Abstract
Objective To review the published literature on current and new treatments for chronic spontaneous urticaria (CSU) and to provide guidance on the potential use of these therapeutics. Data Sources A PubMed search was performed to include Englishlanguage articles with the keywords chronic spontaneous urticaria, pathophysiology, quality of life, and treatments with a preference to those written in the last 5 years. Clinicaltrials.gov was reviewed for recent relevant clinical trials related to potential CSU therapeutics. Study Selections Literature was included if it provided information related to the current understanding of the pathophysiology and management of CSU, as well as potential novel therapeutics currently in development. Results CSU has a significant effect on patients’ quality of life. Current therapies include antihistamines, leukotriene receptor antagonists, omalizumab and immunosuppressants, however, additional treatments are needed. New therapeutics under investigation include IgG1 anti-IgE monoclonal antibody (ligelizumab), CRTH2/DP2 antagonists (AZD1981), Btk inhibitors (fenebrutinib), anti-siglec-8 monoclonal antibody (AK002) and topical syk inhibitors (GSK2646264). Here we review the mechanisms of action as well as recently published data from clinical trials regarding the efficacy and safety of these treatments. Conclusion The development of new treatments for CSU will importantly lead to improved options for patients and may assist with improving our understanding of disease pathophysiology.
Introduction
Chronic Urticaria (CU) is defined as urticaria present either continuously or intermittently (present on most days of the week) for at least 6 weeks. CU may be divided into chronic inducible urticaria, (CIndU), also known as physical urticaria, and chronic spontaneous urticaria (CSU), previously recognized as chronic idiopathic urticaria (CIU). Etiologies of chronic inducible urticaria include dermatographism, as well as cold, delayed pressure, solar, heat, vibratory, cholinergic, contact and aquagenic urticaria. In comparison, CSU is defined as the spontaneous appearance of wheals, angioedema or both for at least 6 weeks due to unknown causes.1, 2 The algorithms for management of CU frequently overlap with CSU. This review will summarize the pathophysiology of CSU, current therapeutic strategies, new treatment options and potential future directions.
The prevalence of CU is estimated to range from 0.5% to 5% in the general population.1 In CSU, the average disease duration is 2 to 5 years3, and 35% to 50% of patients experience spontaneous remission of their urticaria and/or angioedema symptoms in 1 year.4, 5 Of note, a substantial number of CSU patients are affected by angioedema, with nearly 40% experiencing both urticaria and angioedema, and approximately 10% experiencing isolated angioedema without an alternative cause.6 Women are affected nearly twice as often as men, with a peak age of symptom onset between 20 and 40 years.7
Studies have demonstrated an immense burden of CSU on patients’ quality of life. Symptoms of pruritus, urticaria and angioedema lead to unpredictability of attacks, lack of sleep, antihistamine-related fatigue and cosmetic displeasure during episodes. Additionally, many patients continue to experience these effects despite attempts at treatment; less than 50% of patients indicated complete control of symptoms with licensed doses of H1 antihistamines.7 A recent study from van den Elzen et al. demonstrated that up to fourfold antihistamine dosing was insufficient in 54% of subjects with CSU.8 Therefore, there has been an increased focus on identifying the underlying disease mechanisms and the development of novel therapeutics.
Pathophysiology
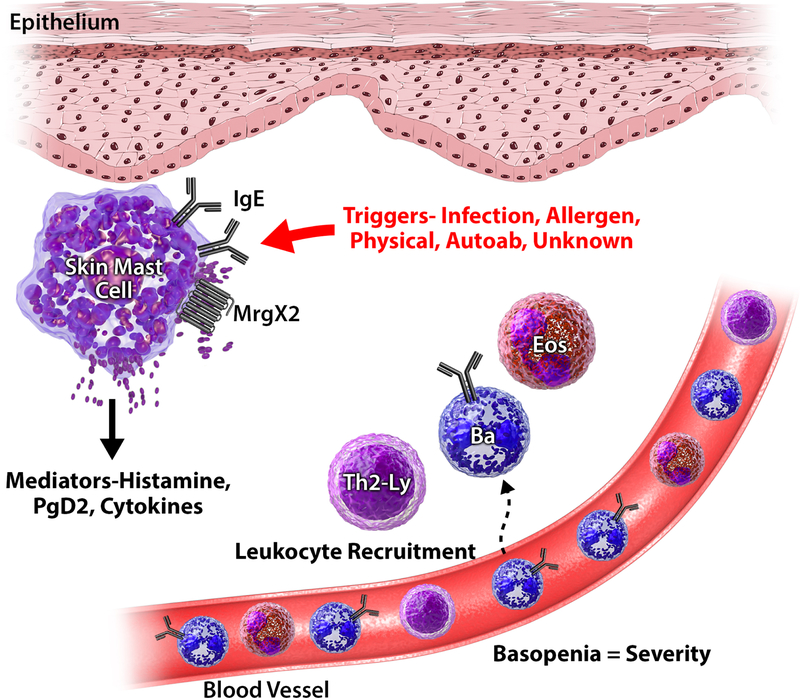
In order to understand the targets of novel therapeutics, it is beneficial to review the cellular and molecular derangements of CSU. The characteristic pruritus, wheals and angioedema present in CSU are thought to be caused by the degranulation of skin mast cells with the release of histamine, proteases and cytokines with the generation of platelet activating factor and other arachidonic acid metabolites (prostaglandin D2, leukotrienes C4, D4, E4). These mediators cause increased vasodilatation and vascular permeability with subsequent interstitial edema, as well as sensory nerve stimulation contributing to the widely recognized swelling, redness and itchiness.9 Lesional biopsies in CSU generally demonstrate perivascular mixed infiltrates of other cells, including basophils, CD4+ lymphocytes, monocytes, neutrophils, and eosinophils with increased IL-4, IL-5 and IFN-γ cellular mRNA expression.10 Additionally, Th2-initiating cytokines including IL-33, IL-25 and TSLP as well as calcitonin gene-related peptide (CGRP) and vascular endothelia growth factor (VEGF) are upregulated in lesional skin biopsies as compared to non lesional and control skin biopsies.11, 12 At this time, mast cells and basophils appear to be the predominant cells involved in the pathophysiology of CSU. In addition, roles for other infiltrating cells such as eosinophils and lymphocytes are less well studied but emerging (Figure 1).
Figure 1:
Pathophysiology of CSU
Mast Cells
Mast cells are derived from CD34+, CD117+, CD13+ pleuripotent progenitor cells which mature under the influence of the local environment. There are two distinct subsets of mast cells, MCT and MCTC. MCT are tryptase positive, chymase negative, T-lymphocyte-dependent cells identified in mucosal tissues (i.e. intestine, lung and nose), while MCTC are tryptase and chymase positive, T lymphocyte-independent cells located predominantly in the skin and gastrointestinal submucosa. Greater than 99% of mast cells in the skin of lesional and non-lesional CSU are of the MC subtype.13 TC Both MCT and MCTC are activated through IgE-dependent stimulation, where IgE bound to FcεRI is crosslinked resulting in a series of intracellular signaling cascades leads to mast cell activation and mediator release. Skin-derived MCTC cells additionally express surface mas-related gene X2 (MrgX2), a G protein-coupled receptor activated by 48/80, VIP and numerous FDA-approved peptidergic drugs, which serves as a secondary mechanism for mast cell activation independent of IgE (Figure 1).14, 15 While it is unclear if the number of MCTC cells differ between CU subjects and healthy controls due to conflicting data11, 13, 14, 16–18, evidence demonstrates that the number of MrgX2+ MCTC cells is significantly greater in skin tissues obtained from CU subjects when compared to healthy controls.14 This observation has led the field to consider the significance of this alternative mechanism of mast cell activation as a potential contributor to CSU.
Basophils
Basophils are also thought to be involved in the pathogenesis of CSU. Basophils have surface FcεRI which bind IgE and are activated following IgE crosslinking, but lack MrgX2 receptors. Basopenia is a unique abnormality identified in active chronic urticaria patients.19, 20 The cause of the basopenia is unknown, although it is hypothesized that it may result from basophil migration from blood to the skin lesions. Greater basopenia is observed with higher baseline urticaria activity scores (UAS), and basopenia resolves with disease remission.21, 22 Post-hoc analysis of phase III trials of omalizumab therapy in CSU subjects demonstrated that improvement of basopenia correlated with clinical improvement in a dose dependent fashion.23 Additional support for basophil involvement in CSU is the alteration in IgE receptor mediated histamine release observed in active disease.24, 25 Two distinct basophil functional phenotypes have been identified in regards to IgE mediated histamine release known as CSU responder (CSU-R) and CSU non-responder (CSU-NR). These phenotypes are based on the pattern of IgE receptor triggered histamine release; CSU-R profiles appear similar to healthy controls with greater than 10% release of total histamine content with optimal, ex-vivo IgE receptor activation, whereas CSU-NR profiles feature less than 10% release of the total histamine content comparatively.26 A third basophil functional phenotype, CSU-NR basopenic (basophils accounting for <0.1% of peripheral blood cells) was recently identified by Rauber et al. with the basophil activation test (BAT); this subtype appears to be clinically the most severely affected with the greatest propensity for autoreactivity.27 The basophil functional phenotype identified by histamine release remain stable during the course of active disease, however, following CSU remission both CSU-R and CSU-NR demonstrated increased sensitivity to IgE receptor triggered histamine release.22, 28 Likewise, overnight culture of healthy donor basophils with active CSU patients’ serum, but not serum taken from CSU patient’s in remission, transfers suppression of IgE receptor histamine response.29 These findings lend evidence to support the role of basophils in the pathophysiology of CSU.
Eosinophils
Eosinophils are significantly increased in skin biopsies from both lesional and non-lesional sites in CSU subjects as compared to healthy controls30, although the significance of eosinophils in CSU is not entirely clear. It has been hypothesized that eosinophils, in combination with mast cells and basophils, may prime the skin for wheal formation.30 A role for eosinophils in CSU has also been linked via the coagulation cascade. Activation of tissue factor (TF) is thought to trigger mast cells, which subsequently degranulate and initiate wheal formation; TF has been co-localized with eosinophil cationic protein (ECP) in CU lesions.31 Vascular endothelia growth factor (VEGF), a potent regulator of angiogenesis and a mediator of vascular permeability, is elevated in CU, and also co-localized with ECP.32 The true role of these mediators however still remains unknown, however, it has been shown that eosinophil granule products can activate the MRGX2 receptor which could be a pathway that is enhanced in patients with CSU.14
Lymphocytes
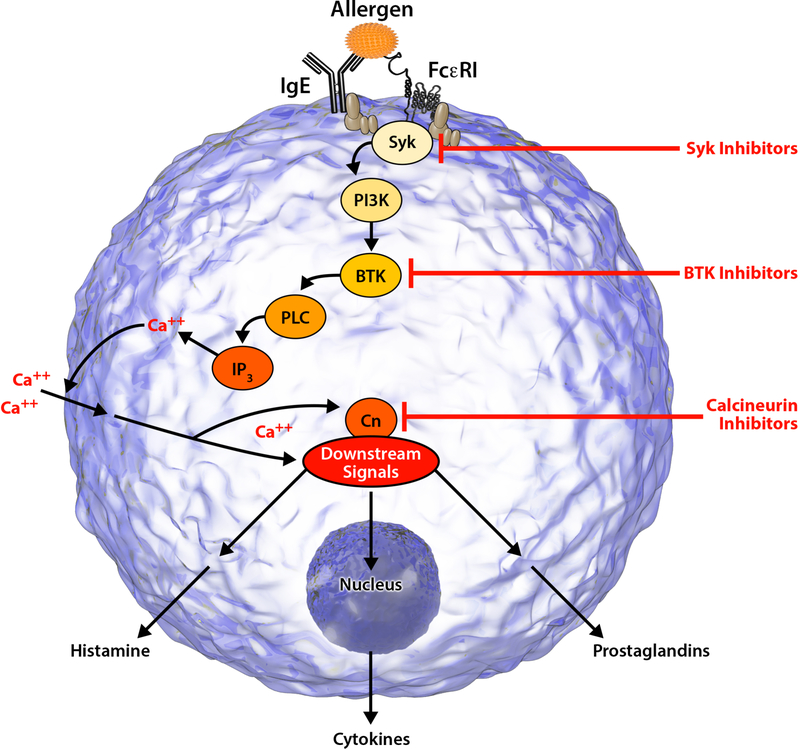
Biopsy specimens in CSU demonstrate a prominence of CD4+ T lymphocytes with few to no B cells. These infiltrating lymphocytes demonstrate characteristics of both Th1 and Th2 cells.6 Recently, there has been increased interest in the role of lymphocytes in CSU pathogenesis given the success of immunosuppressants such as cyclosporine in CSU. Cyclosporine binds to cyclophilin and inhibits the activity of calcineurin to dephosphorylate the nuclear factor of activated T cells (NFAT) (Figure 2). Subsequently, NFAT remains in the cytoplasm and cannot translocate to the nucleus to activate production of inflammatory cytokines such as IL-2, IL-3, IL-4 and TNF-α which have effects on lymphocytes, mast cells and basophils.33 Recently, autoreactive T cells to the IgE receptor have also been identified in CSU patients.34
Figure 2:
Intracellular Signaling Mechanism
Autoimmune Theory
Following the identification of an association between thyroid disease and thyroid autoantibodies with CSU, there was new interest in determining if CSU could represent an autoimmune disorder. The autologous serum skin test (ASST), a test in which autologous serum is intradermally injected with a subsequent positive wheal and flare reaction in positive subjects, was an early assay developed in the 1980s to demonstrate the presence of IgG-anti-IgE or IgG-anti-FcεRI antibodies.35 Positive ASSTs are not unique to CSU subjects (53%) and may be seen in healthy controls. Additionally, even when CSU is in remission ASSTs remain positive, demonstrating a lack of clinical utility.36
Further evidence suggesting the role of autoantibodies in CSU originated from the observation that the sera of patients with CSU and positive ASSTs cause in vitro histamine release from unaffected control basophils. IgG-anti-IgE and IgG-anti-FcεRI antibodies have been identified in CSU subjects, however, similar to the ASST, these are not specific to CSU and may be observed in healthy subjects.36 A recent study from MacGlashan Jr. established more stringent criteria for assessing the presence of autoantibodies in sera, and demonstrated a much lower frequency of functional autoantibodies in CSU of approximately 7%.37 There remains a lack of a standardized assay to define subject with an autoimmune basis in CSU.
Current Treatments
The primary principle of treatment of CSU is to eliminate symptoms, including pruritus, wheals and angioedema. H1 antihistamines function as inverse agonists that combine with and stabilize the inactive conformation of the H1 receptor. A meta-analysis of H1 antihistamine updosing for CSU demonstrated that 63.8% of patients with CSU who were not controlled on onefold dosing responded when the dose was increased fourfold. A large proportion of individuals remained uncontrolled, demonstrating the need for additional clinical treatments.38 Omalizumab, an IgG anti-IgE injectable antibody, was approved for the treatment of antihistamine-refractory CSU for ages 12 and older in 2014. It is well tolerated, quickly administered in the office setting and is approved at either 150mg every 4 weeks and 300mg every 4 weeks. The exact mechanism of action of omalizumab in CSU remains unknown. By selectively binding to free IgE, omalizumab prevents IgE from attaching to FcεRI on mast cells, basophils and as well as other FcεRI bearing cells such as dendritic cells. Omalizumab does not bind to cell surface IgE and therefore cannot cross-link and subsequently activate mast cells or basophils. As free IgE declines, FcεRI decline in number following the internalization and degradation of unoccupied FcεRI.39 Despite many questions regarding the mechanism of symptomatic improvement with omalizumab, how flat-fixed omalizumab dosing achieves clinical improvement, and how omalizumab should be uptitrated or downtitrated in accordance with symptoms, it represents a proven alternative to immunosuppressant use and functions as a steroid-sparing agent in some cases.
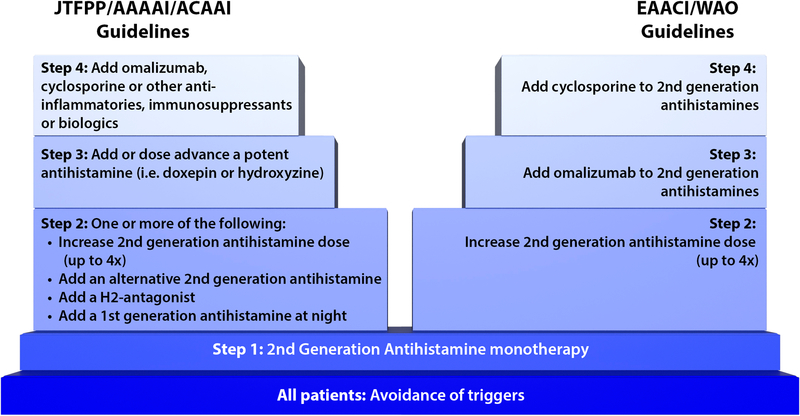
The Joint Task Force on Practice Parameters (JTFPP) in the United States released CSU treatment guidelines in 2014 addressing a stepwise management plan to achieve the goal of complete symptom relief in CSU (Figure 3). Initial therapy includes monotherapy with a second-generation H1 antihistamine. If symptoms persist, a clinician has one of several options available as a next step including fourfold second-generation H1 antihistamine dose advancement, addition of an alternative second-generation H1 antihistamine, addition of an H2 antihistamine, addition of a leukotriene receptor antagonist (LTRA) or addition of a first-generation H1 antihistamine. Step 3 includes dose advancement of a potent antihistamine (i.e. doxepin or hydroxyzine), and lastly step 4 for those with recalcitrant disease recommends the use of omalizumab, cyclosporine or an alternative immunosuppressant.1 Of note, although not included in guideline, additional recent evidence from a case series supports the further exploration of use of montelukast daily as a second-line agent in addition to use of a second-generation H1 antihistamine in the subset of patients with angioedema-predominant chronic spontaneous urticaria.40
Figure 3:
Comparison of JTFPP/AAAAI/ACAAI Guidelines with the EAACI/WAO Guidelines
The EAACI/GA2LEN/EDF/WAO guideline is the most recently updated task force summary available (Figure 3). The first step involves the initiation of a second-generation H1 antihistamine daily. If inadequate control is achieved after a two to four week trial of onefold second-generation H1 antihistamine, then the dose of second-generation H1 antihistamine should be increased up to fourfold. If CSU remains nonresponsive despite the fourfold dose increase, then omalizumab every 4 weeks should be added to the second-generation H1 antihistamine. Immunosuppressants such as cyclosporine are reserved for those with omalizumab nonresponsive disease, due to a higher incidence of adverse events. Per the most recent guideline, LTRAs, H2 antihistamines and dapsone are perceived to have little evidence, and therefore have been removed from the most recent guideline in contrast to the 2014 JTFPP guideline.2
Emerging Therapeutics for Anti-Histamine Refractory Subjects
QGE031 (Ligelizumab)
QGE031 (ligelizumab) is a humanized IgG1 monoclonal antibody which binds to the Cε3 domain of IgE with higher affinity than omalizumab. Results of two phase I randomized, double-blind, placebo-controlled clinical trials indicate that QGE031 demonstrates dose- and time-dependent suppression of free IgE, basophil FcεRI and basophil surface IgE, all of which occur to a greater extent and with longer duration as compared to omalizumab. Additionally, QGE031 suppressed allergen skin prick tests by >95% at 2mg/kg, as compared to a comparable dose of omalizumab which suppressed allergen skin prick testes by 41% (p<0.001).41 In a double-blind, parallel-group, multicenter trial comparing QGE031 against omalizumab in subjects with mild allergic asthma, QGE031 similarly demonstrated dose- and time-dependent suppression of allergen skin prick testing, with both the 72 mg and 240 mg doses demonstrated more pronounced inhibition when compared to omalizumab. All QGE031 doses (24mg, 72mg or 240mg every 2 weeks) also suppressed free IgE lower than the assay limit of quantification, with corresponding decreases in basophil surface IgE and basophil FcεRI.42 Given a common mechanism of action to omalizumab, its use in CSU is under study.
Recently, a dose-finding study of QGE031 as an add-on therapy to evaluate efficacy and safety in patients with CSU was completed ( NCT02477332). PEARL 1 and PEARL 2 are phase 3, multi-center, randomized, double-blind, parallel group studies aimed to establish the efficacy and safety of ligelizumab in adolescent and adult subjects with antihistamine-refractory CSU ( NCT03580369). Over 2000 patients are anticipated to be randomized into 1 of 4 groups including 2 different doses of ligelizumab, omalizumab 300mg every 4 weeks, and a placebo controlled group which will be switched to ligelizumab from week 24 to week 52. The primary outcome is defined as the absolute change in UAS-7 from baseline to week 12. QGE031 may represent a future treatment for those patients with omalizumab-refractory CSU, prior to the use of immunosuppressants.
CRTH2 Antagonist (AZD1981)
Chemoattractant rector-homologous molecule expressed on Th2 cells (CRTH2, DP2) is a receptor for prostaglandin D2 (PGD2) expressed on eosinophils, basophils, some TH2 cells and ILC2 cells. The activation of CRTH2 via PGD2 is thought to cause activation and chemotaxis of basophils and eosinophils.43 In a study of allergic skin diseases, CRTH2 expression on eosinophils was significantly increased in subjects with chronic urticaria in the absence of skin findings, atopic dermatitis and prurigo nodularis as compared to healthy volunteers.44 In contrast, in a study of 23 CSU subjects and 8 non-allergic controls, CRTH2 expression by flow cytometry was significantly lower on blood basophils (Mean Fluorescence Intensity (MFI) 300.2 vs. 398.4, p=0.0178) and eosinophils (MFI 60.27 vs. 70.88, p=0.0439) in CSU subjects. PGD2 stimulation of healthy basophils decreased CRTH2 surface expression via receptor internalization, as also previously observed with eosinophils.43, 45 Based on this, it is speculated that the decreased CRTH2 levels on basophils and eosinophils observed in subjects with active CSU was secondary to in vivo PGD2 activation. Furthermore, this ligand-receptor interaction may one of the causal factors for the migration of eosinophils and basophils in atopic disorders.
There has recently been growing interest in the use of CRTH2 antagonists as novel therapeutics for the management of eosinophilic esophagitis, asthma and allergic rhinitis.46, 47 AZD1981 is an oral, selective, reversible CRTH2 antagonist which recently underwent evaluation in a proof of concept, phase 2, randomized, double-blinded, placebo-controlled, parallel group clinical trial for assessment of safety and efficacy of use in antihistamine-resistant CSU. In regards to the primary endpoint of improvement of urticaria and pruritus as assessed by UAS-7 scores, improvement was noted in both categories by AZD1981 treated and placebo treated subjects, however, the change from baseline in UAS-7 was only statistically significant in AZD1981 treated subjects following a 2 week washout period after 4 full weeks of treatment. In subjects treated with AZD1981 40mg three times daily for 4 weeks, there was a significant increase in CRTH2 expression on blood basophils as compared to baseline, a change not seen in placebo treated subjects.48 With AZD1981 treatment, ex vivo PGD2 induced eosinophil shape was significantly inhibited in the treatment group as compared to the placebo group, and a rise in circulating eosinophils was observed. Additional studies are warranted to further assess optimal treatment duration and dosing.
Fenebrutinib (GDC-0853)
Bruton’s tyrosine kinase (Btk) is a member of the non-receptor (cytoplasmic) tyrosine kinases, involved in signaling through the B-cell receptor (BCR), toll-like receptors (TLRs) and Fc receptors (Figure 2).49 Given the broad range of effect of this tyrosine kinase, a focus has been placed on the development of specific Btk inhibitors for the treatment of various diseases including chronic inflammatory disorders, malignancies and atopic disorders. Ibrutinib, a non-specific oral irreversible Btk inhibitor, significantly decreased previously positive skin prick tests in peanut and/or tree nut subjects and eliminated allergen- mediated BAT responses after 2 doses via rapid inhibition of IgE-dependent activation of mast cells and basophils.50 The practical use of ibrutinib in clinical treatment is limited however, given its non-selective inhibition of numerous other kinases.
GDC-0852 is a highly selective, reversible, noncovalent, oral Btk inhibitor currently undergoing phase 2 clinical trials. Of 7 Btk inhibitors tested against a broad panel of human kinase biochemical assays, GDC-0853 was identified as the most Btk-selective molecule and inhibited only 3 of 286 off-target kinases, demonstrating a theoretical safety advantage.49 In a double-blind, randomized, placebo-controlled phase I study of healthy volunteers treated with GDC-0853, there were no serious or dose-limiting adverse events. Review of dose ascending cohorts and plasma concentrations suggest that a convenient once daily dosing regimen would enable sufficient Btk inhibition.51 Additionally, the use of GDC-0853 has been also assessed in relapsed or refractory B-cell non-Hodgkin lymphoma and chronic lymphocytic leukemia.52
Anti-Siglec-8 (AK002)
Siglecs (sialic acid immunoglobulin-like lectins) are type I transmembrane proteins of the immunoglobulin superfamily with I-type lectin domains in their N-terminal regions present on cells of the immune system. They function to bind to sialylated glycans. The majority of siglecs contain immunoreceptor tyrosine-based motifs (ITIMs) in the intracellular domain, suggesting that these receptors are involved in inhibitory cell signaling.53 Siglec-8 is uniquely expressed on eosinophils, mast cells and basophils, and selectively binds to NeuAcα2–3(6S)Galβ(±Fucα1–3)GlcNAc-, an epitope with both sialic acid and sulfate attached to the same galactose residue. The natural ligands for Siglec-8 have not yet been identified.53 Binding of siglec-8 on eosinophils with monoclonal antibodies (mAbs) led to caspase-3 or ROS dependent apoptosis of the cell.54, 55 Additionally, incubation of human mast cells with Siglec-8 mAbs significantly inhibited FcεRI-dependent histamine and PGD2 release.56 Given these apoptotic and anti- mediator effects, Siglec-8 has become a focus for the development of mAbs and glycan-targeting agents to treat numerous diseases.
A novel humanized non-fucosylated IgG1 monoclonal antibody to Siglec-8, AK002, is currently being explored in clinical trials for the treatment of CSU, eosinophilic gastritis, indolent systemic mastocytosis and severe allergic conjunctivitis.57 Results of a phase 1 ascending dose study of AK002 in healthy volunteers demonstrate complete depletion of blood eosinophils by 1 hour, with an extended duration of eosinophil depletion with increasing doses, peaking at up 84 days of eosinophil depletion with the highest experimental dose of 1.0 mg/kg. AK002 also appeared tolerable with brief mild to moderate infusion reactions observed, and only 1 serious adverse event occurring in 34 subjects.58 A multicenter, open-label phase 2a clinical trial recently explored the efficacy and safety of AK002 in subjects with antihistamine-refractory CSU ( NCT03436797). 13 anti-histamine-resistant omalizumab-naïve CSU subjects and 11 antihistamine and omalizumab resistant CSU subjects enrolled and were treated with 6 intravenous infusions of AK002 at doses of up to 3.0mg/kg. 12 of 13 anti-histamine resistant omalizumab-naïve CSU subjects demonstrated a complete response as per the Urticaria Control Test (UCT) at week 22 (2 weeks after the final infusion) and their average UAS-7 score decreased by 75% at week 22 as compared to baseline.59 6 of 11 anti-histamine and omalizumab resistant subjects demonstrated either a complete or partial response as per the UCT at week 22, and the average UAS-7 score decreased by 50% by week 22 when compared to baseline, demonstrating benefit for some subjects.60
Topical Syk Inhibitor (GSK2646264)
Spleen Tyrosine Kinase (Syk) is member of the non-receptor (cytosolic) tyrosine kinases involved in the signal transduction in B lymphocytes, mast cells and macrophages. Importantly, Syk functions as an integral component of the intracellular FcεRI signaling cascade, inducing mast cell degranulation when activated (Figure 2). Previous in vitro studies have confirmed a reduction in Syk phosphorylation and downstream signal transduction in IgE-sensitized mast cells following treatment with omalizumab, demonstrating this kinase’s critical function.61 In 2006, a novel small molecule inhibitor of the IgE-FcεRI signaling pathway, R112, was identified. As a potent, rapid, reversible inhibitor of Syk, R112 demonstrated an ability to inhibit mast cell degranulation, and both lipid mediator and cytokine production. Given a short pharmacodynamic effect requiring numerous doses per day, R112 was virtually indistinguishable from placebo and not further pursued.62
Novel Syk inhibitors for the topical treatment of inflammatory skin disease were investigated further based on the initially promising characteristics of R112. GSK2646264 demonstrated good selectivity (at least 30-fold) against other kinases evaluated, as well as adequate penetration into the skin.63 Using an anti-IgE-stimulated histamine release ex-vivo human skin model four different strengths (0.1%, 0.5%, 1.0% and 3.0% [wt/wt]) were shown to penetrate the dermis and achieve inhibition of anti-IgE histamine release.64 A phase I trial designed to investigate the safety, local tolerability, pharmacokinetics and pharmacodynamics after single and repeat topical application of GSK2646264 in healthy subjects, subjects with cold urticaria and subjects with CSU was recently completed ( NCT02424799). The results have not been published at this time.
Future Directions
Numerous other potential therapeutics for CSU are currently under investigation. Designed ankyrin repeat proteins (DARPins) are a class of small binding proteins consisting of stacked ankyrin repeat domains capable of binding to target proteins, effectively emulating monoclonal antibodies. Specific DARPins have been identified as capable of binding human FcεRI as well as human IgE, and although these hold promise as potential future treatments, these therapies should be approached with caution given the potential for immunoreactivity.65 Additional biologics and other treatments currently being explored are summarized in Table 1.
Table 1:
Therapeutics under investigation in clinical trials for treatment of CSU
| Study Drug | Mechanism | Phase | Clinicaltrials.gov identifier |
|---|---|---|---|
| Ligelizumab | Anti-IgE | Phase 3 | NCT03580356 |
| AK002 | Anti-Siglec-8 | Phase 2 | NCT03436797 |
| GSK2646264 | Topical SYK inhibitor | Phase 1 | NCT02424799 |
| Fenebrutinib | BTK inhibitor | Phase 2 | NCT03693625 |
| Benralizumab | Anti-IL-5R | Phase 4 | NCT03183024 |
| Mepolizumab | Anti-IL-5 | Phase 1 | NCT03494881 |
| Dupilumab | Anti-IL-4/13 | Phase 2 | NCT03749135 |
Conclusion
This review summarizes the current available treatments for CSU as well as outlines novel therapeutics currently under study in clinical trials. The current U.S. guidelines recommend the use of H1 antihistamines (up to 4 times the upper limit of normal dosing) with the possible use of an LTRA, H2 antihistamines or alternative antihistamines prior to initiation of omalizumab; use of immunosuppressants is reserved for recalcitrant disease. New targets include intracellular signaling molecules such Syk inhibitors and Btk inhibitors, as well as targets of receptors regulating inflammatory cell chemotaxis, such as CRTH2 antagonists. Additional therapies undergoing evaluation include monoclonal antibodies to siglec-8 which are thought to lead to apoptosis of eosinophils and anti-mediator effects on mast cells, as well as a novel humanized IgG1 anti-IgE with higher affinity for IgE than omalizumab. The improved understanding of disease mechanisms will allow better targeting of treatments in CSU and lead to a more personalized approach to therapy.
Acknowledgments
Funding/support: NIH AI116658
Abbreviations
- CU
Chronic Urticaria
- CSU
Chronic Spontaneous Urticaria
- CIndU
Chronic Inducible Urticaria
- mAbs
Monoclonal Antibodies
- FcεRI
High Affinity IgE Receptor
- UAS
Urticaria Activity Score
- UCT
Urticaria Control Test
- MFI
Mean Fluorescence Intensity
- LTRA
Leukotriene Receptor Antagonist
- CSU-R
CSU Responder
- CSU-NR
CSU Non-Responder
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of interest
Kirti J. Johal, MD: none
Sarbjit Saini, MD Disclosures: Grant/Research/Clinical Trial Support: NIH, ITN, Novartis, Regeneron, Genentech.
Consultant/Advisory Boards: Genentech, Novartis, Medimmune, AstraZeneca, Pfizer, Allakos, Eli Lily, GossamerBio.
References
- 1.Bernstein J, Lang D, Khan D. The diagnosis and management of acute and chronic urticaria: 2014 update. Journal of Allergy and Clinical Immunology. 2014;133:1277.e66. [DOI] [PubMed] [Google Scholar]
- 2.Zuberbier T, Aberer W, Asero R, et al. The EAACI/GA2LEN/EDF/WAO guideline for the definition, classification, diagnosis and management of urticaria. Allergy. 2018;73:1393–1414. [DOI] [PubMed] [Google Scholar]
- 3.Toubi E, Kessel A, Avshovich N, et al. Clinical and laboratory parameters in predicting chronic urticaria duration: a prospective study of 139 patients. Allergy. 2004;59:869–873. [DOI] [PubMed] [Google Scholar]
- 4.Kozel MMA, Mekkes JR, Bossuyt PMM, Bos JD. Natural course of physical and chronic urticaria and angioedema in 220 patients. Journal of the American Academy of Dermatology. 2001;45:387–391. [DOI] [PubMed] [Google Scholar]
- 5.Kulthanan K, Jiamton S, Thumpimukvatana N, Pinkaew S. Chronic idiopathic urticaria: prevalence and clinical course. The Journal of Dermatology. 2007;34:294–301. [DOI] [PubMed] [Google Scholar]
- 6.Kaplan AP. Chronic urticaria: Pathogenesis and treatment. Journal of Allergy and Clinical Immunology. 2004;114:465–474. [DOI] [PubMed] [Google Scholar]
- 7.Maurer M, Weller K, Bindslev-Jensen C, et al. Unmet clinical needs in chronic spontaneous urticaria. A GA2LEN task force report. Allergy. 2011;66:317–330. [DOI] [PubMed] [Google Scholar]
- 8.van den Elzen, Mignon T, van Os-Medendorp H, Van den Brink I, et al. Effectiveness and safety of antihistamines up to fourfold or higher in treatment of chronic spontaneous urticaria. Clinical and Translational Allergy. 2017;7:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Saini SS, Kaplan AP. Chronic spontaneous urticaria: The devil’s itch. Journal of Allergy and Clinical Immunology: In Practice. 2018;6:1097–1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ying S, Kikuchi Y, Meng Q, Kay AB, Kaplan AP. TH1/TH2 cytokines and inflammatory cells in skin biopsy specimens from patients with chronic idiopathic urticaria: comparison with the allergen-induced late-phase cutaneous reaction. Journal of Allergy and Clinical Immunology. 2002;109:694–700. [DOI] [PubMed] [Google Scholar]
- 11.Kay AB, Ying S, Ardelean E, et al. Calcitonin gene-related peptide and vascular endothelial growth factor are expressed in lesional but not uninvolved skin in chronic spontaneous urticaria. Clinical & Experimental Allergy. 2014;44:1053–1060. [DOI] [PubMed] [Google Scholar]
- 12.Kay AB, Clark P, Maurer M, Ying S. Elevations in T-helper-2-initiating cytokines (interleukin-33, interleukin-25 and thymic stromal lymphopoietin) in lesional skin from chronic spontaneous (‘idiopathic’) urticaria. British Journal of Dermatology. 2015;172:1294–1302. [DOI] [PubMed] [Google Scholar]
- 13.Smith CH, Kepley C, Schwartz LB, Lee TH. Mast cell number and phenotype in chronic idiopathic urticaria. Journal of Allergy and Clinical Immunology. 1995;96:360–364. [DOI] [PubMed] [Google Scholar]
- 14.Fujisawa D, Kashiwakura J, Kita H, et al. Expression of Mas-related gene X2 on mast cells is upregulated in the skin of patients with severe chronic urticaria. Journal of Allergy and Clinical Immunology. 2014;134:633.e9. [DOI] [PubMed] [Google Scholar]
- 15.McNeil BD, Pundir PP, Meeker S, et al. Identification of a mast-cell-specific receptor crucial for pseudo-allergic drug reactions. Nature. 2015;519:237–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bedard P, Brunet C, Pelletier G, Hebert J. Increased compound 48/80 induced local histamine release from nonlesional skin of patients with chronic urticaria. Journal of Allergy and Clinical Immunology. 1986;78:1121–1125. [DOI] [PubMed] [Google Scholar]
- 17.Elias J, Boss E, Kaplan AP. Studies of the cellular infiltrate of chronic idiopathic urticaria: Prominence of T-lymphocytes, monocytes, and mast cells. Journal of Allergy and Clinical Immunology. 1986;78:914–918. [DOI] [PubMed] [Google Scholar]
- 18.Nettis E, Dambra P, Loria MP, et al. Mast-cell phenotype in urticaria. Allergy. 2001;56:915. [DOI] [PubMed] [Google Scholar]
- 19.Rorsman H Basophilic leucopenia in different forms of urticaria. Allergy. 1962;17:168–184. [DOI] [PubMed] [Google Scholar]
- 20.Grattan C, Walpole D, Francis D, et al. Flow cytometric analysis of basophil numbers in chronic urticaria: basopenia is related to serum histamine releasing activity. Clinical & Experimental Allergy. 1997;27:1417–1424. [DOI] [PubMed] [Google Scholar]
- 21.Grattan C, Dawn G, Gibbs S, Francis D. Blood basophil numbers in chronic ordinary urticaria and healthy controls: diurnal variation, influence of loratadine and prednisolone and relationship to disease activity. Clin Exp Allergy. 2003;33:337–341. [DOI] [PubMed] [Google Scholar]
- 22.Oliver ET, Sterba PM, Saini SS. Interval shifts in basophil measures correlate with disease activity in chronic spontaneous urticaria. Allergy. 2015;70:600–603. [DOI] [PubMed] [Google Scholar]
- 23.Saini SS, Omachi TA, Trzaskoma B, et al. Effect of omalizumab on blood basophil counts in patients with chronic idiopathic/spontaneous urticaria. Journal of Investigative Dermatology. 2017;137:958–961. [DOI] [PubMed] [Google Scholar]
- 24.Greaves MW, Plummer VM, McLaughlan P, Stanworth DR. Serum and cell bound IgE in chronic urticaria. Clinical Allergy. 1974;4:265–271. [DOI] [PubMed] [Google Scholar]
- 25.Kern F, Lichtenstein LM. Defective histamine release in chronic urticaria. Journal of Clinical Investigation. 1976;57:1369–1377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vonakis BM, Vasagar K, Gibbons SP, et al. Basophil FcɛRI histamine release parallels expression of Src-homology 2–containing inositol phosphatases in chronic idiopathic urticaria. Journal of Allergy and Clinical Immunology. 2007;119:441–448. [DOI] [PubMed] [Google Scholar]
- 27.Rauber MM, Pickert J, Holiangu L, Möbs C, Pfützner W. Functional and phenotypic analysis of basophils allows determining distinct subtypes in patients with chronic urticaria. Allergy. 2017;72:1904–1911. [DOI] [PubMed] [Google Scholar]
- 28.Eckman JA, Hamilton RG, Gober LM, Sterba PM, Saini SS. Basophil phenotypes in chronic idiopathic urticaria in relation to disease activity and autoantibodies. Journal of Investigative Dermatology. 2008;128:1956–1963. [DOI] [PubMed] [Google Scholar]
- 29.Sterba P, Hamilton R, Saini S. Suppression of basophil FcɛRI activation by serum from active chronic idiopathic/spontaneous urticaria (CIU/CSU) subjects. The Journal of Investigative Dermatology. 2015;135:1454–1456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kay AB, Ying S, Ardelean E, et al. Elevations in vascular markers and eosinophils in chronic spontaneous urticarial weals with low-level persistence in uninvolved skin. British Journal of Dermatology. 2014;171:505–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cugno M, Marzano AV, Tedeschi A, Fanoni D, Venegoni L, Asero R. Expression of tissue factor by eosinophils in patients with chronic urticaria. International Archives of Allergy and Immunology. 2009;148:170–174. [DOI] [PubMed] [Google Scholar]
- 32.Tedeschi A, Asero R, Marzano AV, et al. Plasma levels and skin-eosinophil-expression of vascular endothelial growth factor in patients with chronic urticaria. Allergy. 2009;64:1616–1622. [DOI] [PubMed] [Google Scholar]
- 33.Kulthanan K, Chaweekulrat P, Komoltri C, et al. Cyclosporine for chronic spontaneous urticaria: A meta-analysis and systematic review. Journal of Allergy and Clinical Immunology: In Practice. 2018;6:586–599. [DOI] [PubMed] [Google Scholar]
- 34.Auyeung P, Mittag D, Hodgkin P, Harrison L. Autoreactive T cells in chronic spontaneous urticaria target the IgE Fc receptor Iα subunit. Journal of Allergy and Clinical Immunology. 2016;138:761–768. [DOI] [PubMed] [Google Scholar]
- 35.Church MK, Kolkhir P, Metz M, Maurer M. The role and relevance of mast cells in urticaria. Immunological Reviews. 2018;282:232–247. [DOI] [PubMed] [Google Scholar]
- 36.Bracken SJ, Abraham S, MacLeod AS. Autoimmune theories of chronic spontaneous urticaria. Frontiers in Immunology. 2019;10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.MacGlashan D Jr. Autoantibodies to IgE and FcεRI and the natural variability of spleen tyrosine kinase expression in basophils. Journal of Allergy and Clinical Immunology. 2019;143:1107.e11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Guillén-Aguinaga S, Jáuregui Presa I, Aguinaga-Ontoso E, Guillén-Grima F, Ferrer M. Updosing nonsedating antihistamines in patients with chronic spontaneous urticaria: a systematic review and meta-analysis. British Journal of Dermatology. 2016;175:1153–1165. [DOI] [PubMed] [Google Scholar]
- 39.Kaplan AP, Giménez-Arnau AM, Saini SS. Mechanisms of action that contribute to efficacy of omalizumab in chronic spontaneous urticaria. Allergy. 2017;72:519–533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Akenroye AT, McEwan C, Saini SS. Montelukast reduces symptom severity and frequency in patients with angioedema-predominant chronic spontaneous urticaria. Journal of Allergy and Clinical Immunology: In Practice. 2018;6:1403–1405. [DOI] [PubMed] [Google Scholar]
- 41.Arm JP, Bottoli I, Skerjanec A, et al. Pharmacokinetics, pharmacodynamics and safety of QGE031 (ligelizumab), a novel high-affinity anti-IgE antibody, in atopic subjects. Clinical & Experimental Allergy. 2014;44:1371–1385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gauvreau G, Arm J, Boulet L, et al. Efficacy and safety of multiple doses of QGE031 (ligelizumab) versus omalizumab and placebo in inhibiting allergen-induced early asthmatic responses. Journal of Allergy and Clinical Immunology. 2016;138:1051–1059. [DOI] [PubMed] [Google Scholar]
- 43.Oliver E, Sterba P, Devine K, Vonakis B, Saini S. Altered expression of chemoattractant receptor-homologous molecule expressed on Th2 cells on blood basophils and eosinophils in patients with chronic spontaneous urticaria. Journal of Allergy and Clinical Immunology. 2016;137:304–306. [DOI] [PubMed] [Google Scholar]
- 44.Yahara H, Satoh T, Miyagishi C, Yokozeki H. Increased expression of CRTH2 on eosinophils in allergic skin diseases. Journal of the European Academy of Dermatology and Venereology. 2010;24:75. [DOI] [PubMed] [Google Scholar]
- 45.Hamada K, Yamada Y, Kamada Y, et al. Prostaglandin D2 and interleukin-5 reduce CRTH2 surface expression on human eosinophils. Allergology International. 2004;53:179–184. [Google Scholar]
- 46.Straumann A, Hoesli S, Bussmann C, et al. Anti-eosinophil activity and clinical efficacy of the CRTH2 antagonist OC000459 in eosinophilic esophagitis. Allergy. 2013;68:375–385. [DOI] [PubMed] [Google Scholar]
- 47.Marone G, Galdiero MR, Pecoraro A, et al. Prostaglandin D2 receptor antagonists in allergic disorders: safety, efficacy, and future perspectives. Expert Opinion on Investigational Drugs. 2019;28:73–84. [DOI] [PubMed] [Google Scholar]
- 48.Oliver ET, Chichester K, Devine K, et al. Effects of an oral CRTH2 antagonist (AZD1981) on eosinophil activity and symptoms in chronic spontaneous urticaria. International Archives of Allergy and Immunology. 2019:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Crawford JJ, Johnson AR, Misner DL, et al. Discovery of GDC-0853: a potent, selective, and noncovalent Bruton’s Tyrosine Kinase Inhibitor in early clinical development. Journal of Medicinal Chemistry. 2018;61:2227–2245. [DOI] [PubMed] [Google Scholar]
- 50.Dispenza MC, Pongracic JA, Singh AM, Bochner BS. Short-term ibrutinib therapy suppresses skin test responses and eliminates IgE-mediated basophil activation in adults with peanut or tree nut allergy. Journal of Allergy and Clinical Immunology. 2018;141:1916.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Herman AE, Chinn LW, Kotwal SG, et al. Safety, pharmacokinetics, and pharmacodynamics in healthy volunteers treated with GDC-0853, a selective reversible Bruton’s Tyrosine Kinase Inhibitor. Clinical Pharmacology & Therapeutics. 2018;103:1020–1028. [DOI] [PubMed] [Google Scholar]
- 52.Byrd JC, Smith S, Wagner-Johnston N, et al. First-in-human phase 1 study of the BTK inhibitor GDC-0853 in relapsed or refractory B-cell NHL and CLL. Oncotarget. 2018;9:13023–13035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kiwamoto T, Kawasaki N, Paulson JC, Bochner BS. Siglec-8 as a drugable target to treat eosinophil and mast cell-associated conditions. Pharmacology and Therapeutics. 2012;135:327–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Nutku E, Aizawa H, Hudson SA, Bochner BS. Ligation of Siglec-8: a selective mechanism for induction of human eosinophil apoptosis. Blood. 2003;101:5014–5020. [DOI] [PubMed] [Google Scholar]
- 55.Kano G, Almanan M, Bochner B, Zimmermann N. Mechanism of Siglec-8–mediated cell death in IL-5–activated eosinophils: Role for reactive oxygen species–enhanced MEK/ERK activation. Journal of Allergy and Clinical Immunology. 2013;132:437–445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yokoi H, Choi O, Hubbard W, et al. Inhibition of FcERI-dependent mediator release and calcium flux from human mast cells by sialic acid-binding immunoglobulin-like lectin 8 engagement. Journal of Allergy and Clinical Immunology. 2008;121:499–505. [DOI] [PubMed] [Google Scholar]
- 57.Allakos I Early preclinical and clinical results demonstrate that AK002 rapidly depletes eosinophils and inhibits mast cell function. 2019;2019.
- 58.Rasmussen HS, Chang AT, Tomasevic N, Bebbington C. A randomized, double-blind, placebo-controlled, ascending dose phase 1 study of AK002, a novel Siglec-8 selective monoclonal antibody, in healthy subjects. Journal of Allergy and Clinical Immunology. 2018;141:AB403. [Google Scholar]
- 59.Allakos I Allakos announces positive phase 2 results in a cohort of xolair-naïve chronic spontaneous urticaria patients. 2019;2019.
- 60.Allakos I Allakos announces positive phase 2 results for AK002 in patients with Xolair refractory chronic spontaneous urticaria and provides additional data from chronic urticaria study cohorts. 2019;2019.
- 61.Serrano-Candelas E, Martinez-Aranguren R, Valero A, et al. Comparable actions of omalizumab on mast cells and basophils. Clinical & Experimental Allergy. 2016;46:92–102. [DOI] [PubMed] [Google Scholar]
- 62.Rossi AB, Herlaar E, Braselmann S, et al. Identification of the Syk kinase inhibitor R112 by a human mast cell screen. Journal of Allergy and Clinical Immunology. 2006;118:749–755. [DOI] [PubMed] [Google Scholar]
- 63.Barker MD, Liddle J, Atkinson FL, et al. Discovery of potent and selective Spleen Tyrosine Kinase inhibitors for the topical treatment of inflammatory skin disease. Bioorganic & Medicinal Chemistry Letters. 2018;28:3458–3462. [DOI] [PubMed] [Google Scholar]
- 64.Ramirez Molina C, Falkencrone S, Skov PS, Hooper-Greenhill E, Barker M, Dickson MC. GSK2646264, a spleen tyrosine kinase inhibitor, attenuates the release of histamine in ex vivo human skin. British Journal of Pharmacology. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Gomez G Current Strategies to Inhibit High AffinityFcεRI-Mediated Signaling for the Treatment of Allergic Disease. Frontiers in immunology. 2019;10. [DOI] [PMC free article] [PubMed] [Google Scholar]