Abstract
Repeated exposure to the acute pro-inflammatory environment that follows ovulation at the ovarian surface and distal fallopian tube over a woman’s reproductive years may increase ovarian cancer risk. To address this, analyses included individual-level data from 558,709 naturally menopausal women across 20 prospective cohorts, among whom 3,246 developed invasive epithelial ovarian cancer (2045 serous, 319 endometrioid, 184 mucinous, 121 clear cell, 577 other/unknown). Cox models were used to estimate multivariable-adjusted hazard ratios (HR) between lifetime ovulatory cycles (LOC) and its components and ovarian cancer risk overall and by histotype. Women in the 90th percentile of LOC (>514 cycles) were almost twice as likely to be diagnosed with ovarian cancer than women in the 10th percentile (<294) [HR (95% confidence interval): 1.92 (1.60–2.30)]. Risk increased 14% per five-year increase in LOC (60 cycles) [(1.10–1.17)]; this association remained after adjustment for LOC components: number of pregnancies and oral contraceptive use [1.08 (1.04–1.12)]. The association varied by histotype, with increased risk of serous [1.13 (1.09–1.17)], endometrioid [1.20 (1.10–1.32)], and clear cell [1.37 (1.18–1.58)], but not mucinous [0.99 (0.88–1.10), P-heterogeneity=0.01] tumors. Heterogeneity across histotypes was reduced [P-heterogeneity=0.15] with adjustment for LOC components [1.08 serous, 1.11 endometrioid, 1.26 clear cell, 0.94 mucinous]. Although the 10-year absolute risk of ovarian cancer is small, it roughly doubles as the number of LOC rises from ~300 to 500. The consistency and linearity of effects strongly support the hypothesis that each ovulation leads to small increases in the risk of most ovarian cancers, a risk which cumulates through life, suggesting this as an important area for identifying intervention strategies.
INTRODUCTION
Ovarian cancer is the most fatal gynecologic cancer. One of the leading hypotheses for epithelial ovarian cancer development is incessant ovulation.(1, 2) Approximately 80% of high-grade serous ovarian cancers likely originate in the fallopian tube (3), which are likely also susceptible to the impact of ovulation. Notably, an acute pro-inflammatory environment is created following ovulation; both the surface of the ovary and distal fallopian tube are bathed in follicular fluid containing inflammatory cytokines, reactive oxygen species, and steroids, creating a DNA damage-rich environment.(4)
The incessant ovulation hypothesis is supported by the consistent positive association between the lifetime number of ovulatory years or cycles and ovarian cancer risk.(5–10) Lending further support, reduced ovarian cancer risk has been observed for reproductive factors that interrupt ovulation (e.g., pregnancy, use of oral contraceptives, and breastfeeding).(8, 11–15) The mechanism as to how the ovulatory process contributes to carcinogenesis is unknown, yet several theories have been proposed, including: an acute proinflammatory environment, altered gonadotropin and/or steroid hormone exposure, or direct damage to the ovarian surface epithelium.(16–18)
It is difficult to measure number of ovulations directly; however, estimates of cumulative lifetime ovulatory cycles (LOC) can be obtained through algorithms that calculate the time between menarche and menopause (menstrual span) subtracting out presumed anovulatory cycles, due to duration of oral contraceptive use and pregnancy. Prior individual studies, however, have not had sufficient numbers to evaluate the role of ovulation on risk independent of the contributors to ovulatory cycle counts over the life course, particularly by histotype. Thus, we investigated the association of LOC overall and independent of its component factors with subsequent risk of ovarian cancer using prospective individual-level data from the Ovarian Cancer Cohort Consortium (OC3). We further evaluated associations by histotype and tumor aggressiveness among high-grade serous tumors (estimated by time between diagnosis and death), given demonstrated heterogeneity in risk factor associations for these tumor subtypes.(19, 20)
METHODS
Study population
The study population included women participating in 20 prospective studies from North America, Europe, Asia, and Australia (Supplementary Table 1). Eligible studies included cohort studies and/or clinical trials with prospective follow-up of women with determination of ovarian cancer endpoints through questionnaire/medical record-based follow-up or confirmation by cancer registries, as well as follow-up for death. Women were excluded from primary analyses if they had a history of cancer (other than non-melanoma skin cancer) at baseline, bilateral oophorectomy prior to study entry, were pre- or peri-menopausal at baseline, or were missing baseline age or age at natural menopause, duration of oral contraceptive use, or number of pregnancies lasting greater than six months (referred to as number of pregnancies in the sections that follow). Our analysis included studies that collected information on age at menarche, age at menopause, number of pregnancies, and oral contraceptive use and the study population was limited to naturally menopausal women at study enrollment (n=558,709), as such women with hysterectomy as their reason for menopause were excluded. All studies obtained ethics approval at their respective institution(s); participants provided either written informed consent or implicit consent through return of the study questionnaire. The OC3 Data Coordinating Center and analytic approaches were approved by the institutional review board of the Brigham and Women’s Hospital.
Exposure definitions
Reproductive factors, including number of pregnancies lasting greater than six months, history and duration of oral contraceptive use, and ages at menarche and menopause, were self-reported at enrollment and previously harmonized as part of a core dataset.(19)
We utilized the following formula to calculate number of LOC:
where ‘menstrual span’ was calculated as the difference between age at natural menopause and age at menarche; ‘OC months’ = duration (in months) of oral contraceptive use; and ‘preg months’=estimated number of months pregnant (calculated from reported number of pregnancies lasting greater than six months*9 months).
Outcome definitions
We included incident epithelial ovarian, peritoneal, or fallopian tube tumors; as described previously.(19) We first evaluated associations of LOC and its component factors with all invasive tumors combined (ovarian, peritoneal, and fallopian tube; n=3,246). Information on histotype was extracted from surgical pathology reports or through cancer registries. The date of or age at death (if applicable) during follow-up was extracted from death registries or reported via family members; all studies reported at least 95% mortality follow-up.(20) We further evaluated associations by the four most common tumor histotype categories: serous (n=2,045, including tumors coded as poorly differentiated), endometrioid (n=319), mucinous (n=184), clear cell (n=121), and a category for missing/unknown histology (n=577). Serous carcinomas are recognized as two distinct diseases (21, 22), low- and high-grade serous carcinoma. We used a combination of histology and tumor grade to further define low-grade serous (grade 1 or well-differentiated; n=70) and high-grade serous (≥ grade 2 or moderately differentiated; n=1375). However, nearly a third of the serous carcinomas (600 of 2045 serous carcinomas) were missing tumor grade. Due to the considerable proportion of unknown grade tumors and the likelihood that these tumors are high-grade, we repeated the analyses excluding only the known low-grade tumors (n=70, leaving n=1975 presumed high-grade serous tumors) and additionally excluding unknown grade (n=600, leaving n=1375 confirmed high-grade serous tumors). We also evaluate associations by tumor aggressiveness among presumed high grade serous tumors (n=1975): highly aggressive (lived <1 year post-diagnosis, n=302), very aggressive (lived 1-<3 years, n=625), moderately aggressive (lived 3-<5 years, n=283), less aggressive (lived 5+ years, n=454), and unknown (n=311).(20)
Statistical methods
We calculated hazard ratios (HR) and 95% confidence intervals (95% CI) using Cox proportional hazards regression to evaluate the association between LOC or its component factors and risk of ovarian cancer. Women entered the analysis at age at study entry and contributed person-time until the age at first diagnosis of ovarian cancer (event), death (censored), or end of follow-up (censored). In primary analyses, we pooled data from all cohorts, stratifying on cohort to account for potential differences in baseline hazards. A priori adjustment factors included baseline age (continuous), body mass index (BMI; <20, 20–24.9, 25–29.9, 30–34.9, 35+ kg/m2), smoking status (never, former, current), and duration of menopausal hormone therapy use (never, >0–5, >5–10, >10 years). We assessed between-study heterogeneity using meta-analysis of cohort-specific estimates.
To estimate the impact of LOC independent of its component factors, we mutually adjusted for oral contraceptive use and pregnancies in our final models. We also evaluated the influence of menstrual span as well as the individual factors age at menarche and age at menopause but could not adjust for all factors simultaneously because of collinearity with calculated LOC. In models adjusting for LOC and pairwise combinations of the individual component factors, oral contraceptive use and number of pregnancies explained the most variation in ovarian cancer risk, therefore we present results for LOC with and without adjustment for oral contraceptive use and number of pregnancies.
We evaluated possible deviations from linearity of the LOC-ovarian cancer association using a five-knot spline with knots at the 10th, 25th, 50th, 75th, and 90th percentiles of LOC. Associations between LOC or its component factors and ovarian cancer case characteristics (e.g., histotypes and tumor aggressiveness) were calculated using fixed effects competing-risks Cox regression.(23) Statistical heterogeneity of associations across case characteristics was assessed via likelihood ratio test comparing a model that assumed different associations for the exposure of interest by case characteristics (full model) to a model with a single estimate for the case characteristics (reduced model).(24) Effect modification by baseline age, oral contraceptives use (never/≤1 year vs. >1-year use), parity (vs. nulliparity)), and commonly measured factors that may influence inflammation (i.e., smoking, BMI, categorized as indicated previously) were evaluated in stratified models, with statistical significance assessed by a likelihood ratio test comparing a model with versus without a multiplicative interaction term. Confounding by other factors that may be associated with LOC and ovarian cancer risk was also assessed, including race, tubal ligation, endometriosis, aspirin/NSAID use, as well as confounding by first degree family history of breast or ovarian cancer.
To understand the pattern of LOC across its component factors better, we generated summary tables of LOC based on commonly observed reproductive characteristics and utilized shading to represent low (blue, reference), moderate (white, HR ~1.4 times compared to blue), and high (red, approximately double risk compared to blue) hazard ratios. We then estimated average absolute risk in each category (blue, white, red) based on the 4 components of LOC using a published calculation to put these associations in context.(25) In brief, the absolute risk calculation was previously developed using data from non-Hispanic white women aged 50 or older from two large population-based US cohorts (NIH-AARP Diet and Health Study and the Prostate, Lung, Colorectal, and Ovarian (PLCO) Cancer Screening Trial).(25) Estimates of relative and absolute risk were combined with age-specific population incidence and competing mortality rates to create the published absolute risk calculator.(25) For ovarian cancer, this model included family history of breast or ovarian cancer, duration of menopausal hormone therapy use, parity, and oral contraceptive use and was validated in a third large population-based US cohort (Nurses’ Health Study cohort).(25) To calculate absolute risk estimates for the current study using the published model, we assumed no history of menopausal hormone therapy use, as that most accurately reflected most of the study population.
Survivor function plots for exposures were parallel suggesting no deviation from proportional hazards. All statistical tests were two-sided, and p-values<0.05 were considered statistically significant; analyses were performed using SAS v9.4 (Cary, North Carolina).
RESULTS
The distribution of baseline characteristics overall and by percentile category of LOC are presented in Table 1. The study population included predominantly white women (>90%). The average baseline age of the study participants increased slightly across LOC category: 58.6, 60.3, 60.8, 61.8, 62.6, 63.5 years for <10th, 10th-<25th, 25-<50th, 50-<75th, 75-<90th, ≥90th percentile of LOC, respectively.
Table 1.
Distribution of baseline demographic and health characteristics by percentile of lifetime ovulatory cycle (LOC) among N=558,709 naturally postmenopausal women from 20 prospective studies in the Ovarian Cancer Cohort Consortium (OC3).
| LOC Quantiles (cutpoints) | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Overall | <10th percentile | 10th-<25th percentile | 25-<50th percentile | 50-<75th percentile | 75-<90th percentile | ≥ 90th percentile | ||||||||
| n=558,709 | (< 294 cycles) | (294-<369) | (369-<435) | (435-<480) | (480-<514) | (≥ 514) | ||||||||
| mean | SD | mean | SD | mean | SD | mean | SD | mean | SD | mean | SD | mean | SD | |
| Baseline age | 61.3 | 6.8 | 58.6 | 7.2 | 60.3 | 6.9 | 60.8 | 6.8 | 61.8 | 6.6 | 62.6 | 6.3 | 63.5 | 5.9 |
| Race | n | % | n | % | n | % | n | % | n | % | n | % | n | % |
| White | 525606 | 94.1 | 52744 | 93.0 | 79285 | 93.5 | 127743 | 94.2 | 136114 | 94.6 | 70759 | 94.4 | 58961 | 94.0 |
| Black | 14967 | 2.7 | 2490 | 4.4 | 2838 | 3.3 | 3568 | 2.6 | 3005 | 2.1 | 1592 | 2.1 | 1474 | 2.4 |
| Other | 14944 | 2.7 | 1120 | 2.0 | 2072 | 2.4 | 3572 | 2.6 | 4045 | 2.8 | 2245 | 3.0 | 1890 | 3.0 |
| Unknown | 3192 | 0.6 | 383 | 0.7 | 584 | 0.7 | 758 | 0.6 | 717 | 0.5 | 383 | 0.5 | 367 | 0.6 |
| BMI (kg/m2) | ||||||||||||||
| <20 | 32236 | 5.8 | 3506 | 6.4 | 5233 | 6.4 | 8263 | 6.3 | 8110 | 5.9 | 4113 | 5.7 | 3011 | 5 |
| 20–24.9 | 227122 | 40.7 | 24156 | 44 | 35467 | 43.3 | 56369 | 43.1 | 58575 | 42.4 | 29752 | 41.5 | 22803 | 38.1 |
| 25–29.9 | 175284 | 31.4 | 17671 | 32.2 | 26078 | 31.9 | 42161 | 32.2 | 45596 | 33 | 23526 | 32.8 | 20252 | 33.9 |
| 30–34.9 | 69390 | 12.4 | 6485 | 11.8 | 10165 | 12.4 | 16388 | 12.5 | 17668 | 12.8 | 9692 | 13.5 | 8992 | 15 |
| 35+ | 33086 | 5.9 | 3127 | 5.7 | 4932 | 6 | 7563 | 5.8 | 8079 | 5.9 | 4623 | 6.4 | 4762 | 8 |
| Smoking status | ||||||||||||||
| Never | 300960 | 53.9 | 28205 | 49.7 | 42115 | 49.7 | 70714 | 52.1 | 79544 | 55.3 | 43223 | 57.6 | 37159 | 59.3 |
| Former | 173448 | 31.0 | 18335 | 32.3 | 28149 | 33.2 | 42931 | 31.7 | 43187 | 30 | 22290 | 29.7 | 18556 | 29.6 |
| Current | 78089 | 14.0 | 9698 | 17.1 | 13573 | 16 | 20548 | 15.1 | 19543 | 13.6 | 8615 | 11.5 | 6112 | 9.7 |
| Oral contraceptive use duration (years) | ||||||||||||||
| Never | 325917 | 58.3 | 10187 | 17.8 | 29261 | 34.3 | 69331 | 50.7 | 101392 | 69.6 | 61389 | 80.1 | 56075 | 86.5 |
| >0–1 | 38855 | 7.0 | 1781 | 3.1 | 3948 | 4.6 | 10244 | 7.5 | 12572 | 8.6 | 6690 | 8.7 | 4801 | 7.4 |
| >1–5 | 86367 | 15.5 | 5934 | 10.4 | 13461 | 15.8 | 30916 | 22.6 | 26613 | 18.3 | 7826 | 10.2 | 3860 | 6 |
| >5–10 | 60034 | 10.8 | 10905 | 19.1 | 22701 | 26.6 | 22248 | 16.3 | 4752 | 3.3 | 683 | 0.9 | 111 | 0.2 |
| 11+ | 47536 | 8.5 | 28347 | 49.6 | 16005 | 18.7 | 4035 | 3 | 277 | 0.2 | 7 | 0 | 1 | 0 |
| Number of pregnancies | ||||||||||||||
| 0 | 68076 | 12.2 | 4685 | 8.2 | 8334 | 9.8 | 12826 | 9.4 | 15211 | 10.4 | 13840 | 18.1 | 13747 | 21.2 |
| 1 | 58587 | 10.5 | 5535 | 9.7 | 9255 | 10.8 | 13192 | 9.6 | 12030 | 8.3 | 11455 | 15 | 8317 | 12.8 |
| 2 | 166498 | 29.8 | 17998 | 31.5 | 23307 | 27.3 | 38164 | 27.9 | 44924 | 30.9 | 22052 | 28.8 | 23438 | 36.1 |
| 3 | 144996 | 26.0 | 14745 | 25.8 | 21142 | 24.8 | 35618 | 26 | 42743 | 29.4 | 18846 | 24.6 | 13680 | 21.1 |
| 4+ | 120552 | 21.6 | 14191 | 24.8 | 23338 | 27.3 | 36974 | 27 | 30698 | 21.1 | 10402 | 13.6 | 5666 | 8.7 |
| Age at menarche | ||||||||||||||
| ≤11 | 129109 | 23.1 | 8759 | 15.4 | 17326 | 20.4 | 23881 | 17.6 | 30529 | 21.2 | 20041 | 26.7 | 28573 | 45.6 |
| 12 | 124327 | 22.3 | 11323 | 20 | 17100 | 20.2 | 28155 | 20.8 | 32600 | 22.7 | 19721 | 26.3 | 15428 | 24.6 |
| 13 | 160214 | 28.7 | 15532 | 27.4 | 24898 | 29.4 | 41096 | 30.3 | 43206 | 30 | 23078 | 30.8 | 12404 | 19.8 |
| 14 | 80824 | 14.5 | 9862 | 17.4 | 13204 | 15.6 | 22425 | 16.5 | 22552 | 15.7 | 8216 | 11 | 4565 | 7.3 |
| 15+ | 64235 | 11.5 | 11261 | 19.8 | 12251 | 14.5 | 20084 | 14.8 | 14994 | 10.4 | 3923 | 5.2 | 1722 | 2.7 |
| Age at menopause | ||||||||||||||
| ≤40 | 41306 | 7.4 | 22479 | 39.6 | 17009 | 20.1 | 1818 | 1.3 | 0 | 0 | 0 | 0 | 0 | 0 |
| 41–45 | 53678 | 9.6 | 9862 | 17.4 | 21765 | 25.7 | 21384 | 15.8 | 666 | 0.5 | 1 | 0 | 0 | 0 |
| 46–50 | 192029 | 34.4 | 16723 | 29.5 | 26808 | 31.6 | 74764 | 55.1 | 64634 | 44.9 | 8841 | 11.8 | 259 | 0.4 |
| 51–55 | 231921 | 41.5 | 7173 | 12.6 | 18143 | 21.4 | 34176 | 25.2 | 74468 | 51.8 | 60700 | 81 | 37261 | 59.4 |
| 56+ | 39775 | 7.1 | 500 | 0.9 | 1054 | 1.2 | 3499 | 2.6 | 4113 | 2.9 | 5437 | 7.3 | 25172 | 40.2 |
| Menopausal hormone use duration (years) | ||||||||||||||
| Never | 319826 | 57.2 | 27449 | 54 | 45117 | 59.1 | 77020 | 62 | 86657 | 65.4 | 45922 | 66.7 | 37661 | 66.4 |
| >0–5 | 111317 | 19.9 | 11540 | 22.7 | 16918 | 22.1 | 28242 | 22.7 | 28957 | 21.8 | 14547 | 21.1 | 11113 | 19.6 |
| >5–10 | 47269 | 8.5 | 6188 | 12.2 | 8259 | 10.8 | 11781 | 9.5 | 10969 | 8.3 | 5277 | 7.7 | 4795 | 8.5 |
| 11+ | 31056 | 5.6 | 5656 | 11.1 | 6100 | 8 | 7110 | 5.7 | 5950 | 4.5 | 3086 | 4.5 | 3154 | 5.6 |
SD = standard deviation
Cells may not sum to total because of missing data (BMI, n missing=21,591 (3.9%); smoking, n missing=6212 (1.1%), menopausal hormone use, n missing=49,241 (8.8%))
In models evaluating the overall LOC effect (without adjustment for component factors), women in the 90th percentile of LOC (≥514) were almost twice as likely to be diagnosed with ovarian cancer during follow-up than women in the 10th percentile (<294 cycles) [HR (95% confidence interval (CI): 1.92 (1.60–2.30), p-trend<0.0001] (Table 2). The association between LOC and ovarian cancer risk was log-linear (Figure 1); associations between LOC and individual histotypes (i.e., serous, endometrioid, mucinous, and clear cell) were also predominantly log-linear.
Table 2.
Associations between categorical and continuous total number of lifetime ovulatory cycles (LOC) and ovarian cancer risk among naturally menopausal women from the Ovarian Cancer Cohort Consortium (OC3).
| Percentile category of LOC | Number of LOC | LOC Years | N events (cases) | Person-years | HR* | (95% CI) |
|---|---|---|---|---|---|---|
| <10th percentile | < 294 | < 24.5 | 214 | 657,188 | 1.00 | Reference |
| 10th-<25th percentile | 294-<369 | 24.5-<30.8 | 405 | 1,001,723 | 1.19 | (1.00–1.43) |
| 25-<50th percentile | 369-<435 | 30.8-<36.3 | 767 | 1,634,435 | 1.36 | (1.15–1.60) |
| 50th-<75th percentile | 435-<480 | 36.3-<40.0 | 917 | 1,750,003 | 1.60 | (1.36–1.88) |
| 75-<90th percentile | 480-<514 | 40.0-<42.8 | 488 | 909,033 | 1.67 | (1.40–1.99) |
| ≥90th percentile | ≥ 514 | ≥ 42.8 | 459 | 744,342 | 1.92 | (1.60–2.30) |
| p-trend<0.0001 | ||||||
| Continuous LOC | HR* | (95% CI) | ||||
| Per 5-year increase in LOC (60 LOC) | 1.14 | (1.10–1.17) | ||||
| Per 5-year increase in LOC (60 LOC) adjusted for oral contraceptive use and pregnancy† | 1.08 | (1.04–1.12) | ||||
Hazard ratios (HR) and 95% confidence intervals (CI) were estimated from Cox proportional hazards models stratified on study cohort and adjusted for baseline age (continuous), body mass index (<20, 20–24.9, 25–29.9, 30–34.9, ≥35 kg/m2), smoking status (never, former, current), and duration of menopausal hormone therapy use (never, ≤5, >5–10, >10 years).
Models additionally adjusted for duration of oral contraceptive use and pregnancy history.
Figure 1.
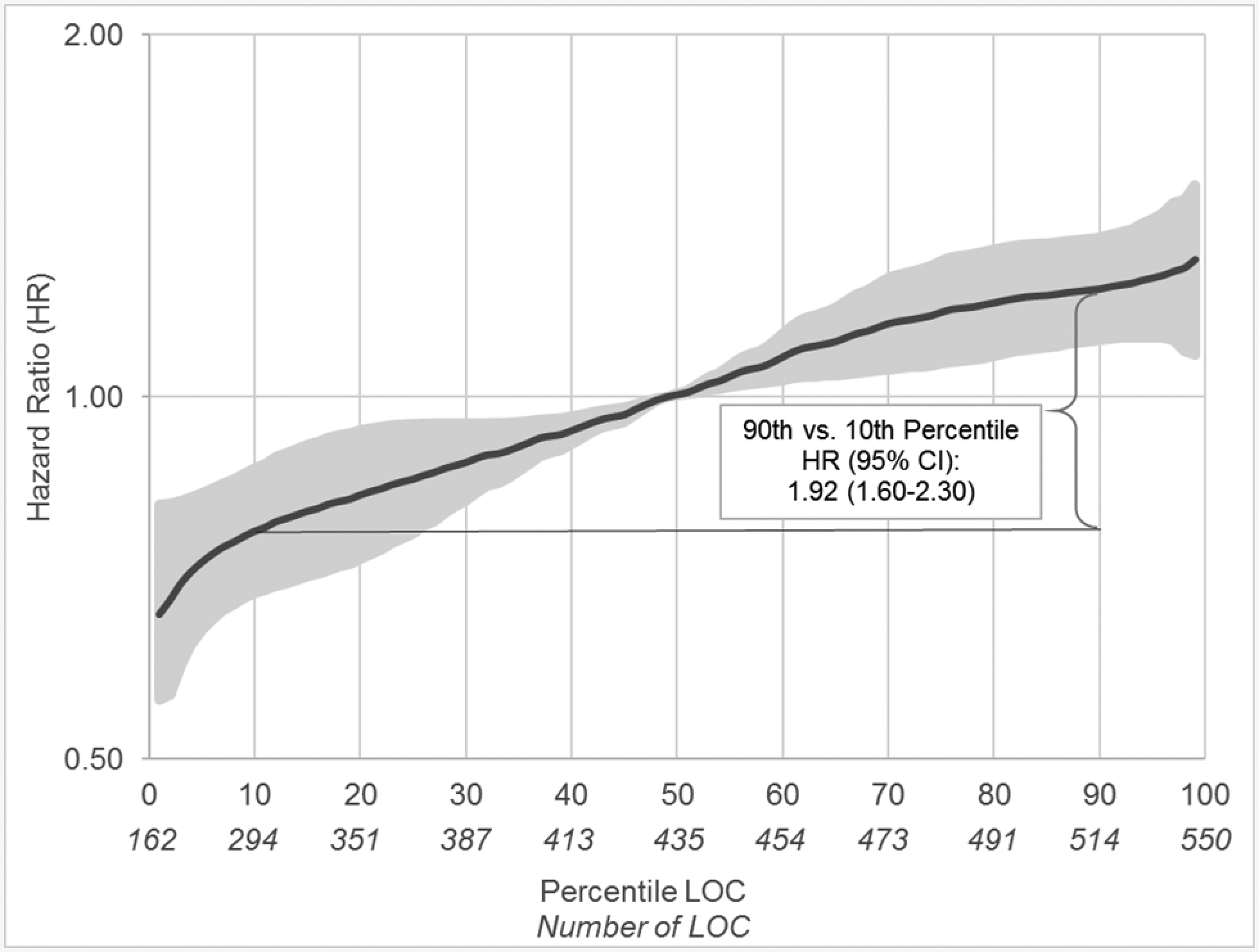
The associations between total number of lifetime ovulatory cycles (LOC) and ovarian cancer risk among naturally menopausal women based on a 5-knot spline (knots at 10th, 25th, 50th, 75th and 90th percentiles): pooled analysis of individual-level data from 20 cohort studies participating in the Ovarian Cancer Cohort Consortium (OC3).
For each five-year increase in LOC (60 cycles), ovarian cancer risk increased by 14% [95% CI: 1.10–1.17]. There was no between study heterogeneity. Adjusting for LOC-components, duration of oral contraceptive use and pregnancy, the LOC-ovarian cancer association remained but was attenuated [per 5 years of LOC: 1.08 (1.04–1.12)] (Table 2). The association was heterogenous by histotype [P heterogeneity=0.01] (Table 3); each five-year increase in LOC was associated with increased risk of serous [1.13 (1.09–1.17)], endometrioid [1.20 (1.10–1.32)], and clear cell [1.37 (1.18–1.58)], but not mucinous [0.99 (0.88–1.10)] tumors. Interestingly, after further adjusting for number of pregnancies lasting greater than six months and oral contraceptive use, hazard ratios were not significantly heterogeneous across histotype [P heterogeneity=0.15] across histotypes [serous 1.08 (1.03–1.12), endometrioid 1.11 (0.99–1.26), clear cell 1.26 (1.08–1.48), mucinous 0.94 (0.81–1.08)], although associations were only statistically significant for serous and clear cell tumors (Table 3). Associations were similar limiting to presumed high-grade serous [1.12 (1.08–1.17); adjusted for pregnancy and oral contraceptive use: 1.07 (1.02–1.11)] and confirmed high-grade serous tumors [1.10 (1.06–1.15); adjusted 1.05 (1.00–1.11), results not tabled].
Table 3.
Associations between continuous total lifetime number of ovulatory cycles and ovarian cancer by histotype considering mutual adjustment for LOC component factors: duration of oral contraceptive use and pregnancies, Ovarian Cancer Cohort Consortium (OC3).
| Serous (n=2,045) | Endometrioid (n=319) | Mucinous (n=184) | Clear Cell (n=121) | Other/Unknown (n=577) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| HR* | (95% CI) | HR* | (95% CI) | HR* | (95% CI) | HR* | (95% CI) | HR* | (95% CI) | P-Het† | |
| Per 5-year increase in LOC (60 LOC) | 1.13 | (1.09–1.17) | 1.20 | (1.10–1.32) | 0.99 | (0.88–1.10) | 1.37 | (1.18–1.58) | 1.14 | (1.06–1.22) | 0.01 |
| Per 5-year increase in LOC (60 LOC) adjusted for duration of oral contraceptive use and pregnancy‡ | 1.08 | (1.03–1.12) | 1.11 | (0.99–1.26) | 0.94 | (0.81–1.08) | 1.26 | (1.08–1.48) | 1.08 | (0.99–1.18) | 0.15 |
Hazard ratios (HR) and 95% confidence intervals (CI) were estimated from competing risk [Gates et al. 2010]. Cox proportional hazards models stratified on study cohort and adjusted for baseline age (continuous), body mass index (<20, 20–24.9, 25–29.9, 30–34.9, ≥35 kg/m2), smoking status (never, former, current) and duration of menopausal hormone therapy use (never, ≤5, >5–10, >10 years). Competing risk models were based on fixed covariate effects.
The P value for heterogeneity (P het) was calculated using a two-sided likelihood ratio test. [Gates et al., 2010]
Models additionally adjusted for duration of oral contraceptive use and number of pregnancies.
The risk of presumed high-grade serous ovarian carcinoma per five-year increase in LOC was not statistically heterogeneous across categories of tumor aggressiveness [P heterogeneity=0.60] (Supplementary Table 2).
LOC-ovarian cancer associations were not significantly modified by age, prior oral contraceptive use, parity, or by factors potentially related to inflammation [i.e., smoking, BMI] (Table 4). LOC-ovarian cancer associations were unchanged in analyses adjusting for additional potential confounders listed above.
Table 4.
Risk of ovarian cancer per 5-year increase in number of lifetime ovulatory cycles (LOC, n=60 cycles) across categories of age, oral contraceptive use, parity, body mass index, and smoking status.
| Ovarian cancer risk per 5-year increase in LOC | |||
|---|---|---|---|
| HR* | (95% CI) | P interaction† | |
| 40–49 years old | 1.19 | (0.88–1.60) | 0.99 |
| 50–59 years old | 1.13 | (1.08–1.19) | |
| 60+ years old | 1.14 | (1.10–1.18) | |
| Oral contraceptive use | |||
| Never or ≤1 year of use | 1.10 | (1.06–1.15) | 0.63 |
| >1 year of use | 1.15 | (1.10–1.21) | . |
| Parity | |||
| Nulliparous | 1.11 | (1.02–1.19) | 0.24 |
| Parous | 1.13 | (1.10–1.17) | |
| Body mass index | |||
| <20 kg/m2 | 1.16 | (1.03–1.30) | 0.90 |
| 20–24.9 kg/m2 | 1.13 | (1.08–1.19) | |
| 25–29.9 kg/m2 | 1.14 | (1.08–1.19) | |
| 30–34.9 kg/m2 | 1.13 | (1.04–1.23) | |
| 35+ kg/m2 | 1.18 | (1.05–1.32) | |
| Smoking status | |||
| Never | 1.16 | (1.12–1.21) | 0.47 |
| Former | 1.11 | (1.05–1.16) | |
| Current | 1.13 | (1.05–1.22) | |
Hazard ratios (HR) and 95% confidence intervals (CI) were estimated from Cox proportional hazards models stratified on study cohort and adjusted for baseline age (continuous), body mass index (<20, 20–24.9, 25–29.9, 30–34.9, ≥35 kg/m2), smoking status (never, former, current), and duration of menopausal hormone therapy use (never, ≤5, >5–10, >10 years).
P interaction from likelihood ratio test.
The pattern of LOC across commonly observed component factors is graphically presented in Figure 2. Based on the categorical model in Table 2, the relative risk varies almost two-fold comparing the 90th (red shading) to 10th (blue shading) percentile of number of LOC. The range of LOC exposure values observed in the current study is most clearly reflected when considering menstrual span in the context of no oral contraceptive use and no pregnancy history (top left panel of Figure 2). Accounting for increasing number of pregnancies (moving left to right across panels) and increasing duration of oral contraceptive use (moving top to bottom across panels) changes the pattern of relative risk to a scenario where virtually all menstrual span combinations reflect values less than 294 LOC representing the lowest absolute risk (bottom right panel of Figure 2). Using these variations in reproductive history and assuming no history of menopausal hormone therapy use, the 10-year absolute risk of ovarian cancer averaged approximately 0.31% (range: 0.21%−0.44%) for 55-year-old women with fewer than 300 LOC (blue); in contrast the 10-year absolute risk averaged approximately 0.59% (0.40%−0.84%) for 55-year-old women with 500 or more LOC (red). The 10-year absolute risk for a 55-year-old woman with the median number of LOC (n~435) averaged approximately 0.42% (range: 0.28%−0.59%) (white).
Figure 2.
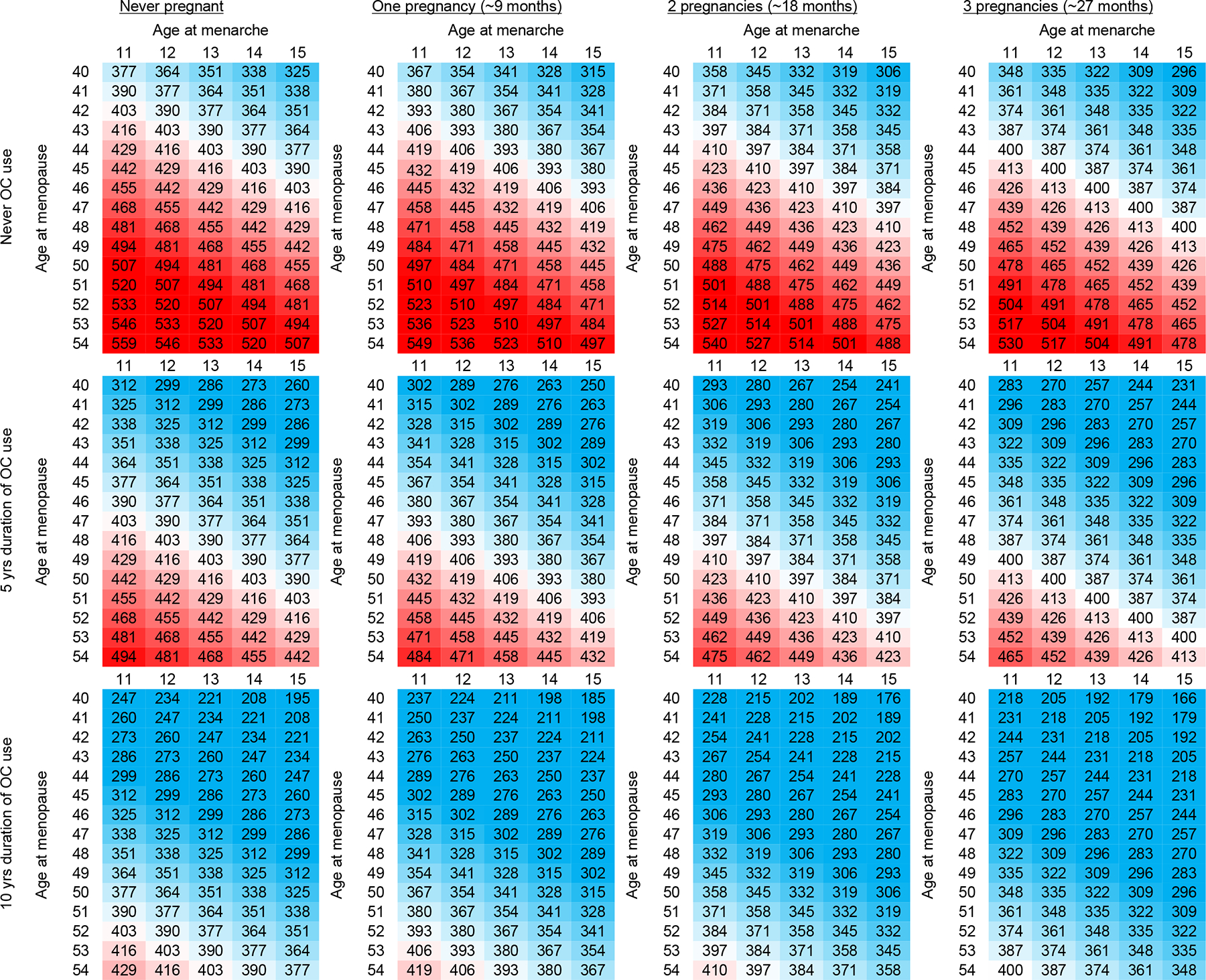
Graphical display of calculated values of total number of lifetime ovulatory cycles (LOC) among naturally postmenopausal women based on common reproductive characteristics, shading is based on LOC cutpoints corresponding to relative risk: low/blue (reference, hazard ratio=1.0), moderate/white (hazard ratio~1.4), and high/red (hazard ratio~2.0); and reflects the following range of absolute risks: lowest [blue,10-year absolute risk for 55-year-old woman: average 0.31% (min-max) (0.21–0.44)] and highest ovarian cancer risk (red:10-year absolute risk for 55-year-old woman: average 0.59% (min-max) (0.40–0.84)).
DISCUSSION
The present analysis represents the largest prospective study to date of LOC and ovarian cancer risk. The results not only confirm prior studies in showing that higher LOC is associated with an increase in subsequent ovarian cancer risk, they provide data that increases are specific to serous and clear cell and suggestive for endometrioid histotypes. While there was statistical heterogeneity in the risk estimates by histotype when considering LOC overall, after accounting for the impact of oral contraceptive use and pregnancies, the effect sizes were more similar for these three histotypes. The results further show a striking dose-response curve that is largely log-linear. Although the 10-year absolute risk of ovarian cancer is small, it roughly doubles as the number of cycles rises from 300 to 500. We estimated that the 10-year absolute risk of ovarian cancer for a 55-year-old postmenopausal woman varies from approximately 0.31% to 0.59% across the spectrum of LOC values calculated herein. Together with data from other study designs, these results suggest a unifying underlying mechanism of action, such as inflammation that occurs with each ovulation.(4)
The association between LOC and ovarian cancer risk has been evaluated in a number of prior studies (5–10, 24, 26–33), with a comparison of the different algorithms reported in a recent study.(33) Risk estimates varied across these studies; however, most showed an increased risk for greater number of LOCs. In studies that evaluated associations by quantiles, risk estimates were in the range of 1.6 to 1.9 for comparing the top to bottom tertile and 2.1–2.8 comparing the top to bottom quartile of LOC. Most of the published ovarian cancer studies evaluated LOC (or ovulatory years) without further adjustment for the components of LOC, although some studies adjusted for at least one component factor. As a result, it is not well established whether LOC is associated with ovarian cancer risk beyond the contributions made by the factors that define LOC. In the current study, however, we demonstrated that higher LOC is associated with a subsequent increase in the risk of ovarian cancer, independent of the associations with duration of oral contraceptive use and number of pregnancies, or more generally irrespective of the cause of anovulation.
Evaluations of the LOC association by histotype (24, 32) and tumor aggressiveness are limited; both published studies reported increased risk of serous and endometrioid tumors. Neither study evaluated risks for clear cell tumors given limited numbers. Given that the current paradigm regarding the origin of ovarian cancers suggests that the majority of serous, endometrioid, and clear cell tumors do not originate from the ovarian surface epithelium (3), it is likely that the increased risk with increasing LOC for these histotypes may be due in part to increased exposure to an acute proinflammatory environment associated with a greater number of LOC.(34) Altered gonadotropin/steroid hormone exposure may also be relevant to clear cell and endometrioid tumors given evidence suggesting etiologic heterogeneity of androgen and estrogen exposure with endometrioid or non-serous histotypes, and null associations for serous tumors.(35–37) Alternatively, we cannot rule out other unknown mechanisms that may explain associations between parity and/or oral contraceptive use on ovarian cancer risk reductions. The absence of heterogeneity in the LOC-histotype associations after adjustment for number of pregnancies and duration of oral contraceptive use likely suggests that the heterogeneity was contributed by these factors, however, it could also reflect an inability to detect heterogeneity given the small effect sizes.
The prospective design of the pooled studies in this analysis precludes recall bias. Additional strengths of the study include the large sample size, the ability to identify deaths as well as capture losses to follow-up, and the ability to account for many known and suspected risk factors for ovarian cancer. Further, we limited our evaluation to naturally postmenopausal women, which allowed us to compute more reliable estimates of lifetime number of menstrual cycles.
Measurement error is inherent to any estimator of LOC and our method is no exception. Multiple algorithms have been used to calculate LOC; the majority include a calculation of menstrual span and then subtract an estimate of cycles when no ovulations are occurring (i.e., on oral contraceptives, pregnant) (5–10, 24, 26–32) as we did. Some algorithms have also subtracted other times when ovulation is suppressed/ceased including 1) breast feeding (5, 7–10, 24, 29, 30, 32), 2) pregnancy loss (7, 27, 29, 30), 3) preterm birth (7), and 3) amenorrhea (either postpartum or missed/irregular periods) (6, 8, 28, 31, 32), and some have accounted for average cycle length.(6, 10, 30) A recent summary of these published algorithms, however, demonstrated that they are all highly correlated (correlations≥0.88, average correlation across algorithms=0.96) (33), therefore we used a calculation that would maximize the number of potential studies that could be evaluated while minimizing missing data. For example, information on breast-feeding duration was only collected in 8 out of 20 cohorts, representing less than 32% of postmenopausal women included. As such, the calculation we used in the current analysis is likely comparable with other methods on a relative scale but may not reflect absolute values of LOC. Information on irregular menstrual cycles, polycystic ovarian syndrome, early pregnancy losses, etc., were not included in our exposure definition. Additional limitations include potential for residual confounding by age-related factors; however, we did not observe substantial differences in associations across age-strata. We utilized a published absolute risk estimate for illustrative purposes (25); however, this model was developed using US white women, and as such may have limited generalizability to non-white/non-US study populations.
In conclusion, higher numbers of LOC were associated with increased risk of ovarian cancer overall and serous and clear cell tumors, independent of the associations with oral contraceptive use duration and number of pregnancies. Our findings support the hypothesis that ovulation may be a common etiologic factor for most types of ovarian cancer, suggesting this as an important area for identifying intervention strategies. It is plausible, that individual mechanisms for the components used to estimate LOC may also influence other factors beyond ovulation. Future research should examine, in detail, the common biologic mechanisms by which ovulation events influence ovarian cancers.
Supplementary Material
Statement of significance:
Although ovarian cancer is rare, risk of most ovarian cancers doubles as the number of lifetime ovulatory cycles increases from ~300 to 500. Thus, identifying an important area for cancer prevention research.
Acknowledgements:
This work was supported by Department of Defense Ovarian Cancer Research Program grant W81XWH-12-1-0561. Additional research funding and support included: The intramural research program of the National Cancer Institute, NIH, DHHS (Breast Cancer Detection and Demonstration Project Follow-up Study; NIH-AARP Diet and Health Study; Prostate, Lung, Colorectal and Ovarian Cancer Screening Study; Z01 CP010128); Intramural Research Program of the American Cancer Society (Cancer Prevention Study II); Breast Cancer Now and the Institute of Cancer Research (UKBGS, Generations Study); R01 CA077398 (California Teachers Study); R01 CA039742 (Iowa Women’s Health Study); R01 CA164973 (Multiethnic Cohort); VicHealth and Cancer Council Victoria, and Australian National Health and Medical Research Council grants 209057, 396414, and 1074383 (Melbourne Collaborative Cohort Study); UM1 CA186107, P01 CA087969, (Nurses’ Health Study); UM1 CA182934, P30 CA016087 and P30 ES000260 (NYU Women’s Health Study); NIEHS Intramural Research Program (Sisters Study, Project Z01-ES044005 to DPS); Swe-nt VR 2017-00644 for the Swedish Infrastructure for Medical Population-based Life-course Environmental Research (SIMPLER) and the Swedish Cancer Foundation (Swedish Mammography Cohort); K22 CA193860 to HRH; K05CA154337 from the National Cancer Institute (NCI) and Office of Dietary Supplements (VITamins And Lifestyle Cohort); CA047988, HL043851, HL080467, HL099355, and CA182913 (Women’s Health Study); and the coordination of EPIC is financially supported by the European Commission (DG-SANCO) and the International Agency for Research on Cancer. The national cohorts are supported by Danish Cancer Society (Denmark); Ligue Contre le Cancer, Institut Gustave Roussy, Mutuelle Générale de l’Education Nationale, Institut National de la Santé et de la Recherche Médicale (INSERM) (France); German Cancer Aid, German Cancer Research Center (DKFZ), Federal Ministry of Education and Research (BMBF) (Germany); the Hellenic Health Foundation (Greece); Associazione Italiana per la Ricerca sul Cancro-AIRC-Italy and National Research Council (Italy); Dutch Ministry of Public Health, Welfare and Sports (VWS), Netherlands Cancer Registry (NKR), LK Research Funds, Dutch Prevention Funds, Dutch ZON (Zorg Onderzoek Nederland), World Cancer Research Fund (WCRF), Statistics Netherlands (The Netherlands); Nordforsk (Norway); Health Research Fund (FIS), PI13/00061 to Granada; PI13/01162 to EPIC-Murcia, Regional Governments of Andalucía, Asturias, Basque Country, Murcia and Navarra, ISCIII RETIC (RD06/0020) (Spain); Swedish Cancer Society, Swedish Research Council and County Councils of Skåne and Västerbotten (Sweden); Cancer Research UK (14136 to EPIC-Norfolk; C570/A16491 and C8221/A19170 to EPIC-Oxford), Medical Research Council (1000143 to EPIC-Norfolk, MR/M012190/1 to EPIC-Oxford) (United Kingdom).
The Nurses’ Health Study would like to acknowledge the Channing Division of Network Medicine and thank the following state cancer registries for their assistance: AL, AZ, AR, CA, CO, CT, DE, FL, GA, ID, IL, IN, IA, KY, LA, ME, MD, MA, MI, NE, NH, NJ, NY, NC, ND, OH, OK, OR, PA, RI, SC, TN, TX, VA, WA, WY. The authors assume full responsibility for analyses and interpretation of these data. The UKBGS thanks Breast Cancer Now and the Institute of Cancer Research (ICR) for support and funding. The ICR acknowledges National Health Service funding to the National Institute for Health Research Biomedical Research Centre. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Previously presented: Abstract/Poster of preliminary results presented at Rivkin Ovarian Cancer Meeting, Seattle, Washington, September 13, 2018
Competing interest statement: All authors report no competing interests/conflicts of interest.
REFERENCES
- 1.Fathalla MF. Incessant ovulation--a factor in ovarian neoplasia? Lancet 1971;2(7716):163. [DOI] [PubMed] [Google Scholar]
- 2.Fathalla MF. Incessant ovulation and ovarian cancer - a hypothesis re-visited. Facts Views Vis Obgyn 2013;5(4):292–7. [PMC free article] [PubMed] [Google Scholar]
- 3.Kurman RJ, Shih Ie M. The Dualistic Model of Ovarian Carcinogenesis: Revisited, Revised, and Expanded. Am J Pathol 2016;186(4):733–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ness RB, Cottreau C. Possible role of ovarian epithelial inflammation in ovarian cancer. J Natl Cancer Inst 1999;91(17):1459–67. [DOI] [PubMed] [Google Scholar]
- 5.Schildkraut JM, Bastos E, Berchuck A. Relationship between lifetime ovulatory cycles and overexpression of mutant p53 in epithelial ovarian cancer. J Natl Cancer Inst 1997;89(13):932–8. [DOI] [PubMed] [Google Scholar]
- 6.Purdie DM, Bain CJ, Siskind V, Webb PM, Green AC. Ovulation and risk of epithelial ovarian cancer. Int J Cancer 2003;104(2):228–32. [DOI] [PubMed] [Google Scholar]
- 7.Terry KL, Titus-Ernstoff L, McKolanis JR, Welch WR, Finn OJ, Cramer DW. Incessant ovulation, mucin 1 immunity, and risk for ovarian cancer. Cancer Epidemiol Biomarkers Prev 2007;16(1):30–5. [DOI] [PubMed] [Google Scholar]
- 8.Tung KH, Goodman MT, Wu AH, McDuffie K, Wilkens LR, Kolonel LN, et al. Reproductive factors and epithelial ovarian cancer risk by histologic type: a multiethnic case-control study. Am J Epidemiol 2003;158(7):629–38. [DOI] [PubMed] [Google Scholar]
- 9.Moorman PG, Schildkraut JM, Calingaert B, Halabi S, Vine MF, Berchuck A. Ovulation and ovarian cancer: a comparison of two methods for calculating lifetime ovulatory cycles (United States). Cancer Causes Control 2002;13(9):807–11. [DOI] [PubMed] [Google Scholar]
- 10.Webb PM, Green A, Cummings MC, Purdie DM, Walsh MD, Chenevix-Trench G. Relationship between number of ovulatory cycles and accumulation of mutant p53 in epithelial ovarian cancer. J Natl Cancer Inst 1998;90(22):1729–34. [DOI] [PubMed] [Google Scholar]
- 11.Michels KA, Pfeiffer RM, Brinton LA, Trabert B. Modification of the Associations Between Duration of Oral Contraceptive Use and Ovarian, Endometrial, Breast, and Colorectal Cancers. JAMA Oncol 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Michels KA, Brinton LA, Pfeiffer RM, Trabert B. Oral Contraceptive Use and Risks of Cancer in the NIH-AARP Diet and Health Study. Am J Epidemiol 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Titus-Ernstoff L, Perez K, Cramer DW, Harlow BL, Baron JA, Greenberg ER. Menstrual and reproductive factors in relation to ovarian cancer risk. Br J Cancer 2001;84(5):714–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gwinn ML, Lee NC, Rhodes PH, Layde PM, Rubin GL. Pregnancy, breast feeding, and oral contraceptives and the risk of epithelial ovarian cancer. J Clin Epidemiol 1990;43(6):559–68. [DOI] [PubMed] [Google Scholar]
- 15.Luan NN, Wu QJ, Gong TT, Vogtmann E, Wang YL, Lin B. Breastfeeding and ovarian cancer risk: a meta-analysis of epidemiologic studies. Am J Clin Nutr 2013;98(4):1020–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fleming JS, Beaugie CR, Haviv I, Chenevix-Trench G, Tan OL. Incessant ovulation, inflammation and epithelial ovarian carcinogenesis: revisiting old hypotheses. Mol Cell Endocrinol 2006;247(1–2):4–21. [DOI] [PubMed] [Google Scholar]
- 17.Cramer DW, Welch WR. Determinants of ovarian cancer risk. II. Inferences regarding pathogenesis. J Natl Cancer Inst 1983;71(4):717–21. [PubMed] [Google Scholar]
- 18.Risch HA. Hormonal etiology of epithelial ovarian cancer, with a hypothesis concerning the role of androgens and progesterone. J Natl Cancer Inst 1998;90(23):1774–86. [DOI] [PubMed] [Google Scholar]
- 19.Wentzensen N, Poole EM, Trabert B, White E, Arslan AA, Patel AV, et al. Ovarian Cancer Risk Factors by Histologic Subtype: An Analysis From the Ovarian Cancer Cohort Consortium. J Clin Oncol 2016;34(24):2888–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fortner RT, Poole EM, Wentzensen NA, Trabert B, White E, Arslan AA, et al. Ovarian cancer risk factors by tumor aggressiveness: an analysis from the Ovarian Cancer Cohort Consortium. Int J Cancer 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Soslow RA. Histologic Subtypes of Ovarian Carcinoma. International Journal of Gynecological Pathology 2008;27:161–174. [DOI] [PubMed] [Google Scholar]
- 22.Vang R, Shih I-M, Kurman RJ. Ovarian Low-grade and High-grade Serous Carcinoma: Pathogenesis, Clinicopathologic and Molecular Biologic Features, and Diagnostic Problems. Advances in Anatomic Pathology 2009;16:267–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lunn M, McNeil D. Applying Cox regression to competing risks. Biometrics 1995;51(2):524–32. [PubMed] [Google Scholar]
- 24.Gates MA, Rosner BA, Hecht JL, Tworoger SS. Risk factors for epithelial ovarian cancer by histologic subtype. Am J Epidemiol 2010;171(1):45–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pfeiffer RM, Park Y, Kreimer AR, Lacey JV Jr., Pee D, Greenlee RT, et al. Risk prediction for breast, endometrial, and ovarian cancer in white women aged 50 y or older: derivation and validation from population-based cohort studies. PLoS Med 2013;10(7):e1001492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Casagrande JT, Louie EW, Pike MC, Roy S, Ross RK, Henderson BE. “Incessant ovulation” and ovarian cancer. Lancet 1979;2(8135):170–3. [DOI] [PubMed] [Google Scholar]
- 27.La Vecchia C, Franceschi S, Gallus G, Decarli A, Liberati A, Tognoni G. Incessant ovulation and ovarian cancer: a critical approach. Int J Epidemiol 1983;12(2):161–4. [DOI] [PubMed] [Google Scholar]
- 28.Siskind V, Green A, Bain C, Purdie D. Beyond ovulation: oral contraceptives and epithelial ovarian cancer. Epidemiology 2000;11(2):106–10. [DOI] [PubMed] [Google Scholar]
- 29.Odukogbe AA, Adebamowo CA, Adeniji AO, Omigbodun AO, Olayemi O, Oladokun A, et al. Total ovulating period: any contribution to ovarian carcinogenesis? Afr J Med Med Sci 2005;34(3):307–9. [PubMed] [Google Scholar]
- 30.Pelucchi C, Galeone C, Talamini R, Bosetti C, Montella M, Negri E, et al. Lifetime ovulatory cycles and ovarian cancer risk in 2 Italian case-control studies. Am J Obstet Gynecol 2007;196(1):83 e1–7. [DOI] [PubMed] [Google Scholar]
- 31.Schildkraut JM, Moorman PG, Bland AE, Halabi S, Calingaert B, Whitaker R, et al. Cyclin E overexpression in epithelial ovarian cancer characterizes an etiologic subgroup. Cancer Epidemiol Biomarkers Prev 2008;17(3):585–93. [DOI] [PubMed] [Google Scholar]
- 32.Peres LC, Moorman PG, Alberg AJ, Bandera EV, Barnholtz-Sloan J, Bondy M, et al. Lifetime number of ovulatory cycles and epithelial ovarian cancer risk in African American women. Cancer Causes Control 2017;28(5):405–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yang HP, Murphy KR, Pfeiffer RM, George N, Garcia-Closas M, Lissowska J, et al. Lifetime Number of Ovulatory Cycles and Risks of Ovarian and Endometrial Cancer Among Postmenopausal Women. Am J Epidemiol 2016;183(9):800–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Richards JS, Russell DL, Ochsner S, Espey LL. Ovulation: new dimensions and new regulators of the inflammatory-like response. Annu Rev Physiol 2002;64:69–92. [DOI] [PubMed] [Google Scholar]
- 35.Ose J, Poole EM, Schock H, Lehtinen M, Arslan AA, Zeleniuch-Jacquotte A, et al. Androgens Are Differentially Associated with Ovarian Cancer Subtypes in the Ovarian Cancer Cohort Consortium. Cancer Res 2017;77(14):3951–3960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Trabert B, Brinton LA, Anderson GL, Pfeiffer RM, Falk RT, Strickler HD, et al. Circulating Estrogens and Postmenopausal Ovarian Cancer Risk in the Women’s Health Initiative Observational Study. Cancer Epidemiol Biomarkers Prev 2016;25(4):648–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Trabert B, Michels KA, Anderson GL, Brinton LA, Falk RT, Geczik AM, et al. Circulating androgens and postmenopausal ovarian cancer risk in the Women’s Health Initiative Observational Study. Int J Cancer 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


