Abstract
Personality traits such as Neuroticism and Conscientiousness are associated with Alzheimer disease (AD) pathophysiology in cognitively normal (CN) and impaired individuals, and may represent potential risk or resilience factors, respectively. This study examined the cross-sectional relationship between personality traits and regional tau deposition using positron emission tomography (PET) in cognitively normal older adults. A cohort of CN (Clinical Dementia Rating (CDR) 0, n=128) older adults completed the NEO Five-Factor Inventory to assess traits of Neuroticism, Extroversion, Openness, Agreeableness, and Conscientiousness and underwent tau-PET and β-amyloid (Aβ)-PET imaging. We utilized linear regression models, adjusting for age, sex, geriatric depression score, and Aβ to evaluate the association between each of the personality traits and regional tau-PET accumulation. Elevated Neuroticism scores were associated with higher tau-PET accumulation in the amygdala (p=.002), entorhinal cortex (p=.012), and inferior temporal cortex (p=.016), as well as with a composite tau-PET measure (p=.002). In contrast, Extroversion, Openness, Agreeableness, and Conscientiousness were not associated with tau deposition in any of these regions (p’s >0.160). Our results indicate that increased Neuroticism is associated with higher tau pathophysiology in regions known to be vulnerable to AD pathophysiology in CN participants. High Neuroticism scores may therefore serve as a potential risk factor for tau accumulation. Alternatively, personality can change with the onset of AD, thus increased tau levels may affect Neuroticism scores. While future longitudinal studies are needed to determine directionality, our findings suggest early associations between Neuroticism and tau accumulation in CN adults.
Keywords: Personality, Neuroticism, Alzheimer disease, Tau, Neurodegeneration
INTRODUCTION
Personality represents a stable organization of character and temperament of a person. It can be quantified for study using personality inventories such as the Neuroticism, Extroversion, and Openness to experience Five-Factor Inventory (NEO; Costa & McCrae, 1985). The NEO provides estimates of Neuroticism, Extraversion, Conscientiousness, Agreeableness, and Openness. Prior work has demonstrated ties between personality, measured by the NEO, and physical activity (Rhodes & Smith, 2006), longevity (Friedman et al., 1993), obesity (Brummett et al., 2006), depression (Chioqueta & Stiles, 2005), and Alzheimer disease (Duchek, Balota, Storandt, & Larsen, 2007; Tautvydaite, Antonietti, Henry, von Gunten, & Popp, 2017). Thus, it appears that certain personality traits are associated with both diverse health habits and health outcomes.
With respect to AD, previous work has noted personality changes in cognitively impaired individuals (Dawson, Welsh-Bohmer, & Siegler, 2000; Petry, Cummings, Hill, & Shapira, 1988) and that these changes may precede the clinical diagnosis of dementia (Balsis, Carpenter, & Storandt, 2005). Work by Duchek and colleagues (2007) has indicated that self-reported neurotic traits are higher in individuals with very mild AD dementia compared with cognitively normal (CN) adults. Similarly, in this study, they also found lower self-reported Openness to experience scores in individuals in the very mild and mild AD dementia cohorts compared to CN controls.
Rather than personality traits being altered with the onset of impairment, a resilient personality profile, including high conscientiousness, may be associated with a lower risk of developing symptomatic dementia. Conversely, a risk-related personality profile, including higher neuroticism, may be associated with a greater likelihood of developing symptomatic dementia. Specifically, Wilson and colleagues (2007) found that individuals with a high Conscientiousness score had an 89% reduction in their risk for AD. Another study, utilizing a meta-analytic approach (Terracciano et al., 2014), reported higher Neuroticism and lower Conscientiousness associated with a threefold increased risk of AD.
Qualities of anxiety, depression, impulsiveness, and vulnerability to stress are often used to describe Neuroticism (John & Srivastava, 1999). These qualities may confer risk for psychological illness and AD dementia, suggesting a possible common mechanism linking stress, neuroticism and AD pathology. However, there remains a dearth of studies investigating the role of personality traits as risk and resilience factors for AD within CN cohorts.
Neuroimaging techniques have been implemented in prior studies to investigate the relationship between personality traits and AD-related neuropathology. A prior study (Jackson, Balota, & Head, 2011) has shown that there are associations between Neuroticism and Conscientiousness and cortical volumes in CN individuals, such that higher Neuroticism and lower Conscientiousness scores corresponded to reductions in cortical volumes. Additionally, recent work (Tautvydaite, Antonietti, et al., 2017; Tautvydaite, Kukreja, et al., 2017) has evaluated the relationship between personality and in vivo cerebrospinal fluid levels of amyloid-beta (Aβ) and tau in a heterogeneous sample of older adults with and without dementia. Tautvydaite and colleagues (2017) found CSF AD biomarkers of phosphorylated tau and Aβ are associated with increases in Neuroticism and decreases in Conscientiousness, further bolstering the link between personality traits and AD.
Prior work using in vivo imaging has also demonstrated that tau, rather than Aβ, is more strongly tied to measures of cognition and symptomatic AD (Brier et al., 2016). Importantly, pathologic tau begins to accumulate in individuals before any clinical symptoms can be detected (Aschenbrenner, Gordon, Benzinger, Morris, & Hassenstab, 2018; Schultz et al., 2018). The introduction of tau-PET ligands that bind to neurofibrillary tangles (NFT)(Johnson et al., 2016) provide a new in vivo biomarker of tau pathology. The association between personality and PET measures of tau pathophysiology in cognitively normal (CN) individuals remains unexamined. Therefore, this study examined the cross-sectional relationship between personality traits, focusing on Neuroticism and Conscientiousness, and regional tau-PET deposition, as well as the stability of the personality traits over time in CN older adults.
METHODS
MATERIALS AND METHODS
Participants
Data from 128 participants from local studies at the Knight Alzheimer Disease Research Center, Washington University in St Louis (including the Adult Children Study and the Healthy Aging and Senile Dementia Study) were used. Inclusion criteria included: having undergone Aβ- and tau-PET scans, completion of a personality assessment, and Geriatric Depression Scale (GDS), and normal cognition (defined by having a Clinical Dementia Rating (CDR) (Morris, 1997) score of 0 at the visit closest to the personality assessment. The Washington University in St. Louis Institutional Review Board approved all procedures and each participant provided signed informed consent for the study.
Personality Measure
The NEO Five Factor Inventory Test (NEO-FFI) was administered to each participant (P. T. Costa & McCrae, 1992b). This 60-item questionnaire measures the personality traits within five factors: Neuroticism, Extraversion, Openness, Agreeableness and Conscientiousness. Every item is rated on a 1–5 scale from ‘strongly agree’ to ‘strongly disagree’. The NEO-FFI is a commonly used personality trait questionnaire, which is highly correlated with the full-length Revised NEO Personality Inventory and has strong internal consistency (P. T. Costa & McCrae, 1992b). The descriptive statistics for the NEO-FFI factors are presented in Table 1. The mean scores on the NEO-FFI for the present sample are consistent with mean scores obtained from a similar adult sample (Duchek et al., 2007). Based on the available literature, we focus on Neuroticism and Conscientiousness as the personality factors of primary interest; Extraversion, Openness, and Agreeableness were also included for completeness. Additionally, a subset of our cohort had two or more total NEO-FFI assessments (N=113), collected prior to the tau-PET imaging, which were used to determine the stability of the personality measures.
Table 1.
Participant characteristics
| Characteristic | Value |
|---|---|
| Cross-sectional cohort (N=128) | |
| Age, Years | 66.7 (8.1) |
| Female, % | 60.9 |
| APOE4 positive, % | 32.5 |
| Mini Mental State Exam§^ | 29.4 (0.9) |
| Geriatric Depression Scale* | 0.98 (1.3) |
| Summary tau-PET SUVR, | 1.12 (0.2) |
| Summary beta-amyloid-PET SUVR | 1.15 (0.5) |
| beta-amyloid- positive, % (n) | 21.0 (27) |
| NEO Factors, raw score (SD), T score | |
| Neuroticism† | 13.6 (7.5), 42.9 |
| Extroversion† | 30.2 (6.3), 54.3 |
| Agreeableness† | 35.5 (5.2), 55.4 |
| Conscientiousness† | 35.3 (6.1), 51.2 |
| Openness† | 29.3 (5.9), 53.9 |
| Longitudinal Subset (N=113) | |
| Age, Years | 67.6 (7.7) |
| Female, % | 61.9 |
| APOE4 positive, % | 31.9 |
| Mini Mental State Exam§ | 29.4 (0.9) |
| Geriatric Depression Scale* | 0.89 (1.1) |
| Summary tau-PET SUVR | 1.19 (0.2) |
| Summary beta-amyloid-PET SUVR | 1.18 (0.6) |
| NEO Factors, raw score (SD), T score | |
| Neuroticism† | 13.6 (7.4), 42.9 |
| Extroversion† | 30.3 (6.4), 54.5 |
| Agreeableness† | 35.5 (5.1), 55.4 |
| Conscientiousness† | 35.3 (6.1), 51.2 |
| Openness† | 29.1 (6.1), 53.9 |
| Number of NEO assessments, N (%), 2/3/4/5/6/7 | 40 (35)/ 28 (25)/ 25 (22)/ 18 (16)/ 1 (1)/ 1 (1) |
| Average time between NEO assessments, Years | 3.67 (2.2) |
Characteristics reported for cross-sectional sample are from study visit closest in time to the tau-PET imaging date. Characteristics reported for the subset with longitudinal data are from the baseline study visit. Mean (SD) is presented unless otherwise noted.
Raw scores are on scale of 0–60
Scored on a scale of 0–30
Scored on a scale of 0–10
Note that from 2 participants APOE4 genotype was not available.
MRI
Imaging data were acquired on a Siemens Biograph mMR (n=125) or Trio 3T scanner (n=3). T1-weighted images were acquired using a magnetization prepared rapid acquisition gradient echo (MPRAGE) sequence with repetition time = 2300 ms, echo time = 2.95 ms, flip angle = 9°, 176 slices, in plane resolution 240 × 256, and slice thickness = 1.2 mm acquired in sagittal orientation. Images underwent volumetric segmentation using FreeSurfer 5.3 (http://freesurfer.net) (Fischl et al., 2004) and Desikan Killany atlas to identify regions of interest (ROIs) used in the PET analyses.
PET imaging
Tau-PET
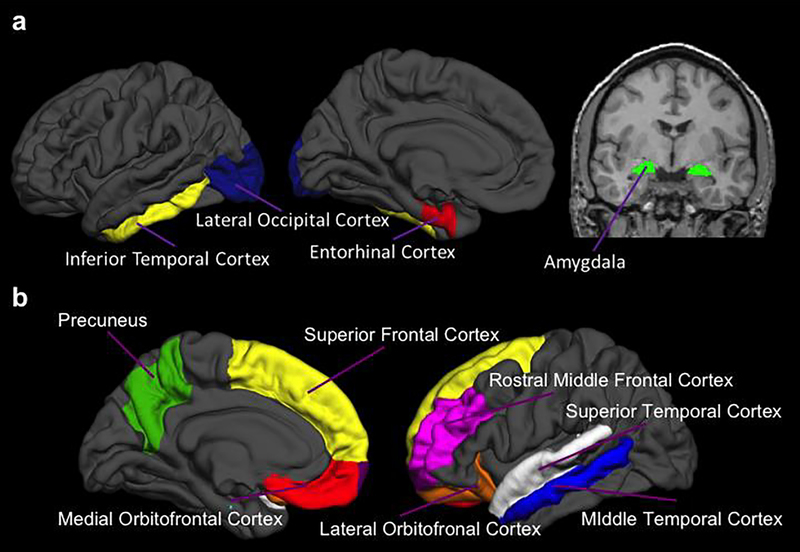
Tau-PET imaging was performed using 18F-AV-1451 (flortaucipir), a radioligand that preferentially binds to neurofibrillary tau. Scans were acquired on a PET/CT scanner. Data were processed using a Freesurfer ROI approach as done in prior work using flortaucipir (M. R. Brier et al., 2016; Gordon et al., 2016; Schultz et al., 2018; Wang et al., 2016). In each ROI, data from the 80–100 minute post-injection window were converted to standardized uptake value ratio (SUVRs) using the cerebellar grey matter as the reference region and partial volume corrected using a regional spread function approach (Su et al., 2015). We focused on SUVR averaged from FreeSurfer ROIs including the bilateral entorhinal cortex, amygdala, inferior temporal cortex, and lateral occipital regions (Figure 1a). These bilateral regions have previously been identified as the best in discriminating CN from AD participants (Mishra et al., 2017). For our main analyses, a summary composite measure was created using the average of these four regions. The average time between NEO-FFI assessment and tau-PET imaging was a mean (SD) of 3.44 (3.31) months.
Fig. 1. Regions of interest.
Selected regions of interest included in the composite tau-PET measure (a), and Aβ-PET measure (b).
Aβ-PET
Participants underwent Aβ-PET imaging with 18F-AV-45 (florbetapir). Scans were acquired on a PET/MR scanner and attenuation corrected with corresponding PET/CT. FreeSurfer ROI data between the 50–70 minute post-injection window were converted to SUVRs using the cerebellar grey matter as the reference region and partial volume corrected using a regional spread function approach (Su et al., 2013). As previously described, a summary Aβ deposition measure which represents regions with earliest Aβ burden was created using the average across the left and right ROIs from FreeSurfer areas corresponding to the lateral orbitofrontal, medial orbitofrontal, rostral middle frontal, superior frontal, superior temporal, middle temporal, and precuneus regions bilaterally (Figure 1b) (Su et al., 2016; Su et al., 2013). The summary Aβ deposition measure was treated as a continuous measure. For descriptive purposes only we split our sample based on a partial volume corrected florbetapir SUVR cutoff of 1.22 (Schultz et al., 2018). The average time between NEO-FFI assessment and Aβ-PET imaging was a mean (SD) of 4.12 (5.91) months.
Statistical Analyses
Association between tau-PET and personality
First, to determine whether there were global associations between tau-PET and each of the personality traits (Neuroticism, Extraversion, Openness, Agreeableness and Conscientiousness), we fitted a linear regression model for the composite tau-PET measure with each of the five personality factors as the independent variable of interest. Additionally, all models included covariates for age, sex, and GDS. We included GDS as a covariate in order to ensure that participants with high Neuroticism did not have increases in tau deposition due to depressive symptoms.
Next, to determine where there were region-specific effects of personality on NFT burden, we repeated our analyses for each of the four individual regions that comprise the composite tau-PET measure, including the entorhinal cortex, amygdala, inferior temporal cortex, and lateral occipital region, with each of the five personality factors as the independent variable of interest.
Association between Aβ-PET and personality
Since the relationship between Aβ and personality in AD as well as Aβ load and tau levels in AD have been reported in previous studies (Schultz et al., 2018; Tautvydaite, Antonietti, et al., 2017) we also evaluated the relationship between Aβ and personality. We fitted a linear regression model for the composite Aβ-PET SUVR, with the independent variable of interest being each of the five personality factors, after adjusting for age, sex, and GDS. For analyses that showed an association between Aβ-PET SUVRs and personality factors, we repeated the original linear regression models evaluating the relationship between tau-PET and that personality factor, including Aβ-PET SUVRs as a covariate to ensure that participants with high Neuroticism and low Conscientiousness did not have more tau deposition due to greater Aβ deposition.
Stability of NEO factors
Although we are unable to evaluate the longitudinal relationship between tau-PET SUVRs and personality traits in our current cohort, and therefore have limited ability to infer causation, we were able to investigate the stability of the NEO-FFI assessments in our CN study sample. To determine the stability of the personality measures in a subset of our cohort who had two or more total NEO-FFI assessments (N=113) collected prior to flortaucipir imaging, we ran a linear mixed effects model for each of the five personality factors to determine if the factors significantly changed over time. Fixed effect terms included the time from the first NEO-FFI assessment (in years) and a random slope and intercept terms for each participant. We additionally examined the intraclass correlation coefficient (ICC) for each factor for the first two NEO-FFI assessments available.
The demographics for the longitudinal cohort are reported in Table 1. The average number of NEO-FFI assessments was 2.23 visits and the time interval between first and last NEO-FFI assessment span between 2.74 and 13.53 years.
Only findings with values of p ≤ 0.05 (2-tailed) were considered significant. Cross-sectional analyses and longitudinal analyses were conducted with lme4 package in R.
RESULTS
Participant characteristics
Table 1 details the relevant characteristics of the participants. The average age of the cross-sectional sample was 67.5 ± 8.7 years and 29.7% were APOE4 positive.
Association between composite tau-PET measure and personality
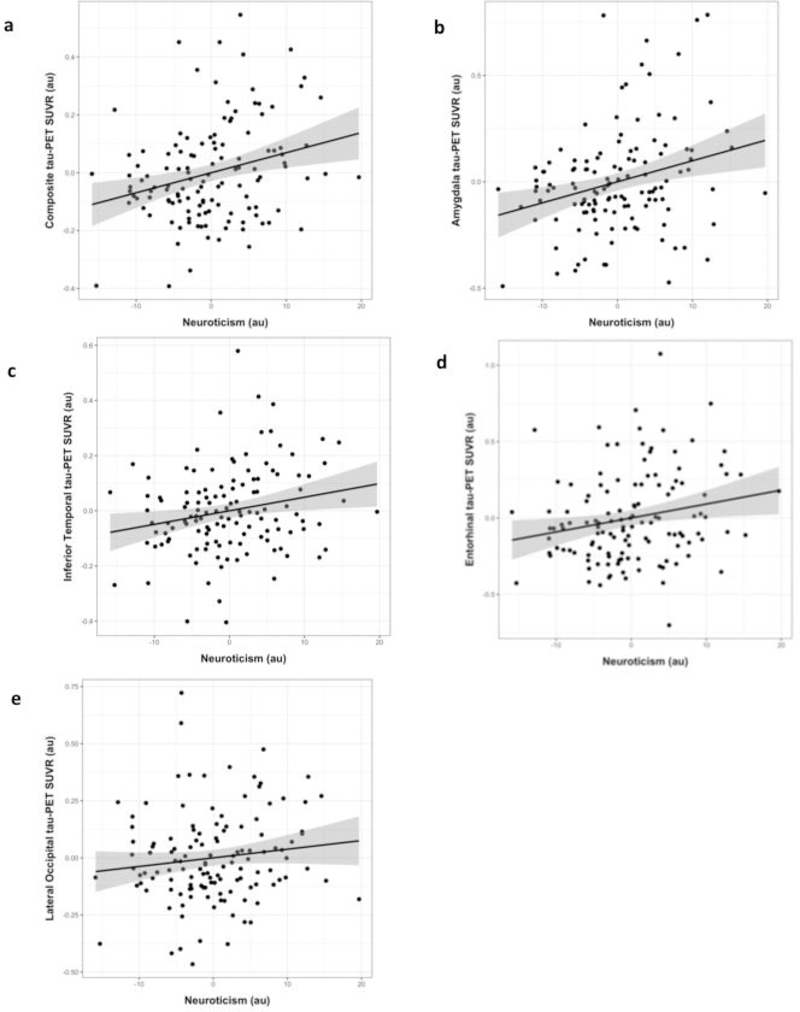
There was a significant positive association between Neuroticism and our composite measure of flortaucipir SUVR (B=0.007, p=0.002)(Figure 2). There were no associations between Extroversion, Openness, Agreeableness, or Conscientiousness and our composite flortaucipir SUVR measure (p’s≥0.298)(Table 2).
Fig. 2. Association between Neuroticism and regional tau-PET.
Covariate-adjusted residuals from linear regression models examining the relationship between Neuroticism and tau-PET SUVR in the composite (a), amygdala (b), inferior temporal cortex (c), entorhinal cortex (d), and lateral occipital cortex (e). PET= Positron emission tomography; SUVR= Standardized uptake value ratio.
Table 2.
Association between tau-PET and personality traits
| NEO Factor | Composite Tau | Amygdala Tau | Entorhinal Tau | Inferior Temporal Tau | Lateral Occipital Tau | Composite Aβ | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| B (SE) | p | B (SE) | p | B (SE) | p | B (SE) | p | B (SE) | p | B (SE) | p | |
| Neuroticism | .007 (.002) | 0.002 | .010 (.003) | 0.002 | .009 (.004) | 0.012 | .005 (.002) | 0.016 | .004 (.003) | 0.154 | .014 (.007) | 0.038 |
| Extroversion | −.002 (.003) | 0.417 | −.005 (.004) | 0.218 | .002 (.004) | 0.693 | −.003 (.002) | 0.160 | −.002 (.003) | 0.422 | −.002 (.002) | 0.491 |
| Openness | −.0004 (.003) | 0.894 | .002 (.004) | 0.572 | −.002 (.005) | 0.704 | −.001 (.002) | 0.559 | −.0004 (.003) | 0.891 | .002 (.002) | 0.512 |
| Agreeableness | −.002 (.003) | 0.484 | −.005 (.005) | 0.296 | −.001 (.005) | 0.844 | −.002 (.003) | 0.475 | −.001 (.004) | 0.759 | .001 (.003) | 0.612 |
| Conscientiousness | −.003 (.003) | 0.298 | −.004 (.004) | 0.327 | −.003 (.005) | 0.236 | −.001 (.002) | 0.785 | −.001 (.003) | 0.654 | −.002 (.002) | 0.308 |
Association between each of the five personality traits and composite or regional tau-PET SUVR and composite Aβ-PET SUVR levels. Unstandardized regression coefficients (B) and p-values correspond to multivariate models, adjusted for age, sex, and geriatric depression scale score. PET= Positron emission tomography; SUVR= Standardized uptake value ratio.
Association between tau-PET ROIs and personality
There were significant positive associations between Neuroticism and flortaucipir SUVR in the entorhinal cortex (B=0.009, p=0.012), inferior temporal lobe (B=0.005, p=0.016), and amygdala (B=0.010, p=0.002), but not in the lateral occipital cortex (B= 0.004, p=0.154) (Figure 2). There were no associations between regional tau-PET SUVRS and Openness (p’s≥0.572), Agreeableness (p’s≥0.296), Extraversion (p’s≥0.160), or Conscientiousness (p’s≥0.236) factors (Table 2).
Association between composite Aβ-PET measure and personality
As expected, there was a strong relationship between tau-PET and Aβ-PET SUVRs, after adjusting for age and sex, in our sample (B[SE]=0.156[.027], p=6.73e-08). Additionally, there was a positive association between Neuroticism scores and the composite Aβ-PET SUVRs (B[SE]=0.014 [.007], p=0.038). There was no relationship between Extroversion, Openness, Agreeableness, or Conscientiousness and Aβ-PET SUVRs (p’s≥0.189, Table 2).
Next, to determine whether Aβ deposition was driving the relationship between tau-PET SUVRs and Neuroticism scores, we repeated our analyses, with Aβ-PET SUVRs included in the models as a covariate. After adjusting for Aβ-PET SUVRs, results remained essentially the same for our composite measure of flortaucipir SUVR (B=0.005, p=0.018) and flortaucipir amygdala SUVRs (B=0.007, p=0.014), but were non-significant for the entorhinal cortex (B=0.006, p=0.104), and inferior temporal cortex (B=0.004, p=0.064).
Stability of NEO factors
In a subset of our sample (N=113) with longitudinal NEO-FFI assessments, Neuroticism and Openness scores showed a decrease over time (B=−0.140, p=0.025 and B=−0.140, p=0.003, respectively), while Agreeableness (B=0.054, p=0.245), Extroversion (B=−0.004, p=0.927), and Conscientiousness (B=0.013, p=0.749) scores remained stable. These results are presented in Table 3 and Supplementary Figure 1. ICC for Neuroticism, Openness, Agreeableness, Extroversion, and Conscientiousness scores were 0.832, 0.886, 0.780, 0.887, and 0.883, respectively.
Table 3.
Stability of NEO factors
| NEO Factor | B (SE) | p |
|---|---|---|
| Neuroticism | −0.140 (.061) | .025 |
| Conscientiousness | 0.013 (.039) | .749 |
| Openness | −0.140 (.037) | <.001 |
| Extroversion | −0.004 (.046) | .927 |
| Agreeableness | 0.054 (.046) | .245 |
Change in personality traits over time. Unstandardized regression coefficients B values and p-values from linear mixed effects model. Fixed effect terms included the time from the first NEO assessment (in years), and random slope and intercept terms, for each participant.
DISCUSSION
This study showed that, in a cohort of CN older adults, Neuroticism is associated with increased tau and Aβ levels, detected by tau- and Aβ-PET. Specifically, individuals with higher Neuroticism scores had higher flortaucipir SUVR in regions including the amygdala, entorhinal cortex, and inferior temporal cortex, as well as a composite measure of tauopathy. Similarly, higher Neuroticism scores were associated with higher Aβ deposition. These relationships were selective, as there were no associations between Agreeableness, Openness, Extroversion, or Conscientiousness, with tau-PET or Aβ-PET SUVRs. Importantly, the relationship between Neuroticism and tau was not driven by depressive symptoms.
Furthermore, when we re-examined the relationship between neuroticism and tau-PET SUVRs, accounting for Aβ levels, results remained the same for select regions, including the amygdala, with a trend towards significant for the inferior temporal cortex tau-PET SUVRs. However, after adjusting for Aβ levels, there was no association between Neuroticism and entorhinal tau-PET. This could suggest that Aβ may be moderating the effect of tau on personality traits in some regions, but not others. Larger cohort studies with sufficient ranges of Aβ levels are needed to test the interaction between personality traits and Aβ on flortaucipir PET SUVRs.
Initial studies from our group and others report relationships between personality traits and cognitive functioning in older individuals with (Duchek et al., 2007) and without (Jackson et al., 2011) dementia. For example, Duchek and colleagues (2007) examined the differences in personality traits in the earliest stages of AD and found that informant ratings of Neuroticism, Extraversion, Openness, Agreeableness, and Conscientiousness were able to discriminate CN controls from individuals with very mild AD. Furthermore, informant ratings of Neuroticism and Conscientiousness discriminated these groups as well as was associated with a composite episodic memory measure. These results suggest that personality, including Neuroticism, may serve as an early marker for onset of AD. Interestingly, our sample had relatively low scores for Neuroticism, albeit within a normal range, suggesting the associations could be even more pronounced if the cohort had higher levels of Neuroticism.
Additionally, Jackson and colleagues (2011) have examined the relationship between personality traits and common pathophysiological features of cognitive dysfunction and dementia, including grey and white matter volumes. They found that higher Neuroticism was associated with smaller regional volumes and greater decreases in volume with advancing age, and conversely, higher Conscientiousness was associated with larger regional volumes and less decline in volumes with aging. While these results and others (Zufferey et al., 2017) point to a potential detrimental effect of Neuroticism on age-related volume loss in CN individuals a major limitation of these past studies is that they did not screen for presence of AD-related pathology including Aβ or tau, which are known to accumulate in the brain decades proceeding any clinically meaningful cognitive deficits in healthy control individuals.
Animal models have shown that prolonged stress, a trait of individuals with high neuroticism, has negative effects on neural integrity, particularly in medial temporal lobe regions such as the hippocampus and amygdala. Specifically, chronic stress has been shown to lead to dendritic atrophy, synaptic loss, and suppression of neurogenesis in the hippocampus (Radley & Morrison, 2005) and alterations of limbic connectivity (Poeggel et al., 2003). In human studies, results suggest that stress and anxiety may confer risk for psychological illness and dementia through interaction with the hypothalamus-pituitary-adrenal (HPA)-axis. The HPA axis is required for stress adaptations and mechanisms involved in regulating function of the HPA axis and sensitivity to stress include the amygdala and medial temporal regions such as hippocampus and entorhinal cortex (Herman et al., 2016). Similar to animal studies mentioned above these limbic and medial temporal regions have been shown to be vulnerable to negative effects of abnormal HPA axis functioning in humans (Valli et al., 2016).
The results of our study highlight region-specific associations of higher Neuroticism with higher in vivo tau-PET SUVRs bilaterally in the amygdala, entorhinal cortex and inferior temporal cortex, which are HPA axis-associated brain regions. Since these regions are known to be the most sensitive to early accumulation of tau, it may be that chronic stress, expressed by a neurotic personality, creates region-specific vulnerability for future tauopathy. However, intricate molecular and behavioral animal and human translational studies are needed to explore this mechanism in further detail. Furthermore, PET tracer limitations, including non-specific off-target binding, make it difficult to disentangle the associations between Neuroticism and tau within subcortical structure of HPA-associated areas including hypothalamus and pituitary regions in vivo.
A limitation of this study is the cross-sectional nature of the imaging data, therefore we cannot determine causality. Prior work has shown that personality measures are stable over time (P. T. Costa & McCrae, 1992a, 1992b), but this may not be true in the case of pathological aging. However, a recent study in the Baltimore Longitudinal Study of Aging cohort (A. Terracciano, An, Sutin, Thambisetty, & Resnick, 2017) analyzed longitudinal personality trait data from over 2,000 individuals and examined whether the trajectory of personality traits was dependent on conversion to MCI or dementia from baseline. They found that MCI and AD individuals had higher mean levels of Neuroticism and lower Conscientiousness, compared to non-impaired older adults. Importantly, personality traits remained stable even in those who converted to MCI or dementia; there were no differences in trajectory of personality traits in individuals who converted to MCI or dementia, compared to non-impaired older adults. These data suggest that longitudinal rates of change in personality traits do not occur as a function of underlying proteinopathy, and that change in personality may not be a useful tool in identifying individuals at risk for conversion. Instead, these data, and results from the current study suggest cross-sectional assessment of personality traits may provide a behavioral risk factor for individuals who may be at increased risk for developing dementia.
Personality traits, including Neuroticism, have been reported to decrease with advancing age in some (Donnellan & Lucas, 2008; A. Terracciano, McCrae, Brant, & Costa, 2005), but not all (P. J. Costa & RR, 1988) prior studies. We do not yet have enough longitudinal tau-PET data to directly compare change in tau pathophysiology to change in personality factors in our cohort. Therefore, we are unable to infer causality of the cross-sectional results we report. However, we do have longitudinal NEO-FFI assessments, collected prior to the tau-PET data, and examined whether the personality traits changed over time. Using a linear mixed effects model to leverage all our longitudinal data, we found Neuroticism and Openness decreased over time, while Extroversion, Agreeableness, and Conscientiousness remained stable. In contrast, when comparing longitudinal assessments over the first two visits with an ICC, all of the NEO-FFI appeared relatively stable (0.780–0.887). The difference in results obtained from the linear mixed effects model and the ICC may reflect that the linear mixed effects model took into account all longitudinal visits (2 to 7 visits; between 2.74 and 13.53 years of follow-up) while the ICC utilized data from the first two visits (average of 3.67 years between first two visits). Larger samples and samples with concurrent longitudinal tau measures are needed to better understand the implications of decreasing neuroticism on tau pathophysiology in CN adults.
Overall, the present study provides evidence for a link between Neuroticism and tau deposition in CN individuals, independent of depressive symptoms or Aβ burden. While others have reported associations between Neuroticism and cognitive decline and dementia, our findings suggest that Neuroticism is associated with early pathophysiologic features of neurodegenerative diseases, including AD. While we cannot draw strong conclusions regarding causation in this study, our findings, in the context of the literature, suggest that Neuroticism is related to tau burden in the absence of cognitive impairment. This work makes a critical contribution to the efforts to determine the risk and resilience of AD-related pathophysiology that certain personality traits confer.
Supplementary Material
Supplementary Fig. 1. Neuroticism and Openness decrease over time. Spaghetti plot showing longitudinal Neuroticism (a), Conscientiousness (b), Openness (c), Extroversion (d), and Agreeableness (e) scores over time.
Acknowledgements
The authors acknowledge the financial support of Fred Simmons and Olga Mohan, the Charles F. and Joanne Knight Alzheimer’s Research Initiative, the Hope Center for Neurological Disorders, the Mallinckrodt Institute of Radiology, the American Society for Neuroradiology, and the Barnes-Jewish Hospital Foundation (BJHF), the Paula and Rodger Riney Fund, the BJHF Willman Scholar Fund, and the Daniel J Brennan MD Fund. This research was additionally funded by BrightFocus Foundation grants A2017272S and A2017330S; Alzheimer’s Association Research Grant AARG -17-532945; Arizona Alzheimer’s Research Consortium; National Science Foundation grant DGE-1745038; and National Institutes of Health grants P50AG005681, P01AG026276, P01AG003991, R01AG055444, R01AG031581, UL1TR000448, R01EB009352, 1P30NS098577, and K01AG053474-01A1. Avid Radiopharmaceuticals (a wholly owned subsidiary of Eli Lilly) provided doses of 18Fflorbetapir, partial funding for 18F-florbetapir scanning, precursor for 18F-flortaucipir, and technology transfer for manufacturing of 18F-flortaucipir. The authors thank their participants, without whom this study would not have been possible.
Funding:
The authors acknowledge the financial support of Fred Simmons and Olga Mohan, the Charles F. and Joanne Knight Alzheimer’s Research Initiative, the Hope Center for Neurological Disorders, the Mallinckrodt Institute of Radiology, the American Society for Neuroradiology, and the Barnes-Jewish Hospital Foundation (BJHF), the BJHF Paula and Rodger O. Riney Fund, the BJHF Willman Scholar Fund, and the Daniel J Brennan Fund. This research was additionally funded by BrightFocus Foundation grants A2017272S and A2017330S; Alzheimer’s Association Research Grant AARG -17-532945; Arizona Alzheimer’s Research Consortium; National Science Foundation grant DGE-1745038; and National Institutes of Health grants P50AG005681, P01AG026276, P01AG003991, R01AG055444, R01AG031581, UL1TR000448, R01EB009352, 1P30NS098577, and K01AG053474-01A1. Avid Radiopharmaceuticals (a wholly owned subsidiary of Eli Lilly) provided doses of 18F-florbetapir, partial funding for 18F-florbetapir scanning, precursor for 18F-flortaucipir, and technology transfer for manufacturing of 18F-flortaucipir.
Footnotes
Conflicts of interest:
John C. Morris, Tammie L.S. Benzinger, and Brian A. Gordon report participation in clinical trials sponsored by Eli Lilly, Roche, and Biogen. Avid Radiopharmaceuticals (a wholly owned subsidiary of Eli Lilly provided doses of 18F-florbetapir, partial funding for 18F-florbetapir scanning, precursor for 18F-flortaucipir and technology transfer for manufacturing of 18F-flortaucipir). None of the authors, nor their family members, own stock or have equity interest (outside of mutual funds or other externally directed accounts) in any pharmaceutical or biotechnology company.
Data Availability:
Data that support the findings of this study are available from the Knight ADRC at https://knightadrc.wustl.edu/Research/ResourceRequest.htm.
Publisher's Disclaimer: This Author Accepted Manuscript is a PDF file of a an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
REFERENCES
- Aschenbrenner AJ, Gordon BA, Benzinger TLS, Morris JC, & Hassenstab JJ (2018). Influence of tau PET, amyloid PET, and hippocampal volume on cognition in Alzheimer disease. Neurology, 91(9), e859–e866. doi: 10.1212/WNL.0000000000006075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balsis S, Carpenter BD, & Storandt M (2005). Personality Change Precedes Clinical Diagnosis of Dementia of the Alzheimer Type. The Journals of Gerontology: Series B, 60(2), P98–P101. doi: 10.1093/geronb/60.2.P98 [DOI] [PubMed] [Google Scholar]
- Barnes DE, & Yaffe K (2011). The projected effect of risk factor reduction on Alzheimer’s disease prevalence. Lancet Neurol, 10(9), 819–828. doi: 10.1016/S1474-4422(11)70072-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boots EA, Schultz SA, Oh JM, Larson J, Edwards D, Cook D, … Okonkwo OC (2015). Cardiorespiratory fitness is associated with brain structure, cognition, and mood in a middle-aged cohort at risk for Alzheimer’s disease. Brain Imaging Behav, 9(3), 639–649. doi: 10.1007/s11682-014-9325-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brier MR, Gordon B, Friedrichsen K, McCarthy J, Stern A, Christensen J, … Ances BM (2016). Tau and Abeta imaging, CSF measures, and cognition in Alzheimer’s disease. Sci Transl Med, 8(338), 338ra366. doi: 10.1126/scitranslmed.aaf2362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brier MR, Gordon B, Friedrichsen K, McCarthy J, Stern A, Christensen J, … Ances BM (2016). Tau and Aβ imaging, CSF measures, and cognition in Alzheimer’s disease. Science translational medicine, 8(338), 338ra366–338ra366. doi: 10.1126/scitranslmed.aaf2362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brummett BH, Babyak MA, Williams RB, Barefoot JC, Costa PT, & Siegler IC (2006). NEO personality domains and gender predict levels and trends in body mass index over 14 years during midlife. Journal of Research in Personality, 40(3), 222–236. doi: 10.1016/i.irp.2004.12.002 [DOI] [Google Scholar]
- Chioqueta AP, & Stiles TC (2005). Personality traits and the development of depression, hopelessness, and suicide ideation. Personality and Individual Differences, 38(6), 1283–1291. doi: 10.1016/j.paid.2004.08.010 [DOI] [Google Scholar]
- Costa PJ, & RR M (1988). Personality in adulthood: a six-year longitudinal study of self-reports and spouse ratings on the NEO Personality Inventory. J Pers Soc Psychol., 54(5), 853–863. [DOI] [PubMed] [Google Scholar]
- Costa PT, & McCrae RR (1992a). Four ways five factors are basic. Personality and Individual Differences, 13(6), 653–665. doi: 10.1016/0191-8869(92)90236-I [DOI] [Google Scholar]
- Costa PT, & McCrae RR (1992b). NEO PI-R professional manual. Odessa, FL: Psychological Assessment Resources. [Google Scholar]
- Dawson DV, Welsh-Bohmer KA, & Siegler IC (2000). Premorbid personality predicts level of rated personality change in patients with Alzheimer disease. Alzheimer Dis Assoc Disord, 14(1), 11–19. [DOI] [PubMed] [Google Scholar]
- Donnellan MB, & Lucas RE (2008). Age differences in the Big Five across the life span: evidence from two national samples. Psychol Aging, 23(3), 558–566. doi: 10.1037/a0012897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duchek JM, Balota DA, Storandt M, & Larsen R (2007). The power of personality in discriminating between healthy aging and early-stage Alzheimer’s disease. J Gerontol B Psychol Sci Soc Sci, 62(6), P353–361. [DOI] [PubMed] [Google Scholar]
- Fischl B, van der Kouwe A, Destrieux C, Halgren E, Segonne F, Salat DH, … Dale AM (2004). Automatically parcellating the human cerebral cortex. Cereb Cortex, 14(1), 11–22. [DOI] [PubMed] [Google Scholar]
- Friedman HS, Tucker JS, Tomlinson-Keasey C, Schwartz JE, Wingard DL, & Criqui MH (1993). Does childhood personality predict longevity? Journal of Personality and Social Psychology, 65(1), 176–185. doi: 10.1037/0022-3514.65.1.176 [DOI] [PubMed] [Google Scholar]
- Gordon BA, Friedrichsen K, Brier M, Blazey T, Su Y, Christensen J, … Benzinger TL (2016). The relationship between cerebrospinal fluid markers of Alzheimer pathology and positron emission tomography tau imaging. Brain, 139(Pt 8), 2249–2260. doi: 10.1093/brain/aww139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herman JP, McKlveen JM, Ghosal S, Kopp B, Wulsin A, Makinson R, … Myers B (2016). Regulation of the hypothalamic-pituitary-adrenocortical stress response. Comprehensive Physiology, 6(2), 603–621. doi: 10.1002/cphy.c150015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson J, Balota DA, & Head D (2011). Exploring the relationship between personality and regional brain volume in healthy aging. Neurobiol Aging, 32(12), 2162–2171. doi: 10.1016/j.neurobiolaging.2009.12.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- John OP, & Srivastava S (1999). The Big Five Trait taxonomy: History, measurement, and theoretical perspectives In Handbook of personality: Theory and research, 2nd ed. (pp. 102–138). New York, NY, US: Guilford Press. [Google Scholar]
- Johnson KA, Schultz A, Betensky RA, Becker JA, Sepulcre J, Rentz D, … Sperling R (2016). Tau positron emission tomographic imaging in aging and early Alzheimer disease. Ann Neurol, 79(1), 110–119. doi: 10.1002/ana.24546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishra S, Gordon BA, Su Y, Christensen J, Friedrichsen K, Jackson K, … Benzinger TLS (2017). AV-1451 PET imaging of tau pathology in preclinical Alzheimer disease: Defining a summary measure. Neuroimage. doi: 10.1016/j.neuroimage.2017.07.050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris JC (1997). Clinical dementia rating: a reliable and valid diagnostic and staging measure for dementia of the Alzheimer type. Int Psychogeriatr, 9 Suppl 1, 173–176; discussion 177–178. [DOI] [PubMed] [Google Scholar]
- Petry S, Cummings JL, Hill M, & Shapira J (1988). Personality alterations in dementia of the alzheimer type. Archives of Neurology, 45(11), 1187–1190. doi: 10.1001/archneur.1988.00520350025009 [DOI] [PubMed] [Google Scholar]
- Poeggel G, Helmeke C, Abraham A, Schwabe T, Friedrich P, & Braun K (2003). Juvenile emotional experience alters synaptic composition in the rodent cortex, hippocampus, and lateral amygdala. Proc Natl Acad Sci U S A, 100(26), 16137–16142. doi: 10.1073/pnas.2434663100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radley JJ, & Morrison JH (2005). Repeated stress and structural plasticity in the brain. Ageing Res Rev, 4(2), 271–287. doi: 10.1016/j.arr.2005.03.004 [DOI] [PubMed] [Google Scholar]
- Rhodes RE, & Smith NE (2006). Personality correlates of physical activity: a review and meta-analysis. Br J Sports Med, 40(12), 958–965. doi: 10.1136/bjsm.2006.028860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz SA, Boots EA, Almeida RP, Oh JM, Einerson J, Korcarz CE, … Okonkwo OC (2015). Cardiorespiratory Fitness Attenuates the Influence of Amyloid on Cognition. J Int Neuropsychol Soc, 21(10), 841–850. doi: 10.1017/S1355617715000843 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz SA, Gordon BA, Mishra S, Su Y, Perrin RJ, Cairns NJ, … Benzinger TLS (2018). Widespread distribution of tauopathy in preclinical Alzheimer’s disease. Neurobiol Aging, 72, 177–185. doi: 10.1016/j.neurobiolaging.2018.08.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz SA, Larson J, Oh J, Koscik R, Dowling MN, Gallagher CL, … Okonkwo OC (2015). Participation in cognitively-stimulating activities is associated with brain structure and cognitive function in preclinical Alzheimer’s disease. Brain Imaging Behav, 9(4), 729–736. doi: 10.1007/s11682-014-9329-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su Y, Blazey TM, Owen CJ, Christensen JJ, Friedrichsen K, Joseph-Mathurin N, … Dominantly Inherited Alzheimer, N. (2016). Quantitative Amyloid Imaging in Autosomal Dominant Alzheimer’s Disease: Results from the DIAN Study Group. PLoS One, 11(3), e0152082. doi: 10.1371/journal.pone.0152082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su Y, Blazey TM, Snyder AZ, Raichle ME, Marcus DS, Ances BM, … Dominantly Inherited Alzheimer, N. (2015). Partial volume correction in quantitative amyloid imaging. Neuroimage, 107, 55–64. doi: 10.1016/j.neuroimage.2014.11.058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su Y, D’Angelo GM, Vlassenko AG, Zhou G, Snyder AZ, Marcus DS, … Benzinger TL (2013). Quantitative analysis of PiB-PET with FreeSurfer ROIs. PLoS One, 8(11), e73377. doi: 10.1371/journal.pone.0073377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tautvydaite D, Antonietti JP, Henry H, von Gunten A, & Popp J (2017). Relations between personality changes and cerebrospinal fluid biomarkers of Alzheimer’s disease pathology. J Psychiatr Res, 90, 12–20. doi: 10.1016/j.jpsychires.2016.12.024 [DOI] [PubMed] [Google Scholar]
- Tautvydaite D, Kukreja D, Antonietti JP, Henry H, von Gunten A, & Popp J (2017). Interaction between personality traits and cerebrospinal fluid biomarkers of Alzheimer’s disease pathology modulates cognitive performance. Alzheimers Res Ther, 9(1), 6. doi: 10.1186/s13195-017-0235-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terracciano A, An Y, Sutin AR, Thambisetty M, & Resnick SM (2017). Personality Change in the Preclinical Phase of Alzheimer Disease. JAMA Psychiatry, 74(12), 1259–1265. doi: 10.1001/jamapsychiatry.2017.2816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terracciano A, McCrae RR, Brant LJ, & Costa PT (2005). Hierarchical linear modeling analyses of the NEO-PI-R scales in the Baltimore Longitudinal Study of Aging. Psychol Aging, 20(3), 493–506. doi: 10.1037/0882-7974.20.3.493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terracciano A, Sutin AR, An Y, O’Brien RJ, Ferrucci L, Zonderman AB, & Resnick SM (2014). Personality and risk of Alzheimer’s disease: New data and meta-analysis. Alzheimer’s & Dementia, 10(2), 179–186. doi: 10.1016/i.ialz.2013.03.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valli I, Crossley NA, Day F, Stone J, Tognin S, Mondelli V, … McGuire P (2016). HPA-axis function and grey matter volume reductions: imaging the diathesis-stress model in individuals at ultra-high risk of psychosis. Translational Psychiatry, 6, e797. doi:10.1038/tp.2016.6810.1038/tp.2016.68https://www.nature.com/articles/tp201668#supplementary-informationhttps://www.nature.com/articles/tp201668#supplementary-information [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Benzinger TL, Su Y, Christensen J, Friedrichsen K, Aldea P, … Ances BM (2016). Evaluation of Tau Imaging in Staging Alzheimer Disease and Revealing Interactions Between beta-Amyloid and Tauopathy. JAMA Neurol, 73(9), 1070–1077. doi: 10.1001/jamaneurol.2016.2078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson RS, Schneider JA, Arnold SE, Bienias JL, & Bennett DA (2007). Conscientiousness and the incidence of alzheimer disease and mild cognitive impairment. Archives of General Psychiatry, 64(10), 1204–1212. doi: 10.1001/archpsyc.64.10.1204 [DOI] [PubMed] [Google Scholar]
- Zufferey V, Donati A, Popp J, Meuli R, Rossier J, Frackowiak R, … Kherif F (2017). Neuroticism, depression, and anxiety traits exacerbate the state of cognitive impairment and hippocampal vulnerability to Alzheimer’s disease. Alzheimers Dement (Amst), 7, 107–114. doi: 10.1016/j.dadm.2017.05.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Fig. 1. Neuroticism and Openness decrease over time. Spaghetti plot showing longitudinal Neuroticism (a), Conscientiousness (b), Openness (c), Extroversion (d), and Agreeableness (e) scores over time.