Abstract
We established that loss of miR-15a/16-1 genes on chromosome 13q14 is the most common alteration in Chronic Lymphocytic Leukemia (CLL) and that miR-15/16 are crucial negative regulator of BCL-2, an antiapoptotic gene overexpressed in most CLLs and in many other malignancies. We have also shown that miR-15/16 target ROR1, a cell surface receptor for Wnt5a which can enhance growth/survival of CLL cells. Interestingly, ROR1 is expressed by many cancers, but not by normal adult tissues. Moreover, Venetoclax, the anti-BCL-2 drug, and Cirmtuzumab, the monoclonal antibody against ROR1, are synergistic in killing CLL cells.
Since an additional miR-15/16 locus exists on chromosome 3q25 (miR-15b/16-2), we generated a knocked out mouse model to study its the role in cancer. We observed that the KO mice developed predominantly CLL. Thus, we generated a double knock out mouse model where both miR-15/16 loci were deleted. Surprisingly we observed that 77% of double KO mice developed Acute Myeloid Leukemia (AML). Based on these evidences, we anticipate that also AMLs with low miR-15/16 expression, overexpression of BCL2 and expression of ROR1, would show an excellent response to a combination therapy with venetoclax and monoclonal antibodies against ROR1, since both drugs target the same malignant cells that have lost miR-15/16.
Keywords: CLL, AML, miR-15/16, BCL-2, ROR1, Venetoclax, Cirmtuzumab
1. Introduction
In the last few years, two drugs have been developed to target specifically Chronic Lymphocytic Leukemia (CLL) cells: Venetoclax and Cirmtuzumab (Rassenti et al., 2017).
The design of Venetoclax, was based on the discovery of the key role of the antiapoptotic B-cell lymphoma 2 gene (BCL-2) and its dysregulation in follicular lymphomas and CLL (Calin et al., 2002; Croce and Reed, 2016; Tsujimoto et al., 1985; Tsujimoto and Croce, 1986; Tsujimoto et al., 1984). BCL-2 is a master regulator of cell survival (Croce and Reed, 2016) and Venetoclax was developed to kill malignant cells driven by the overexpression this protein (Pekarsky et al., 2018; Pekarsky and Croce, 2019).
The development of Cirmtuzumab, was based on the discovery that the tyrosine kinase-like orphan receptor 1 (ROR1), a receptor of Wnt-5a, is frequently expressed on the surface of CLL cells but not in normal somatic tissues (Cui et al., 2016). Since Wnt5a-ROR1 binding on CLL cell surface initiates the non-canonical Wnt-5a pathway that promotes cell proliferation, Cirmtuzumab was developed as a humanized monoclonal antibody to compete with Wnt5a in the binding of ROR1, thus preventing the initiation of the proliferation signal (Pekarsky and Croce, 2019; Yu et al., 2017; Zhou et al., 2017).
2. BCL-2 and Venetoclax
In 1984 we investigated the t(14;18) chromosomal translocations, the hallmark of follicular lymphoma. These studies led to the discovery and characterization of the B-cell lymphoma 2 gene (BCL-2). In follicular lymphoma, the t(14;18) translocation places the BCL-2 gene from chromosome 18 next to the immunoglobulin heavy chain locus on chromosome 14, prompting to an excessive transcription and expression of BCL-2 (Tsujimoto et al., 1985; Tsujimoto et al., 1984). The mechanism of action of this oncogene, however, remained unknown until 1988 when a functional study revealed that, in IL-3-dependent haematopoietic progenitor, exogenous expression of Bcl-2 increased cell survival when the essential grow factor was removed. In this experiment, both BCL-2 overexpressing cells and control cells went into cycle arrest when IL-3 was removed. However, while control cells died within 72 hours, cells overexpressing BCL-2 did not (Vaux et al., 1988). These evidences indicated that BCL-2 overexpression supports cancer cell survival by preventing cell death rather than enhancing proliferation. This effect was noted in human cancers as well. For instance, in small low grade breast cancer with positive estrogen receptor/progesterone receptor (ER/PR) status and negative human epidermal growth factor receptor 2 (HER2) status, high expression of BCL-2 correlates with favorable prognostics (Dawson et al., 2010). However, high expression of BCL-2 is an independent unfavorable prognostic factor in patients with ER-negative/PR-negative or triple-negative breast cancer (Abdel-Fatah et al., 2013; Honma et al., 2015). Likewise, in prostate cancer, the expression level of Bcl-2 is increased during progression from androgen-dependent to androgen-independent growth stage (Lin et al., 2007; McDonnell et al., 1992). In this context, the survival advantage provided by BCL-2 overexpression, facilitate the accumulation of additional lesions that may lead to full malignant transformation. BCL-2 transgenic mice, show accumulation of B lymphoid cells, but the incidence of lymphoma is low and delayed (McDonnell et al., 1989) while Myc transgenic mice develop lymphomas, but only after the 6th week of age and following a period of benign lymphocytosis (Harris et al., 1988; Langdon et al., 1986), double transgenics for Bcl-2 and c-Myc, show a very rapid lymphoma development, caused by the combination of Myc-driven enhanced proliferation, with the Bcl-2-driven apoptosis evasion (Strasser et al., 1990).
To generate an effective anti-Bcl-2 compound is essential to understand its mechanism of action. High expression of BCL-2 protect cells from the effects of cellular stresses that would otherwise induce apoptosis (Fulda, 2010; Fulda et al., 2010). The mechanism can be summarized as Bcl-2 binding and inactivating Bcl-2-related proteins which would trigger apoptosis by damaging the mitochondrial outer membrane (Kroemer et al., 2007). Bcl-2 related proteins contain highly conserved BCL-2 homology (BH) domains and are divided in 3 subgroups (Gillies and Kuwana, 2014): (1) Apoptosis initiators, or BH3 proteins, such as Bim, Bid, Puma and Bad, which detect cellular stress and activate apoptosis; (2) Apoptosis effectors, or multi-BH proteins, such as Bax or Bak, which permeabilize the mitochondrial outer membrane; and (3) Antiapoptotic members, such as Bcl-2 Mcl-1, Bcl-xl, Bfl-1/a1, Bcl-w, Bcl-b, which bind and inactivate BH3 and multi-BH proteins to halt apoptosis. This system is designed to finely regulate apoptosis initiation by inducing or inhibiting Bak and Bax in response to cell conditions. Thus, maintaining the correct balance in the expression of all these proteins is essential to ensure apoptosis activation in response to cell damage. The observation that BCL-2 overexpression prevents apoptosis by binding to BH3-protein, led to the generation of BH3-mimetic compounds that would hinder Bcl-2 to allow apoptosis initiation (Hata et al., 2015). It took over 20 years from our discoveries on BCL-2, to the generation of the first anti-Bcl-2 drugs, ABT-737 and ABT-263, developed by the pharmaceutical company Abbott the in the early 2000. Unfortunately, these compounds, would not only inhibit Bcl-2 but also Bcl-xL, which is essential for the survival of platelets, causing serious thrombocytopenia (Croce and Reed, 2016). Thus, a specific Bcl-2-inhibitor, ABT-199 (Venetoclax), was generated in 2013, that showed striking success in causing CLL cell death (Croce and Reed, 2016; Souers et al., 2013). In 2016, 32 years after the discovery of BCL-2 and 11 years after the elucidation of its microRNA-driven dysregulation mechanism in CLL, FDA approved the use of Venetoclax as a single treatment agent for relapsed/refractory CLL (Croce and Reed, 2016; Stilgenbauer et al., 2018) and in May 2019, Venetoclax was approved as a Chemotherapy-Free combination regimen for previously untreated CLL patients (Fischer et al., 2019). Remarkably, in 2018, the FDA also granted accelerated approval to use Venetoclax in combination with Azacitidine, Decitabine or Cytarabine to treat newly-diagnosed AML patients who are ineligible for chemotherapy (DiNardo et al., 2019).
3. ROR1 and Cirmtuzumab
ROR1 is transmembrane protein within the receptor tyrosine kinase (RTK) family. ROR1 was initially discovered in early 1990 in a neuroblastoma cell line as a cell surface receptor with a key role in the neurite growth in the central nervous system (Reddy et al., 1997). ROR1 gene is highly expressed during early embryonic development, playing an important role in regulating embryonic muscle and skeletal development (Borcherding et al., 2014). During fetal development though, the expression of ROR1 is turned off, thus normal adult cells and tissues do not express this protein. In sharp contrast with the negligible expression of ROR1 in normal adult tissues, significant expression of ROR1 has been detected in several cancers, suggesting that this transmembrane protein could be considered as a tumor-specific cells surface antigen (Zhang et al., 2012). Expression of ROR1 was undoubtedly associated with ovarian cancer stem cells (Zhang et al., 2014) and CLL (Cui et al., 2016) and it seems to play a functional role in promoting migration/invasion by activating the non-canonical Wnt5a pathway (Hasan et al., 2019). Studies on ROR1 expression in CLL patients showed that only ~10% of CLL patients have leukemic cells (CD19/CD5 double positive) expressing negligible level of ROR1 like normal CD19+ B cells (Rassenti et al., 2017). In ~90% of patients (Rassenti et al., 2017), ROR1 is highly and consistently expressed in malignant cells (~ 97% of CD19/CD5 double positive B-CLL cells show high expression of ROR1 as surface antigen) (Uhrmacher et al., 2011). No significant difference in ROR1 expression on CD19/CD5 double positive cell surface was found when comparing ROR1+ indolent and ROR1+ aggressive patients and its expression on CLL cell surface is not influenced by treatment. Indeed even after 6 cycles of therapy, the residual CD19/CD5 double positive leukemic cells are still ROR1 positive (Uhrmacher et al., 2011). This indicates that ROR1 can be a diagnostic marker not only for initial diagnosis but also as treatment-independent indicator of the clinical stage and minimal residual disease. Thus, in 2016, a humanized mAb specific for ROR1 (UC-961 or Cirmtuzumab) was developed and preclinical studies showed that cirmtuzumab is specific and safe (Choi et al., 2015). Cirmtuzumab is currently being evaluated in clinical trials for therapy in CLL and since ROR1 is broadly expressed in many types of cancer, but not their normal tissue counterparts, breast cancer clinical trials have started to test effectiveness of Cirmtuzumab in combination with chemotherapy agents (Zhang et al., 2019).
4. miR-15/16 expression in leukemia
In 2000, we investigated the deletion affecting chromosome 13q often observed in patients with chronic lymphocytic leukemia. This genomic region was also found deleted or mutated in other malignancies (Chen et al., 2001; Dong et al., 2001) and thus we believed that it was hosting an important tumor suppressor gene. Surprisingly, in 2002, we discovered that, instead, a cluster of two microRNA genes, miR-15a and miR-16–1, was the target of 13q deletions in CLL (Calin et al., 2002). Remarkably, functional analysis showed that microRNA are non-coding RNAs capable to bind the 3’ UTRs of mRNAs in a sequence specific fashion (Ambros, 2004) or guide Poly(A)-specific Ribonuclease (PARN) to a target specific mRNA (Gomez-Cambronero et al., 2018), inducing mRNA decay and/or inhibition of translation. Thus, microRNAs were already thought to be involved in cellular processes as negative regulators of gene expression. However, we were the first to demonstrate that an alteration in the non-coding genome was effectively involved in cancer pathogenesis (Calin et al., 2002). Indeed, we proved that the deletion of a microRNA can lead to the aberrant overexpression of important genes by showing, in 2005, that miR-15/16 function as tumor suppressors in CLL by directly targeting BCL-2: the loss of these negative regulators of BCL-2 expression in 13q- CLL results in inhibition of apoptosis and CLL onset (Cimmino et al., 2005). More recently we discovered that ROR1 is also a target of miR-15/16 (Rassenti et al., 2017) and we showed that, in patients where ROR1 expression is lower, miR-15/16 expression is higher. Furthermore, we confirmed that CLL cells expressing high levels of ROR1 also expressed high levels of BCL-2 (Rassenti et al., 2017). Several reports revealed that loss of miR-15a/16–1 expression is a hallmark of almost all CLL cases: about 55% of CLL cases lose miR-15a/16–1 expression as a consequence of the chromosomal deletion at 13q14 detectable by FISH analysis (Döhner et al., 2000); about 15% of CLL cases that do not show such chromosomal abnormality, carry smaller deletions (not FISH detectable) or mutations in the miR-15a/16–1 genomic region (Calin et al., 2005); lastly we proved that p53 is a positive activator of miR-15/16, and since p53 is deleted/mutated in 7–10% of CLL samples, loss of p53 may lead to down-regulation of miR-15/16 (Fabbri et al., 2011). Thus, up to 80–90% of CLL cases show a low expression of miR-15/16 (Rassenti et al., 2017). This evidences are consistent with the observation that ~90% of CLL patients bear leukemic cells showing a high expression of ROR1. All of these striking results indicate that, in CLL, lack of miR-15/16 cells causes a combination of Bcl-2-driven apoptosis evasion and ROR1-driven enhanced proliferation that lead to cancer development and progression. Since, in this scenario, the selection of a BCL-2 or a ROR1 mutant clone resistant to monotherapy is a possible event, we believe that CLL patients should be treated with a combination therapy of Venetoclax and Cirmtuzumab, to elicit enhanced anticancer effect and protect from selection of resistant subclones. Indeed this formulation would target two cancer drivers upregulated as a consequence of the same alteration: miR-15/16 loss. In support of this idea, we proved that Cirmtuzumab could enhance the in vitro cytotoxicity of Venetoclax for CLL cells with high-level ROR1 (Rassenti et al., 2017). Since BCL2 and ROR1 dysregulation has been observed also in other malignancies such as breast, prostate and ovarian cancer (Abdel-Fatah et al., 2013; Bonci et al., 2008; Lagadinou et al., 2013; Lin et al., 2007; Zhang et al., 2014), we believe that this formulation would be effective for treatment of several other types of cancers in reducing the possibility of resistance/relapse to virtually zero.
Interestingly, an additional locus of miR-15/16 exists on chromosome 3 (3q25), encoding for miR-15b/16–2. Thus, in 2015 we generated a knocked out mouse model to study its role in lymphomagenesis. These mice developed mainly CLL and few diffuse large B cell lymphoma at higher penetrance and earlier age than the miR-15a/16–1 KO mice described by Klein et al (Klein et al., 2010; Lovat et al., 2015). In humans, mir-16–1 and miR16–2 mature sequences are exactly the same while miR15a and miR15b have slightly different sequences but maintain the same “seed region” responsible for mRNA targeting, thus we were able to investigate the expression of miR-15/16 locus on 3q25 in CLL, by evaluating the expression of miR-15b in B-CLL cells from 13q- and 11q-deleted patients (Lovat et al., 2015). We compared these results with miR-15b expression in normal CD19+ B cells: mir-15b is downregulated 1.6 folds in 13q-CLL vs normal CD19+ B cells and 3.4-folds in 11q-CLL vs normal CD19+ B cells. Additional analysis revealed that in 13q− CLLs patients, the lack of miR-15a/16–1 cluster seem to be compensated by modulating the expression of pri-miR-15b and miR-15b, suggesting a possible level of miRNA “regulation” that warrant further study. Following these experiments, we generated a double knocked out mouse where both miR-15/16 loci were deleted (Lovat et al., 2018). Surprisingly, these mice developed an aggressive AML (77%) and, more infrequently, B cell lymphomas (23%). This was an unexpected exciting result, indicating that the combined loss of miR-15a, miR-15b, and miR-16 and overexpression of their targets starting from animal development lead to acute myeloid leukemia. Remarkably, previous studies suggested that, in AML patients, BCL-2 is overexpressed in Leukemia Stem Cells (LSCs) (Lagadinou et al., 2013), and showed that overexpression of Bcl-2 or Mcl-1 is associated to chemoresistance, failure to achieve complete remission and relapse (Ricciardi et al., 2017). Our miR-15/16 double KO AML mouse model suggests that BCL-2 overexpression in LSCs is very likely induced by the absence of miR-15/16 which could also induce ROR1 expression as well. Thus, since relapse in AML is typically caused by the development of treatment-resistant clones derived from LSCs, a Venetoclax-Cirmtuzumab combination treatment could be successful in targeting LSCs, and prevent the occurrence of drug resistance development and relapse.
5. Future perspectives and concluding remarks: identification of novel mechanism of gene expression dysregulation in cancer, efficient stratification of patient and multi-target combination therapy
In this review, we discussed the role of microRNA dysregulation in cancer development, and its involvement in the identification of genes that can be targets for novel drugs. It is important to highlight that the dysregulation of other small non-coding RNAs may affect gene expression regulation in cancer as well (Pekarsky and Croce, 2019). For instance, our most recent studies showed that a novel class of small non-coding RNAs generated through the processing steps of tRNA molecules and named tsRNAs, can inhibit key genes in the development of CLL and possibly other cancers (Balatti et al., 2017a; Balatti et al., 2017b; Balatti et al., 2015; Pekarsky et al., 2016). The mechanism of action of tsRNAs is still under investigation but they seem to be able to affect gene expression both pre and post transcriptionally (Pekarsky et al., 2016). These molecules could represent additional markers for diagnosis and their study could lead to the identification to novel targets for therapy, as previously done for microRNAs.
MiR-15a/16–1 deletion on chromosome 13q14 is a driver event for the development of CLL since it leads to the overexpression of BCL-2 and of ROR1, an anti-apoptotic gene and an initiator of proliferation signals, respectively (Cimmino et al., 2005; Rassenti et al., 2017). Thus, the discovery of miR-15/16 dysregulation in CLL provided the basis for development of Venetoclax and Cirmtuzumab. Remarkably, in the last decade, many other types of cancers were shown to be ROR1 positive (Zhang et al., 2012) and to overexpress BCL-2 (Abdel-Fatah et al., 2013; Lin et al., 2007), while the expression of miR-15/16 is still under investigation. Furthermore, the mouse model where the additional locus for miR-15/16 on chromosome 3q25 (miR-15b/16–2) is knocked out along with the MiR-15a/16–1 locus, develops for the most part Acute Myeloid Leukemia (Lovat et al., 2018). Based on these observation, the study of genetic alterations involving miR-15/16 in CLL-B cells from CLL patients and LSC from AML patients may represent a valuable tool to stratify patients for more effective therapy. MiR-15/16 main target genes, BCL-2 and ROR1 should be therapeutically pursued (Pekarsky et al., 2018) in patients where miR-15/16 are downregulated. Indeed, a combination therapy of Venetoclax and Cirmtuzumab would be quite efficient because it would target both drivers upregulated as a consequence of the same alteration: miR-15/16 loss. Additionally, both drugs show very modest side effects. Lastly, it is important to notice that the identification of miR-15/16 as the cause of the dysregulation of these two druggable targets, also offers the opportunity to identify additional targets for the development of new compounds.
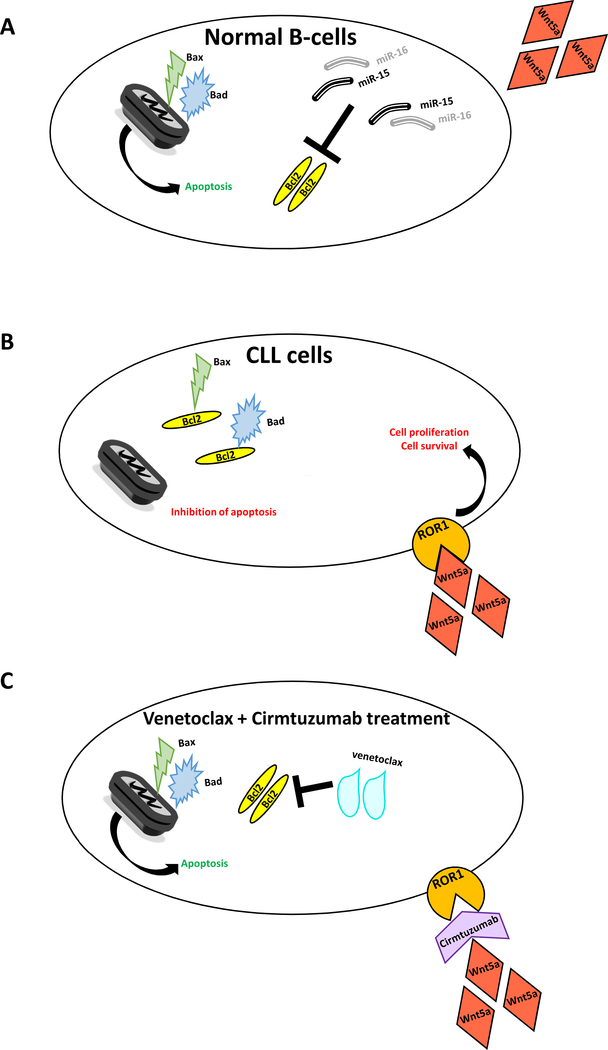
Figure 1.
In normal B cells, Mir-15/16 target Bcl2. BAC and BAD can activate apoptosis. ROR1 is not expressed thus Wnt5a cannot activate the proliferation pathway (A). In leukemic cells, lack of miR-15/16 is the cause of Bcl2 overexpression that leads to apoptosis inhibition. ROR1 is expressed in the surface of leukemic cells thus wnt5a activates the proliferation pathway (B). Therapeutic implication of combined treatment with Venetoclax, that targets Bcl2 and Cirmtuzumab that binds to ROR1: the antiapoptotic pathway is initiated and the cell proliferation pathway is inhibited (C).
Acknowledgements
This work was supported by the National Cancer Institute grant R35 CA197706 (CMC)
Footnotes
Conflict of interest
There are no conflict of interest for any of the authors.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abdel-Fatah TM, Perry C, Dickinson P, Ball G, Moseley P, Madhusudan S, Ellis IO, Chan SY, 2013. Bcl2 is an independent prognostic marker of triple negative breast cancer (TNBC) and predicts response to anthracycline combination (ATC) chemotherapy (CT) in adjuvant and neoadjuvant settings. Ann Oncol 24(11), 2801–2807. [DOI] [PubMed] [Google Scholar]
- Ambros V, 2004. The functions of animal microRNAs. Nature 431(7006), 350–355. [DOI] [PubMed] [Google Scholar]
- Balatti V, Nigita G, Veneziano D, Drusco A, Stein GS, Messier TL, Farina NH, Lian JB, Tomasello L, Liu CG, Palamarchuk A, Hart JR, Bell C, Carosi M, Pescarmona E, Perracchio L, Diodoro M, Russo A, Antenucci A, Visca P, Ciardi A, Harris CC, Vogt PK, Pekarsky Y, Croce CM, 2017a.. tsRNA signatures in cancer. Proc Natl Acad Sci U S A 114(30), 8071–8076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balatti V, Pekarsky Y, Croce CM, 2017b.. Role of the tRNA-Derived Small RNAs in Cancer: New Potential Biomarkers and Target for Therapy. Adv Cancer Res 135, 173–187. [DOI] [PubMed] [Google Scholar]
- Balatti V, Rizzotto L, Miller C, Palamarchuk A, Fadda P, Pandolfo R, Rassenti LZ, Hertlein E, Ruppert AS, Lozanski A, Lozanski G, Kipps TJ, Byrd JC, Croce CM, Pekarsky Y, 2015. TCL1 targeting miR-3676 is codeleted with tumor protein p53 in chronic lymphocytic leukemia. Proc Natl Acad Sci U S A 112(7), 2169–2174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonci D, Coppola V, Musumeci M, Addario A, Giuffrida R, Memeo L, D’Urso L, Pagliuca A, Biffoni M, Labbaye C, Bartucci M, Muto G, Peschle C, De Maria R, 2008. The miR-15a-miR-16–1 cluster controls prostate cancer by targeting multiple oncogenic activities. Nat Med 14(11), 1271–1277. [DOI] [PubMed] [Google Scholar]
- Borcherding N, Kusner D, Liu GH, Zhang W, 2014. ROR1, an embryonic protein with an emerging role in cancer biology. Protein Cell 5(7), 496–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calin GA, Dumitru CD, Shimizu M, Bichi R, Zupo S, Noch E, Aldler H, Rattan S, Keating M, Rai K, Rassenti L, Kipps T, Negrini M, Bullrich F, Croce CM, 2002. Frequent deletions and down-regulation of micro- RNA genes miR15 and miR16 at 13q14 in chronic lymphocytic leukemia. Proc Natl Acad Sci U S A 99(24), 15524–15529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calin GA, Ferracin M, Cimmino A, Di Leva G, Shimizu M, Wojcik SE, Iorio MV, Visone R, Sever NI, Fabbri M, Iuliano R, Palumbo T, Pichiorri F, Roldo C, Garzon R, Sevignani C, Rassenti L, Alder H, Volinia S, Liu CG, Kipps TJ, Negrini M, Croce CM, 2005. A MicroRNA signature associated with prognosis and progression in chronic lymphocytic leukemia. N Engl J Med 353(17), 1793–1801. [DOI] [PubMed] [Google Scholar]
- Chen C, Frierson HF, Haggerty PF, Theodorescu D, Gregory CW, Dong JT, 2001. An 800-kb region of deletion at 13q14 in human prostate and other carcinomas. Genomics 77(3), 135–144. [DOI] [PubMed] [Google Scholar]
- Choi MY, Widhopf GF, Wu CC, Cui B, Lao F, Sadarangani A, Cavagnaro J, Prussak C, Carson DA, Jamieson C, Kipps TJ, 2015. Pre-clinical Specificity and Safety of UC-961, a First-In-Class Monoclonal Antibody Targeting ROR1. Clin Lymphoma Myeloma Leuk 15 Suppl, S167–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cimmino A, Calin GA, Fabbri M, Iorio MV, Ferracin M, Shimizu M, Wojcik SE, Aqeilan RI, Zupo S, Dono M, Rassenti L, Alder H, Volinia S, Liu CG, Kipps TJ, Negrini M, Croce CM, 2005. miR-15 and miR-16 induce apoptosis by targeting BCL2. Proc Natl Acad Sci U S A 102(39), 13944–13949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Croce CM, Reed JC, 2016. Finally, An Apoptosis-Targeting Therapeutic for Cancer. Cancer Res 76(20), 5914–5920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui B, Ghia EM, Chen L, Rassenti LZ, DeBoever C, Widhopf GF, Yu J, Neuberg DS, Wierda WG, Rai KR, Kay NE, Brown JR, Jones JA, Gribben JG, Frazer KA, Kipps TJ, 2016. High-level ROR1 associates with accelerated disease progression in chronic lymphocytic leukemia. Blood 128(25), 2931–2940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson SJ, Makretsov N, Blows FM, Driver KE, Provenzano E, Le Quesne J, Baglietto L, Severi G, Giles GG, McLean CA, Callagy G, Green AR, Ellis I, Gelmon K, Turashvili G, Leung S, Aparicio S, Huntsman D, Caldas C, Pharoah P, 2010. BCL2 in breast cancer: a favourable prognostic marker across molecular subtypes and independent of adjuvant therapy received. Br J Cancer 103(5), 668–675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiNardo CD, Pratz K, Pullarkat V, Jonas BA, Arellano M, Becker PS, Frankfurt O, Konopleva M, Wei AH, Kantarjian HM, Xu T, Hong WJ, Chyla B, Potluri J, Pollyea DA, Letai A, 2019. Venetoclax combined with decitabine or azacitidine in treatment-naive, elderly patients with acute myeloid leukemia. Blood 133(1), 7–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong JT, Boyd JC, Frierson HF, 2001. Loss of heterozygosity at 13q14 and 13q21 in high grade, high stage prostate cancer. Prostate 49(3), 166–171. [DOI] [PubMed] [Google Scholar]
- Döhner H, Stilgenbauer S, Benner A, Leupolt E, Kröber A, Bullinger L, Döhner K, Bentz M, Lichter P, 2000. Genomic aberrations and survival in chronic lymphocytic leukemia. N Engl J Med 343(26), 1910–1916. [DOI] [PubMed] [Google Scholar]
- Fabbri M, Bottoni A, Shimizu M, Spizzo R, Nicoloso MS, Rossi S, Barbarotto E, Cimmino A, Adair B, Wojcik SE, Valeri N, Calore F, Sampath D, Fanini F, Vannini I, Musuraca G, Dell’Aquila M, Alder H, Davuluri RV, Rassenti LZ, Negrini M, Nakamura T, Amadori D, Kay NE, Rai KR, Keating MJ, Kipps TJ, Calin GA, Croce CM, 2011. Association of a microRNA/TP53 feedback circuitry with pathogenesis and outcome of B-cell chronic lymphocytic leukemia. JAMA 305(1), 59–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer K, Al-Sawaf O, Bahlo J, Fink AM, Tandon M, Dixon M, Robrecht S, Warburton S, Humphrey K, Samoylova O, Liberati AM, Pinilla-Ibarz J, Opat S, Sivcheva L, Le Dû K, Fogliatto LM, Niemann CU, Weinkove R, Robinson S, Kipps TJ, Boettcher S, Tausch E, Humerickhouse R, Eichhorst B, Wendtner CM, Langerak AW, Kreuzer KA, Ritgen M, Goede V, Stilgenbauer S, Mobasher M, Hallek M, 2019. Venetoclax and Obinutuzumab in Patients with CLL and Coexisting Conditions. N Engl J Med 380(23), 2225–2236. [DOI] [PubMed] [Google Scholar]
- Fulda S, 2010. Evasion of apoptosis as a cellular stress response in cancer. Int J Cell Biol 2010, 370835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fulda S, Gorman AM, Hori O, Samali A, 2010. Cellular stress responses: cell survival and cell death. Int J Cell Biol 2010, 214074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillies LA, Kuwana T, 2014. Apoptosis regulation at the mitochondrial outer membrane. J Cell Biochem 115(4), 632–640. [DOI] [PubMed] [Google Scholar]
- Gomez-Cambronero J, Fite K, Miller TE, 2018. How miRs and mRNA deadenylases could post-transcriptionally regulate expression of tumor-promoting protein PLD. Adv Biol Regul 68, 107–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris AW, Pinkert CA, Crawford M, Langdon WY, Brinster RL, Adams JM, 1988. The E mu-myc transgenic mouse. A model for high-incidence spontaneous lymphoma and leukemia of early B cells. J Exp Med 167(2), 353–371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasan MK, Rassenti L, Widhopf GF, Yu J, Kipps TJ, 2019. Wnt5a causes ROR1 to complex and activate cortactin to enhance migration of chronic lymphocytic leukemia cells. Leukemia 33(3), 653–661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hata AN, Engelman JA, Faber AC, 2015. The BCL2 Family: Key Mediators of the Apoptotic Response to Targeted Anticancer Therapeutics. Cancer Discov 5(5), 475–487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honma N, Horii R, Ito Y, Saji S, Younes M, Iwase T, Akiyama F, 2015. Differences in clinical importance of Bcl-2 in breast cancer according to hormone receptors status or adjuvant endocrine therapy. BMC Cancer 15, 698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein U, Lia M, Crespo M, Siegel R, Shen Q, Mo T, Ambesi-Impiombato A, Califano A, Migliazza A, Bhagat G, Dalla-Favera R, 2010. The DLEU2/miR-15a/16–1 cluster controls B cell proliferation and its deletion leads to chronic lymphocytic leukemia. Cancer Cell 17(1), 28–40. [DOI] [PubMed] [Google Scholar]
- Kroemer G, Galluzzi L, Brenner C, 2007. Mitochondrial membrane permeabilization in cell death. Physiol Rev 87(1), 99–163. [DOI] [PubMed] [Google Scholar]
- Lagadinou ED, Sach A, Callahan K, Rossi RM, Neering SJ, Minhajuddin M, Ashton JM, Pei S, Grose V, O’Dwyer KM, Liesveld JL, Brookes PS, Becker MW, Jordan CT, 2013. BCL-2 inhibition targets oxidative phosphorylation and selectively eradicates quiescent human leukemia stem cells. Cell Stem Cell 12(3), 329–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langdon WY, Harris AW, Cory S, Adams JM, 1986. The c-myc oncogene perturbs B lymphocyte development in E-mu-myc transgenic mice. Cell 47(1), 11–18. [DOI] [PubMed] [Google Scholar]
- Lin Y, Fukuchi J, Hiipakka RA, Kokontis JM, Xiang J, 2007. Up-regulation of Bcl-2 is required for the progression of prostate cancer cells from an androgen-dependent to an androgen-independent growth stage. Cell Res 17(6), 531–536. [DOI] [PubMed] [Google Scholar]
- Lovat F, Fassan M, Gasparini P, Rizzotto L, Cascione L, Pizzi M, Vicentini C, Balatti V, Palmieri D, Costinean S, Croce CM, 2015. miR-15b/16–2 deletion promotes B-cell malignancies. Proc Natl Acad Sci U S A 112(37), 11636–11641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovat F, Fassan M, Sacchi D, Ranganathan P, Palamarchuk A, Bill M, Karunasiri M, Gasparini P, Nigita G, Distefano R, Veneziano D, Dorrance AM, Garzon R, Croce CM, 2018. Knockout of both miR-15/16 loci induces acute myeloid leukemia. Proc Natl Acad Sci U S A 115(51), 13069–13074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonnell TJ, Deane N, Platt FM, Nunez G, Jaeger U, McKearn JP, Korsmeyer SJ, 1989. bcl-2-immunoglobulin transgenic mice demonstrate extended B cell survival and follicular lymphoproliferation. Cell 57(1), 79–88. [DOI] [PubMed] [Google Scholar]
- McDonnell TJ, Troncoso P, Brisbay SM, Logothetis C, Chung LW, Hsieh JT, Tu SM, Campbell ML, 1992. Expression of the protooncogene bcl-2 in the prostate and its association with emergence of androgen-independent prostate cancer. Cancer Res 52(24), 6940–6944. [PubMed] [Google Scholar]
- Pekarsky Y, Balatti V, Croce CM, 2018. BCL2 and miR-15/16: from gene discovery to treatment. Cell Death Differ 25(1), 21–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pekarsky Y, Balatti V, Palamarchuk A, Rizzotto L, Veneziano D, Nigita G, Rassenti LZ, Pass HI, Kipps TJ, Liu CG, Croce CM, 2016. Dysregulation of a family of short noncoding RNAs, tsRNAs, in human cancer. Proc Natl Acad Sci U S A 113(18), 5071–5076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pekarsky Y, Croce CM, 2019. Noncoding RNA genes in cancer pathogenesis. Adv Biol Regul 71, 219–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rassenti LZ, Balatti V, Ghia EM, Palamarchuk A, Tomasello L, Fadda P, Pekarsky Y, Widhopf GF, Kipps TJ, Croce CM, 2017. MicroRNA dysregulation to identify therapeutic target combinations for chronic lymphocytic leukemia. Proc Natl Acad Sci U S A 114(40), 10731–10736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy UR, Phatak S, Allen C, Nycum LM, Sulman EP, White PS, Biegel JA, 1997. Localization of the human Ror1 gene (NTRKR1) to chromosome 1p31-p32 by fluorescence in situ hybridization and somatic cell hybrid analysis. Genomics 41(2), 283–285. [DOI] [PubMed] [Google Scholar]
- Ricciardi MR, Mirabilii S, Licchetta R, Piedimonte M, Tafuri A, 2017. Targeting the Akt, GSK-3, Bcl-2 axis in acute myeloid leukemia. Adv Biol Regul 65, 36–58. [DOI] [PubMed] [Google Scholar]
- Souers AJ, Leverson JD, Boghaert ER, Ackler SL, Catron ND, Chen J, Dayton BD, Ding H, Enschede SH, Fairbrother WJ, Huang DC, Hymowitz SG, Jin S, Khaw SL, Kovar PJ, Lam LT, Lee J, Maecker HL, Marsh KC, Mason KD, Mitten MJ, Nimmer PM, Oleksijew A, Park CH, Park CM, Phillips DC, Roberts AW, Sampath D, Seymour JF, Smith ML, Sullivan GM, Tahir SK, Tse C, Wendt MD, Xiao Y, Xue JC, Zhang H, Humerickhouse RA, Rosenberg SH, Elmore SW, 2013. ABT-199, a potent and selective BCL-2 inhibitor, achieves antitumor activity while sparing platelets. Nat Med 19(2), 202–208. [DOI] [PubMed] [Google Scholar]
- Stilgenbauer S, Eichhorst B, Schetelig J, Hillmen P, Seymour JF, Coutre S, Jurczak W, Mulligan SP, Schuh A, Assouline S, Wendtner CM, Roberts AW, Davids MS, Bloehdorn J, Munir T, Böttcher S, Zhou L, Salem AH, Desai M, Chyla B, Arzt J, Kim SY, Verdugo M, Gordon G, Hallek M, Wierda WG, 2018. Venetoclax for Patients With Chronic Lymphocytic Leukemia With 17p Deletion: Results From the Full Population of a Phase II Pivotal Trial. J Clin Oncol 36(19), 1973–1980. [DOI] [PubMed] [Google Scholar]
- Strasser A, Harris AW, Bath ML, Cory S, 1990. Novel primitive lymphoid tumours induced in transgenic mice by cooperation between myc and bcl-2. Nature 348(6299), 331–333. [DOI] [PubMed] [Google Scholar]
- Tsujimoto Y, Cossman J, Jaffe E, Croce CM, 1985. Involvement of the bcl-2 gene in human follicular lymphoma. Science 228(4706), 1440–1443. [DOI] [PubMed] [Google Scholar]
- Tsujimoto Y, Croce CM, 1986. Analysis of the structure, transcripts, and protein products of bcl-2, the gene involved in human follicular lymphoma. Proceedings of the National Academy of Sciences of the United States of America 83(14), 5214–5218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsujimoto Y, Finger LR, Yunis J, Nowell PC, Croce CM, 1984. Cloning of the chromosome breakpoint of neoplastic B cells with the t(14;18) chromosome translocation. Science 226(4678), 1097–1099. [DOI] [PubMed] [Google Scholar]
- Uhrmacher S, Schmidt C, Erdfelder F, Poll-Wolbeck SJ, Gehrke I, Hallek M, Kreuzer KA, 2011. Use of the receptor tyrosine kinase-like orphan receptor 1 (ROR1) as a diagnostic tool in chronic lymphocytic leukemia (CLL). Leuk Res 35(10), 1360–1366. [DOI] [PubMed] [Google Scholar]
- Vaux DL, Cory S, Adams JM, 1988. Bcl-2 gene promotes haemopoietic cell survival and cooperates with c-myc to immortalize pre-B cells. Nature 335(6189), 440–442. [DOI] [PubMed] [Google Scholar]
- Yu J, Chen L, Cui B, Wu C, Choi MY, Chen Y, Zhang L, Rassenti LZ, Widhopf Ii GF, Kipps TJ, 2017. Cirmtuzumab inhibits Wnt5a-induced Rac1 activation in chronic lymphocytic leukemia treated with ibrutinib. Leukemia 31(6), 1333–1339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang S, Chen L, Wang-Rodriguez J, Zhang L, Cui B, Frankel W, Wu R, Kipps TJ, 2012. The onco-embryonic antigen ROR1 is expressed by a variety of human cancers. Am J Pathol 181(6), 1903–1910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang S, Cui B, Lai H, Liu G, Ghia EM, Widhopf GF, Zhang Z, Wu CC, Chen L, Wu R, Schwab R, Carson DA, Kipps TJ, 2014. Ovarian cancer stem cells express ROR1, which can be targeted for anti-cancer-stem-cell therapy. Proc Natl Acad Sci U S A 111(48), 17266–17271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang S, Zhang H, Ghia EM, Huang J, Wu L, Zhang J, Lam S, Lei Y, He J, Cui B, Widhopf GF, Yu J, Schwab R, Messer K, Jiang W, Parker BA, Carson DA, Kipps TJ, 2019. Inhibition of chemotherapy resistant breast cancer stem cells by a ROR1 specific antibody. Proc Natl Acad Sci U S A 116(4), 1370–1377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y, Kipps TJ, Zhang S, 2017. Wnt5a Signaling in Normal and Cancer Stem Cells. Stem Cells Int 2017, 5295286. [DOI] [PMC free article] [PubMed] [Google Scholar]