Abstract
Objective:
The objectives of the present work were to optimize and validate the synthesis and stability of 14(R,S)-[18F]fluoro-6-thia-heptadecanoic acid ([18F]FTHA) and 16-[18F]fluoro-4-thia-palmitic acid ([18F]FTP) under cGMP conditions for clinical applications.
Methods:
Benzyl-14-(R,S)-tosyloxy-6-thiaheptadecanoate and methyl 16-bromo-4-thia-palmitate were used as precursors for the synthesis of [18F]FTHA and [18F]FTP, respectively. For comparison, a fatty acid analog lacking a thia-substitution, 16-[18F]fluoro-palmitic acid ([18F]FP), was synthesized from the precursor methyl 16-bromo-palmitate. A standard nucleophilic reaction using cryptand (Kryptofix /K222, 8.1 mg), potassium carbonate (K2CO3, 4.0 mg) and 18F-fluoride were employed for the 18F-labeling and potassium hydroxide (0.8 M) was used for the post-labeling ester hydrolysis. The final products were purified via reverse phase semi-preparative HPLC and concentrated via trap and release on a C-18 plus solid phase extraction cartridge. The radiochemical purities of the [18F]fluorothia fatty acids and [18F]FP were examined over a period of 4 h post-synthesis using an analytical HPLC. All the syntheses were optimized in an automated TRACERlab FX-N Pro synthesizer. Liquid chromatography mass spectrometry (LCMS) and high resolution mass spectrometry (HRMS) was employed to study the identity and nature of side products formed during radiosynthesis and as a consequence of post-synthesis radiation induced oxidation.
Results:
Radiosyntheses of [18F]FTHA, [18F]FTP and [18F]FP were achieved in moderate (8-20% uncorrected) yields. However, it was observed that the HPLC-purified [18F]fluorothia fatty acids, [18F]FTHA and [18F]FTP at higher radioactivity concentrations (>1.11 GBq/mL, 30 mCi/mL) underwent formation of 18F-labeled side products over time but [18F]FP (lacking a sulfur heteroatom) remained stable up to 4 h post-synthesis. Various radiation protectors like ethanol and ascorbic acid were examined to minimize the formation of side products formed during [18F]FTHA and [18F]FTP synthesis but showed only limited to no effect. Analysis of the side products by LCMS showed formation of sulfoxides of both [18F]FTHA and [18F]FTP. The identity of the sulfoxide side product was further confirmed by synthesizing a non-radioactive reference standard of the sulfoxide analog of FTP and matching retention times on HPLC and molecular ion peaks on LC/HRMS. Radiation-induced oxidation of the sulfur heteroatom was mitigated by dilution of product with isotonic saline to reduce the radioactivity concentration to <0.518 GBq/mL (14 mCi/mL).
Conclusions
Successful automated synthesis of [18F]fluorothia fatty acids were carried out in cGMP facility for their routine production and clinical applications. Instability of [18F]fluorothia fatty acids were observed at radioactivity concentrations exceeding 1.11 GBq/mL (30 mCi/mL) but mitigated through dilution of the product to <0.518 GBq/mL (14 mCi/mL). The identities of the side products formed were established as the sulfoxides of the respective thia fatty acids caused by radiation-induced oxidation of the sulfur heteroatom.
Keywords: [18F]fluorothia fatty acid, [18F]FTHA, [18F]FTP, [18F]FP and radiation induced oxidation
Introduction
[18F]Fluorothia fatty acids are promising positron emission tomography (PET) probes for noninvasive estimation of fatty acid oxidation (FAO) rates in living tissues. Over the years, [18F]fluorothia fatty acids have shown considerable potential to address FAO dependent processes in the clinical patients with metabolic disorders1–3, cardiomyopathies2–3, acute coronary syndrome3, heart failure2–3 and cancer4. It has been well documented in the literature that the position of the sulfur heteroatom of thia fatty acids determines there metabolic fate 5. Whereas even-substituted thia fatty acis (e.g. 4-thia and 6-thia) undergo partial β-oxidation, odd-substituted thia fatty acids are β-oxidized to 3-thia fatty acyl-CoA within the mitochondria but cannot undergo further metabolism. 3-Thia fatty acids are metabolized via ω-oxidation and sulfur oxidation in endoplasmic reticulum followed by peroxisomal β-oxidation to sulfoxy dicarboxylic acids5. Due to the clinical potential of [18F]FTHA6–7 and [l8F]FTP1 for imaging of FAO rate, we embarked on optimization and validation of their radiosyntheses in our cGMP facility to produce sufficient quantities for multiple doses over several hours. Interestingly, during this study, it was found that the presence of the sulfur heteroatom on these [18F]fluorothia fatty acids play an important role on radiochemical stability when producing batches at higher radioactivity levels. Herein, we describe automated cGMP production of [18F]FTHA and [18F]FTP, and investigations on the mechanism of radiation-induced instability of these [18F]fluorothia fatty acids utilizing liquid chromatography mass spectrometry (LCMS) analysis. Furthermore, various excipients were tested to mitigate the effects of radiolysis. The chemical structures of the 18F-labeled fatty acids in this study are depicted in the Fig. 1.
Figure-1:
Chemical structures of 18F-labeled fatty acids.
2. Materials and methods
2.1. Chemicals and reagents
All chemicals and solvents were purchased from Sigma-Aldrich (St. Louis, MO) and used as received. Anhydrous solvents were also purchased from Sigma-Aldrich in “sure seal” bottles. TLC analysis of reaction mixtures and products was performed on Merck silica gel 60 F254 TLC plates. Cryptand (Kryptofix 2.2.2), benzyl-14-(R,S)-tosyloxy-6-thiaheptadecanoate (FTHA precursor) and 14-(R,S)fluoro-6-thiaheptadecanoic acid (FTHA reference standard)8 were purchased from ABX (Radeberg, Germany). Methyl 3-(12-bromododecylthio)propionate (FTP precursor) and 3-(12-fluorododecylthio)propionic acid (FTP reference standard) were synthesized as reported earlier9–12. Methyl 16-bromohexadecanoate was purchased from Sigma-Aldrich.
2.2. HPLC Method and conditions
The HPLC mobile phase was MeOH/H2O/Acetic Acid in 88:12:0.4 v:v ratio for all three fatty acids [18F]FTHA, [18F]FTP and [18F]FP. For preparative HPLC, a Phenomenex Luna 5μ,C-18(2), 100Å, 250 x 10mm column with a flow rate of 5 mL/min (Rt=16.2 min ) was used. For analytical HPLC, a Luna 5μ, C-8(2), 100Å, 150 x 10mm column with a flow rate of 0.7 mL/min was employed and retention time (Rt) of ~6.2 min was found for the product and ~4.0 min for the unknown (side product), in case of [18F]FTHA and [18F]FTP, whereas, retention time (Rt) of ~7.6 min was found for [18F]FP.
2.3. General procedure for18F-labeling
We used benzyl-14-(R,S)-tosyloxy-6-thiaheptadecanoate as a precursor for synthesis of 14(R,S)-[18F]fluoro-6-thia-heptadecanoic acid ([18F]FTHA) probe, whereas, methyl 3-(12-bromododecylthio)propionate and methyl 16-bromohexadecanoate were used as precursors for the synthesis of 16-[18F]fluoro-4-thia-palmitic acid ([18F]FTP) and 16-[18F]fluoro palmitic acid ([18F]FP) probes, respectively. Nucleophilic [18F] fluorination and subsequent hydrolysis of the ester was performed with slight modification to the previously described methods8, 11–12. Briefly, cyclotron-produced [18F]fluoride was dried down under helium at 100 °C in a 20 mL reactor vial containing Kryptofix 2.2.2 (8.1 mg), acetonitrile (0.65 mL), and K2CO3 (4 mg) solution in water (0.15 mL) on the TRACERlab FX N Pro radiosynthesis module. The residue was further dried by azeotropic distillation with acetonitrile (2 x 1.0 mL). A solution of the precursor (approximately 3 mg) in acetonitrile (1.8 mL) was added, and subjected to heating at 80 °C for 10 min. Subsequent hydrolysis of the resulting [18F]fluoro-ester was performed in the same vessel by the addition of 0.4 mL 0.4M KOH followed by heating at 80 °C for 5 min. The mixture was then cooled, acidified with acetic acid (1.2 N, 0.4 mL in 1.5 mL of HPLC mobile phase), and injected on to the semi-preparative HPLC column (Phenomenex Luna 5μ,C-18(2),100Å, 250 x 10 mm column (Rt=16.2 min)). An in-line ultraviolet detector (225 nm) was used to monitor the elution of unlabeled materials. The [18F]fluoro fatty acid fraction was diluted in 50 mL water and trapped on a C-18 Sep-Pak cartridge (Accel C-18 light, Waters, Milford, MA), followed by washing of the Sep-Pak with 10 mL water. The product was eluted from the Sep-Pak using 2.5 mL solution 1:1 water: ethanol (v/v) in to product vial containing 10 mL of 0.9 % NaCl solution. The obtained product was filtered through a 0.22 μm filter (Millex-GS, Millipore, and Bedford, MA USA) and analyzed for radiochemical purity by radio-HPLC in the system described above. For radiochemical stability studies, formulations were performed with and without addition of 0.9 % NaCl solution, also described in next section. As quality control measures, we performed appearance, pH, sterility, endotoxins, half-life, residual solvent analysis, radiochemical purity and molar activity analyses.
2.4.1. Method of stability analysis of [18F]FTHA, [18F]FTP, and [18F]FP
After final formulation of radiolabeled [18F]FTHA, [18F]FTP, and [18F]FP, the [18F]fluorothia fatty acids were subjected on analytical HPLC as described in the previous section (2.2) to measure the radiochemical purity of the product over 4 h post-synthesis. Radiochemical purity (%) was measured as relative percentage of product [18F]fluorothia fatty acid peak area to the sum of all detected radioactive peak areas.
2.4.2. Method of formulation of high and low radioactivity concentrations of [18F]FTHA, [18F]FTP and [18F]FP
Experiments were designed to understand the effects of radiation on the formation of radiolabeled side products as a function of radioactivity concentration of [18F]fluorothia fatty acids. To obtain two radioactivity concentrations (high and low) from the same radiolabeling reaction, we divided the HPLC-purified product before the trap and release (C-18) step. The HPLC-purified product peak (5 mL) was collected in a round bottom flask containing 50 mL of deionized water (as normally performed), but before trapping the labeled product on the C-18 Sep-Pak, the whole 55 mL of solution was divided into two fractions: 1) larger fraction (40 mL), and 2) smaller fraction (15 mL). These two fractions were separately loaded on two separate C-18 Sep-Paks to produce high and low radioactivity concentration products. All C-18 trapped high and low concentration products ([18F]FTHA, [18F]FTP and [18F]FP) were eluted with 3 mL of ethanol: deionized water (1:1) before examining their radiochemical stability over time using radio-HPLC as described earlier in section 2.2. Details of these steps are delineated in Fig. 2.
Figure 2:

Experimental design to study the effect of radiation on stability of [18F]fluorothia fatty acids.
2.5. Method of mechanistic study using [19F]fluorothia fatty acids
Due to the low mass levels associated with standard 18F radiolabeling of thia fatty acids, a mechanistic study was carried out with [19F]fluorothia fatty acids to mimic similar radiation exposure and produce measurable quantities of side products for analysis by LCMS. To do this, both [19F]FTHA(1.7 mg) and [19F]FTP (2.6 mg) were dissolved in 2.5 mL of ethanol separately in 10 mL glass vials. Freshly cyclotron produced aqueous 18F-fluoride solutions (2.5 mL, ~ 7.4-9.3 GBq (200-250 mCi)) were directly added in the vials containing [19F]FTHA (1.7 mg) and [19F]FTP (2.6 mg) reference standards without any purification. Both solutions were left at room temperature for 24 hours. After a day (24 h post-radiation exposure), 1.0 mL of each solution was separately subjected on HPLC for purification using same method and condition used during standard [18F]fluorothia fatty acid synthesis as described in section 2.2. Peaks were collected at similar retention times of side product (3.2-4.8 min Rt) and [18F]fluorothia fatty acid products (5.5-7.0 min) as seen on the UV detector. The collected samples were analyzed by LCMS.
2.6. Method of LCMS analysis of collected fractions
LCMS analysis of collected peak fractions were performed on a Thermo Fisher instrument, (Thermo QE-negative ESI, full scan m/z100-700 PRM mode with isolation window of 1 Da having scan resolution: 70000). The column used for LCMS was Waters HSS-C18, 2.1 mm x 150 mm, 1.8 μm, at 40 °C with a flow rate of 0.4 mL/min. A gradient mobile phase was employed with mobile phase A (70 % acetonitrile with 1 mM NH4OAc) and solution B (99% acetonitrile with 1 mM NH4OAc). The highest peaks obtained on LC were analyzed for mass spectrometry.
2.7. Synthesis of FTP-sulfoxide (4):
As a reference standard for a sulfoxy fluorothia fatty acid, FTP-sulfoxide (4) was achieved by a control oxidation of 3-(12-fluorododecylthio)propionic acid (FTP reference standard) with 30 % H2O2 in acetonitrile as described in Scheme-1. In brief, 35 mg (0.12 mmol) of 3-(12-fluorododecylthio)propionic acid was dissolved in anhydrous acetonitrile (3 mL) and 2.5 equivalent of 30 % H2O2 (0.1 mL) was added in the reaction. The reaction mixture was stirred at 35 °C for 60 min. Progress of reaction was monitored by thin layer chromatography using 10 % methanol-chloroform. After completion of reaction, the product was isolated by extraction with chloroform and the organic phase was dried using anhydrous sodium sulfate, filtered and concentrated using rotary evaporator. The obtained product was analyzed by NMR, HRMS and HPLC without any further purification as purity was over 99 % (based on HPLC). The yield of compound 4 was (13.6 mg) found to be 37 % as an off-white solid. 1H NMR (23 °C, 599.77 MHz, CDCl3) δ ppm: : 4.49 (dt, 2H, J1 = 47.3 Hz, J2 = 6.2 Hz), 3.09 (m, 1H), 2.97-2.93 (m, 3H), 2.82 (m, 1H), 2.72 (m, 1H), 1.80-1.71 (m, 2H), 1.69-1.67 (m, 4H), 1.42-1.40 (m, 2H), 1.35-1.30 (brs, 12H), HRMS (ES): Calculated for C15H28O3SF [M+-H] 307.1743 and found 307.1743
Scheme-1:

Synthesis of FTP-sulfoxide.
2.8. Statistical analysis
All values are given as mean ± standard deviation.
3. Results and discussion
3.1. Radiosynthesis of [18F]FTHA, [18F]FTP and [18F]FP
Radiosynthesis of [l8F]FTHA, [18F]FTP and [18F]FP were performed using the TRACERlab FX N Pro radiosynthesis module in a cGMP environment. The production yields were quite consistent for each tracers; for [18F]FTHA the uncorrected yield was 10.9±2.2 %, [18F]FTP uncorrected yield was 7.7±4.5 % , [18F]FP showed a bit better uncorrected yield of 18.4±1.5 % (Table 1). The overall time of radiosynthesis for all radiotracers was 62-65 min. Molar activities of [18F]FTHA and [18F]FTP were found to be 149±104 GBq/μmol (n=3), (4.02±2.8 Ci/μmol) and 86±19 GBq/μmol (n=2), (2.32±0.51 Ci/μmol) at end of synthesis, respectively.
Table 1.
Decay corrected and uncorrected yields of [18F]FTHA, [18F]FTP and [18F]FP.
| Fatty acids | Uncorrected Yield (%, at end of synthesis) | Decay Corrected Yield (%, at end of synthesis) |
|---|---|---|
| [18F]FTHA (n=7) | 10.9±2.2 | 16.9±4.1 |
| [18F]FTP (n=2) | 7.7±4.5 | 11.4±6.6 |
| [18F]FP (n=2) | 18.4±1.5 | 28.1±3.3 |
3.2. Radiation induced instability of [18F]FTHA and [18F]FTP
Instability of 18F-labeled thia fatty acids was observed immediately after completion of radiosynthesis. It is critical to mention that the automated radiosyntheses were performed with 37-74 GBq (1.0-2.0 Ci) of starting 18F-radioactivity to meet the clinical demands of [18F]FTHA (~5.55-9.25 GBq or 150-250 mCi). As per our clinical requirements, we anticipated a 4 h stability period of [18F]fluorothia fatty acids would be sufficient. In our initial study, purified and formulated [18F]FTHA was injected on analytical radio-HPLC soon after (10 min post synthesis) the completion of radiosynthesis. The samples were found to contain [18F]FTHA in <93.5 % radiochemical purity at 10 min post-radiosynthesis. It was surprising to find the HPLC purified product having such low radiochemical purity so early after synthesis. Further analysis over time of the [18F]FTHA product showed radiochemical purity decreasing to as low as 62.8 % at 4 h post-radiosynthesis (Fig. 3). Interestingly, there was an emergence of a single radioactive unknown peak at Rt3.9-4.2 min (less lipophilic than [18F]FTHA), which grew over time. Initially, we suspected it could be a free 18F-fluoride a result of a side product due to β-elimination, but independent analysis of free 18F-fluoride under the same HPLC conditions ruled out this possibility. To mitigate radiation-induced instability of [18F]FTHA, we tested the addition of various excipients and formulating media including bovine serum albumin (BSA) (0.9%), human serum albumin (HSA,) (5%), intralipid (0.7 mL) and ascorbic acid (25 μL of 200 mg/mL) but all of these attempts gave unsatisfactory results. Only ascorbic acid (5 mg total) showed some resistance but was not enough to significantly slow the [18F]FTHA conversion to the side product over 4 h to meet the >95 % radiochemical purity release criterion.
Figure 3:

Representative radio HPLC trace of [18F]FTHA (B, Rt 6.07-6.76 min) and appearance of unknown peak (A, Rt 3.9-4.2 min) at 10 min and at 240 min (4 h) post synthesis.
We synthesized [18F]FTP using the same radiochemistry conditions and evaluated its stability over time. Not surprising, [18F]FTP also showed a similar side product formation over time that eluted slightly ahead of the [18F]FTP product on HPLC (Fig. 4). These unexpected results prompted us to synthesize and evaluate the stability of a [18F]fluoro fatty acid 16-[18F]fluoro palmitic acid ([18F]FP) lacking thia-substitution to compare to our results with [18F]FTHA and [18F]FTP. Radiosynthesis of [18F]FP showed absolutely no decomposition of the product over time, even at high radioactivity concentration (4.03 GBq/ mL or 108.9 mCi/mL) (Fig. 5). The relative instability of [18F]fluorothia fatty acids in comparison to [18F]FP was evidence that the presence of a sulfur heteroatom in the former could be attributed to the formation of a more polar side product. However, ours and others previous experiences with acceptable stability of low radioactivity concentrations of fluorothia fatty acids 5, 8–9, 12–13 gave us another clue that the rate of formation of [18F]fluorothia fatty acid side product could be related to the higher radioactivity concentrations used in scaling up the radiosynthesis for clinical production. By this time, we suspected that radiation induced instability was related to both the thia fatty acid structure and radioactivity concentration in the formulated product. Therefore, we designed experiments to better understand the threshold of radiation exposure which could trigger the formation of polar impurities and cause instability of fluorothia fatty acids. To rule out the effects of variation in radiosynthesis from batch to batch, we decided to use the same [18F]fluorothia fatty acid product and divide it into two parts just before trapping it on the C-18 Sep-Pak in a way to get two different (high and low) radioactivity concentrations as described in method section 2.4.2. The results obtained for [18F]FTHA (Table 2, Fig. 6) showed that the high radioactivity formulation (2.37 GBq/mL or 64 mCi/mL) had high decomposition rate. The radiochemical purity dropped to 89.8 % within first 10 min post-radiosynthesis and further to 62.8 % at 4 h. [18F]FTHA produced from the same radiosynthesis but formulated as a low radioactivity concentration (0.89 GBq/ mL or 24 mCi/mL) showed a lower rate of decomposition; radiochemical purity dropped to 86.7 % at 4 h. We therefore reduced the radioactivity concentration of the formulation further to note a more profound improvement on stability of [18F]FTHA. At a radioactivity concentration 1.254 GBq/mL (33.9 mCi/mL) the purity of [18F]FTHA was found to be 94.0 % with in first 10 min post synthesis and purity reduced to 87.2 % only in 4 h. Furthermore, when radioactivity concentration was even further reduced to 0.52 GBq/mL or 14.0 mCi/mL, the radiochemical purity of [18F]FTHA was found to be 94.6 % within first 10 min of post radiosynthesis and purity remained over 90 % even at 4 h. These findings clearly showed the degrading effect of radiation on the radiochemical purity of [18F]FTHA. Similar studies were performed with other two PET probes [18F]FTP and [18F]FP and results are presented in Tables 3–4 and also in Fig. 7.
Figure 4:

Representative radio HPLC trace of [18F]FTP (B, Rt 5.97-6.13 min) and appearance of unknown peak (A, Rt 4.3-4.6 min) at 10 min and at 240 min (4 h) post synthesis.
Figure 5:
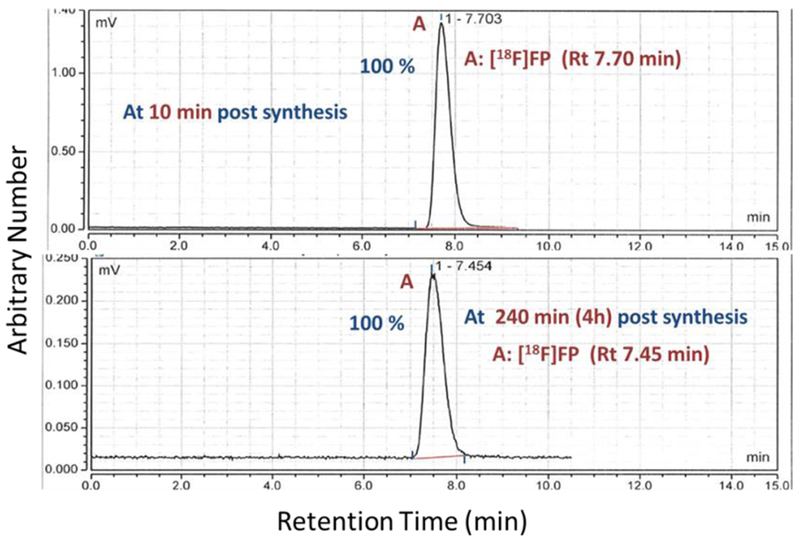
Representative radio HPLC trace of [18F]FP (A, Rt 7.45-7.7 min) and absence of any unknown peak at 10 min and at 240 min (4 h) post synthesis.
Table 2:
Instability of [18F]FTHA at different radioactivity concentration and growth of unknown side product over time.
| SN | Fatty acid | Radioactivity Concentration (GBq/mL or mCi/mL) | Time of Post synthesis analysis | Peak I (Unknown) | Peak II (Product) | |||
|---|---|---|---|---|---|---|---|---|
| Retention Time (min) | Relative Percentage | Retention Time | Relative Percentage | |||||
| 1 | [18F]FTHA | High conc. (2.37 GBq/mL or 64.0 mCi/mL at time zero) | 2.2 GBq/mL (60.1 mCi/mL) | 10 min | 4.08 | 10.2% | 6.38 | 89.8% |
| 1.6 GBq/mL (43.8 mCi/mL) | 60 min | - | - | - | - | |||
| 1.1 GBq/mL (30.0 mCi/mL) | 120 min | 4.08 | 28.3% | 6.33 | 71.7% | |||
| 0.76 GBq/mL (20.5 mCi/mL) | 180 min | 4.07 | 34.3% | 6.34 | 65.7% | |||
| 0.52 GBq/mL (14.0 mCi/mL) | 240 min | 4.11 | 37.2% | 6.76 | 62.8% | |||
| Low conc. (0.89 GBq/mL or 24.0 mCi/mL at time zero) | 0.74 GBq/mL (19.9 mCi/mL) | 30 min | 4.22 | 11.5% | 6.41 | 88.5% | ||
| 0.60 GBq/mL (16.4 mCi/mL) | 60 min | 4.04 | 10.0% | 6.35 | 90.0% | |||
| 0.41 GBq/mL (11.2 mCi/mL) | 120 min | 4.02 | 14.2% | 6.20 | 85.8% | |||
| 0.29 GBq/mL (7.7 mCi/mL) | 180 min | 4.08 | 13.8% | 6.34 | 86.2% | |||
| 0.20 GBq/mL (5.27 mCi/mL) | 240 min | 4.16 | 13.3% | 6.79 | 86.7% | |||
| 2 | [18F]FTHA | High conc. (1.25 GBq/mL or 33.9 mCi/mL at time zero) | 1.2 GBq/mL (31.8 mCi/mL) | 10 min | 4.05 | 6.0% | 6.15 | 94.0% |
| 0.86 GBq/mL (23.2 mCi/mL) | 60 min | 4.05 | 9.1% | 6.12 | 90.9% | |||
| 0.59 GBq/mL (15.9 mCi/mL) | 120 min | 4.08 | 9.4% | 6.12 | 90.6% | |||
| 0.40 GBq/mL (10.9 mCi/mL) | 180 min | 4.13 | 11.9% | 6.20 | 88.1% | |||
| 0.28 GBq/mL (7.5 mCi/mL) | 240 min | 4.03 | 12.8% | 6.14 | 87.2% | |||
| Low conc. (0.52 GBq/mL or 14.0 mCi/mL at time zero) | 0.43 GBq/mL (11.6 mCi/mL) | 30 min | 3.57 | 5.4% | 5.62 | 94.6% | ||
| 0.36 GBq/mL or 9.6 mCi/mL | 60 min | 4.05 | 8.8% | 6.14 | 91.2% | |||
| 0.24 GBq/mL (6.5 mCi/mL) | 120 min | 4.03 | 8.3% | 6.18 | 91.7% | |||
| 0.17 GBq/mL (4.5 mCi/mL) | 180 min | - | - | - | - | |||
| 0.11 GBq/mL (3.0 mCi/mL) | 240 min | 4.09 | 9.8% | 6.12 | 90.2% | |||
Figure 6:
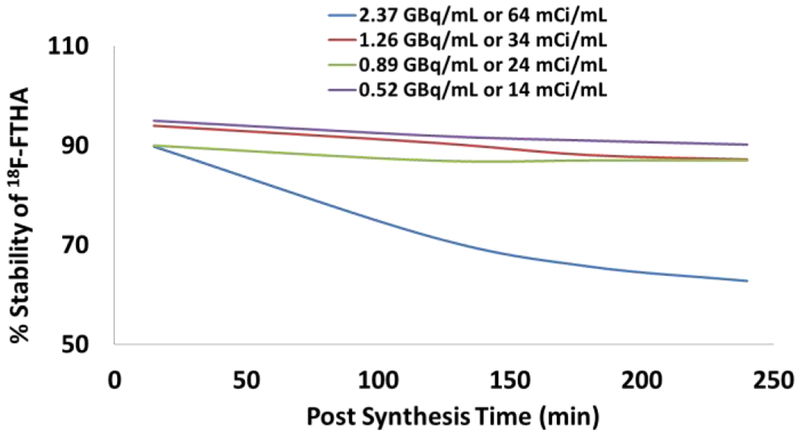
Radioactivity concentration dependent instability of [18F]FTHA over time (0-240 min) post synthesis.
Table 3:
Instability of [18F]FTP at different radioactivity concentration and growth of unknown side product over time.
| SN | Fatty acid | Radioactivity Concentration (GBq/mL or mCi/mL) | Time of Post synthesis analysis | Peak I (Unknown) | Peak II (Product) | |||
|---|---|---|---|---|---|---|---|---|
| Retention Time (min) | Relative Percentage | Retention Time | Relative Percentage | |||||
| 1 | [18F]FTP | High conc. (1.395 GBq/mL or 37.7 mCi/mL at time zero) | 1.3 GBq/mL (35.4 mCi/mL) | 10 min | 4.53 | 4.2% | 6.13 | 95.8% |
| 0.96 GBq/mL (25.8 mCi/mL) | 60 min | - | - | - | - | |||
| 0.66 GBq/mL (17.7 mCi/mL) | 120 min | 4.45 | 15.5% | 6.13 | 84.5% | |||
| 0.45 GBq/mL (12.1 mCi/mL) | 180 min | 4.33 | 16.4% | 5.97 | 83.6% | |||
| 0.31 GBq/mL ( 8.3 mCi/mL) | 240 min | 4.36 | 17.7% | 5.98 | 82.3% | |||
| Low conc. (0.53 GBq/mL or 14.3 mCi/mL at time zero) | 0.44 GBq/mL (11.8 mCi/mL) | 30 min | 4.54 | 2.6% | 6.09 | 97.4% | ||
| 0.36 GBq/mL (9.8 mCi/mL) | 60 min | - | - | - | - | |||
| 0.25 GBq/mL (6.7 mCi/mL) | 120 min | 4.53 | 6.3% | 6.13 | 93.7% | |||
| 0.17 GBq/mL (4.6 mCi/mL) | 180 min | 4.35 | 10.8% | 5.97 | 89.2% | |||
| 0.12 GBq/mL (3.1 mCi/mL) | 240 min | 4.35 | 10.3% | 5.96 | 89.7% | |||
Table 4:
Stability of [18F]FP at different radioactivity concentration over time.
| SN | Fatty acid | Radioactivity Concentration (GBq/mL or mCi/mL) | Time of Post synthesis analysis | Peak I (Unknown) | Peak II (Product) | |||
|---|---|---|---|---|---|---|---|---|
| Retention Time (min) | Relative Percentage | Retention Time | Relative Percentage | |||||
| 1 | [18F]FP | High conc. (4.03 GBq/mL or 108.9 mCi/mL at time zero) | 3.8 GBq/mL (102.2 mCi/mL) | 10 min | Not detected | 7.71 | 100 % | |
| 2.8 GBq/mL (74.6 mCi/mL) | 60 min | 7.68 | 100 % | |||||
| 1.9 GBq/mL (51.1 mCi/mL) | 120 min | 7.52 | 100 % | |||||
| 1.3 GBq/mL (35.0 mCi/mL) | 180 min | 7.52 | 100 % | |||||
| 0.9 GBq/mL (24.0 mCi/mL) | 240 min | 7.53 | 100 % | |||||
| Low conc. (0.226 GBq/mL or 6.1 mCi/mL at time zero) | 0.19 GBq/mL (5.0 mCi/mL) | 30 min | 7.59 | 100 % | ||||
| 0.16 GBq/mL (4.2 mCi/mL) | 60 min | 7.58 | 100 % | |||||
| 0.11 GBq/mL (2.9 mCi/mL) | 120 min | 7.58 | 100 % | |||||
| 0.70 GBq/mL (2.0 mCi/mL) | 180 min | 7.61 | 100 % | |||||
| 0.05 GBq/mL (1.34 mCi/mL) | 240 min | 7.59 | 100 % | |||||
| 2 | [18F]FP | High conc. (3.43 GBq/mL or 92.7 mCi/mL at time zero) | 3.2 GBq/mL (87.0 mCi/mL) | 10 min | 7.70 | 100 % | ||
| 2.4 GBq/mL (63.8 mCi/mL) | 60 min | 7.68 | 100 % | |||||
| 1.6 GBq/mL (43.5 mCi/mL) | 120 min | 7.50 | 100 % | |||||
| 1.1 GBq/mL (29.6 mCi/mL) | 180 min | 7.50 | 100 % | |||||
| 0.76 GBq/mL (20.4 mCi/mL) | 240 min | 7.45 | 100 % | |||||
| Low conc. (0.51 GBq/mL or 13.8 mCi/mL at time zero) | 0.42 GBq/mL (11.4 mCi/mL) | 30 min | 7.59 | 100 % | ||||
| 0.35 GBq/mL (9.5 mCi/mL) | 60 min | 7.60 | 100 % | |||||
| 0.24 GBq/mL (6.5 mCi/mL) | 120 min | 7.60 | 100 % | |||||
| 0.16 GBq/mL (4.4 mCi/mL) | 180 min | 7.58 | 100 % | |||||
| 1.1 GBq/mL (3.0 mCi/mL) | 240 min | 7.58 | 100 % | |||||
Figure 7:

Radioactivity concentration dependent stability of thia/non-thia 18F-fatty acids and growth of unknown side product over time (0-240 min) post synthesis. (A) [18F]FTHA, (B) [18F]FTP, (C) [18F]FP.
To further understand the structure dependent effect of radiation on fluorothia fatty acids, we compared the data on [18F]FTP (Table 3) with [18F]FTHA (Table 2). There are three differences in chemical structures of [18F]FTHA and [18F]FTP (Figure 1). [18F]FTHA is a C-17 chain fatty acid, having sulfur heteroatom substitution at C-6 and 18F-labeling at C-14 (secondary carbon). Whereas, [18F]FTP has a C-16 chain, with sulfur substitution at C-4, and 18F-labeling on the terminal 16th carbon atom (primary carbon). In spite of these structural differences between the two [18F]fluorothia fatty acids they both behaved similarly with regards to radiation induced instability. The higher concentration formulation (1.395 GBq/mL or 37.7 mCi/mL) of [18F]FTP, showed 95.8% purity with in first 10 min post radiosynthesis but purity was reduced to 82.3 % at 4h. Whereas, the low radioactivity formulation (0.53 GBq/mL or 14.3 mCi/mL) of [18F]FTP showed 97.4 % purity within first 10 min post radiosynthesis and purity remained close to 89.7 % even at 4 h time point. These data were similar with those previously discussed for [18F]FTHA.
Even though there are structural differences between [18F]FTHA and [18F]FTP both have been affected by radiation induced formation of a side product. However, when a non thia fluoro fatty acid [18F]FP was synthesized in similar conditions, no sign of formation of side product was observed over time even at high radioactivity concentrations (4.03 GBq/mL or 108.9 mCi/mL) as presented in Table 4. It is evident that the mechanism of formation of side products of [18F]fluorothia fatty acids at high radioactivity formulation involves the presence of the sulfur heteroatom in fluorothia fatty acids and certainly pinpoints the role or susceptibility of sulfur atom in radiolysis-induced formation of side product. Moreover, the non-radioactive standards of [18F]FTHA and [18F]FTP were found to remain stable at room temperatures for weeks, further supporting the role of radiation on formation of side product.
3.3. Characterization of unknown side product
Although formation of the polar side products of the [18F]fluorothia fatty acids were significantly mitigated by reducing the radioactivity concentration to <0.518 GBq/mL (14 mCi/mL), it was important to characterize the identity of the side product to provide a mechanistic understanding of the radiolytic decomposition process. After ruling out the possibility of presence of free fluoride [18F], our first thought was the side products being possible breakdown fragments of labeled fluorothia fatty acids. However, characterization of a side product based on a standard 18F-labeling synthesis was challenging due to the extremely small mass (cold/nonradioactive mass) involved. Besides, selection of right technique to do this job was also challenging as many other techniques may not be informative enough and may even require relatively higher quantity of the analyte. Whereas, due to inherent similarities between radiolysis and mass spectrometry14–16 and need for only small quantity of analyte for the study, we chose LCMS as a tool to decipher the identity and nature of the side products. Detailed mass fragmentation patterns of nonradioactive FTP and FTHA were performed to better understand the possible cleavage patterns of fluorothia fatty acids, which are outlined in Figs. 8–9. Aided by an understanding of cleavage patterns of the fluorothia fatty acids, we performed a mechanistic study in which nonradioactive FTP and FTHA reference standards were directly exposed to aqueous solutions at high radioactivity concentrations of [18F]fluoride (18F~ 7.4-9.3 GBq or 200-250 mCi) as described earlier in section 2.5.
Figure 8:
LCMS and plausible mass fragmentation pattern of non-radioactive FTP.
Figure 9:
Plausible mass fragmentation pattern of non-radioactive FTHA.
After decay of radioactivity, the radiation exposed nonradioactive FTP and FTHA reference standards were subjected to HPLC (same conditions and column). Peak fractions were collected in the same regions of side products (a bit before and later) and desired products determined from the [18F]fluorothia fatty acid syntheses. Collected fractions of both “side product” and “desired product” fractions were sent for LCMS analysis. On LCMS the retention time (Rt) for FTP and FTHA reference standards were 1.52 min and 2.25 min, respectively. Whereas, their respective “side product” fractions showed retention times (Rt’s) of 0.853 min and 0.963 min, respectively (see supporting documents). LCMS data for the “desired product” fractions (intact FTHA and FTP) showed the same retention time and mass as of the reference standards of these fluorothia fatty acids. However, LCMS data for “side product” fractions showed an early retention time compared to their parent reference standard analogous to the earlier radioactive peaks in the [18F]fluorothia fatty acid syntheses. The similar nature of side products observed during our stability analysis on analytical HPLC confirmed the relatively polar nature of the side products compared to their parent compounds. Contrary to our expectation, the mass of the side products were found to be a bit higher than their parent compounds ruling out the possibility of fragmentation as the means of decomposition (Fig. 10). A careful analysis of mass spectra of the side products showed an addition of 16 amu to the parent mass indicating oxidation of the parent compound. As discussed earlier, formation of side product was evidently linked to radiation and the sulfur heteroatom. Therefore, it became apparent that oxidation must have occurred on the sulfur atom, which indeed is the most susceptible site for oxidation on fluorothia fatty acids. Thus, the LCMS data clearly suggested radiation-induced formation of sulfoxides of both FTHA and FTP as side products over time and this effect was more profound at higher radioactivity concentrations.
Figure 10:
LCMS spectrum of collected side product fraction from control study and its characterization as a FTHA-sulfoxide.
Although the LCMS data strongly implicated the sulfoxide nature of the side products, we decided to chemically synthesize a sulfoxide derivative of one of the fluorothia fatty acids to verify the chemical identity of the newly discovered side products. To do this, we synthesized the sulfoxide derivative of FTP as described in method section 2.7 and also outlined in Scheme 1. FTP-sulfoxide was characterized by NMR and HRMS before injecting it on HPLC using exactly same HPLC condition and column which was used during stability analysis. As expected, the retention time of FTP-sulfoxide equivalently matched the retention time of the radiolysis-induced side product of [18F]FTP (Fig. 11), further endorsing the sulfoxide nature of the side products. Formation of radiation induced sulfoxide was also reported in literature with other sulfur containing radiopharmaceuticals like L-[11C]methionine17.
Figure 11:

Comparison of the retention times (Rt) of radioactive side product and chemically synthesized nonradioactive FTP-sulfoxide.[ A: Unknown rad peak (Rt 4.45 min), A*: Unknown UV peak (Rt 4.01 min), A**: Chemically synthesized FTP-sulfoxide UV peak (Rt 3.67 min), B: [18F]FTP rad peak (Rt 6.12 min), and B*:[19F]FTP UV peak (Rt 5.59 min)].
Conclusions
The [18F]Fluorothia fatty acids, [18F]FTHA and [18F]FTP, were synthesized successfully under cGMP conditions at higher radioactivity concentrations 1.11 GBq/mL(30 mCi/mL). Upon stability analysis, the two formulated [18F]fluorothia fatty acids each gave rise to a more polar 18F-labeled side product whereas no side products were formed by the non-thia fluoro fatty acid [18F]FP. The identity of the side products were investigated by a mechanistic study using LCMS. The findings strongly support the notion that radiolysis-induced oxidation of the sulfur heteroatom is responsible for conversion of [18F]fluorothia fatty acids to their respective sulfoxides. In the case of [18F]FTP, the nonradioactive reference standard of FTP-sulfoxide was synthesized and shown to have equivalent retention time on HPLC as the 18F-labeled side product of [18F]FTP. Formation of the observed side products were significantly mitigated by reducing radioactivity concentration of the product with sterile saline to <0.518 GBq/mL (14 mCi/mL).
Supplementary Material
Acknowledgments
The authors wish to thank Ms. Mai Petterson for acquisition of LCMS data. This work was financially supported by Department of Radiology, Mayo Clinic and CTSA Mayo Clinic Rochester. LCMS was performed at Mayo Clinic Metabolomics Core facility, which is financially supported by U24DK100469 and UL1TR000135 grants.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Mather KJ, DeGrado TR. Imaging of myocardial fatty acid oxidation. Biochim Biophys Acta. 2016, 1861(10), 1535–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fillmore N, Mori J, Lopaschuk GD, Mitochondrial fatty acid oxidation alterations in heart failure, ischaemic heart disease and diabetic cardiomyopathy. Br J Pharmacol. 2014, 171(8): 2080–2090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lopaschuk GD, Ussher JR, Folmes CD, Jaswal JS, Stanley WC. Myocardial Fatty Acid Metabolism in Health and Disease. Physiol. Rev, 2010, 90, 207–258. [DOI] [PubMed] [Google Scholar]
- 4.Pandey MK, Bansal A, DeGrado TR. Fluorine-18 labeled thia fatty acids for PET imaging of fatty acid oxidation in heart and cancer. Heat Metab. 2011, 51-15–19. [Google Scholar]
- 5.Skrede S, Sørensen HN, Larsen LN, Steineger HH, Høvik K, Spydevold OS, Horn R, Bremer J. Thia fatty acids, metabolism and metabolic effects. Biochim Biophys Acta. 1997, 1344(2), 115–31. [DOI] [PubMed] [Google Scholar]
- 6.Takala TO, Nuutila P, Pulkki K, Oikonen V, Grönroos T, Savunen T, Vähäsilta T, Luotolahti M, Kallajoki M, Bergman J, Forsback S, Knuuti J. 14(R,S)-[18F]Fluoro-6-thia-heptadecanoic acid as a tracer of free fatty acid uptake and oxidation in myocardium and skeletal muscle. Eur J Nucl Med Mol Imaging. 2002, 29(12): 1617–22 [DOI] [PubMed] [Google Scholar]
- 7.Din MU, Saari T, Raiko J, Kudomi N, Maurer SF, Lahesmaa M, Fromme T, Amir EZ, Klingenspor M, Solin O, Nuutila P, Virtanen KA, Postprandial Oxidative Metabolism of Human Brown Fat Indicates Thermogenesis. Cell Metab, 2018, 28(2), 207–216.e3 [DOI] [PubMed] [Google Scholar]
- 8.DeGrado TR. Synthesis of 14 (R,S)-[18F]fluoro-6-thia-heptadecanoic acid (FTHA). J. Labelled Compd. Radiopharm,1991, 29, 989–995. [Google Scholar]
- 9.DeGrado TR, Wang S, Holden JE, Nickles RJ, Taylor M, Stone CK, Synthesis and preliminary evaluation of 18F-labeled 4-thia palmitate as a PET tracer of myocardial fatty acid oxidation. Nucl. Med. Biol, 2000, 27, 221–231. [DOI] [PubMed] [Google Scholar]
- 10.DeGrado TR, Bhattacharyya F, Pandey MK, Belanger AP, Wang S. Synthesis and preliminary evaluation of 18-18F-fluoro-4-thia-oleate as a PET probe of fatty acid oxidation. J. Nucl. Med, 2010, 51(8), 1310–1317. [DOI] [PubMed] [Google Scholar]
- 11.Belanger AP, Pandey MK, Degrado TR. Microwave-assisted radiosynthesis of [18F]fluorinated fatty acid analogs. Nucl. Med. Biol, 2011, 38(3), 435–441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pandey MK, Belanger AP, Wang S, DeGrado TR. Structure Dependence of Long-Chain [18F]Fluorothia Fatty Acids as Myocardial Fatty Acid Oxidation Probes. J Med Chem., 2012, 55, 10674–10684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Savisto N, Viljanen T, Kokkomäki E, Bergman J, Solin O. Automated production of [18F]FTHA according to GMP. J. Labelled Compd. Radiopharm, 2018, 61(2):84–93 [DOI] [PubMed] [Google Scholar]
- 14.McLafferty FW. Mass Spectrometric Analysis: Molecular Rearrangements. Anal Chem. 1959, 31(1), 82–87. [Google Scholar]
- 15.Burr JG A correlation between Mass Spectra and Radiolysis Data. J. Phys. Chem, 1957, 61 (11), 1483–1485. [Google Scholar]
- 16.McDonell WR, Newton AS. The Radiation Chemistry of the Aliphatic Alcohols . J. Am. Chem. Soc, 1954, 76 (18), 4651–4658. [Google Scholar]
- 17.Woods M , Leung L, Frantzen K, Garrick JG, Zhang Z, Zhang C, English W, Wilson D, Bénard F, Lin KS. Improving the stability of 11C–labeled L-methionine with ascorbate. EJNMMI Radiopharmacy and Chemistry (2017) 2:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.





