Abstract
PURPOSE
We aimed to investigate genomic correlates underlying extremes of survivorship in metastatic colorectal cancer (CRC) and their applicability in informing survival in distinct subsets of metastatic CRC patients.
EXPERIMENTAL DESIGN
We examined differences in oncogenic somatic alterations between metastatic CRC cohorts demonstrating extremes of survivorship following complete metastasectomy: ≤2-year (n=17) and ≥10-year (n=18) survivors. Relevant genomic findings, and their association with overall survival (OS), were validated in two independent datasets of 935 stage IV and 443 resected stage I-IV patients.
RESULTS
In the extremes-of-survivorship cohort, significant co-occurrence of KRAS hotspot mutations and TP53 alterations was observed in ≤2-year survivors (P<0.001). When validating these findings in the independent cohort of 935 stage IV patients, incorporation of the cumulative effect of any oncogenic Ras/B-raf (i.e., either KRAS, NRAS, or BRAF) and TP53 alteration generated three prognostic clusters: (1) TP53-altered alone (median OS 132m); (2) Ras/B-raf-altered alone (65m) or Ras/B-raf- and TP53 pan-wildtype (60m); and (3) co-altered Ras/B-raf-TP53 (40m; P<0.0001). Co-altered Ras/B-raf-TP53 was independently associated with mortality (HR 2.47, 95%CI 1.91–3.21, P<0.001). This molecular profile predicted survival in the second independent cohort of 443 resected stage I-IV patients. Co-altered Ras/B-raf-TP53 was associated with worse OS in patients with liver (n=490) and lung (n=172), but not peritoneal surface (n=149), metastases. Moreover, co-altered Ras/B-raf-TP53 tumors were significantly more likely to involve extrahepatic metastatic sites with limited salvage options.
CONCLUSIONS
Genomic analysis of extremes of survivorship following CRC metastasectomy identifies a prognostic role for co-altered Ras/B-raf-TP53 and its association with distinct patterns of CRC metastasis.
INTRODUCTION
Although metachronous or synchronous metastases develop in over half of patients with colorectal cancer (CRC), only 15–20% are deemed resectable at presentation (1). Following complete resection of colorectal liver metastases (CRLM), for instance, approximately 20% are cured (2). In the largest study to date on actual ≥10-year survivors after CRLM resection (n=102), no single clinical factor was sufficiently discriminatory to preclude or predict long-term cure (2). Potential molecular underpinnings of such extremes of survivorship after colorectal metastasectomy, and its applicability in informing clinical outcome in broader populations with metastatic CRC, have not been previously explored.
Well-studied genetic features of metastatic CRC include APC alterations (3,4), 18q loss of heterozygosity (5), microsatellite instability (MSI) (6), loss of SMAD4 (7), activating mutations of BRAF (8) and KRAS/NRAS (9,10) oncogenes, and deleterious TP53 mutations (11). Only a small proportion of these molecular alterations, however, are therapeutically actionable (12). In particular, Ras/B-raf and TP53 alterations, observed oncogenic drivers in more than half of metastatic CRC patients (11,13), have been challenging to target. This therapeutic impasse has been attributed, in part, to the clinical heterogeneity of metastatic CRC—for instance, divergent outcomes in patients with metastasis to the liver, lung, or peritoneal surface (14). While increasing evidence supports the association between alterations in BRAF (15,16), KRAS/NRAS (17), TP53 (18,19), SMAD4 (20), or relevant gene combinations thereof (21), and survival in metastatic CRC, their role in informing prognosis in patient subsets with distinct patterns of organ-specific metastasis remain unclear. As such, clinicians have relied largely on Ras/B-raf mutation status alone, regardless of metastatic pattern, to infer prognosis and make therapeutic decisions in contemporary practice.
In the present study, utilizing the Memorial Sloan Kettering-Integrated Mutation Profiling of Actionable Cancer Targets (MSK-IMPACT) targeted exome capture and deep sequencing platform (22), we hypothesized that a distinct molecular profile may be associated with extremes of survivorship in patients with completely resected metastatic CRC. After evaluating for such a molecular profile in an extreme outlier test set, we validated and extended these findings in two additional independent cohorts of 935 stage IV and 443 completely resected stage I-IV CRC patients. We then explored whether this molecular profile could discriminate between populations of metastatic CRC patients with distinct patterns of organ-specific metastasis.
MATERIALS AND METHODS
Selection of exploratory and validation datasets
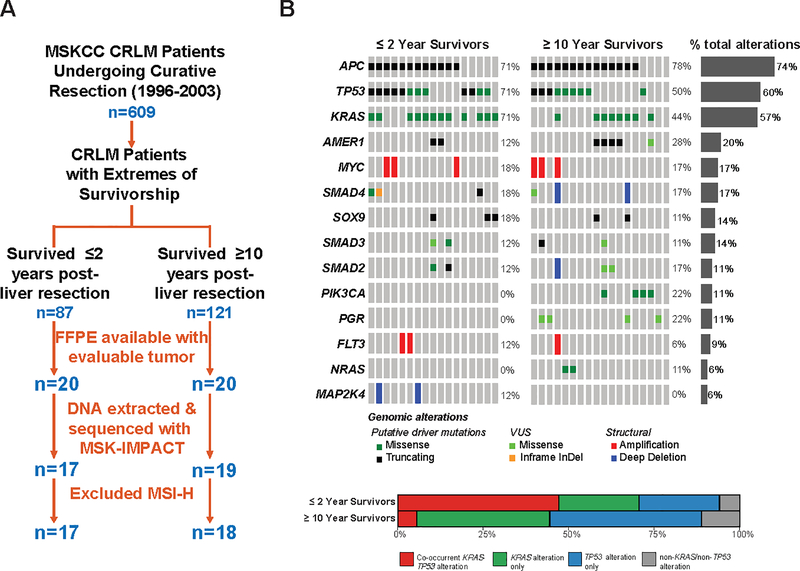
Patients who underwent curative-intent resection of CRLM at Memorial Sloan-Kettering Cancer Center (MSKCC) from 1996 to 2003 (n=609) were identified from a prospectively maintained hepatobiliary surgery database (23) and stratified into extremes-of-survivorship cohorts based on disease-specific survival—short term (≤2-year; n=87) and long-term (≥10-year; n=121) survivors. Of these, 17 patients in ≤2-year and 19 patients in ≥10-year cohorts had evaluable DNA extracted from archived formalin-fixed paraffin embedded tumor tissue for MSK-IMPACT analysis (Figure 1A). Descriptive statistics were performed, and these cohorts were compared across clinicodemographic and molecular characteristics.
Figure 1:
A. CONSORT diagram of selection of extreme outlier test set (n=35) investigating molecular profile associated with extremes of survivorship in resected colorectal liver metastasis (CRLM); B. (Upper) OncoPrint representation of the 14 most frequently altered genes in ≤2-year and ≥10-year survivorship cohorts following CRLM resection. Types of gene alteration grouped by putative driver mutations, variants of undetermined significance (VUS), or structural alterations are shown in the adjoining color legend. Somatic alteration frequencies in recurrently altered genes corresponding to specific genes are shown in the adjacent histograms. (Lower) Proportion of patients in ≤2-year and ≥10-year survivorship cohorts with co-occurrent KRAS-TP53 alterations, KRAS alterations alone, TP53 alterations alone, or non-KRAS/non-TP53 alterations.
To validate and extend genomic findings from this extreme outlier test set, we selected 935 tumors from stage IV CRC patients treated at MSKCC and sequenced with MSK-IMPACT between April 2014 and September 2016 (validation cohort #1; see Supplementary Methods and Supplementary Table S1). The clinical characteristics of this cohort have been described previously; notably, two-thirds of patients presented with synchronous metastasis and half (49.6%) underwent complete metastasectomy (24). Diagnosis of metastasis was established intraoperatively or radiographically. The pattern of distant organ-specific metastasis was adjudicated by first site of detected metastasis, and categorized as liver (n=490, 66.3% resected), lung (n=172, 26.7% resected), peritoneum/ovary (n=149, 31.5% resected), or other (e.g., bone, brain, etc.; n=124).
An independent cohort of completely resected stage I-IV CRC patients derived from the Cartes d’Identité des Tumeurs (CIT) French dataset (n=443), a majority of whom had stage I-III disease (88%), was employed as validation cohort #2. Clinicopathologic characteristics and survival outcomes of this cohort have been previously reported (13). Genomic information on KRAS G12/13 hotspots, BRAFV600E, and TP53 exon 4–9 hotspot mutations was available for analysis; relapse-free survival (RFS) was the survival metric available in this cohort. Patterns of metastatic spread were unavailable for this dataset. The clinical and genomic data are available publicly (13).
This study was approved by the institutional review board at MSKCC, all investigations were conducted in accordance with the Declaration of Helsinki, and written informed consent was obtained from all patients included in the study.
Genomic Sequencing and Analysis
Sequencing of DNA from primary tumor or specified metastasis and matched normal controls was performed with MSK-IMPACT, a hybridization capture-based next-generation sequencing assay (22). Sequencing output was processed as previously described (24) and as noted in Supplementary Methods.
Tumor mutation burden per sample was calculated as the total number of nonsynonymous mutations divided by the actual number of bases analyzed. MSIsensor scores were calculated and represented the unstable proportion of all tested microsatellites (25). All sequenced samples in this study were classified as microsatellite stable (MSS; MSIsensor <10), microsatellite instability-high (MSI-H; MSIsensor ≥10 or TMB ≥25/Mb) (25,26), or those containing POLE/POLD1 exonuclease domain hotspots (known POLE-mutants observed: P286R, S459F, or V411L; POLD1-mutant: S478N) (27). Given the favorable prognosis as well as the relative rarity of MSI-H and POLE/POLD1-mutant cases in metastatic CRC (24,28), this study focused on identifying oncogenic alleles exclusively in the MSS subtype and their association with survival.
Comparison of genomic alterations
Genomic alterations were filtered for known driver variants using a priori knowledge via OncoKB, a precision oncology knowledgebase that curates the effects of cancer variants and their potential clinical actionability (http://oncokb.org) (29). Two-sided Fisher’s exact test was performed to compare the frequency of oncogenic alterations between respective cohorts. Multiple hypothesis correction to account for false discovery rate was performed using the Benjamini-Hochberg method (30). For survival analyses, only those genes altered in ≥5 cases were included for analysis.
Statistical Analysis
Two-tailed t-tests were used to compare continuous variables, while the Fisher’s exact test was used to compare categorical variables. Overall survival (OS) was examined from date of liver resection (for extremes of survivorship cohort, n=35) or date of diagnosis of metastatic disease (for validation cohort #1, n=935) to date of death or last available follow-up. RFS for validation cohort #2 (n=443) was defined as time from surgery to first recurrence and censored at 5 years. Kaplan-Meier survival curves were generated and compared using the log-rank test. Multivariable survival models were computed using Cox proportional hazards regression. Adjusted multivariate survival analyses were performed by controlling for covariates significant in the Cox model using a conditional survival adjustment approach (31). Only false discovery rate-adjusted p-values were reported and values ≤0.05 were considered statistically significant. Statistical analyses were performed using R v3.4.1 (https://cran.r-project.org/) and SAS v9.4 (Cary, NC).
RESULTS
Clinical characteristics of the extremes-of-survivorship cohort
Among resected CRLM patients surviving ≤2 (n=17) or ≥10 (n=19) years with molecular profiling data, all but one patient had MSS tumors. The single patient with somatic hypermutation (i.e., MSIsensor score 47.3, MSI-H) was a ≥10-year survivor and excluded from further analysis (Figure 1A).
In this extreme outlier test set of patients with resected MSS CRLM (n=35), a majority of patients were >50 years old (83%), male (58%), and had left-sided primary tumors (61%). Approximately half of patients received neoadjuvant 5-FU-based systemic therapy (51%) and underwent liver-directed therapy with hepatic arterial infusion (HAI) chemotherapy (46%). Compared with ≥10-year survivors, the ≤2-year survivors had higher mean clinical risk scores (i.e., composite of five clinical determinants of recurrence after CRLM resection (32)), a higher proportion of T3/T4 primaries, a larger number of resected liver tumors, greater size of the largest tumor, and a higher incidence of bilobar metastases (Table 1).
Table 1:
Comparison of demographic and clinical characteristics between resected colorectal liver metastasis patients surviving ≤2 years (n=17) or ≥10 years (n=18) with evaluable DNA for MSK-IMPACT analysis (SD: standard deviation; CEA: carcinoembryonic antigen; cm: centimeter).
| Variable | Overall cohort (n=35) | ≤2-year survivors (n=17) | ≥10-year survivors (n=18) | p-value |
|---|---|---|---|---|
| Age at diagnosis, mean (SD), years | 60.1 (12.9) | 58.1 (16.4) | 61.9 (8.8) | 0.38 |
| Male, n (%) | 20 (57%) | 8 (47%) | 12 (67%) | 0.19 |
| Caucasian, n (%) | 30 (86%) | 14 (82%) | 16 (89%) | 0.65 |
| Preoperative CEA, mean (SD), μg/L | 33.8 (65.4) | 44.4 (79.6) | 22.3 (46.3) | 0.41 |
| Right-sided primary, n (%) | 12 (34%) | 6 (35%) | 6 (33%) | 0.91 |
| T3/T4 primary, n (%) | 29 (83%) | 17 (100%) | 12 (67%) | 0.02 |
| Node-positive primary, n (%) | 19 (54%) | 10 (59%) | 9 (50%) | 0.53 |
| Bilobar liver metastasis, n (%) | 9 (26%) | 7 (41%) | 2 (11%) | 0.035 |
| Size of largest liver metastasis, mean (SD), cm | 4.6 (2.7) | 5.9 (3.0) | 3.4 (1.7) | 0.004 |
| Neoadjuvant 5-FU-based chemotherapy, n (%) | 18 (51%) | 9 (53%) | 9 (50%) | 1.00 |
| Number of resected liver metastases, mean (SD) | 2.6 (2.0) | 3.4 (2.4) | 1.9 (1.5) | 0.031 |
| Margin-negative resection, n (%) | 27 (77%) | 11 (65%) | 16 (89%) | 0.11 |
| Clinical Risk Scorea, mean (SD) | 2.1 (1.3) | 2.7 (1.3) | 1.6 (1.2) | 0.013 |
| Adjuvant hepatic artery infusion pump chemotherapyb, n (%) | 16 (46%) | 7 (41%) | 9 (50%) | 0.74 |
| Adjuvant systemic therapy alone, n (%) | 16 (46%) | 7 (41%) | 9 (50%) | 0.74 |
| Duration of adjuvant therapy, mean (SD), weeks | 47.1 (25.1) | 58.1 (30.4) | 37.2 (13.5) | 0.01 |
Clinical Risk Score: composite score (1 point each) for synchronous liver metastases, disease-free interval <12 months, bilobar metastases, node-positive primary, ≥1 metastasis, and tumor >5 cm)31
All patients who received adjuvant hepatic arterial infusion pump chemotherapy also received systemic chemotherapy
Molecular profile associated with extremes of survivorship
In the extremes-of-survivorship test set, the fourteen most frequently altered genes included members of the RTK/RAS, cell cycle, and WNT/β-catenin signaling pathways. The most frequent somatic gene alterations were APC (74.3%), TP53 (60.0%%), and KRAS (57.1%). No statistically significant differences existed among somatic alterations for any individual gene between the ≤2-year and ≥10-year cohorts either when examining all alterations (i.e., variants of unknown significance and known oncogenic drivers; Figure 1B) or when filtering by known oncogenic driver alterations only (29). Moreover, no differences existed in tumor mutation burden (mutations/MB) between ≤2-year and ≥10-year extremes-of-survivorship cohorts.
In ≤2-year survivors, KRAS missense mutations and MYC amplifications exhibited significant mutual exclusivity (P=0.015). In ≥10-year survivors, AMER1 (i.e., encodes for APC membrane recruitment protein 1, regulating Wnt signaling) truncating mutations demonstrated significant co-occurrence with KRAS missense mutations (P=0.023), while AMER1 truncating mutations and TP53 inactivating alterations were significantly mutually exclusive (P=0.041).
In comparing alteration profiles between the cohorts, KRAS exon 2 (i.e., G12/13) hotspot mutations and TP53 variants were significantly more often co-occurrent in ≤2-year survivors compared with ≥10-year survivors (67% vs. 0%, P<0.001). Conversely, KRAS and TP53 alterations were more frequently mutually exclusive in the ≥10-year compared with ≤2-year survivor cohorts (88.9% vs. 41.2%, P=0.005) (Figure 1B). When stratifying the exploratory cohort into groups with or without co-occurrent KRAS and TP53 alterations, median OS (34.8 vs. 227.2 months, P=0.015) and RFS (9.3 vs. not reached months, P=0.002) was significantly worse in patients with co-occurrent KRAS-TP53 alterations; Supplementary Figure S1).
Validation of molecular profile in an independent cohort of 935 metastatic CRC patients
Our group has previously reported on the genomic landscape of metastatic CRC utilizing MSK-IMPACT in 1,134 patient samples (24). From this cohort, we analyzed the 935 patients with MSS tumors (validation cohort #1) to further evaluate the association of co-occurring KRAS-TP53 alterations with extremes of survivorship (Supplementary Methods, Supplementary Table S1). In validation cohort #1, median age was 53 years, 52.8% were male, 25.8% had right-sided primaries, 68.7% presented with synchronous metastatic disease, 39.5% had more than one distinct site of metastasis (at time of diagnosis), 52.2% had any chemotherapy exposure prior to sequencing, 49.6% underwent metastasectomy, and 39.4% received HAI chemotherapy.
At a median follow-up of 28.5 (range 0.3–292.9) months, median and 5-year OS was 66.5 months and 52.7%, respectively. Patients with co-occurring KRAS-TP53 alterations (n=272) had significantly worse median OS compared with patients without co-altered KRAS-TP53 (n=663) (41.0 vs. 72.7 months, P<0.0001; Figure 2A). Further prognostic discrimination was achieved by stratifying validation cohort #1 into co-occurring KRASALT-TP53ALT, single-altered KRASALT-TP53WT or KRASWT-TP53ALT, and non-KRAS non-TP53 altered (KRASWT/TP53WT) subgroups (Figure 2B). In the subset of patients with liver as first site of metastasis (n=490), co-altered KRASALT/TP53ALT (n=123) was associated with significantly worse OS compared with either TP53 (n=269) or KRAS (n=64) alterations alone (58.3 vs. 147.4 vs. 108.9 months, P≤0.05). Median OS did not statistically differ between KRASWT/TP53ALTand KRASALT/TP53WTcohorts (P=0.95) (Figure 2C).
Figure 2:
A. Stratification of MSKCC validation cohort (n=935) of metastatic CRC patients by presence or absence of co-occurrent KRAS and TP53 alterations; B. Further prognostic refinement by stratification of validation cohort (n=935) by co-occurrence of KRAS and TP53 alterations, singly altered KRAS or TP53, and non-KRAS/non-TP53 alterations; C. Recapitulation of the prognostic performance of this molecular profile in mCRC patients with liver as first site of metastasis (n=490). Number at risk at each time point indicated in adjoining risk table; D. OncoPrint representation of the 13 most frequently altered genes in ≤2-year (n=167) and ≥8-year (n=69) survivorship cohorts in the MSKCC validation cohort. Types of gene alteration grouped by putative driver mutations or structural alterations are shown in the adjoining color legend.
We observed that survival in the co-occurrent KRASALT/TP53ALT and KRASWT/TP53WT cohorts were statistically similar, both in the overall as well as liver metastasis datasets (Figures 2B & C). Further interrogation of the KRASWT/TP53WT cohort (n=57) revealed enrichment for oncogenic alterations in BRAF (21%) and NRAS (14%). We have previously demonstrated that KRAS, BRAF, and NRAS alterations were each independently predictive of survival despite adjusting for primary tumor sidedness in metastatic CRC (24). Other altered genes in this KRASWT/TP53WT cohort were APC (63%), SMAD4 (25%), and SOX9 (18%) (Figure 2D).
Cumulative effect of co-altered Ras/B-raf and TP53 refines prognostication and is independently associated with worse survival
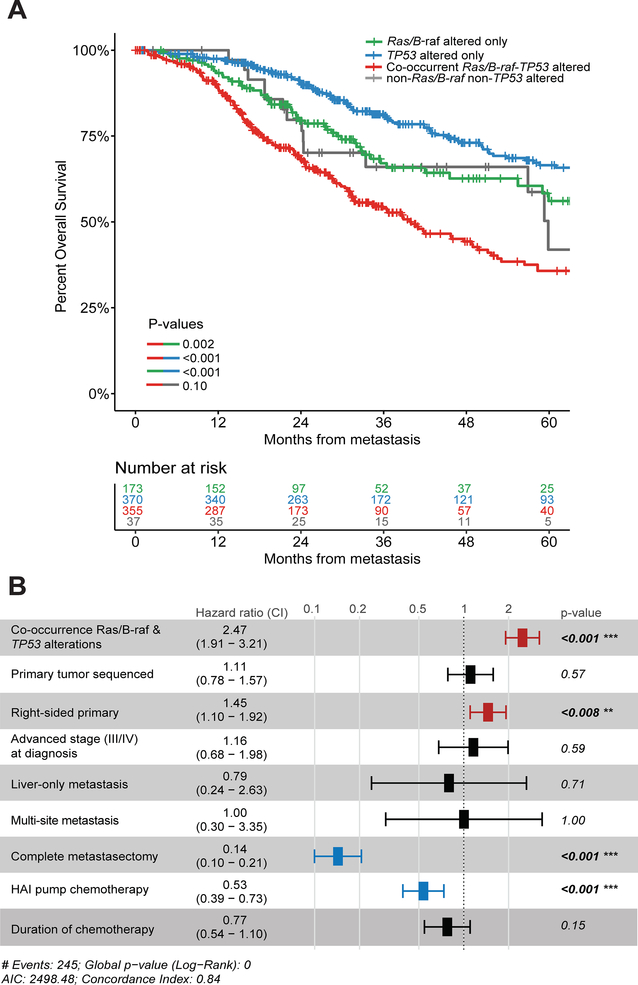
Based on these findings, we incorporated the cumulative effect of any Ras/B-raf alteration (i.e., either KRAS, NRAS, or BRAF) and TP53 alterations on OS in validation cohort #1 to determine if such clustering might refine prognostication. As such, three prognostically distinct subgroups emerged. Patients with TP53 alterations alone demonstrated the best prognosis (median OS 132.0 months), while patients with Ras/B-raf alterations alone (median OS 64.7 months) and pan-wildtype tumors (neither Ras/B-raf nor TP53 alterations; median OS 59.9 months) had an intermediate prognosis. Patients with co-altered Ras/B-raf and TP53 demonstrated the worst prognosis (median OS 40.0 months; P<0.0001) (Figure 3A). Adjusted multivariate survival analyses constructed using a conditional survival adjustment approach confirmed association of co-altered Ras/B-raf-TP53 with lowest OS in both tested models (Supplementary Figure S2).
Figure 3:
A. Stratification of MSKCC validation cohort (n=935) by co-occurrence of Ras/B-raf (i.e., any alterations in KRAS, BRAF, or NRAS) and TP53 alterations, Ras/B-raf alterations alone, TP53 alterations alone, and non-Ras/B-raf/non-TP53 alterations. Number at risk at each time point indicated in adjoining risk table; B. Forest plot indicating Cox proportional-hazards model for overall survival; bar-and-whisker plot depicts hazard ratio and 95% confidence interval, with corresponding p-values shown alongside.
Next, co-altered Ras/B-raf-TP53 and clinicopathologic variables previously shown to be independently associated with OS in this cohort (24) were entered in a Cox proportional hazards regression model. Co-occurring Ras/B-raf and TP53 alterations were independently associated with mortality (hazard ratio 2.47, 95% CI 1.91 to 3.21, P<0.001). Variables associated with improved risk-adjusted OS were left-sided primary tumor location, performance of complete metastasectomy, and receipt of HAI chemotherapy (Figure 3B).
Validation of molecular profile in a second independent cohort of stage I-IV CRC patients
We evaluated the prognostic relevance of co-altered Ras/B-raf-TP53 in an independent French multicenter cohort of resected stage I-IV CRC patients (validation cohort #2, n=443). Patients with Ras/B-raf and TP53 co-occurrence (n=67) demonstrated significantly worse median RFS (37.1 months) compared with patients with Ras/B-raf alterations alone (n=127; median RFS not reached) or TP53 alterations alone (n=111; median RFS not reached) (P=0.017; Supplementary Figure S3).
Association of molecular profile with survival in distinct patterns of metastasis
We next examined if this molecular clustering achieved prognostic discrimination in CRC cohorts with distinct patterns of organ-specific metastasis. Using validation cohort #1 (n=935), we identified patients with liver as first site of metastasis (n=490). We noted that co-altered Ras/B-raf-TP53 was associated with worse OS (median OS 51.6 months) compared with TP53-altered only (187.1 months), Ras/B-raf-altered only (70.0 months), or pan-wildtype cohorts (P<0.001). These findings were recapitulated in patients with lung metastasis (n=172) — co-altered Ras/B-raf-TP53 remained associated with worse OS compared with either Ras/B-raf-altered alone, TP53-altered alone, or pan-wildtype tumors (P=0.019). However, in patients with peritoneal surface (peritoneum/ovary; n=149) metastasis, the presence of co-altered Ras/B-raf-TP53 was not associated with worse OS compared with either Ras/B-raf-altered alone or TP53-altered alone subsets (P=0.15; Figure 4A).
Figure 4:
Prognostic relevance of co-occurrent Ras/B-raf and TP53 alterations in metastatic CRC patients with: A. (from left to right) Liver, lung, and peritoneal surface as first site of metastasis. Number at risk at each time point indicated in adjoining risk table. B. Sankey diagram illustrating relative flow of first site of metastasis from respective genomic subgroups in metastatic CRC: TP53-altered alone (n=370), Ras/B-raf-altered alone (n=173), and co-altered Ras/B-raf-TP53 (n=355). Peritoneal surface refers to peritoneum, ovary, or omentum.
We then constructed a Sankey diagram to illustrate the flow of first organ of metastasis in the three relevant genomic subgroups of interest: co-altered Ras/B-raf-TP53, Ras/B-raf-altered alone, and TP53-altered alone. Metastatic CRC tumors harboring co-occurring Ras/B-raf and TP53 alterations were significantly more likely to involve extrahepatic metastatic sites (e.g., peritoneal surface, lung, brain, bone, etc.) compared with Ras/B-raf-altered or TP53-altered alone tumors (54.6% vs. 42.9%, P=0.002). Conversely, tumors with TP53 alterations alone were significantly more likely to metastasize to liver compared with Ras/B-raf-altered tumors (79.7% vs. 69.2%, P=0.001; Figure 4B).
DISCUSSION
To our knowledge, this is the first study to characterize and validate a distinct molecular profile associated with extremes of survivorship in patients undergoing curative-intent metastasectomy of metastatic colorectal cancer. Furthermore, we extend these genomic findings in two large and clinically diverse cohorts of 935 stage IV CRC and 443 completely resected stage I-IV CRC patients to emphasize broad applicability and prognostic relevance. We demonstrate that co-occurrent oncogenic alterations in TP53 and Ras/B-raf signaling members KRAS, NRAS, or BRAF predict survival beyond conventional clinical or pathologic features in microsatellite-stable metastatic CRC. We then leverage this molecular clustering to demonstrate its prognostic discrimination in patient subsets with distinct patterns of organ-specific metastasis—co-altered Ras/B-raf-TP53 was associated with worse OS in patients with liver and lung, but not peritoneal surface, metastasis.
These clinical findings are mechanistically supported by preclinical evidence. Cellular transformation in CRC is a synergistic process driven by loss-of-function TP53 alterations and oncogenic Ras/B-raf activation (33), in part due to the regulatory role of the p53 tumor suppressor on Ras/B-raf signaling (34). Beyond sufficiency to induce tumorigenesis, co-occurrence of TP53 and Ras/B-raf signaling alterations abrogate cellular senescence (35), promote metastasis-defining processes such as invasion and motility (36), and generate a highly metastatic phenotype in vivo. While convergence of the p53 and RTK-RAS pathways in the regulation of cell fate is well studied, its biologic relevance in the clinical arena is incompletely understood. In prior work, analysis of 123 locally advanced rectal cancers resected following neoadjuvant chemoradiotherapy demonstrated that co-occurrence of KRAS-TP53 mutations was associated with chemoradiotherapy resistance (37). A recent single-institution study utilizing a 50-gene sequencing platform indicated that co-altered KRAS-TP53 was an independent predictor of shorter OS in 401 patients with resected CRLM (38). The absence of large-scale validation in the latter study, however, limits its generalizability to a broader metastatic CRC population.
Our study extends these early efforts in understanding the clinical impact of Ras/B-raf-TP53 cooperativity in several ways. First, by drawing on two large independent validation datasets, this study underscores the clinical relevance of co-occurring alterations in TP53 and Ras/B-raf signaling members KRAS, NRAS, and BRAF in diverse metastatic CRC patient subsets. Second, this molecular profile reconciles the distinct outcomes of CRC patients with mutually exclusive KRAS (17,39), BRAF (16,40), and NRAS (10) mutations, with or without TP53 alterations, into a clinically relevant model while maintaining prognostic discrimination between these respective subgroups. Third, within the context of Ras/B-raf and TP53-driven molecular stratification described herein, this analysis deepens our understanding of the clinical implications of pivotal genomic alterations beyond oncogenic Ras/B-raf and TP53. This is exemplified by conflation of outcomes between Ras/B-raf-altered only and pan-wildtype (non-Ras/B-raf, non-TP53 altered) cohorts. In the latter subgroup, prognostically relevant and/or potentially actionable alterations include ARID1A mutations (41,42), PIK3CA missense mutations (43,44), ERBB2 amplification (45), and SMAD4 alterations (20,21). In particular, the role of altered SMAD4—which comprised 25% of the pan-wildtype cohort—deserves discussion. In light of recent data from Kawaguchi et al. indicating the prognostic cooperativity of SMAD4, Ras/B-raf, and TP53 in patients with resected CRLM (21), an exploratory analysis in our validation cohort #1 revealed prognostic superiority of co-altered SMAD4-Ras/B-raf-TP53 compared with co-altered Ras/B-raf-TP53 alone (see Supplementary Figure S4). Subgroup analyses attempting to confirm these findings in cohorts with distinct patterns of metastasis, however, were underpowered and less reliable. Notwithstanding, these data warrant further exploration as they complement recent findings from independent mCRC cohorts.
We draw upon the molecular clustering from the extremes-of-survivorship cohort to uncover a novel association between co-altered Ras/B-raf-TP53 and worse survival in patients with liver and lung, but not peritoneal surface, metastases. The divergent clinical presentations and ensuing outcomes in patients with these patterns of metastasis are well documented (46,47). The more frequent presentation of co-altered Ras/B-raf-TP53 tumors with extrahepatic metastasis, particularly to sites with limited opportunities for therapeutic salvage (e.g., peritoneal surface, bone, brain, etc.) may suggest a putative molecular basis for the clinical heterogeneity observed in metastatic CRC. Conversely, the lack of prognostic discrimination offered by this genomic clustering in patients with peritoneal surface metastasis suggests the need for alternative biomarkers in this disease setting. Our findings warrant future validation since genomically annotated metastatic CRC datasets with granular information on patterns of metastatic spread are currently unavailable. As such, the data herein open the door for future investigation into the interplay between this molecular phenotype and factors governing differential metastatic organotropism, such as integrin-mediated tumor interaction with extracellular matrix components (48), metastatic colonization by cancer stem cells (49), or immunoregulatory networks in pre-metastatic niches (50).
Our study has several limitations. First, given the retrospective design, we cannot eliminate biases in patient selection for resection in the examined cohorts. Second, the low DNA extraction yield from archived tumors in the extreme outlier test cohort may have rendered the ensuing analysis underpowered to detect other genomic differences (e.g., SMAD4) between ≤2-year and ≥10-year survivors. Third, classifying the first organ of metastasis (e.g., in validation cohort #1) is challenging in a subset of patients presenting with disseminated disease. Fourth, it is also possible that the peritoneal surface-metastatic cohort was underpowered to detect subtle differences in survival between prognostic subgroups. Fifth, this study does not address the relationship of such genomic clustering with response to treatment (i.e., as a predictive biomarker). Finally, patients with metastatic CRC sequenced at our tertiary referral cancer center could be enriched for non-traditional biology or treatment paradigms (e.g., hepatic arterial infusion chemotherapy for liver metastasis) that may confound these results. Notwithstanding, the findings reported herein suggest that prognostic considerations in CRC must extend beyond the isolated contributions of KRAS/NRAS, BRAF, or TP53 to a model inclusive of all these genetic elements. Such molecular stratification could be incorporated into future trial design in order to allow more precise selection of CRC patients for elements of multimodality therapy, with the goal of further improving contemporary survival outcomes.
Supplementary Material
TRANSLATIONAL RELEVANCE.
In this study, we identify the genomic underpinnings associated with extremes of survivorship following complete colorectal cancer metastasectomy, and validate these findings in two large independent cohorts (n=935, 443) of metastatic colorectal cancer patients. Co-alteration of oncogenic TP53 alterations with either KRAS, NRAS, or BRAF (i.e., Ras/B-raf) mutations was associated with significantly worse survival compared with alterations in either gene group alone. These data suggest that molecular prognostication in metastatic colorectal cancer should extend beyond the isolated contributions of KRAS, BRAF, or TP53 to a model inclusive of all these genetic elements. We then leverage this molecular clustering to demonstrate its prognostic discrimination in patient subsets with distinct patterns of organ-specific metastasis—co-altered Ras/B-raf-TP53 was associated with worse survival in patients with liver and lung, but not peritoneal surface, metastasis. The more frequent presentation of co-altered Ras/B-raf-TP53 tumors with extrahepatic metastasis, particularly to sites with limited opportunities for therapeutic salvage (e.g., peritoneal surface, bone, brain, etc.) may suggest a putative molecular basis for the clinical heterogeneity observed in metastatic colorectal cancer.
ACKNOWLEDGMENTS
Financial Support: This work was supported by the National Cancer Institute at the National Institutes of Health (P30 CA008748 to J. Smith); The American Society of Colon and Rectal Surgeons Career Development Award to J. Smith; Roslyn Faculty Research Award to J. Smith; American Society of Colon and Rectal Surgeons Limited Project Grant to J. Smith; Franklin Martin MD Faculty Research Fellowship from the American College of Surgeons to J. Smith; the Wasserman Colon/Rectal Cancer Fund to J. Smith; the Colorectal Cancer Alliance and the Chris4Life Research Award and in part by a Stand Up To Cancer Colorectal Cancer Dream Team Translational Research Grant (Grant Number: SU2C-AACR-DT22-17). Stand Up To Cancer is a division of the Entertainment Industry Foundation. Research grants are administered by the American Association for Cancer Research, the scientific partner of SU2C.
Conflict of Interest Disclosure: Dr. Garcia-Aguilar has received support from Medtronic, Johnson and Johnson, and Intuitive Surgical. Dr. Smith received travel support for fellowship education from Intuitive Surgical Inc. and has served as a clinical advisor for Guardant Health, Inc. Dr. Michael Berger serves on the advisory board for Roche. Dr. Nancy Kemeny has received support from Amgen. Dr. Rona Yaeger has received research support from Array BioPharma, Genentech, GlaxoSmithKline, and Novartis, and has served as an advisory board member for GlaxoSmithKline.
REFERENCES
- 1.Siegel RL, Miller KD, Fedewa SA, Ahnen DJ, Meester RGS, Barzi A, et al. Colorectal cancer statistics, 2017. CA Cancer J Clin 2017;67(3):177–93 doi 10.3322/caac.21395. [DOI] [PubMed] [Google Scholar]
- 2.Tomlinson JS, Jarnagin WR, DeMatteo RP, Fong Y, Kornprat P, Gonen M, et al. Actual 10-year survival after resection of colorectal liver metastases defines cure. J Clin Oncol 2007;25(29):4575–80 doi 10.1200/JCO.2007.11.0833. [DOI] [PubMed] [Google Scholar]
- 3.Cottrell S, Bicknell D, Kaklamanis L, Bodmer WF. Molecular analysis of APC mutations in familial adenomatous polyposis and sporadic colon carcinomas. Lancet 1992;340(8820):626–30. [DOI] [PubMed] [Google Scholar]
- 4.Powell SM, Zilz N, Beazer-Barclay Y, Bryan TM, Hamilton SR, Thibodeau SN, et al. APC mutations occur early during colorectal tumorigenesis. Nature 1992;359(6392):235–7 doi 10.1038/359235a0. [DOI] [PubMed] [Google Scholar]
- 5.Ogino S, Nosho K, Irahara N, Shima K, Baba Y, Kirkner GJ, et al. Prognostic significance and molecular associations of 18q loss of heterozygosity: a cohort study of microsatellite stable colorectal cancers. J Clin Oncol 2009;27(27):4591–8 doi 10.1200/JCO.2009.22.8858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Samowitz WS, Curtin K, Ma KN, Schaffer D, Coleman LW, Leppert M, et al. Microsatellite instability in sporadic colon cancer is associated with an improved prognosis at the population level. Cancer Epidemiol Biomarkers Prev 2001;10(9):917–23. [PubMed] [Google Scholar]
- 7.Forcet C, Ye X, Granger L, Corset V, Shin H, Bredesen DE, et al. The dependence receptor DCC (deleted in colorectal cancer) defines an alternative mechanism for caspase activation. Proc Natl Acad Sci U S A 2001;98(6):3416–21 doi 10.1073/pnas.051378298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Iacopetta B, Li WQ, Grieu F, Ruszkiewicz A, Kawakami K. BRAF mutation and gene methylation frequencies of colorectal tumours with microsatellite instability increase markedly with patient age. Gut 2006;55(8):1213–4 doi 10.1136/gut.2006.095455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Andreyev HJ, Norman AR, Cunningham D, Oates JR, Clarke PA. Kirsten ras mutations in patients with colorectal cancer: the multicenter “RASCAL” study. J Natl Cancer Inst 1998;90(9):675–84. [DOI] [PubMed] [Google Scholar]
- 10.Cercek A, Braghiroli MI, Chou JF, Hechtman JF, Kemeny N, Saltz L, et al. Clinical Features and Outcomes of Patients with Colorectal Cancers Harboring NRAS Mutations. Clin Cancer Res 2017;23(16):4753–60 doi 10.1158/1078-0432.CCR-17-0400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cancer Genome Atlas N. Comprehensive molecular characterization of human colon and rectal cancer. Nature 2012;487(7407):330–7 doi 10.1038/nature11252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Graham DM, Coyle VM, Kennedy RD, Wilson RH. Molecular Subtypes and Personalized Therapy in Metastatic Colorectal Cancer. Curr Colorectal Cancer Rep 2016;12:141–50 doi 10.1007/s11888-016-0312-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Marisa L, de Reynies A, Duval A, Selves J, Gaub MP, Vescovo L, et al. Gene expression classification of colon cancer into molecular subtypes: characterization, validation, and prognostic value. PLoS Med 2013;10(5):e1001453 doi 10.1371/journal.pmed.1001453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Riihimaki M, Hemminki A, Sundquist J, Hemminki K. Patterns of metastasis in colon and rectal cancer. Sci Rep 2016;6:29765 doi 10.1038/srep29765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Barras D, Missiaglia E, Wirapati P, Sieber OM, Jorissen RN, Love C, et al. BRAF V600E Mutant Colorectal Cancer Subtypes Based on Gene Expression. Clin Cancer Res 2017;23(1):104–15 doi 10.1158/1078-0432.CCR-16-0140. [DOI] [PubMed] [Google Scholar]
- 16.Margonis GA, Buettner S, Andreatos N, Kim Y, Wagner D, Sasaki K, et al. Association of BRAF Mutations With Survival and Recurrence in Surgically Treated Patients With Metastatic Colorectal Liver Cancer. JAMA Surg 2018:e180996 doi 10.1001/jamasurg.2018.0996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vauthey JN, Zimmitti G, Kopetz SE, Shindoh J, Chen SS, Andreou A, et al. RAS mutation status predicts survival and patterns of recurrence in patients undergoing hepatectomy for colorectal liver metastases. Ann Surg 2013;258(4):619–26; discussion 26–7 doi 10.1097/SLA.0b013e3182a5025a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.de Jong KP, Gouw AS, Peeters PM, Bulthuis M, Menkema L, Porte RJ, et al. P53 mutation analysis of colorectal liver metastases: relation to actual survival, angiogenic status, and p53 overexpression. Clin Cancer Res 2005;11(11):4067–73 doi 10.1158/1078-0432.CCR-04-2389. [DOI] [PubMed] [Google Scholar]
- 19.Mollevi DG, Serrano T, Ginesta MM, Valls J, Torras J, Navarro M, et al. Mutations in TP53 are a prognostic factor in colorectal hepatic metastases undergoing surgical resection. Carcinogenesis 2007;28(6):1241–6 doi 10.1093/carcin/bgm012. [DOI] [PubMed] [Google Scholar]
- 20.Mizuno T, Cloyd JM, Vicente D, Omichi K, Chun YS, Kopetz SE, et al. SMAD4 gene mutation predicts poor prognosis in patients undergoing resection for colorectal liver metastases. Eur J Surg Oncol 2018;44(5):684–92 doi 10.1016/j.ejso.2018.02.247. [DOI] [PubMed] [Google Scholar]
- 21.Kawaguchi Y, Kopetz S, Newhook TE, De Bellis M, Chun YS, Tzeng CD, et al. Mutation Status of RAS, TP53, and SMAD4 is Superior to Mutation Status of RAS Alone for Predicting Prognosis after Resection of Colorectal Liver Metastases. Clin Cancer Res 2019. doi 10.1158/1078-0432.CCR-19-0863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cheng DT, Mitchell TN, Zehir A, Shah RH, Benayed R, Syed A, et al. Memorial Sloan Kettering-Integrated Mutation Profiling of Actionable Cancer Targets (MSK-IMPACT): A Hybridization Capture-Based Next-Generation Sequencing Clinical Assay for Solid Tumor Molecular Oncology. J Mol Diagn 2015;17(3):251–64 doi 10.1016/j.jmoldx.2014.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.House MG, Ito H, Gonen M, Fong Y, Allen PJ, DeMatteo RP, et al. Survival after hepatic resection for metastatic colorectal cancer: trends in outcomes for 1,600 patients during two decades at a single institution. J Am Coll Surg 2010;210(5):744–52, 52–5 doi 10.1016/j.jamcollsurg.2009.12.040. [DOI] [PubMed] [Google Scholar]
- 24.Yaeger R, Chatila WK, Lipsyc MD, Hechtman JF, Cercek A, Sanchez-Vega F, et al. Clinical Sequencing Defines the Genomic Landscape of Metastatic Colorectal Cancer. Cancer Cell 2018;33(1):125–36 e3 doi 10.1016/j.ccell.2017.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Niu B, Ye K, Zhang Q, Lu C, Xie M, McLellan MD, et al. MSIsensor: microsatellite instability detection using paired tumor-normal sequence data. Bioinformatics 2014;30(7):1015–6 doi 10.1093/bioinformatics/btt755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stadler ZK, Battaglin F, Middha S, Hechtman JF, Tran C, Cercek A, et al. Reliable Detection of Mismatch Repair Deficiency in Colorectal Cancers Using Mutational Load in Next-Generation Sequencing Panels. J Clin Oncol 2016;34(18):2141–7 doi 10.1200/JCO.2015.65.1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Church DN, Briggs SE, Palles C, Domingo E, Kearsey SJ, Grimes JM, et al. DNA polymerase epsilon and delta exonuclease domain mutations in endometrial cancer. Hum Mol Genet 2013;22(14):2820–8 doi 10.1093/hmg/ddt131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Domingo E, Freeman-Mills L, Rayner E, Glaire M, Briggs S, Vermeulen L, et al. Somatic POLE proofreading domain mutation, immune response, and prognosis in colorectal cancer: a retrospective, pooled biomarker study. Lancet Gastroenterol Hepatol 2016;1(3):207–16 doi 10.1016/S2468-1253(16)30014-0. [DOI] [PubMed] [Google Scholar]
- 29.Chakravarty D, Gao J, Phillips SM, Kundra R, Zhang H, Wang J, et al. OncoKB: A Precision Oncology Knowledge Base. JCO Precis Oncol 2017;2017 doi 10.1200/PO.17.00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Benjamini Y, Cohen R. Weighted false discovery rate controlling procedures for clinical trials. Biostatistics 2017;18(1):91–104 doi 10.1093/biostatistics/kxw030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nieto FJ, Coresh J. Adjusting survival curves for confounders: a review and a new method. Am J Epidemiol 1996;143(10):1059–68 doi 10.1093/oxfordjournals.aje.a008670. [DOI] [PubMed] [Google Scholar]
- 32.Fong Y, Fortner J, Sun RL, Brennan MF, Blumgart LH. Clinical score for predicting recurrence after hepatic resection for metastatic colorectal cancer: analysis of 1001 consecutive cases. Ann Surg 1999;230(3):309–18; discussion 18–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hanahan D, Weinberg RA. The hallmarks of cancer. Cell 2000;100(1):57–70. [DOI] [PubMed] [Google Scholar]
- 34.Buganim Y, Solomon H, Rais Y, Kistner D, Nachmany I, Brait M, et al. p53 Regulates the Ras circuit to inhibit the expression of a cancer-related gene signature by various molecular pathways. Cancer Res 2010;70(6):2274–84 doi 10.1158/0008-5472.CAN-09-2661. [DOI] [PubMed] [Google Scholar]
- 35.Morton JP, Timpson P, Karim SA, Ridgway RA, Athineos D, Doyle B, et al. Mutant p53 drives metastasis and overcomes growth arrest/senescence in pancreatic cancer. Proc Natl Acad Sci U S A 2010;107(1):246–51 doi 10.1073/pnas.0908428107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zheng S, El-Naggar AK, Kim ES, Kurie JM, Lozano G. A genetic mouse model for metastatic lung cancer with gender differences in survival. Oncogene 2007;26(48):6896–904 doi 10.1038/sj.onc.1210493. [DOI] [PubMed] [Google Scholar]
- 37.Garcia-Aguilar J, Chen Z, Smith DD, Li W, Madoff RD, Cataldo P, et al. Identification of a biomarker profile associated with resistance to neoadjuvant chemoradiation therapy in rectal cancer. Ann Surg 2011;254(3):486–92; discussion 92–3 doi 10.1097/SLA.0b013e31822b8cfa. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chun YS, Passot G, Yamashita S, Nusrat M, Katsonis P, Loree JM, et al. Deleterious Effect of RAS and Evolutionary High-risk TP53 Double Mutation in Colorectal Liver Metastases. Ann Surg 2017. doi 10.1097/SLA.0000000000002450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Taieb J, Zaanan A, Le Malicot K, Julie C, Blons H, Mineur L, et al. Prognostic Effect of BRAF and KRAS Mutations in Patients With Stage III Colon Cancer Treated With Leucovorin, Fluorouracil, and Oxaliplatin With or Without Cetuximab: A Post Hoc Analysis of the PETACC-8 Trial. JAMA Oncol 2016;2(5):643–53 doi 10.1001/jamaoncol.2015.5225. [DOI] [PubMed] [Google Scholar]
- 40.Roth AD, Tejpar S, Delorenzi M, Yan P, Fiocca R, Klingbiel D, et al. Prognostic role of KRAS and BRAF in stage II and III resected colon cancer: results of the translational study on the PETACC-3, EORTC 40993, SAKK 60–00 trial. J Clin Oncol 2010;28(3):466–74 doi 10.1200/JCO.2009.23.3452. [DOI] [PubMed] [Google Scholar]
- 41.Bitler BG, Aird KM, Garipov A, Li H, Amatangelo M, Kossenkov AV, et al. Synthetic lethality by targeting EZH2 methyltransferase activity in ARID1A-mutated cancers. Nat Med 2015;21(3):231–8 doi 10.1038/nm.3799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Putra J, Suriawinata AA. Clinical Significance of Loss of ARID1A Expression in Colorectal and Small Intestinal Carcinoma. Clin Transl Gastroenterol 2015;6:e131 doi 10.1038/ctg.2015.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mei ZB, Duan CY, Li CB, Cui L, Ogino S. Prognostic role of tumor PIK3CA mutation in colorectal cancer: a systematic review and meta-analysis. Ann Oncol 2016;27(10):1836–48 doi 10.1093/annonc/mdw264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Baselga J, Im SA, Iwata H, Cortes J, De Laurentiis M, Jiang Z, et al. Buparlisib plus fulvestrant versus placebo plus fulvestrant in postmenopausal, hormone receptor-positive, HER2-negative, advanced breast cancer (BELLE-2): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol 2017;18(7):904–16 doi 10.1016/S1470-2045(17)30376-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sartore-Bianchi A, Trusolino L, Martino C, Bencardino K, Lonardi S, Bergamo F, et al. Dual-targeted therapy with trastuzumab and lapatinib in treatment-refractory, KRAS codon 12/13 wild-type, HER2-positive metastatic colorectal cancer (HERACLES): a proof-of-concept, multicentre, open-label, phase 2 trial. Lancet Oncol 2016;17(6):738–46 doi 10.1016/S1470-2045(16)00150-9. [DOI] [PubMed] [Google Scholar]
- 46.Khattak MA, Martin HL, Beeke C, Price T, Carruthers S, Kim S, et al. Survival differences in patients with metastatic colorectal cancer and with single site metastatic disease at initial presentation: results from South Australian clinical registry for advanced colorectal cancer. Clin Colorectal Cancer 2012;11(4):247–54 doi 10.1016/j.clcc.2012.06.004. [DOI] [PubMed] [Google Scholar]
- 47.Qiu M, Hu J, Yang D, Cosgrove DP, Xu R. Pattern of distant metastases in colorectal cancer: a SEER based study. Oncotarget 2015;6(36):38658–66 doi 10.18632/oncotarget.6130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Enns A, Korb T, Schluter K, Gassmann P, Spiegel HU, Senninger N, et al. Alphavbeta5-integrins mediate early steps of metastasis formation. Eur J Cancer 2005;41(7):1065–72 doi 10.1016/j.ejca.2004.12.031. [DOI] [PubMed] [Google Scholar]
- 49.Malanchi I, Santamaria-Martinez A, Susanto E, Peng H, Lehr HA, Delaloye JF, et al. Interactions between cancer stem cells and their niche govern metastatic colonization. Nature 2011;481(7379):85–9 doi 10.1038/nature10694. [DOI] [PubMed] [Google Scholar]
- 50.Liu Y, Cao X. Characteristics and Significance of the Pre-metastatic Niche. Cancer Cell 2016;30(5):668–81 doi 10.1016/j.ccell.2016.09.011. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.