RPE transplantation and its role in retinal disease. Prog. Retin. Eye Res 26, 598–635. [DOI] [PubMed] [Google Scholar]
- da Cruz L, Fynes K, Georgiadis O, Kerby J, Luo YH, Ahmado A, Vernon A, Daniels JT, Nommiste B, Hasan SM, Gooljar SB, Carr AF, Vugler A, Ramsden CM, Bictash M, Fenster M, Steer J, Harbinson T, Wilbrey A, Tufail A, Feng G, Whitlock M, Robson AG, Holder GE, Sagoo MS, Loudon PT, Whiting P, Coffey PJ, 2018. Phase 1 clinical study of an embryonic stem cell-derived retinal pigment epithelium patch in age-related macular degeneration. Nat. Biotechnol 36, 328–337. [DOI] [PubMed] [Google Scholar]
- Das T, del Cerro M, Jalali S, Rao VS, Gullapalli VK, Little C, Loreto DA, Sharma S, Sreedharan A, del Cerro C, Rao GN, 1999. The transplantation of human fetal neuroretinal cells in advanced retinitis pigmentosa patients: results of a long-term safety study. Exp. Neurol 157, 58–68. [DOI] [PubMed] [Google Scholar]
- Davila ML, Riviere I, Wang X, Bartido S, Park J, Curran K, Chung SS, Stefanski J, Borquez-Ojeda O, Olszewska M, Qu J, Wasielewska T, He Q, Fink M, Shinglot H, Youssif M, Satter M, Wang Y, Hosey J, Quintanilla H, Halton E, Bernal Y, Bouhassira DCG, Arcila ME, Gonen M, Roboz GJ, Maslak P, Douer D, Frattini MG, Giralt S, Sadelain M, Brentjens R, 2014. Efficacy and toxicity management of 19-28z CAR T cell therapy in B cell acute lymphoblastic leukemia. Sci. Transl. Med 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis RJ, Alam NM, Zhao C, Muller C, Saini JS, Blenkinsop TA, Mazzoni F, Campbell M, Borden SM, Charniga CJ, Lederman PL, Aguilar V, Naimark M, Fiske M, Boles N, Temple S, Finnemann SC, Prusky GT, Stern JH, 2017. The developmental stage of adult human stem cell-derived retinal pigment epithelium cells influences transplant efficacy for vision rescue. Stem Cell Reports 9, 42–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis RJ, Blenkinsop TA, Campbell M, Borden SM, Charniga CJ, Lederman PL, Frye AM, Aguilar V, Zhao C, Naimark M, Kiehl TR, Temple S, Stern JH, 2016. Human RPE stem cell-derived RPE preserves photoreceptors in the Royal College of Surgeons rat: Method for quantifying the area of photoreceptor sparing. J. Ocul. Pharmacol. Ther 32, 304–309. [DOI] [PubMed] [Google Scholar]
- Decembrini S, Koch U, Radtke F, Moulin A, Arsenijevic Y, 2014. Derivation of traceable and transplantable photoreceptors from mouse embryonic stem cells. Stem Cell Reports 2, 853–865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decembrini S, Martin C, Sennlaub F, Chemtob S, Biel M, Samardzija M, Moulin A, Behar-Cohen F, Arsenijevic Y, 2017. Cone genesis tracing by the Chrnb4-EGFP mouse line: Evidences of cellular material fusion after cone precursor transplantation. Mol. Ther 25, 634–653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- del Cerro M, Ison JR, Bowen GP, Lazar E, del Cerro C, 1991. Intraretinal grafting restores visual function in light-blinded rats. Neuroreport 2, 529–532. [DOI] [PubMed] [Google Scholar]
- del Cerro M, Notter MF, del Cerro C, Wiegand SJ, Grover DA, Lazar E, 1989. Intraretinal transplantation for rod-cell replacement in light-damaged retinas. J. Neural Transplant. 1, 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- del Cerro M, Notter MF, Grover DA, Gash DM, Jiang LQ, del Cerro C, 1988. Retinal transplants into adult eyes affected by phototoxic retinopathy. Prog. Brain Res 78, 125–130. [DOI] [PubMed] [Google Scholar]
- Deng WL, Gao ML, Lei XL, Lv JN, Zhao H, He KW, Xia XX, Li LY, Chen YC, Li YP, Pan D, Xue T, Jin ZB, 2018. Gene Correction Reverses Ciliopathy and Photoreceptor Loss in iPSC-Derived Retinal Organoids from Retinitis Pigmentosa Patients. Stem Cell Reports 10, 1267–1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dias MF, Joo K, Kemp JA, Fialho SL, da Silva Cunha A Jr., Woo SJ, Kwon YJ, 2017. Molecular genetics and emerging therapies for retinitis pigmentosa: Basic research and clinical perspectives. Prog. Retin. Eye Res [DOI] [PubMed] [Google Scholar]
- Diniz B, Thomas P, Thomas B, Ribeiro R, Hu Y, Brant R, Ahuja A, Zhu D, Liu L, Koss M, Maia M, Chader G, Hinton DR, Humayun MS, 2013. Subretinal implantation of retinal pigment epithelial cells derived from human embryonic stem cells: Improved survival when implanted as a monolayer. Invest. Ophthalmol. Vis. Sci 54, 5087–5096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dominici M, Le Blanc K, Mueller I, Slaper-Cortenbach I, Marini F, Krause D, Deans R, Keating A, Prockop D, Horwitz E, 2006. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy 8, 315–317. [DOI] [PubMed] [Google Scholar]
- Drukker M, Katchman H, Katz G, Even-Tov Friedman S, Shezen E, Hornstein E, Mandelboim O, Reisner Y, Benvenisty N, 2006. Human embryonic stem cells and their differentiated derivatives are less susceptible to immune rejection than adult cells. Stem Cells 24, 221–229. [DOI] [PubMed] [Google Scholar]
- Ejstrup R, Kiilgaard JF, Tucker BA, Klassen HJ, Young MJ, La Cour M, 2010. Pharmacokinetics of intravitreal glial cell line-derived neurotrophic factor: Experimental studies in pigs. Exp. Eye Res 91, 890–895. [DOI] [PubMed] [Google Scholar]
- Enzmann V, Yolcu E, Kaplan HJ, Ildstad ST, 2009. Stem cells as tools in regenerative therapy for retinal degeneration. Arch. Ophthalmol 127, 563–571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans JB, Syed BA, 2013. New hope for dry AMD? Nat Rev Drug Discov 12, 501–502. [DOI] [PubMed] [Google Scholar]
- Faktorovich EG, Steinberg RH, Yasumura D, Matthes MT, LaVail MM, 1990. Photoreceptor degeneration in inherited retinal dystrophy delayed by basic fibroblast growth factor. Nature 347, 83–86. [DOI] [PubMed] [Google Scholar]
- FDA, 2016. Maco Biotech Collection - Sterile cord clood collection unit. The Food and Drug Administration. [Google Scholar]
- FDA, 2017a. Code of Federal Regulations Title 21. The Food and Drug Adminstration. [Google Scholar]
- FDA, 2017b. FDA Warns About US Stem Cell Therapies. The Food and Drug Administration. [Google Scholar]
- FDA, 2017c. Regulatory considerations for human cells, tissues, and cellular and tissue-based products: Minimal manipulation and homologous use. The Food and Drug Administration. [Google Scholar]
- FDA, 2017d. Same surgical procedure exception under 21 CFR 1271.15(b): Questions and answers regarding the scope of the exception. The Food and Drug Administration. [Google Scholar]
- FDA, 2018. FDA seeks permanent injunctions against two stem cell clinics. The Food and Drug Adminstration. [Google Scholar]
- FDA, February 28 1997. Proposed Approach to Regulation of Cellular and Tissue-Based Products. The Food and Drug Adminstration. [DOI] [PubMed] [Google Scholar]
- Frasson M, Picaud S, Leveillard T, Simonutti M, Mohand-Said S, Dreyfus H, Hicks D, Sabel J, 1999. Glial cell line-derived neurotrophic factor induces histologic and functional protection of rod photoreceptors in the rd/rd mouse. Invest. Ophthalmol. Vis. Sci 40, 2724–2734. [PubMed] [Google Scholar]
- Friedenstein AJ, Petrakova KV, Kurolesova AI, Frolova GP, 1968. Heterotopic of bone marrow. Analysis of precursor cells for osteogenic and hematopoietic tissues. Transplantation 6, 230–247. [PubMed] [Google Scholar]
- Fu Y, Foden JA, Khayter C, Maeder ML, Reyon D, Joung JK, Sander JD, 2013. High-frequency off-target mutagenesis induced by CRISPR-Cas nucleases in human cells. Nat. Biotechnol 31, 822–826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujii M, Sunagawa GA, Kondo M, Takahashi M, Mandai M, 2016. Evaluation of micro electroretinograms recorded with multiple electrode array to assess focal retinal function. Sci. Rep 6, 30719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gagliardi G, Ben M'Barek K, Goureau O, 2019. Photoreceptor cell replacement in macular degeneration and retinitis pigmentosa: A pluripotent stem cell-based approach. Prog. Retin. Eye Res 71, 1–25. [DOI] [PubMed] [Google Scholar]
- Galderisi U, Giordano A, 2014. The gap between the physiological and therapeutic roles of mesenchymal stem cells. Med. Res. Rev 34, 1100–1126. [DOI] [PubMed] [Google Scholar]
- Gamm DM, Wong R, and the, A.G.I.W.P., 2015. Report on the National Eye Institute Audacious Goals initiative: Photoreceptor regeneration and integration workshop. Transl Vis Sci Technol 4, 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gasparini SJ, Llonch S, Borsch O, Ader M, 2019. Transplantation of photoreceptors into the degenerative retina: Current state and future perspectives. Prog. Retin. Eye Res 69, 1–37. [DOI] [PubMed] [Google Scholar]
- George M, Nazari H, Mitra D, Clegg DO, Hinton DR, Humayan MS, 2017. Scaffolds for cell transplantation, in: Schwartz SD, Nagiel A, Lanza R (Eds.), Cellular Therapies for Retinal Disease. Springer International Publishing, pp. 45–54. [Google Scholar]
- Gimble JM, Katz AJ, Bunnell BA, 2007. Adipose-derived stem cells for regenerative medicine. Circ. Res 100, 1249–1260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golestaneh N, Chu Y, Xiao YY, Stoleru GL, Theos AC, 2017. Dysfunctional autophagy in RPE, a contributing factor in age-related macular degeneration. Cell Death Dis. 8, e2537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Cordero A, Kruczek K, Naeem A, Fernando M, Kloc M, Ribeiro J, Goh D, Duran Y, Blackford SJI, Abelleira-Hervas L, Sampson RD, Shum IO, Branch MJ, Gardner PJ, Sowden JC, Bainbridge JWB, Smith AJ, West EL, Pearson RA, Ali RR, 2017. Recapitulation of Human Retinal Development from Human Pluripotent Stem Cells Generates Transplantable Populations of Cone Photoreceptors. Stem Cell Reports 9, 820–837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Cordero A, West EL, Pearson RA, Duran Y, Carvalho LS, Chu CJ, Naeem A, Blackford SJI, Georgiadis A, Lakowski J, Hubank M, Smith AJ, Bainbridge JWB, Sowden JC, Ali RR, 2013. Photoreceptor precursors derived from three-dimensional embryonic stem cell cultures integrate and mature within adult degenerate retina. Nat. Biotechnol 31, 741–747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodell MA, Nguyen H, Shroyer N, 2015. Somatic stem cell heterogeneity: diversity in the blood, skin and intestinal stem cell compartments. Nat. Rev. Mol. Cell Biol 16, 299–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gouras P, Du J, Gelanze M, Lopez R, Kwun R, Kjeldbye H, Krebs W, 1991. Survival and synapse formation of transplanted rat rods. J. Neural Transplant. Plast 2, 91–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamel C, 2006. Retinitis pigmentosa. Orphanet J. Rare Dis 1, 40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Handa JT, 2012. How does the macula protect itself from oxidative stress? Mol. Aspects Med 33, 418–435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Handa JT, Cano M, Wang L, Datta S, Liu T, 2017. Lipids, oxidized lipids, oxidation-specific epitopes, and Age-related Macular Degeneration. Biochim. Biophys. Acta 1862, 430–440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hankin MH, Lund RD, 1987. Specific target-directed axonal outgrowth from transplanted embryonic rodent retinae into neonatal rat superior colliculus. Brain Res 408, 344–348. [DOI] [PubMed] [Google Scholar]
- Hanley JA, Lippman-Hand A, 1983. If nothing goes wrong, is everything all right? Interpreting zero numerators. JAMA 249, 1743–1745. [PubMed] [Google Scholar]
- Hanus J, Zhao F, Wang S, 2016. Current therapeutic developments in atrophic age-related macular degeneration. Br. J. Ophthalmol 100, 122–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartong DT, Berson EL, Dryja TP, 2006. Retinitis pigmentosa. Lancet 368, 1795–1809. [DOI] [PubMed] [Google Scholar]
- Hendrickson A, Bumsted-O'Brien K, Natoli R, Ramamurthy V, Possin D, Provis J, 2008. Rod photoreceptor differentiation in fetal and infant human retina. Exp. Eye Res 87, 415–426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirami Y, Osakada F, Takahashi K, Okita K, Yamanaka S, Ikeda H, Yoshimura N, Takahashi M, 2009. Generation of retinal cells from mouse and human induced pluripotent stem cells. Neurosci. Lett 458, 126–131. [DOI] [PubMed] [Google Scholar]
- Ho AC, Chang TS, Samuel M, Williamson P, Willenbucher RF, Malone T, 2017. Experience With a Subretinal Cell-based Therapy in Patients With Geographic Atrophy Secondary to Age-related Macular Degeneration. Am. J. Ophthalmol 179, 67–80. [DOI] [PubMed] [Google Scholar]
- Homma K, Okamoto S, Mandai M, Gotoh N, Rajasimha HK, Chang YS, Chen S, Li W, Cogliati T, Swaroop A, Takahashi M, 2013. Developing rods transplanted into the degenerating retina of Crx-knockout mice exhibit neural activity similar to native photoreceptors. Stem Cells 31, 1149–1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hori J, Ng TF, Shatos M, Klassen H, Streilein JW, Young MJ, 2003. Neural progenitor cells lack immunogenicity and resist destruction as allografts. Stem Cells 21, 405–416. [DOI] [PubMed] [Google Scholar]
- Hu Y, Liu L, Lu B, Zhu D, Ribeiro R, Diniz B, Thomas PB, Ahuja AK, Hinton DR, Tai YC, Hikita ST, Johnson LV, Clegg DO, Thomas BB, Humayun MS, 2012. A novel approach for subretinal implantation of ultrathin substrates containing stem cell-derived retinal pigment epithelium monolayer. Ophthalmic Res 48, 186–191. [DOI] [PubMed] [Google Scholar]
- Hudson KL, Collins FS, 2017. The 21st Century Cures Act - A View from the NIH. N. Engl. J. Med 376, 111–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humayun MS, de Juan E Jr., del Cerro M, Dagnelie G, Radner W, Sadda SR, del Cerro C, 2000. Human neural retinal transplantation. Invest. Ophthalmol. Vis. Sci 41, 3100–3106. [PubMed] [Google Scholar]
- Hunt NC, Hallam D, Chichagova V, Steel DH, Lako M, 2018. The application of biomaterials to tissue engineering neural retina and retinal pigment epithelium. Advanced healthcare materials 7. [DOI] [PubMed] [Google Scholar]
- Idelson M, Alper R, Obolensky A, Ben-Shushan E, Hemo I, Yachimovich-Cohen N, Khaner H, Smith Y, Wiser O, Gropp M, Cohen MA, Even-Ram S, Berman-Zaken Y, Matzrafi L, Rechavi G, Banin E, Reubinoff B, 2009. Directed Differentiation of Human Embryonic Stem Cells into Functional Retinal Pigment Epithelium Cells. Cell Stem Cell 5, 396–408. [DOI] [PubMed] [Google Scholar]
- Iraha S, Tu HY, Yamasaki S, Kagawa T, Goto M, Takahashi R, Watanabe T, Sugita S, Yonemura S, Sunagawa GA, Matsuyama T, Fujii M, Kuwahara A, Kishino A, Koide N, Eiraku M, Tanihara H, Takahashi M, Mandai M, 2018. Establishment of Immunodeficient Retinal Degeneration Model Mice and Functional Maturation of Human ESC-Derived Retinal Sheets after Transplantation. Stem Cell Reports 10, 1059–1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson M, Gaze RM, 1965. Selection of appropriate tectal connections by regenerating optic nerve fibers in adult goldfish. Exp. Neurol 13, 418–430. [DOI] [PubMed] [Google Scholar]
- Jahn R, Lang T, Südhof TC, 2003. Membrane fusion. Cell 112, 519–533. [DOI] [PubMed] [Google Scholar]
- Jahn R, Scheller RH, 2006. SNAREs - Engines for membrane fusion. Nature Reviews Molecular Cell Biology 7, 631–643. [DOI] [PubMed] [Google Scholar]
- Jayakody SA, Gonzalez-Cordero A, Ali RR, Pearson RA, 2015. Cellular strategies for retinal repair by photoreceptor replacement. Prog. Retin. Eye Res 46, 31–66. [DOI] [PubMed] [Google Scholar]
- Jha BS, Bharti K, 2015. Regenerating Retinal Pigment Epithelial Cells to Cure Blindness: A Road Towards Personalized Artificial Tissue. Curr Stem Cell Rep 1, 79–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin ZB, Okamoto S, Osakada F, Homma K, Assawachananont J, Hirami Y, Iwata T, Takahashi M, 2011. Modeling retinal degeneration using patient-specific induced pluripotent stem cells. PLoS One 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jinek M, Chylinski K, Fonfara I, Hauer M, Doudna JA, Charpentier E, 2012. A programmable dual-RNA-guided DNA endonuclease in adaptive bacterial immunity. Science 337, 816–821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson SO, 2017. Radical new stem cell procedure helps Dubai patients regain sight. The National. [Google Scholar]
- Jonas JB, Witzens-Harig M, Arseniev L, Ho AD, 2010. Intravitreal autologous bone-marrow-derived mononuclear cell transplantation. Acta Ophthalmol 88, e131–132. [DOI] [PubMed] [Google Scholar]
- Joussen AM, Heussen FM, Joeres S, Llacer H, Prinz B, Rohrschneider K, Maaijwee KJ, van Meurs J, Kirchhof B, 2006. Autologous translocation of the choroid and retinal pigment epithelium in age-related macular degeneration. Am. J. Ophthalmol 142, 17–30. [DOI] [PubMed] [Google Scholar]
- Kalos M, Levine BL, Porter DL, Katz S, Grupp SA, Bagg A, June CH, 2011. T cells with chimeric antigen receptors have potent antitumor effects and can establish memory in patients with advanced leukemia. Sci. Transl. Med 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamao H, Mandai M, Ohashi W, Hirami Y, Kurimoto Y, Kiryu J, Takahashi M, 2017. Evaluation of the Surgical Device and Procedure for Extracellular Matrix-Scaffold-Supported Human iPSC-Derived Retinal Pigment Epithelium Cell Sheet Transplantation. Invest. Ophthalmol. Vis. Sci 58, 211–220. [DOI] [PubMed] [Google Scholar]
- Kamao H, Mandai M, Wakamiya S, Ishida J, Goto K, Ono T, Suda T, Takahashi M, Kiryu J, 2014. Objective Evaluation of the Degree of Pigmentation in Human Induced Pluripotent Stem Cell-Derived RPE. Invest Ophthalmol Vis Sci 55, 8309–8318. [DOI] [PubMed] [Google Scholar]
- Kanemura H, Go MJ, Nishishita N, Sakai N, Kamao H, Sato Y, Takahashi M, Kawamata S, 2013. Pigment epithelium-derived factor secreted from retinal pigment epithelium facilitates apoptotic cell death of iPSC. Sci. Rep 3, 2334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanemura H, Go MJ, Shikamura M, Nishishita N, Sakai N, Kamao H, Mandai M, Morinaga C, Takahashi M, Kawamata S, 2014. Tumorigenicity studies of induced pluripotent stem cell (iPSC)-derived retinal pigment epithelium (RPE) for the treatment of age-related macular degeneration. PLoS One 9, e85336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kashani AH, Lebkowski JS, Rahhal FM, Avery RL, Salehi-Had H, Dang W, Lin CM, Mitra D, Zhu D, Thomas BB, Hikita ST, Pennington BO, Johnson LV, Clegg DO, Hinton DR, Humayun MS, 2018. A bioengineered retinal pigment epithelial monolayer for advanced, dry age-related macular degeneration. Sci. Transl. Med 10. [DOI] [PubMed] [Google Scholar]
- Kauper K, McGovern C, Sherman S, Heatherton P, Rapoza R, Stabila P, Dean B, Lee A, Borges S, Bouchard B, Tao W, 2012. Two-year intraocular delivery of ciliary neurotrophic factor by encapsulated cell technology implants in patients with chronic retinal degenerative diseases. Invest. Ophthalmol. Vis. Sci 53, 7484–7491. [DOI] [PubMed] [Google Scholar]
- Kemp P, 2018. The Stem Cell Hard Sell, in: Meisel A (Ed.). BBC World Service [Google Scholar]
- Kern S, Eichler H, Stoeve J, Klüter H, Bieback K, 2006. Comparative analysis of mesenchymal stem cells from bone marrow, umbilical cord blood, or adipose tissue. Stem Cells 24, 1294–1301. [DOI] [PubMed] [Google Scholar]
- Kirschen McLoon L, McLoon SC, Lund RD, 1981. Cultured embryonic retinae transplanted to rat brain: Differentiation and formation of projections to host superior colliculus. Brain Res 226, 15–31. [DOI] [PubMed] [Google Scholar]
- Klassen H, 2016. Stem cells in clinical trials for treatment of retinal degeneration. Expert Opin. Biol. Ther 16, 7–14. [DOI] [PubMed] [Google Scholar]
- Klassen H, Imfeld KL, Ray J, Young MJ, Gage FH, Berman MA, 2003. The immunological properties of adult hippocampal progenitor cells. Vision Res 43, 947–956. [DOI] [PubMed] [Google Scholar]
- Klassen H, Kiilgaard JF, Zahir T, Ziaeian B, Kirov I, Scherfig E, Warfvinge K, Young MJ, 2007a. Progenitor cells from the porcine neural retina express photoreceptor markers after transplantation to the subretinal space of allorecipients. Stem Cells 25, 1222–1230. [DOI] [PubMed] [Google Scholar]
- Klassen H, Lund RD, 1987. Retinal transplants can drive a pupillary reflex in host rat brains. Proc. Natl. Acad. Sci. U. S. A 84, 6958–6960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klassen H, Lund RD, 1990a. Parameters of retinal graft-mediated responses are related to underlying target innervation. Brain Res 533, 181–191. [DOI] [PubMed] [Google Scholar]
- Klassen H, Lund RD, 1990b. Retinal graft-mediated pupillary responses in rats: Restoration of a reflex function in the mature mammalian brain. J. Neurosci 10, 578–587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klassen H, Schwartz MR, Bailey AH, Young MJ, 2001. Surface markers expressed by multipotent human and mouse neural progenitor cells include tetraspanins and non-protein epitopes. Neurosci. Lett 312, 180–182. [DOI] [PubMed] [Google Scholar]
- Klassen H, Schwartz PH, Ziaeian B, Nethercott H, Young MJ, Bragadottir R, Tullis GE, Warfvinge K, Narfstrom K, 2007b. Neural precursors isolated from the developing cat brain show retinal integration following transplantation to the retina of the dystrophic cat. Vet. Ophthalmol 10, 245–253. [DOI] [PubMed] [Google Scholar]
- Klassen H, Ziaeian B, Kirov II, Young MJ, Schwartz PH, 2004a. Isolation of retinal progenitor cells from post-mortem human tissue and comparison with autologous brain progenitors. J. Neurosci. Res 77, 334–343. [DOI] [PubMed] [Google Scholar]
- Klassen HJ, Ng TF, Kurimoto Y, Kirov I, Shatos M, Coffey P, Young MJ, 2004b. Multipotent retinal progenitors express developmental markers, differentiate into retinal neurons, and preserve light-mediated behavior. Invest. Ophthalmol. Vis. Sci 45, 4167–4173. [DOI] [PubMed] [Google Scholar]
- Klimanskaya I, Hipp J, Rezai KA, West M, Atala A, Lanza R, 2004. Derivation and comparative assessment of retinal pigment epithelium from human embryonic stem cells using transcriptomics. Cloning Stem Cells 6, 217–245. [DOI] [PubMed] [Google Scholar]
- Koh S, Chen WJ, Dejneka NS, Harris IR, Lu B, Girman S, Saylor J, Wang S, Eroglu C, 2018. Subretinal Human Umbilical Tissue-Derived Cell Transplantation Preserves Retinal Synaptic Connectivity and Attenuates Muller Glial Reactivity. J. Neurosci 38, 2923–2943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuppermann BD, Boyer DS, Mills B, Yang J, Klassen HJ, 2018. Safety and Activity of a Single, Intravitreal Injection of Human Retinal Progenitor Cells (jCell) for Treatment of Retinitis Pigmentosa (RP). Invest. Ophthalmol. Vis. Sci 59, 2987–2987.30025116 [Google Scholar]
- Kuriyan AE, Albini TA, Townsend JH, Rodriguez M, Pandya HK, Leonard RE, Parrott MB, Rosenfeld PJ, Flynn HW, Goldberg JL, 2017. Vision loss after intravitreal injection of autologous "stem Cells" for AMD. N. Engl. J. Med 376, 1047–1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuroda T, Yasuda S, Kusakawa S, Hirata N, Kanda Y, Suzuki K, Takahashi M, Nishikawa S, Kawamata S, Sato Y, 2012. Highly sensitive in vitro methods for detection of residual undifferentiated cells in retinal pigment epithelial cells derived from human iPS cells. PLoS One 7, e37342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lade DC, 2017. Government website steers patients to unproven medical treatments, Sun Sentinel, Orlando. [Google Scholar]
- Lakowski J, Baron M, Bainbridge J, Barber AC, Pearson RA, Ali RR, Sowden JC, 2010. Cone and rod photoreceptor transplantation in models of the childhood retinopathy Leber congenital amaurosis using flow-sorted Crx-positive donor cells. Hum. Mol. Genet 19, 4545–4559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lakowski J, Gonzalez-Cordero A, West EL, Han YT, Welby E, Naeem A, Blackford SJ, Bainbridge JW, Pearson RA, Ali RR, Sowden JC, 2015. Transplantation of Photoreceptor Precursors Isolated via a Cell Surface Biomarker Panel From Embryonic Stem Cell-Derived Self-Forming Retina. Stem Cells 33, 2469–2482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamba DA, Gust J, Reh TA, 2009. Transplantation of human embryonic stem cell-derived photoreceptors restores some visual function in Crx-deficient mice. Cell Stem Cell 4, 73–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamba DA, Karl MO, Ware CB, Reh TA, 2006. Efficient generation of retinal progenitor cells from human embryonic stem cells. Proc. Natl. Acad. Sci. U. S. A 103, 12769–12774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamba DA, McUsic A, Hirata RK, Wang PR, Russell D, Reh TA, 2010. Generation, purification and transplantation of photoreceptors derived from human induced pluripotent stem cells. PLoS One 5, e8763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavail MM, Li L, Turner JE, Yasumura D, 1992. Retinal pigment epithelial cell transplantation in RCS rats: normal metabolism in rescued photoreceptors. Exp. Eye Res 55, 555–562. [DOI] [PubMed] [Google Scholar]
- Le Blanc K, 2006. Mesenchymal stromal cells: Tissue repair and immune modulation. Cytotherapy 8, 559–561. [DOI] [PubMed] [Google Scholar]
- Lee OK, Kuo TK, Chen WM, Lee KD, Hsieh SL, Chen TH, 2004. Isolation of multipotent mesenchymal stem cells from umbilical cord blood. Blood 103, 1669–1675. [DOI] [PubMed] [Google Scholar]
- Lee SJ, Klein J, Haagenson M, Baxter-Lowe LA, Confer DL, Eapen M, Fernandez-Vina M, Flomenberg N, Horowitz M, Hurley CK, Noreen H, Oudshoorn M, Petersdorf E, Setterholm M, Spellman S, Weisdorf D, Williams TM, Anasetti C, 2007. High-resolution donor-recipient HLA matching contributes to the success of unrelated donor marrow transplantation. Blood 110, 4576–4583. [DOI] [PubMed] [Google Scholar]
- Leung EH, Flynn HW Jr., Albini TA, Medina CA, 2016. Retinal Detachment After Subretinal Stem Cell Transplantation. Ophthalmic Surg Lasers Imaging Retina 47, 600–601. [DOI] [PubMed] [Google Scholar]
- Levin LA, Miller JW, Zack DJ, Friedlander M, Smith LEH, 2017. Special commentary: Early clinical development of cell replacement therapy: Considerations for the National Eye Institute Audacious Goals Initiative. Ophthalmology 124, 926–934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis RA, von Wolff K, Tetz M, Koerber N, Kearney JR, Shingleton BJ, Samuelson TW, 2009. Canaloplasty: Circumferential viscodilation and tensioning of Schlemm canal using a flexible microcatheter for the treatment of open-angle glaucoma in adults. Two-year interim clinical study results. J. Cataract Refract. Surg 35, 814–824. [DOI] [PubMed] [Google Scholar]
- Lewis RA, Von Wolff K, Tetz M, Koerber N, Kearney JR, Shingleton BJ, Samuelson TW, 2011. Canaloplasty: Three-year results of circumferential viscodilation and tensioning of Schlemm canal using a microcatheter to treat open-angle glaucoma. J. Cataract Refract. Surg 37, 682–690. [DOI] [PubMed] [Google Scholar]
- Li N, Li XR, Yuan JQ, 2009. Effects of bone-marrow mesenchymal stem cells transplanted into vitreous cavity of rat injured by ischemia/reperfusion. Graefes Arch. Clin. Exp. Ophthalmol 247, 503–514. [DOI] [PubMed] [Google Scholar]
- Lister R, Pelizzola M, Kida YS, Hawkins RD, Nery JR, Hon G, Antosiewicz-Bourget J, O'Malley R, Castanon R, Klugman S, Downes M, Yu R, Stewart R, Ren B, Thomson JA, Evans RM, Ecker JR, 2011. Hotspots of aberrant epigenomic reprogramming in human induced pluripotent stem cells. Nature 471, 68–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Chen SJ, Li SY, Qu LH, Meng XH, Wang Y, Xu HW, Liang ZQ, Yin ZQ, 2017. Long-Term safety of human retinal progenitor cell transplantation in retinitis pigmentosa patients. Stem Cell Research and Therapy 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lluis F, Cosma MP, 2010. Cell-fusion-mediated somatic-cell reprogramming: a mechanism for tissue regeneration. J. Cell. Physiol 223, 6–13. [DOI] [PubMed] [Google Scholar]
- Lu B, Malcuit C, Wang S, Girman S, Francis P, Lemieux L, Lanza R, Lund R, 2009. Long-term safety and function of RPE from human embryonic stem cells in preclinical models of macular degeneration. Stem Cells 27, 2126–2135. [DOI] [PubMed] [Google Scholar]
- Lund RD, Wang S, Lu B, Girman S, Holmes T, Sauve Y, Messina DJ, Harris IR, Kihm AJ, Harmon AM, Chin FY, Gosiewska A, Mistry SK, 2007. Cells isolated from umbilical cord tissue rescue photoreceptors and visual functions in a rodent model of retinal disease. Stem Cells 25, 602–611. [DOI] [PubMed] [Google Scholar]
- Luo J, Baranov P, Patel S, Ouyang H, Quach J, Wu F, Qiu A, Luo H, Hicks C, Zeng J, Zhu J, Lu J, Sfeir N, Wen C, Zhang M, Reade V, Patel S, Sinden J, Sun X, Shaw P, Young M, Zhang K, 2014. Human retinal progenitor cell transplantation preserves vision. J. Biol. Chem 289, 6362–6371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maaijwee K, Heimann H, Missotten T, Mulder P, Joussen A, van Meurs J, 2007a. Retinal pigment epithelium and choroid translocation in patients with exudative age-related macular degeneration: long-term results. Graefes Arch. Clin. Exp. Ophthalmol 245, 1681–1689. [DOI] [PubMed] [Google Scholar]
- Maaijwee K, Joussen AM, Kirchhof B, van Meurs JC, 2008. Retinal pigment epithelium (RPE)-choroid graft translocation in the treatment of an RPE tear: preliminary results. Br. J. Ophthalmol 92, 526–529. [DOI] [PubMed] [Google Scholar]
- Maaijwee KJ, van Meurs JC, Kirchhof B, Mooij CM, Fischer JH, Mackiewicz J, Kobuch K, Joussen AM, 2007b. Histological evidence for revascularisation of an autologous retinal pigment epithelium--choroid graft in the pig. Br. J. Ophthalmol 91, 546–550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machalinska A, Baumert B, Kuprjanowicz L, Wiszniewska B, Karczewicz D, Machalinski B, 2009. Potential application of adult stem cells in retinal repair-challenge for regenerative medicine. Curr. Eye Res 34, 748–760. [DOI] [PubMed] [Google Scholar]
- Machemer R, Aaberg TM, Freeman HM, Irvine AR, Lean JS, Michels RM, 1991. An updated classification of retinal detachment with proliferative vitreoretinopathy. Am. J. Ophthalmol 112, 159–165. [DOI] [PubMed] [Google Scholar]
- Mack AA, Kroboth S, Rajesh D, Wang WB, 2011. Generation of induced pluripotent stem cells from CD34+ cells across blood drawn from multiple donors with non-integrating episomal vectors. PLoS One 6, e27956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackie AR, Losordo DW, 2011. CD34-positive stem cells: in the treatment of heart and vascular disease in human beings. Tex. Heart Inst. J. 38, 474–485. [PMC free article] [PubMed] [Google Scholar]
- MacLaren RE, Pearson RA, MacNeil A, Douglas RH, Salt TE, Akimoto M, Swaroop A, Sowden JC, Ali RR, 2006. Retinal repair by transplantation of photoreceptor precursors. Nature 444, 203–207. [DOI] [PubMed] [Google Scholar]
- Maeda T, Lee MJ, Palczewska G, Marsili S, Tesar PJ, Palczewski K, Takahashi M, Maeda A, 2013. Retinal pigmented epithelial cells obtained from human induced pluripotent stem cells possess functional visual cycle enzymes in vitro and in vivo. J. Biol. Chem 288, 34484–34493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mali P, Yang L, Esvelt KM, Aach J, Guell M, DiCarlo JE, Norville JE, Church GM, 2013. RNA-guided human genome engineering via Cas9. Science 339, 823–826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandai M, Fujii M, Hashiguchi T, Sunagawa GA, Ito SI, Sun J, Kaneko J, Sho J, Yamada C, Takahashi M, 2017a. iPSC-Derived Retina Transplants Improve Vision in rd1 End-Stage Retinal-Degeneration Mice. Stem Cell Reports 8, 69–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandai M, Watanabe A, Kurimoto Y, Hirami Y, Morinaga C, Daimon T, Fujihara M, Akimaru H, Sakai N, Shibata Y, Terada M, Nomiya Y, Tanishima S, Nakamura M, Kamao H, Sugita S, Onishi A, Ito T, Fujita K, Kawamata S, Go MJ, Shinohara C, Hata K, Sawada M, Yamamoto M, Ohta S, Ohara Y, Yoshida K, Kuwahara J, Kitano Y, Amano N, Umekage M, Kitaoka F, Tanaka A, Okada C, Takasu N, Ogawa S, Yamanaka S, Takahashi M, 2017b. Autologous induced stem-cell-derived retinal cells for macular degeneration. N. Engl. J. Med 376, 1038–1046. [DOI] [PubMed] [Google Scholar]
- May-Simera HL, Wan Q, Jha BS, Hartford J, Khristov V, Dejene R, Chang J, Patnaik S, Lu Q, Banerjee P, Silver J, Insinna-Kettenhofen C, Patel D, Lotfi M, Malicdan M, Hotaling N, Maminishkis A, Sridharan R, Brooks B, Miyagishima K, Gunay-Aygun M, Pal R, Westlake C, Miller S, Sharma R, Bharti K, 2018. Primary Cilium-Mediated Retinal Pigment Epithelium Maturation Is Disrupted in Ciliopathy Patient Cells. Cell reports 22, 189–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGill TJ, Stoddard J, Renner LM, Messaoudi I, Bharti K, Mitalipov S, Lauer A, Wilson DJ, Neuringer M, 2018. Allogeneic iPSC-Derived RPE Cell Graft Failure Following Transplantation Into the Subretinal Space in Nonhuman Primates. Invest. Ophthalmol. Vis. Sci 59, 1374–1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MD Stem Cells, 2018. Press Releases [Google Scholar]
- Mertens J, 2018. YOUR HEALTH: Stem cell therapy that helps restore lost sight. WQAD; 8. [Google Scholar]
- Meyer JS, Howden SE, Wallace KA, Verhoeven AD, Wright LS, Capowski EE, Pinilla I, Martin JM, Tian S, Stewart R, Pattnaik B, Thomson JA, Gamm DM, 2011. Optic vesicle-like structures derived from human pluripotent stem cells facilitate a customized approach to retinal disease treatment. Stem Cells 29, 1206–1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer JS, Shearer RL, Capowski EE, Wright LS, Wallace KA, McMillan EL, Zhang SC, Gamm DM, 2009. Modeling early retinal development with human embryonic and induced pluripotent stem cells. Proc. Natl. Acad. Sci. U. S. A 106, 16698–16703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyagishima KJ, Wan Q, Corneo B, Sharma R, Lotfi MR, Boles NC, Hua F, Maminishkis A, Zhang C, Blenkinsop T, Khristov V, Jha BS, Memon OS, D'Souza S, Temple S, Miller SS, Bharti K, 2016. In Pursuit of Authenticity: Induced Pluripotent Stem Cell-Derived Retinal Pigment Epithelium for Clinical Applications. Stem cells translational medicine 5, 1562–1574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyagishima KJ, Wan Q, Miller SS, Bharti K, 2017. A basis for comparison: sensitive authentication of stem cell derived RPE using physiological responses of intact RPE monolayers. Stem Cell Transl Investig 4. [PMC free article] [PubMed] [Google Scholar]
- Moisseiev E, Smit-McBride Z, Oltjen S, Zhang P, Zawadzki RJ, Motta M, Murphy CJ, Cary W, Annett G, Nolta JA, Park SS, 2016. Intravitreal Administration of Human Bone Marrow CD34+ Stem Cells in a Murine Model of Retinal Degeneration. Invest. Ophthalmol. Vis. Sci 57, 4125–4135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagiel A, Schwartz SD, 2018. Stem Cells for Diseases of the Retina, 3 ed. Elsevier, Cambridge, MA. [Google Scholar]
- Nakano T, Ando S, Takata N, Kawada M, Muguruma K, Sekiguchi K, Saito K, Yonemura S, Eiraku M, Sasai Y, 2012. Self-formation of optic cups and storable stratified neural retina from human ESCs. Cell Stem Cell 10, 771–785. [DOI] [PubMed] [Google Scholar]
- Niemansburg SL, van Delden JJ, Oner FC, Dhert WJ, Bredenoord AL, 2014. Ethical implications of regenerative medicine in orthopedics: an empirical study with surgeons and scientists in the field. Spine J 14, 1029–1035. [DOI] [PubMed] [Google Scholar]
- Nirwan RS, Albini TA, Sridhar J, Flynn HW Jr., Kuriyan AE, 2019. Assessing "Cell Therapy" Clinics Offering Treatments of Ocular Conditions using Direct-to-Consumer Marketing Websites in the United States. Ophthalmology. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Odorico JS, Kaufman DS, Thomson JA, 2001. Multilineage differentiation from human embryonic stem cell lines. Stem Cells 19, 193–204. [DOI] [PubMed] [Google Scholar]
- Ohi Y, Qin H, Hong C, Blouin L, Polo JM, Guo T, Qi Z, Downey SL, Manos PD, Rossi DJ, Yu J, Hebrok M, Hochedlinger K, Costello JF, Song JS, Ramalho-Santos M, 2011. Incomplete DNA methylation underlies a transcriptional memory of somatic cells in human iPS cells. Nat. Cell Biol 13, 541–549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortin-Martinez A, Tsai ELS, Nickerson PE, Bergeret M, Lu Y, Smiley S, Comanita L, Wallace VA, 2017. A Reinterpretation of Cell Transplantation: GFP Transfer From Donor to Host Photoreceptors. Stem Cells 35, 932–939. [DOI] [PubMed] [Google Scholar]
- Osakada F, Ikeda H, Mandai M, Wataya T, Watanabe K, Yoshimura N, Akaike A, Sasai Y, Takahashi M, 2008. Toward the generation of rod and cone photoreceptors from mouse, monkey and human embryonic stem cells. Nat. Biotechnol 26, 215–224. [DOI] [PubMed] [Google Scholar]
- Otani A, Dorrell MI, Kinder K, Moreno SK, Nusinowitz S, Banin E, Heckenlively J, Friedlander M, 2004. Rescue of retinal degeneration by intravitreally injected adult bone marrow-derived lineage-negative hematopoietic stem cells. J. Clin. Invest 114, 765–774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan CK, Heilweil G, Lanza R, Schwartz SD, 2013. Embryonic stem cells as a treatment for macular degeneration. Expert Opin. Biol. Ther 13, 1125–1133. [DOI] [PubMed] [Google Scholar]
- Pang WW, Price EA, Sahoo D, Beerman I, Maloney WJ, Rossi DJ, Schrier SL, Weissman IL, 2011. Human bone marrow hematopoietic stem cells are increased in frequency and myeloid-biased with age. Proc. Natl. Acad. Sci. U. S. A 108, 20012–20017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park SS, 2016. Cell Therapy Applications for Retinal Vascular Diseases: Diabetic Retinopathy and Retinal Vein Occlusion. Invest. Ophthalmol. Vis. Sci 57, ORSFj1–ORSFj10. [DOI] [PubMed] [Google Scholar]
- Park SS, Bauer G, Abedi M, Pontow S, Panorgias A, Jonnal R, Zawadzki RJ, Werner JS, Nolta J, 2014. Intravitreal autologous bone marrow CD34+ cell therapy for ischemic and degenerative retinal disorders: preliminary phase 1 clinical trial findings. Invest. Ophthalmol. Vis. Sci 56, 81–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park SS, Caballero S, Bauer G, Shibata B, Roth A, Fitzgerald PG, Forward KI, Zhou P, McGee J, Telander DG, Grant MB, Nolta JA, 2012. Long-term effects of intravitreal injection of GMP-grade bone-marrow-derived CD34+ cells in NOD-SCID mice with acute ischemia-reperfusion injury. Invest. Ophthalmol. Vis. Sci 53, 986–994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park SS, Moisseiev E, Bauer G, Anderson JD, Grant MB, Zam A, Zawadzki RJ, Werner JS, Nolta JA, 2017. Advances in bone marrow stem cell therapy for retinal dysfunction. Prog. Retin. Eye Res 56, 148–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pastor JC, 1998. Proliferative vitreoretinopathy: An overview. Surv. Ophthalmol 43, 3–18. [DOI] [PubMed] [Google Scholar]
- Pearson RA, Gonzalez-Cordero A, West EL, Ribeiro JR, Aghaizu N, Goh D, Sampson RD, Georgiadis A, Waldron PV, Duran Y, Naeem A, Kloc M, Cristante E, Kruczek K, Warre-Cornish K, Sowden JC, Smith AJ, Ali RR, 2016. Donor and host photoreceptors engage in material transfer following transplantation of post-mitotic photoreceptor precursors. Nature communications 7, 13029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips MJ, Wallace KA, Dickerson SJ, Miller MJ, Verhoeven AD, Martin JM, Wright LS, Shen W, Capowski EE, Percin EF, Perez ET, Zhong X, Canto-Soler MV, Gamm DM, 2012. Blood-derived human iPS cells generate optic vesicle-like structures with the capacity to form retinal laminae and develop synapses. Invest. Ophthalmol. Vis. Sci 53, 2007–2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polak JM, Bishop AE, 2006. Stem cells and tissue engineering: past, present, and future. Ann. N. Y. Acad. Sci 1068, 352–366. [DOI] [PubMed] [Google Scholar]
- Posel C, Moller K, Frohlich W, Schulz I, Boltze J, Wagner DC, 2012. Density gradient centrifugation compromises bone marrow mononuclear cell yield. PLoS One 7, e50293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quyyumi AA, Vasquez A, Kereiakes DJ, Klapholz M, Schaer GL, Abdel-Latif A, Frohwein S, Henry TD, Schatz RA, Dib N, Toma C, Davidson CJ, Barsness GW, Shavelle DM, Cohen M, Poole J, Moss T, Hyde P, Kanakaraj AM, Druker V, Chung A, Junge C, Preti RA, Smith RL, Mazzo DJ, Pecora A, Losordo DW, 2017. PreSERVE-AMI: A Randomized, Double-Blind, Placebo-Controlled Clinical Trial of Intracoronary Administration of Autologous CD34+ Cells in Patients With Left Ventricular Dysfunction Post STEMI. Circ. Res 120, 324–331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radtke ND, Aramant RB, Petry HM, Green PT, Pidwell DJ, Seiler MJ, 2008. Vision improvement in retinal degeneration patients by implantation of retina together with retinal pigment epithelium. Am. J. Ophthalmol 146, 172–182. [DOI] [PubMed] [Google Scholar]
- Rafii S, Lyden D, 2003. Therapeutic stem and progenitor cell transplantation for organ vascularization and regeneration. Nat. Med 9, 702–712. [DOI] [PubMed] [Google Scholar]
- Ratnapriya R, Chew EY, 2013. Age-related macular degeneration-clinical review and genetics update. Clin. Genet 84, 160–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rong AJ, Lam BL, Ansari ZA, Albini TA, 2018. Vision Loss Secondary to Autologous Adipose Stem Cell Injections: A Rising Problem. JAMA Ophthalmol 136, 97–99. [DOI] [PubMed] [Google Scholar]
- Roper J, Tammela T, Cetinbas NM, Akkad A, Roghanian A, Rickelt S, Almeqdadi M, Wu K, Oberli MA, Sánchez-Rivera F, Park YK, Liang X, Eng G, Taylor MS, Azimi R, Kedrin D, Neupane R, Beyaz S, Sicinska ET, Suarez Y, Yoo J, Chen L, Zukerberg L, Katajisto P, Deshpande V, Bass AJ, Tsichlis PN, Lees J, Langer R, Hynes RO, Chen J, Bhutkar A, Jacks T, Yilmaz ÖH, 2017. In vivo genome editing and organoid transplantation models of colorectal cancer and metastasis. Nat. Biotechnol 35, 569–576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan JM, Barry FP, Murphy JM, Mahon BP, 2005. Mesenchymal stem cells avoid allogeneic rejection. Journal of inflammation (London, England) 2, 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saini JS, Temple S, Stern JH, 2016. Human Retinal Pigment Epithelium Stem Cell (RPESC). Adv. Exp. Med. Biol 854, 557–562. [DOI] [PubMed] [Google Scholar]
- Salero E, Blenkinsop TA, Corneo B, Harris A, Rabin D, Stern JH, Temple S, 2012. Adult human RPE can be activated into a multipotent stem cell that produces mesenchymal derivatives. Cell Stem Cell 10, 88–95. [DOI] [PubMed] [Google Scholar]
- Sanges D, Simonte G, Di Vicino U, Romo N, Pinilla I, Nicolas M, Cosma MP, 2016. Reprogramming Muller glia via in vivo cell fusion regenerates murine photoreceptors. J. Clin. Invest 126, 3104–3116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santos-Ferreira T, Llonch S, Borsch O, Postel K, Haas J, Ader M, 2016a. Retinal transplantation of photoreceptors results in donor-host cytoplasmic exchange. Nature communications 7, 13028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santos-Ferreira T, Volkner M, Borsch O, Haas J, Cimalla P, Vasudevan P, Carmeliet P, Corbeil D, Michalakis S, Koch E, Karl MO, Ader M, 2016b. Stem Cell-Derived Photoreceptor Transplants Differentially Integrate Into Mouse Models of Cone-Rod Dystrophy. Invest. Ophthalmol. Vis. Sci 57, 3509–3520. [DOI] [PubMed] [Google Scholar]
- Saraf SS, Cunningham MA, Kuriyan AE, Read SP, Rosenfeld PJ, Flynn HW Jr., Albini TA, 2017. Bilateral retinal detachments after intravitreal injection of adipose-derived 'Stem Cells' in a patient with exudative macular degeneration. Ophthalmic Surgery Lasers and Imaging Retina 48, 772–775. [DOI] [PubMed] [Google Scholar]
- Satarian L, Nourinia R, Safi S, Kanavi MR, Jarughi N, Daftarian N, Arab L, Aghdami N, Ahmadieh H, Baharvand H, 2017. Intravitreal Injection of Bone Marrow Mesenchymal Stem Cells in Patients with Advanced Retinitis Pigmentosa; a Safety Study. Journal of Ophthalmic & Vision Research 12, 58–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitt S, Aftab U, Jiang C, Redenti S, Klassen H, Miljan E, Sinden J, Young M, 2009. Molecular characterization of human retinal progenitor cells. Invest. Ophthalmol. Vis. Sci 50, 5901–5908. [DOI] [PubMed] [Google Scholar]
- Schwank G, Koo BK, Sasselli V, Dekkers JF, Heo I, Demircan T, Sasaki N, Boymans S, Cuppen E, Van Der Ent CK, Nieuwenhuis EES, Beekman JM, Clevers H, 2013. Functional repair of CFTR by CRISPR/Cas9 in intestinal stem cell organoids of cystic fibrosis patients. Cell Stem Cell 13, 653–658. [DOI] [PubMed] [Google Scholar]
- Schwartz SD, Hubschman J-P, Heilwell G, Franco-Cardenas V, Pan CK, Ostrick RM, Mickunas E, Gay R, Klimanskaya I, Lanza R, 2012a. Embryonic stem cell trials for macular degeneration: a preliminary report. The Lancet. [DOI] [PubMed] [Google Scholar]
- Schwartz SD, Hubschman JP, Heilwell G, Franco-Cardenas V, Pan CK, Ostrick RM, Mickunas E, Gay R, Klimanskaya I, Lanza R, 2012b. Embryonic stem cell trials for macular degeneration: a preliminary report. Lancet 379, 713–720. [DOI] [PubMed] [Google Scholar]
- Schwartz SD, Regillo CD, Lam BL, Eliott D, Rosenfeld PJ, Gregori NZ, Hubschman J-P, Davis JL, Heilwell G, Spirn M, Maguire J, Gay R, Bateman J, Ostrick RM, Morris D, Vincent M, Anglade E, Del Priore LV, Lanza R, 2015a. Human embryonic stem cell-derived retinal pigment epithelium in patients with age-related macular degeneration and Stargardt's macular dystrophy: follow-up of two open-label phase 1/2 studies. The Lancet 385, 509–516. [DOI] [PubMed] [Google Scholar]
- Schwartz SD, Regillo CD, Lam BL, Eliott D, Rosenfeld PJ, Gregori NZ, Hubschman JP, Davis JL, Heilwell G, Spirn M, Maguire J, Gay R, Bateman J, Ostrick RM, Morris D, Vincent M, Anglade E, Del Priore LV, Lanza R, 2015b. Human embryonic stem cell-derived retinal pigment epithelium in patients with age-related macular degeneration and Stargardt's macular dystrophy: follow-up of two open-label phase 1/2 studies. Lancet 385, 509–516. [DOI] [PubMed] [Google Scholar]
- Schwartz SD, Tan G, Hosseini H, Nagiel A, 2016. Subretinal transplantation of embryonic stem cell-derived retinal pigment epithelium for the treatment of macular degeneration: An assessment at 4 years. Invest. Ophthalmol. Vis. Sci 57, ORSFc1–ORSFc9. [DOI] [PubMed] [Google Scholar]
- Scruggs BA, Jiao C, Cranston CM, Kaalberg E, Wang K, Russell SR, Wiley LA, Mullins RF, Stone EM, Tucker BA, Sohn EH, 2019. Optimizing Donor Cellular Dissociation and Subretinal Injection Parameters for Stem Cell-Based Treatments. Stem cells translational medicine 8, 797–809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sears AE, Bernstein PS, Cideciyan AV, Hoyng C, Charbel Issa P, Palczewski K, Rosenfeld PJ, Sadda S, Schraermeyer U, Sparrow JR, Washington I, Scholl HPN, 2017. Towards Treatment of Stargardt Disease: Workshop Organized and Sponsored by the Foundation Fighting Blindness. Transl Vis Sci Technol 6, 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seiler MJ, Aramant RB, 2012a. Cell replacement and visual restoration by retinal sheet transplants. Prog. Retin. Eye Res 31, 661–687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seiler MJ, Aramant RB, 2012b. Cell replacement and visual restoration by retinal sheet transplants. Prog. Retin. Eye Res 31, 661–687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selden NR, Al-Uzri A, Huhn SL, Koch TK, Sikora DM, Nguyen-Driver MD, Guillaume DJ, Koh JL, Gultekin SH, Anderson JC, Vogel H, Sutcliffe TL, Jacobs Y, Steiner RD, 2013. Central nervous system stem cell transplantation for children with neuronal ceroid lipofuscinosis. J. Neurosurg. Pediatr 11, 643–652. [DOI] [PubMed] [Google Scholar]
- Sezer O, Possinger K, Metzner B, Illiger HJ, Wattad M, Heit W, Fuss H, Schultze W, 2000. Optimal CD34(+) cell dose in autologous peripheral-blood stem-cell transplantation. J. Clin. Oncol 18, 3319–3320. [DOI] [PubMed] [Google Scholar]
- Sharma A, Burridge PW, McKeithan WL, Serrano R, Shukla P, Sayed N, Churko JM, Kitani T, Wu H, Holmstrom A, Matsa E, Zhang Y, Kumar A, Fan AC, Del Alamo JC, Wu SM, Moslehi JJ, Mercola M, Wu JC, 2017. High-throughput screening of tyrosine kinase inhibitor cardiotoxicity with human induced pluripotent stem cells. Sci. Transl. Med 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen JK, Dong A, Hackett SF, Bell WR, Green WR, Campochiaro PA, 2007. Oxidative damage in age-related macular degeneration. Histol. Histopathol 22, 1301–1308. [DOI] [PubMed] [Google Scholar]
- Shirai H, Mandai M, Matsushita K, Kuwahara A, Yonemura S, Nakano T, Assawachananont J, Kimura T, Saito K, Terasaki H, Eiraku M, Sasai Y, Takahashi M, 2016. Transplantation of human embryonic stem cell-derived retinal tissue in two primate models of retinal degeneration. Proc. Natl. Acad. Sci. U. S. A 113, E81–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sieving PA, Caruso RC, Tao W, Coleman HR, Thompson DJ, Fullmer KR, Bush RA, 2006. Ciliary neurotrophic factor (CNTF) for human retinal degeneration: phase I trial of CNTF delivered by encapsulated cell intraocular implants. Proc. Natl. Acad. Sci. U. S. A 103, 3896–3901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh MS, Aslam SA, Duncan IL, Cramer AO, Barnard AR, MacLaren RE, 2014. Cell fusion following photoreceptor transplantation into the non-degenerate retina. Invest. Ophthalmol. Vis. Sci 55, 3989–3989. [Google Scholar]
- Singh MS, Balmer J, Barnard AR, Aslam SA, Moralli D, Green CM, Barnea-Cramer A, Duncan I, MacLaren RE, 2016. Transplanted photoreceptor precursors transfer proteins to host photoreceptors by a mechanism of cytoplasmic fusion. Nature communications 7, 13537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh MS, Charbel Issa P, Butler R, Martin C, Lipinski DM, Sekaran S, Barnard AR, MacLaren RE, 2013. Reversal of end-stage retinal degeneration and restoration of visual function by photoreceptor transplantation. Proceedings of the National Academy of Sciences 110, 1101–1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh MS, MacLaren RE, 2011. Stem cells as a therapeutic tool for the blind: biology and future prospects. Proc. Biol. Sci 278, 3009–3016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh MS, MacLaren RE, 2018. Stem Cell Treatment for Age-Related Macular Degeneration: the Challenges. Invest. Ophthalmol. Vis. Sci 59, Amd78–amd82. [DOI] [PubMed] [Google Scholar]
- Siqueira RC, Messias A, Gurgel VP, Simoes BP, Scott IU, Jorge R, 2015. Improvement of ischaemic macular oedema after intravitreal injection of autologous bone marrow-derived haematopoietic stem cells. Acta Ophthalmol 93, e174–176. [DOI] [PubMed] [Google Scholar]
- Siqueira RC, Messias A, Voltarelli JC, Messias K, Arcieri RS, Jorge R, 2013. Resolution of macular oedema associated with retinitis pigmentosa after intravitreal use of autologous BM-derived hematopoietic stem cell transplantation. Bone Marrow Transplant. 48, 612–613. [DOI] [PubMed] [Google Scholar]
- Siqueira RC, Messias A, Voltarelli JC, Scott IU, Jorge R, 2011. Intravitreal injection of autologous bone marrow-derived mononuclear cells for hereditary retinal dystrophy: a phase I trial. Retina 31, 1207–1214. [DOI] [PubMed] [Google Scholar]
- Smiley S, Nickerson PE, Comanita L, Daftarian N, El-Sehemy A, Tsai EL, Matan-Lithwick S, Yan K, Thurig S, Touahri Y, Dixit R, Aavani T, De Repentingy Y, Baker A, Tsilfidis C, Biernaskie J, Sauve Y, Schuurmans C, Kothary R, Mears AJ, Wallace VA, 2016. Establishment of a cone photoreceptor transplantation platform based on a novel cone-GFP reporter mouse line. Sci. Rep 6, 22867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobrin L, Seddon JM, 2014. Nature and nurture- genes and environment- predict onset and progression of macular degeneration. Prog. Retin. Eye Res 40, 1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sohn EH, Jiao C, Kaalberg E, Cranston C, Mullins RF, Stone EM, Tucker BA, 2015. Allogenic iPSC-derived RPE cell transplants induce immune response in pigs: a pilot study. Sci. Rep 5, 11791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song MJ, Bharti K, 2016. Looking into the future: Using induced pluripotent stem cells to build two and three dimensional ocular tissue for cell therapy and disease modeling. Brain Res 1638, 2–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer R, Fisher S, Lewis GP, Malone T, 2017. Epiretinal membrane in a subject after transvitreal delivery of palucorcel (CNTO 2476). Clin. Ophthalmol 11, 1797–1803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanzel BV, Liu Z, Somboonthanakij S, Wongsawad W, Brinken R, Eter N, Corneo B, Holz FG, Temple S, Stern JH, Blenkinsop TA, 2014. Human RPE stem cells grown into polarized RPE monolayers on a polyester matrix are maintained after grafting into rabbit subretinal space. Stem Cell Reports 2, 64–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strauss O, 2005. The retinal pigment epithelium in visual function. Physiol. Rev 85, 845–881. [DOI] [PubMed] [Google Scholar]
- Sugita S, Iwasaki Y, Makabe K, Kamao H, Mandai M, Shiina T, Ogasawara K, Hirami Y, Kurimoto Y, Takahashi M, 2016a. Successful Transplantation of Retinal Pigment Epithelial Cells from MHC Homozygote iPSCs in MHC-Matched Models. Stem Cell Reports 7, 635–648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugita S, Iwasaki Y, Makabe K, Kimura T, Futagami T, Suegami S, Takahashi M, 2016b. Lack of T Cell Response to iPSC-Derived Retinal Pigment Epithelial Cells from HLA Homozygous Donors. Stem Cell Reports 7, 619–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sunness JS, 2015. Stem cells in age-related macular degeneration and Stargardt's macular dystrophy. Lancet 386, 29. [DOI] [PubMed] [Google Scholar]
- Takagi S, Mandai M, Hirami Y, Sugita S, Takahashi M, Kurimoto Y, 2018. Frequencies of human leukocyte antigen alleles and haplotypes among Japanese patients with age-related macular degeneration. Jpn. J. Ophthalmol [DOI] [PubMed] [Google Scholar]
- Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichisaka T, Tomoda K, Yamanaka S, 2007. Induction of Pluripotent Stem Cells from Adult Human Fibroblasts by Defined Factors. Cell 131, 861–872. [DOI] [PubMed] [Google Scholar]
- Takahashi M, 2016. [Retinal Cell Therapy Using iPS Cells]. Nippon Ganka Gakkai zasshi 120, 210–224; discussion 225. [PubMed] [Google Scholar]
- Takahashi M, Palmer TD, Takahashi J, Gage FH, 1998. Widespread integration and survival of adult-derived neural progenitor cells in the developing optic retina. Mol. Cell. Neurosci 12, 340–348. [DOI] [PubMed] [Google Scholar]
- Tetz M, Rizzo S, Augustin AJ, 2012. Safety of submacular suprachoroidal drug administration via a microcatheter: Retrospective analysis of European treatment results. Ophthalmologica 227, 183–189. [DOI] [PubMed] [Google Scholar]
- Théry C, Zitvogel L, Amigorena S, 2002. Exosomes: Composition, biogenesis and function. Nature Reviews Immunology 2, 569–579. [DOI] [PubMed] [Google Scholar]
- Thomas BB, Zhu D, Zhang L, Thomas PB, Hu Y, Nazari H, Stefanini F, Falabella P, Clegg DO, Hinton DR, Humayun MS, 2016. Survival and Functionality of hESC-Derived Retinal Pigment Epithelium Cells Cultured as a Monolayer on Polymer Substrates Transplanted in RCS Rats. Invest. Ophthalmol. Vis. Sci 57, 2877–2887. [DOI] [PubMed] [Google Scholar]
- Tomita M, Adachi Y, Yamada H, Takahashi K, Kiuchi K, Oyaizu H, Ikebukuro K, Kaneda H, Matsumura M, Ikehara S, 2002. Bone marrow-derived stem cells can differentiate into retinal cells in injured rat retina. Stem Cells 20, 279–283. [DOI] [PubMed] [Google Scholar]
- Tomita M, Lavik E, Klassen H, Zahir T, Langer R, Young MJ, 2005. Biodegradable polymer composite grafts promote the survival and differentiation of retinal progenitor cells. Stem Cells 23, 1579–1588. [DOI] [PubMed] [Google Scholar]
- Tucker BA, Redenti SM, Jiang C, Swift JS, Klassen HJ, Smith ME, Wnek GE, Young MJ, 2010. The use of progenitor cell/biodegradable MMP2-PLGA polymer constructs to enhance cellular integration and retinal repopulation. Biomaterials 31, 9–19. [DOI] [PubMed] [Google Scholar]
- Turner DL, Cepko CL, 1988. A common progenitor for neurons and glia persists in rat retina late in development. Nature 328, 131–136. [DOI] [PubMed] [Google Scholar]
- Turner DL, Snyder EY, Cepko CL, 1990. Lineage-independent determination of cell type in the embryonic mouse retina. Neuron 4, 833–845. [DOI] [PubMed] [Google Scholar]
- Turner JE, Blair JR, 1986. Newborn rat retinal cells transplanted into a retinal lesion site in adult host eyes. Brain Res 391, 91–104. [DOI] [PubMed] [Google Scholar]
- Turner JE, Seiler M, Aramant R, Blair JR, 1988. Embryonic retinal grafts transplanted into the lesioned adult rat retina. Prog. Brain Res 78, 131–139. [DOI] [PubMed] [Google Scholar]
- Turner L, Knoepfler P, 2016. Selling Stem Cells in the USA: Assessing the Direct-to-Consumer Industry. Cell Stem Cell 19, 154–157. [DOI] [PubMed] [Google Scholar]
- Tzameret A, Sher I, Belkin M, Treves AJ, Meir A, Nagler A, Levkovitch-Verbin H, Barshack I, Rosner M, Rotenstreich Y, 2014. Transplantation of human bone marrow mesenchymal stem cells as a thin subretinal layer ameliorates retinal degeneration in a rat model of retinal dystrophy. Exp. Eye Res 118, 135–144. [DOI] [PubMed] [Google Scholar]
- University of Nebraska Medical Center, 2014. Foundation for the Accreditation of Cellular Therapy [Google Scholar]
- US Clinical Trials.gov, 2018. Stem Cell Ophthalmology Treatment Study (SCOTS) U.S. National Library of Medicine
- Valadi H, Ekström K, Bossios A, Sjöstrand M, Lee JJ, Lötvall JO, 2007. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat. Cell Biol 9, 654–659. [DOI] [PubMed] [Google Scholar]
- Van Hoffelen SJ, Young MJ, Shatos MA, Sakaguchi DS, 2003. Incorporation of murine brain progenitor cells into the developing mammalian retina. Invest. Ophthalmol. Vis. Sci 44, 426–434. [DOI] [PubMed] [Google Scholar]
- van Meurs J, van Romunde, van Zeeburg ETJ, Pertile G, 2018. Inflammatory destruction of the RPE of an autologous RPE and choroid graft? Invest Ophthalmol Vis Sci 59, 849. [Google Scholar]
- Venditti A, Battaglia A, Del Poeta G, Buccisano F, Maurillo L, Tamburini A, Del Moro A, Epiceno AM, Martiradonna M, Caravita T, Santinelli S, Adorno G, Picardi A, Zinno F, Lanti A, Bruno A, Suppo G, Franchi A, Franconi G, Amadori S, 1999. Enumeration of CD34+ hematopoietic progenitor cells for clinical transplantation: comparison of three different methods. Bone Marrow Transplant. 24, 1019–1027. [DOI] [PubMed] [Google Scholar]
- Verbakel SK, van Huet RAC, Boon CJF, den Hollander AI, Collin RWJ, Klaver CCW, Hoyng CB, Roepman R, Klevering BJ, 2018. Non-syndromic retinitis pigmentosa. Prog. Retin. Eye Res [DOI] [PubMed] [Google Scholar]
- Vrtovec B, Poglajen G, Sever M, Lezaic L, Socan A, Haddad F, Wu JC, 2013. CD34+ stem cell therapy in nonischemic dilated cardiomyopathy patients. Clin. Pharmacol. Ther 94, 452–458. [DOI] [PubMed] [Google Scholar]
- Vrtovec B, Sever M, Jensterle M, Poglajen G, Janez A, Kravos N, Zemljic G, Cukjati M, Cernelc P, Haddad F, Wu JC, Jorde UP, 2016. Efficacy of CD34+ Stem Cell Therapy in Nonischemic Dilated Cardiomyopathy Is Absent in Patients With Diabetes but Preserved in Patients With Insulin Resistance. Stem cells translational medicine 5, 632–638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waldron PV, Di Marco F, Kruczek K, Ribeiro J, Graca AB, Hippert C, Aghaizu ND, Kalargyrou AA, Barber AC, Grimaldi G, Duran Y, Blackford SJI, Kloc M, Goh D, Zabala Aldunate E, Sampson RD, Bainbridge JWB, Smith AJ, Gonzalez-Cordero A, Sowden JC, Ali RR, Pearson RA, 2018. Transplanted Donor- or Stem Cell-Derived Cone Photoreceptors Can Both Integrate and Undergo Material Transfer in an Environment-Dependent Manner. Stem Cell Reports 10, 406–421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W, Lee SJ, Scott PA, Lu X, Emery D, Liu Y, Ezashi T, Roberts MR, Ross JW, Kaplan HJ, Dean DC, 2016. Two-Step Reactivation of Dormant Cones in Retinitis Pigmentosa. Cell reports 15, 372–385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Zhang D, Shen B, Zhang Y, Gu P, 2018. Stem/progenitor cells and biodegradable scaffolds in the treatment of retinal degenerative diseases. Current Stem Cell Research and Therapy 13, 160–173. [DOI] [PubMed] [Google Scholar]
- Warfvinge K, Kiilgaard JF, Klassen H, Zamiri P, Scherfig E, Streilein W, Prause JU, Young MJ, 2006. Retinal progenitor cell xenografts to the pig retina: immunological reactions. Cell Transplant. 15, 603–612. [DOI] [PubMed] [Google Scholar]
- Warfvinge K, Kiilgaard JF, Lavik EB, et al. , 2005. Retinal progenitor cell xenografts to the pig retina: Morphologic integration and cytochemical differentiation. Arch. Ophthalmol 123, 1385–1393. [DOI] [PubMed] [Google Scholar]
- Warfvinge K, Schwartz PH, Kiilgaard JF, la Cour M, Young MJ, Scherfig E, Klassen H, 2011. Xenotransplantation of Human Neural Progenitor Cells to the Subretinal Space of Nonimmunosuppressed Pigs. Journal of Transplantation 2011, 948740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss JN, Benes SC, Levy S, 2016a. Stem Cell Ophthalmology Treatment Study (SCOTS): improvement in serpiginous choroidopathy following autologous bone marrow derived stem cell treatment. Neural regeneration research 11, 1512–1516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss JN, Levy S, Benes SC, 2015a. Stem Cell Ophthalmology Treatment Study (SCOTS) for retinal and optic nerve diseases: a case report of improvement in relapsing auto-immune optic neuropathy. Neural regeneration research 10, 1507–1515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss JN, Levy S, Benes SC, 2016b. Stem Cell Ophthalmology Treatment Study (SCOTS): bone marrow-derived stem cells in the treatment of Leber's hereditary optic neuropathy. Neural regeneration research 11, 1685–1694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss JN, Levy S, Benes SC, 2017. Stem Cell Ophthalmology Treatment Study: bone marrow derived stem cells in the treatment of non-arteritic ischemic optic neuropathy (NAION). Stem Cell Investig 4, 94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss JN, Levy S, Malkin A, 2015b. Stem Cell Ophthalmology Treatment Study (SCOTS) for retinal and optic nerve diseases: a preliminary report. Neural regeneration research 10, 982–988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen R, Tao W, Li Y, Sieving PA, 2012. CNTF and retina. Prog. Retin. Eye Res 31, 136–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wert KJ, Lin JH, Tsang SH, 2014. General pathophysiology in retinal degeneration. Dev. Ophthalmol 53, 33–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West EL, Pearson RA, Duran Y, Gonzalez-Cordero A, MacLaren RE, Smith AJ, Sowden JC, Ali RR, 2012. Manipulation of the recipient retinal environment by ectopic expression of neurotrophic growth factors can improve transplanted photoreceptor integration and survival. Cell Transplant. 21, 871–887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whiteley SJO, Klassen H, Coffey PJ, Young MJ, 2001. Photoreceptor rescue after low-dose intravitreal IL-1 β injection in the RCS rat. Exp. Eye Res 73, 557–568. [DOI] [PubMed] [Google Scholar]
- Wilson DJ, Neuringer M, Stoddard J, Renner LM, Bailey S, Lauer A, McGill TJ, 2017. Subretinal Cell-Based Therapy: An Analysis of Surgical Variables to Increase Cell Survival. Retina (Philadelphia, Pa.) 37, 2162–2166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong WL, Su X, Li X, Cheung CM, Klein R, Cheng CY, Wong TY, 2014. Global prevalence of age-related macular degeneration and disease burden projection for 2020 and 2040: a systematic review and meta-analysis. Lancet Glob Health 2, e106–116. [DOI] [PubMed] [Google Scholar]
- www.biotimeinc.com, Biotime: Producs and Pipeline. Webpage accessed May 26, 2019.
- Yang J, Lewis G, Lu B, Turovets N, Luna G, Girman S, Bauer G, Fisher S, Wang S, Klassen H, 2013. Translational development of human retinal progenitor cells for treatment of retinitis pigmentosa. Invest. Ophthalmol. Vis. Sci 54, 2237–2237. [Google Scholar]
- Yao J, Ko CW, Baranov PY, Regatieri CV, Redenti S, Tucker BA, Mighty J, Tao SL, Young MJ, 2015. Enhanced differentiation and delivery of mouse retinal progenitor cells using a micropatterned biodegradable thin-film polycaprolactone scaffold. Tissue engineering. Part A 21, 1247–1260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young MJ, Ray J, Whiteley SJ, Klassen H, Gage FH, 2000. Neuronal differentiation and morphological integration of hippocampal progenitor cells transplanted to the retina of immature and mature dystrophic rats. Mol. Cell. Neurosci 16, 197–205. [DOI] [PubMed] [Google Scholar]
- Yu J, Vodyanik MA, Smuga-Otto K, Antosiewicz-Bourget J, Frane JL, Tian S, Nie J, Jonsdottir GA, Ruotti V, Stewart R, Slukvin II, Thomson JA, 2007. Induced pluripotent stem cell lines derived from human somatic cells. Science 318, 1917–1920. [DOI] [PubMed] [Google Scholar]
- Zarbin M, 2016. Cell-Based Therapy for Degenerative Retinal Disease. Trends Mol. Med 22, 115–134. [DOI] [PubMed] [Google Scholar]
- Zhao T, Zhang ZN, Rong Z, Xu Y, 2011. Immunogenicity of induced pluripotent stem cells. Nature 474, 212–215. [DOI] [PubMed] [Google Scholar]
- Zhong X, Gutierrez C, Xue T, Hampton C, Vergara MN, Cao LH, Peters A, Park TS, Zambidis ET, Meyer JS, Gamm DM, Yau KW, Canto-Soler MV, 2014a. Generation of three-dimensional retinal tissue with functional photoreceptors from human iPSCs. Nature Communications 5, 4047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong XF, Gutierrez C, Xue T, Hampton C, Vergara MN, Cao LH, Peters A, Park TS, Zambidis ET, Meyer JS, Gamm DM, Yau KW, Canto-Soler MV, 2014b. Generation of three-dimensional retinal tissue with functional photoreceptors from human iPSCs. Nature Communications 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu J, Reynolds J, Garcia T, Cifuentes H, Chew S, Zeng X, Lamba DA, 2018. Generation of Transplantable Retinal Photoreceptors from a Current Good Manufacturing Practice-Manufactured Human Induced Pluripotent Stem Cell Line. Stem cells translational medicine 7, 210–219. [DOI] [PMC free article] [PubMed] [Google Scholar]